Abstract
We genotyped Borrelia burgdorferi strains detected in larvae of Ixodes scapularis removed from songbirds and compared them with those found in host-seeking I. scapularis nymphs sampled throughout the eastern United States. Birds are capable of transmitting most known genotypes, albeit at different frequencies than expected based on genotypes found among host-seeking nymphs.
Borrelia burgdorferi, the etiologic agent of Lyme disease (5), is a vector-borne spirochete transmitted by certain species of Ixodes (Acari: Ixodidae) ticks. In eastern North America, B. burgdorferi is maintained in a sylvatic cycle consisting of vertebrate reservoir hosts and immature stages of the black-legged tick, Ixodes scapularis: larval ticks acquire spirochetes while feeding on infected hosts, maintain infection through molt, and infect naïve hosts as nymphs. The genotypic diversity of B. burgdorferi has been characterized in North America (4, 12), and this variation has been linked with differential pathogenicity in mice (19) and humans (20). Mammals, notably the white-footed mouse (Peromyscus leucopus) (17), are the primary reservoirs for B. burgdorferi and are capable of transmitting most genotypic variants (3, 10). Songbirds are commonly parasitized by immature I. scapularis ticks, and field studies have shown that most North American bird species are capable of infecting I. scapularis larvae with B. burgdorferi during feeding (1). Laboratory studies have demonstrated high reservoir competence for some bird species (16) but not others (14), and some species show apparent variation in competence (8, 14), potentially owing to pathogen strain- or genotype-specific differences in immune response. Although a wide variety of songbirds are capable of transmitting B. burgdorferi to ticks (1), few data exist showing genotype diversity of B. burgdorferi strains detected in bird-derived I. scapularis larvae. To determine the ability of birds to support and transmit diverse B. burgdorferi lineages, we compared the B. burgdorferi genotype frequency profile detected in bird-derived I. scapularis larvae to the genotype frequencies in a large sample of host-seeking I. scapularis nymphs collected throughout the eastern United States.
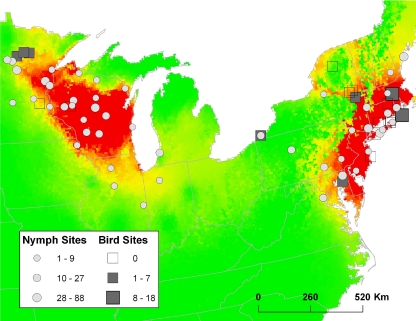
In collaboration with bird biologists in 12 eastern states, we collected ticks from songbirds at 20 sites in 2007 and 2008 (Fig. 1). Birds were captured in mist nets and released immediately following data and tick collection. We extracted DNA from all ticks using Qiagen (Qiagen, Inc., Valencia, CA) DNeasy blood and tissue kits using the recommended protocols but substituting Roche proteinase K (Roche Applied Science, Indianapolis, IN) for Qiagen proteinase K. We used nested PCR to amplify a portion of the B. burgdorferi 16S-23S rRNA intergenic spacer (IGS) region (4) and bidirectionally sequenced the resulting amplicons. The B. burgdorferi amplicons were compared to a BLAST library containing reference genotypes (18) obtained from host-seeking I. scapularis nymphs collected throughout the eastern United States from 2004 to 2006 (6, 7) (Fig. 1). Sequences that did not provide unambiguous chromatogram peaks for all nucleotides or did not match to previously identified genotypes at all loci were excluded from analysis. We compared the genotype frequencies in bird-derived larvae and host-seeking nymphs by Fisher's exact test in SAS, version 9.2 (SAS Systems, Cary, NC), with P values determined by Monte Carlo estimation. Because B. burgdorferi genotype frequencies differ among population foci (7), we performed analyses for midwestern and northeastern subsets of data in addition to global analyses.
FIG. 1.
Numbers of unambiguous IGS sequences recovered from bird-derived Ixodes scapularis larvae and host-seeking I. scapularis nymphs. Map shading represents areas of highest (red) and lowest (green) predicted risk of I. scapularis-borne disease (data from Diuk-Wasser et al. [6]). One bird sampling site (Pierre, SD), from which no B. burgdorferi-infected larvae were recovered, is not shown.
We detected B. burgdorferi in 103 out of 622 bird-derived I. scapularis larvae collected from 53 individual birds. All PCR products were sequenced, and 65 larvae, collected from 36 birds of 13 species, produced unambiguous chromatogram peaks at all nucleotides. Of these sequences, 63 matched to previously identified genotypes (Table 1), one differed from all previously identified sequence types at 3 of 812 loci, and another differed at 5 of 812 loci. We detected 15 previously described genotypes in bird-derived I. scapularis larvae and 25 different genotypes (Table 1) (1 genotype was found in a single sample and 1 was found in two samples) in host-seeking I. scapularis nymphs (18) from the same broad geographic region (Fig. 1). Global comparisons indicate significantly different genotype frequencies in host-seeking (n = 729) and bird-derived (n = 63) I. scapularis ticks (100,000 Monte Carlo samples, P < 0.0001). Similarly, within both the Northeast (48 bird ticks and 352 nymphs) and Midwest (15 bird ticks and 377 nymphs) sample groups, genotype frequencies differed between bird-derived and host-seeking ticks (P = 0.001 and P = 0.02, respectively).
TABLE 1.
Numbers of each B. burgdorferi IGS genotype detected in host-seeking I. scapularis nymphs and bird-derived I. scapularis larvae sampled in the northeastern and midwestern United States
| IGS genotypea | Detection of B. burgdorferi IGS genotype in: |
||||
|---|---|---|---|---|---|
| Host-seeking nymphs (n = 729) |
Bird-derived larvae (n = 63) |
||||
| Total no. (no. in Midwest, in Northeast) | Overall frequency estimate | Total no. (no. in Midwest, in Northeast) | Overall frequency estimate | 95% confidence intervalb | |
| 1 | 51 (8, 43) | 0.07 | 8 (0, 8) | 0.13 | 0.06-0.22 |
| 2 | 94 (14, 80) | 0.13 | 3 (0, 3) | 0.05 | 0.01-0.12 |
| 3 | 34 (0, 34) | 0.05 | 8 (0, 8) | 0.13 | 0.06-0.22 |
| 4 | 36 (2, 34) | 0.05 | 9 (0, 9) | 0.14 | 0.07-0.24 |
| 5 | 54 (38, 16) | 0.07 | 3 (0, 3) | 0.05 | 0.01-0.12 |
| 6 | 48 (39, 9) | 0.07 | 10 (6, 4) | 0.16 | 0.08-0.26 |
| 7 | 24 (12, 12) | 0.03 | 0 (0, 0) | 0.00 | |
| 8 | 39 (7, 32) | 0.05 | 0 (0, 0) | 0.00 | |
| 9 | 17 (4, 13) | 0.02 | 8 (2, 6) | 0.13 | 0.06-0.22 |
| 10 | 3 (3, 0) | 0.00 | 0 (0, 0) | 0.00 | |
| 11 | 11 (10, 1) | 0.02 | 1 (0, 1) | 0.02 | 0.01-0.07 |
| 12 | 82 (68, 14) | 0.11 | 3 (1, 2) | 0.05 | 0.01-0.12 |
| 13 | 2 (2, 0) | 0.00 | 0 (0, 0) | 0.00 | |
| 14 | 69 (66, 3) | 0.09 | 2 (1, 1) | 0.03 | 0.01-0.10 |
| 17 | 36 (20, 16) | 0.05 | 2 (1, 1) | 0.03 | 0.01-0.10 |
| 18 | 27 (27, 0) | 0.04 | 0 (0, 0) | 0.00 | |
| 20 | 15 (13, 2) | 0.02 | 0 (0, 0) | 0.00 | |
| 22 | 17 (17, 0) | 0.02 | 1 (1, 0) | 0.02 | 0.01-0.07 |
| 23 | 14 (13, 1) | 0.02 | 3 (3, 0) | 0.05 | 0.01-0.12 |
| 24 | 13 (1, 12) | 0.02 | 1 (0, 1) | 0.02 | 0.01-0.07 |
| 26 | 12 (0, 12) | 0.02 | 1 (0, 1) | 0.02 | 0.01-0.07 |
| 27 | 1 (0, 1) | 0.00 | 0 (0, 0) | 0.00 | |
| 28 | 18 (1, 17) | 0.03 | 0 (0, 0) | 0.00 | |
| 29 | 9 (9, 0) | 0.01 | 0 (0, 0) | 0.00 | |
| 30 | 3 (3, 0) | 0.00 | 0 (0, 0) | 0.00 | |
The IGS genotypes listed in this table are according to Travinsky et al. (18); GenBank sequence accession numbers may be found therein.
Binomial log-likelihood confidence intervals were calculated for each bird-derived genotype frequency estimate; ranges in boldface do not contain the frequency estimate for host-seeking nymphs.
Our results indicate that birds can become infected with and are capable of transmitting to I. scapularis larvae most of the B. burgdorferi genotypes that are regularly detected in host-seeking nymphs, although at different frequencies than would be expected based on host-seeking nymph samples. For example, genotypes 4, 6, and 9 were recovered from birds more often than expected by chance alone, and genotype 2 was detected in bird-derived larvae less frequently than would be expect by chance (Table 1). The differences in genotype frequencies among sample types could arise for several reasons. First, B. burgdorferi genotype frequencies in host-seeking ticks vary over space (7) and time (15). Although our avian sampling sites were generally spatially congruent with the nymphal collection sites (Fig. 1), stochastic sampling effects or interannual variation could influence the detected frequency of different genotypes in bird-derived larvae, as well as in host-seeking nymphs.
Second, differences in genotype frequencies in bird-derived larvae and host-seeking nymphs could result from host specialization of certain B. burgdorferi genotype groups to particular host taxa. Brisson and Dykhuizen (3) suggested that host specialization accounted for differences in genotype frequency among B. burgdorferi strains detected in larvae collected from diverse mammalian species and those present in host-seeking nymphs, which should represent the expected “background” genotypic diversity at a given site. However, Hanincova et al. (10) demonstrated that spatiotemporal scale influences observed genotype frequencies and concluded that caution is warranted in drawing conclusions about host specificity. One of the most commonly detected genotypes in birds (IGS 9) in our study was not encountered in a sample of 205 mammal-derived ticks (10), suggesting possible specialization of this genotype to avian hosts.
Wang et al. (19) and Hanincova et al. (11) demonstrated that there are apparent fitness differences among B. burgdorferi genotypes in a particular host type, and Wormser et al. (20) reported strain-specific variation in B. burgdorferi virulence for humans. Host specialization in North America may or may not be defined as incompatibility of certain genospecies with host taxa, as proposed for B. burgdorferi (sensu lato) transmission cycles in Europe (13). Although some genotypes are absent from or rare in certain taxa, mammals and birds are apparently capable of transmitting a range of B. burgdorferi genotypic variants.
How birds vary in their competency as reservoirs for B. burgdorferi strains and genotypes of different pathogenicity for humans may be important to public health. Allelic associations between IGS and ospC, a plasmid-borne gene thought to be a mammalian virulence factor (9), exist at smaller spatial scales, but it may not be prudent to infer ospC genotype from IGS genotype (18). However, none of the genotypes that are overrepresented in birds are classified as ribosomal spacer type I, the B. burgdorferi genotype group that tends to cause disseminating infections in humans (20). As birds likely play a critical role in the rate and direction of B. burgdorferi dispersal (1, 2), understanding the relationships between host and pathogen will help to predict spatiotemporal shifts in genotype frequencies and human risk of Lyme disease.
Acknowledgments
This research was funded in part by NIH awards R03 AI-076856-01, U54 AI-065359, and R21 AI-065848, by the Yale Institute for Biospheric Studies Faculty Support Endowment, by USDA-ARS Cooperative Agreement 58-0790-5-068, and by the G. Harold and Leila Y. Mathers Charitable Foundation. Additional support was provided through NIH fellowship 5T32 AI-07404.
We thank Bridgit Travinsky for assistance with molecular analysis and a large number of biologists who sampled birds and collected ticks for us. J. Childs and D. Fish provided valuable comments on an earlier version of the manuscript. We also acknowledge D. Fish, P. Cislo, P. Krause, N. Lloyd, and L. Rollend for their valuable thoughts, insights, and expertise.
Footnotes
Published ahead of print on 22 October 2010.
REFERENCES
- 1.Brinkerhoff, R. J., C. M. Folsom-O'Keefe, K. Tsao, and M. A. Diuk-Wasser. 2009. Do birds affect Lyme disease risk? Range expansion of the vector-borne pathogen, Borrelia burgdorferi. Front. Ecol. Environ. doi: 10.1890/090062. [DOI]
- 2.Brinkerhoff, R. J., C. M. Folsom-O'Keefe, H. M. Streby, S. J. Bent, K. Tsao, D. Fish, and M. A. Diuk-Wasser. Regional variation in tick parasitism on migrating North American songbirds: implications for the spread of the Lyme pathogen, Borrelia burgdorferi. J. Med. Entomol., in press. [DOI] [PubMed]
- 3.Brisson, D., and D. E. Dykhuizen. 2004. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168:713-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunikis, J., U. Garpmo, J. Tsao, J. Berglund, D. Fish, and A. G. Barbour. 2004. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 150:1741-1755. [DOI] [PubMed] [Google Scholar]
- 5.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease—a tick-borne spirochetosis? Science 216:1317-1319. [DOI] [PubMed] [Google Scholar]
- 6.Diuk-Wasser, M. A., G. Vourc'h, P. Cislo, A. G. Hoen, F. Melton, S. A. Hamer, M. Rowland, G. J. Hickling, J. I. Tsao, A. G. Barbour, U. Kitron, J. Piesman, and D. Fish. 2010. Field and climate-based model for predicting the density of host-seeking nymphal Ixodes scapularis, an important vector of tick-borne disease agents in the eastern United States. Global Ecol. Biogeogr. 19:504-514. [Google Scholar]
- 7.Gatewood, A. G., K. A. Liebman, G. Vourc'h, J. Bunikis, S. A. Hamer, R. Cortinas, F. Melton, P. Cislo, U. Kitron, J. Tsao, A. G. Barbour, D. Fish, and M. A. Diuk-Wasser. 2009. Climate and tick seasonality predict Borrelia burgdorferi genotype distribution. Appl. Environ. Microbiol. 75:2476-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg, H. S., P. A. Buckley, M. G. Balmforth, E. Zhioua, S. Mitra, and F. G. Buckley. 2005. Reservoir competence of native North American birds for the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 42:445-449. [DOI] [PubMed] [Google Scholar]
- 9.Grimm, D. K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection in mammals. Proc. Nat. Acad. Sci. U. S. A. 101:3142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanincova, K., K. Kurtenbach, M. A. Diuk-Wasser, B. Brei, and D. Fish. 2006. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg. Infect. Dis. 12:604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanincova, K., N. H. Ogden, M. A. Diuk-Wasser, C. J. Pappas, D. Fish, I. Schwartz, and K. Kurtenbach. 2008. Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl. Environ. Microbiol. 74:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoen, A. G., G. Margos, S. J. Bent, M. A. Diuk-Wasser, A. Barbour, K. Kurtenbach, and D. Fish. 2009. Phylogeography of Borrelia burgdorferi in the eastern United States reflects multiple independent Lyme disease emergence events. Proc. Natl. Acad. Sci. U. S. A. 106:15013-15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurtenbach, K., S. De Michelis, S. Etti, S. M. Schafer, H.-S. Sewell, V. Brade, and P. Kraiczy. 2002. Host association of Borrelia burgdorferi sensu lato—the key role of host complement. Trends Microbiol. 10:74-79. [DOI] [PubMed] [Google Scholar]
- 14.Mather, T. N., S. R. Telford, A. B. Maclachlan, and A. Spielman. 1989. Incompetence of catbirds as reservoirs for the Lyme disease spirochete (Borrelia burgdorferi). J. Parasitol. 75:66-69. [PubMed] [Google Scholar]
- 15.Qiu, W. G., D. E. Dykhuizen, M. S. Acosta, and B. J. Luf. 2002. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics 160:833-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richter, D., A. Spielman, N. Komar, and F. R. Matuschka. 2000. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 6:133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmidt, K. A., and R. S. Ostfeld. 2001. Biodiversity and the dilution effect in disease ecology. Ecology 82:609-619. [Google Scholar]
- 18.Travinsky, B., J. Bunikis, and A. G. Barbour. 2010. Geographic differences in genetic locus linkages for Borrelia burgdorferi. Emerg. Infect. Dis. doi: 10.3201/eid1607.091452. [DOI] [PMC free article] [PubMed]
- 19.Wang, G., C. Ojami, H. Wu, V. Sakesenberg, R. Iyer, D. Liveris, S. A. McClain, G. P. Wormser, and I. Schwartz. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wormser, G. P., D. Brisson, D. Liveris, K. Hanincova, S. Sandigursky, J. Nowakowski, R. B. Nadelman, S. Ludin, and I. Schwartz. 2008. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J. Infect. Dis. 198:1358-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]