Abstract
Fungi in the basidiomycetous genus Amanita owe their high mammalian toxicity to the bicyclic octapeptide amatoxins such as α-amanitin. Amatoxins and the related phallotoxins (such as the heptapeptide phalloidin) are encoded by members of the “MSDIN” gene family and are synthesized on ribosomes as short (34- to 35-amino-acid) proproteins. Antiamanitin antibodies and confocal microscopy were used to determine the cellular and subcellular localizations of amanitin accumulation in basidiocarps (mushrooms) of the Eastern North American destroying angel (Amanita bisporigera). Consistent with previous studies, amanitin is present throughout the basidiocarp (stipe, pileus, lamellae, trama, and universal veil), but it is present in only a subset of cells within these tissues. Restriction of amanitin to certain cells is especially marked in the hymenium. Several lines of evidence implicate a specific prolyl oligopeptidase, A. bisporigera POPB (AbPOPB), in the initial processing of the amanitin and phallotoxin proproteins. The gene for AbPOPB is restricted taxonomically to the amatoxin-producing species of Amanita and is clustered in the genome with at least one expressed member of the MSDIN gene family. Immunologically, amanitin and AbPOPB show a high degree of colocalization, indicating that toxin biosynthesis and accumulation occur in the same cells and possibly in the same subcellular compartments.
More than 90% of all fatal mushroom poisonings are due to species of Amanita in section Phalloideae, such as the death cap (A. phalloides) and the destroying angel species complex (A. bisporigera, A. verna, A. virosa, and A. ocreata) (3). The toxic agents are a family of bicyclic octapeptides known as amatoxins, which survive cooking and the human digestive tract and are actively taken up by liver and other cells. The biochemical target of the amatoxins is RNA polymerase II, the major enzyme transcribing protein-coding mRNAs in eukaryotic cells (4). The 50% lethal dose (LD50) of α-amanitin for humans is ∼0.1 mg/kg, and one mushroom can contain a fatal dose of 10 to 12 mg (36). Irreversible liver failure and death may result at 3 to 7 days after ingestion.
The amatoxins (and the chemically related phallotoxins such as phallacidin and phalloidin) are synthesized on ribosomes and not, like all other known fungal cyclic peptides, by nonribosomal peptide synthetases (10, 33). Amatoxins are translated as 35-amino-acid proproteins and must undergo several posttranslational modifications, including proteolytic cleavage, cyclization, hydroxylation, and formation of a unique tryptophan-cysteine cross bridge called tryptathionine (19). The amatoxin and phallotoxin genes are members of a large family, called the “MSDIN” family, that are predicted to encode proproteins of 34 to 37 amino acids with conserved upstream and downstream sequences flanking a hypervariable region of 7 to 10 amino acids. The hypervariable region gives rise to the linear peptides corresponding to the mature toxins (18). Certain species of Amanita have thus evolved the genetic potential to synthesize a large variety of small, cyclic peptides using the same fundamental biochemical mechanism.
In the MSDIN family of genes, both the amino acid immediately preceding the toxin region and the last amino acid in the toxin region itself are invariant Pro residues (10). All known amatoxins and phallotoxins, as well as other cyclic peptides that have been identified in A. phalloides, contain at least one Pro (36). A peptidase that cleaves a synthetic phalloidin peptide at the carboxy side of both Pro residues to release the linear phalloidin heptapeptide was purified from the phalloidin-producing mushroom Conocybe apala (also called C. lactea or C. albipes) (18). This processing peptidase was identified as a member of the prolyl oligopeptidase (POP) family of proteases (6, 29). Due to limiting amounts of biological material (Amanita does not form fruiting bodies in culture) and the instability of the enzyme, it has not yet been possible to determine if a POP enzyme also processes the amanitins in species of Amanita.
Temporal and structural sequestration are commons feature of secondary metabolism in microorganisms and plants, serving to prevent these often biologically active compounds from interfering with primary metabolism and to ensure their presence at the ecologically most appropriate time and place (16). Mushrooms (here defined as the basidiocarps of macrofungi in the phylum Basidiomycota) produce a number of biologically active secondary metabolites, and some mechanism of separation from primary metabolism would seem to be essential for both the amatoxins and the phallotoxins, since their sites of action (RNA polymerase II and F-actin, respectively) are present in all eukaryotic cells.
Many secondary metabolites are known to be synthesized in specialized cells in other organisms, such as plant trichomes and cone snail secretory ducts (16, 34). However, little is known about the relationship between chemical and structural cellular specialization in relation to natural product production in macrofungi. Besides the amatoxins and phallotoxins, examples of biologically active molecules from mushrooms include psilocybin, lovastatin, muscarine, ibotenic acid, muscimol, strobilurins, pleuromutilin, and illudins (1, 27). Specialized mushroom structures known or suspected to contain toxic compounds include cystidia in genera such as Inocybe, Russula, and Strobilurus, which are poisonous to mammals or insects (13, 15, 17, 23). Conocybe apala, which makes phalloidin, has specialized secretory cells that are toxic to nematodes (12). Pleurotus cystidiosus makes specialized cells (toxocysts) consisting of a liquid droplet containing nematocidal compounds surrounded by an elastic adherent envelope (30). Differential cytological staining indicates that many mushrooms contain biochemically specialized hyphae, called secretory hyphae, that are intermingled with the structural hyphae (5). However, the relationship between structure and chemistry, i.e., whether the active compounds are actually located in the specialized cell types, has rarely been established for any mushroom.
In this study, we used a specific antiamanitin antibody to localize the sites of amanitin accumulation in relation to the cellular distribution of A. bisporigera POPB (AbPOPB), an enzyme implicated in the posttranslational processing of the amatoxin proproteins. The results indicate that amanitins are synthesized and stored in a subset of cells found throughout the mushrooms.
MATERIALS AND METHODS
Biological material.
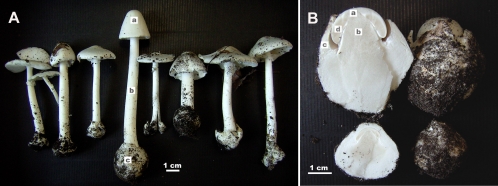
Mushrooms of the Eastern North American destroying angel (Amanita bisporigera) were collected in Ingham County, Michigan, in July and August of 2008 and 2009 (Fig. 1). Very young fruiting bodies (button stage, still enclosed in the universal veil, and not yet emerged above the ground) were also collected when possible (Fig. 1). Mushrooms were fixed in 4% paraformaldehyde within 2 to 4 h of collection. All the mushrooms used in this study were analyzed by high-pressure liquid chromatography (HPLC) to confirm the presence of amanitins (7, 11). Amanitin concentrations varied from mushroom to mushroom, and samples with higher concentrations were chosen for the immunohistochemical analyses.
Fig. 1.
(A) Mature basidiocarps of Amanita bisporigera used in this study (collected in Ingham County, MI, in August, 2009). (B) Immature basidiocarps collected before emergence from the ground. Intact basidiocarps are shown on the right and longitudinal sections through the same basidiocarps on the left. Structures: a, pileus (cap); b, stipe (stem); c, universal veil, giving rise to the volva at the base of the stem as indicated in panel A; d, lamella (gill).
Amatoxin- and phallotoxin-lacking species of Amanita (A. citrina and A. muscaria) were also collected in the same location as controls. The other Amanita species used as a source of DNA for blotting were previously described (10).
Immunological and microscopic methods.
To prepare paraffin sections, blocks of tissue (∼3-mm cubes) were fixed in 4% paraformaldehyde for 1 h at room temperature. Following graded dehydration in ethanol, tissue blocks were transferred to xylene and incubated for 10 min with stirring. This step was repeated, and tissue blocks were then embedded in paraffin by standard methods. Sectioning to 5- to 10-μm thickness was performed with a rotary microtome (Leica RM2155). Selected sections were mounted on slides and the paraffin removed by washing twice in xylene (5 min for each wash). Xylene was then removed by washing the slides in ethanol twice (5 min for each wash), after which a graded rehydration step was conducted in ethanol to phosphate-buffered saline (PBS). The slides were boiled for 20 min in a staining dish in the presence of 100 mM sodium citrate buffer, pH 6.0. After cooling at room temperature for 20 min, the slides were washed twice in PBS for 5 min.
Before hybridization, tissue sections on the slides were incubated with blocking buffer (3% dry milk in PBS, 0.1% Tween 20, and whole goat polyclonal IgG [catalog no. sc-6654; Santa Cruz Biotechnology, Santa Cruz, CA]) at a 1:30 dilution for 4 h at room temperature.
Primary antibodies were prepared in the same blocking buffer at a 1:30 dilution. For dual staining, the two primary antibodies were added at the same time. The slides plus primary antibody were incubated typically for 4 h in a humidified chamber. The AbPOPB antibody signal in mature mushrooms was consistently weaker than that in immature mushrooms. Therefore, tissue sections were incubated with anti-AbPOPB and antiamanitin antibodies for 15 h instead of the usual 4 h. The slides were then rinsed twice for 5 min each time in PBS and incubated with secondary antibody, diluted according to the manufacturer's instructions in blocking buffer, in a humidified chamber for 2 h. The slides were rinsed twice for 10 min each in PBS, mounted with 1 drop of Citifluor (Citifluor Ltd., Leicester, United Kingdom), and viewed with an Olympus FV1000D confocal laser scanning microscope.
The antiamanitin antibody was obtained from Heinz Faulstich (Heidelberg, Germany) (14). The antibody had been raised in rabbits and purified by affinity chromatography. It can detect 1.5 ng/ml α-amanitin in human serum and urine. It reacts with α- and γ-amanitin but not with β-amanitin or phallotoxins (14). Most specimens of A. bisporigera produce α-, β-, γ-, and ε-amanitin as well as several phallotoxins (21).
The antibodies against AbPOPA and AbPOPB were raised in mouse (Cocalico Biologicals, Reamstown, PA). Whole serum was used for immunoblotting and immunohistology. A monoclonal antibody against a synthetic actin C-terminal peptide was produced in mouse (A3853; Sigma-Aldrich). The secondary antibodies used were Alexa Fluor 488 goat anti-mouse IgG (A11029; Invitrogen) and Alexa Fluor 594 goat anti-rabbit IgG (A11037; Invitrogen).
The specificity of the antibody was tested by immunoblotting against inclusion bodies of Escherichia coli expressing AbPOPB and against total extracts of A. bisporigera. Total protein was obtained by grinding 0.5 g frozen basidiocarps of A. bisporigera in liquid nitrogen. To the resulting powder was added 2 ml of a stock solution of protease inhibitors. The stock solution was made by dissolving five tablets of Complete Mini EDTA-free protease inhibitor (Roche Applied Science, Mannheim, Germany) in 5 ml water and adding an additional 5 ml of protease inhibitor cocktail (P2714; Sigma) diluted with water according to the manufacturer's instructions. The homogenate was centrifuged at 14,000 × g in 1.5-ml microtubes for 15 min. The supernatant was fractionated by SDS-PAGE (premade gels of 7.5% acrylamide in Tris-HCl; Bio-Rad, Richmond, CA). The SDS-PAGE markers were Bio-Rad Precision Plus (Dual Color). In order to minimize proteolytic degradation of the POP proteins, the entire extraction procedure was performed in less than 30 min and all solutions were kept on ice or at 4°C. Proteins were transferred to nitrocellulose membranes (catalog no. 162-0115; Bio-Rad) and detected using ECL Western blotting substrate according to the manufacturer's instructions (catalog no. 32106; Pierce, Rockford, IL). Anti-POP antiserum was incubated with the membrane for 30 min. The membranes were subsequently exposed on Hyperfilm MP (Amersham/GE Healthcare, Buckinghamshire, United Kingdom) for 15 to 60 s.
Isolation and expression of prolyl oligopeptidase genes.
For cloning AbPOPA and AbPOPB, PCR primers corresponding to the N and C termini of both putative genes (forward primers, 5′-GAAACGAGAGGCGAAGTCAAGGTG-3′ and 5′-TCAAATGAAGTAGACGAATGGAC-3′; reverse primers, 5′-AAGTGGATGACGATTATGCGGCAG-3′ and 5′-CACACGGATGAGCAATGGATGAG-3′) were used in both combinations and the amplicons cloned into E. coli DH5α. These primers were based on the partial genomic sequence of A. bisporigera (10). The amplicons were cloned into E. coli DH5α and sequenced. The full sequences of the genes were obtained from lambda clones (see below). Full-length cDNA copies were obtained by 5′ and 3′ rapid amplification of cDNA ends (RACE) (18) using primers 5′-ATGTCTCGCCGAACTCGCCGCCTCCTC-3′ (5′-RACE) and 5′-GATTGGGTATTTGGCGCAGAAGTCACG-3′ (3′-RACE) for AbPOPA and primers 5′-GTGACGAACGATGAACATTGGAACTT-3′ and 5′-GAAGATGGTAAATATGTGGCCCTGTA-3′ for AbPOPB. Sequences generated from the RACE reactions were used to assemble full-length cDNAs of AbPOPA and AbPOPB.
AbPOPA and AbPOPB cDNAs were cloned into the pET21 vector between the BamHI and NotI sites. T-7-tagged versions of both genes were also generated. The expression constructs were transformed into E. coli strain BL21(DE3) (Novagen, EMD Chemicals, Inc., Darmstadt, Germany), and three positive clones for each construct were selected. Expression was induced in a 50-ml culture at an optical density at 600 nm (OD600) of 0.7 with 0.4 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) following the manufacturer's protocols (Novagen, 11th ed.). The cells were disrupted by freeze-thaw treatment and treated with 0.5 U/ml Benzonase nuclease.
Both expressed POP proteins were present in inclusion bodies and yielded no detectable activity with the chromogenic POP substrate Z-Gly-Pro-pNA (Sigma-Aldrich) at 1 mM (18). Instead, we purified the inclusion bodies of expressed AbPOPA and AbPOPB. The untagged POP proteins were then separated by SDS-PAGE, and gel slices containing the POP proteins were used for antibody production. Anti-AbPOPA antibody was raised in chicken and anti-AbPOPB antibody was raised in mouse by Cocalico Biologicals (Reamstown, PA), using their standard protocols.
Nucleic acid manipulations.
DNA for blotting was digested with PstI and electrophoresed in 0.7% agarose. Hybridizations were performed overnight at 65°C in 4× SET (20× SET is 3 M NaCl, 0.6 M Tris, and 0.04 M EDTA, pH 7.4), 0.1% sodium pyrophosphate, 0.2% SDS, 10% dextran sulfate, and 625 μg/ml heparin. Full-length cDNAs of AbPOPA and AbPOPB were labeled with [32P]dCTP to use as probes. To construct the genomic lambda library, genomic DNA of A. bisporigera was partially digested with BfucI and electrophoresed in a 0.5% agarose gel at 5 V/cm. DNA fragments of 12 to 20 kb were recovered with the QIAEX II gel extraction kit (catalog no. 20021; Qiagen, Valencia, CA). The fragments were cloned into the λBlueSTAR vector (Novagen). The resulting library was screened with AbPOPA and AbPOPB cDNA probes by standard methods (28). Positive clones were subcloned into E. coli strain BMC25.8 and sequenced.
Nucleotide sequence accession numbers.
Sequence data from this article can be found in the GenBank/EMBL database under the following accession numbers: HQ225840 for AbPOPA, HQ225841 for AbPOPB, and EU196153 (updated) for MSD-10 of A. bisporigera.
RESULTS
Immunolocalization of amanitin.
In order to investigate the types of tissue in which amanitin is distributed within a basidiocarp, samples from several representative tissue types, including pileus (cap), lamella (gill), stipe (stem), and universal veil, were examined (Fig. 1). Multiple basidiocarps of different developmental stages and various locations were examined.
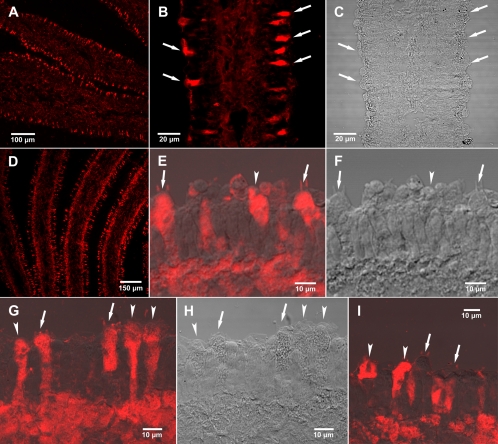
The most intense and specific antiamanitin signals were found in a subset of cells located in the hymenium, i.e., on the lamella edges where the basidia are formed (Fig. 2A to C). In some samples, there was a strong signal from the subhymenial layer in addition to the cells of the hymenium (Fig. 2D). Under higher magnification of a single lamella, the presence of amanitin in only certain hymenial cells and in the subhymenium was clear (Fig. 2E and G). Some of the hymenial cells containing amanitin were true basidia (arrows) because they have sterigmata (the slender projections on the basidia to which basidiospores are attached before they are released), whereas others might be either sterile cells or basidia at different stages of maturity (arrowheads) (Fig. 2E to I). Not all basidia contained amanitin, nor did all sterile cells. Figure 2I shows hymenial cells in which two basidia with sterigmata did not show the presence of amanitin, whereas several adjacent sterile cells did. The trama (inner part of the lamella) consistently showed weaker staining than the hymenium or the subhymenium (Fig. 2A, B, and D).
Fig. 2.
Amanitin immunostaining of lamellae (gills) of A. bisporigera observed by confocal laser scanning microscopy (CLSM). (A) Low-magnification CLSM view showing cross-section of two lamellae and part of a third. (B) Higher magnification of a single lamella by CLSM. (C) Same gill section as in panel B viewed by differential interference contrast (DIC). (D) A different basidiocarp, showing a cross-section of three lamellae and parts of two others by CLSM. (E) Antiamanitin immunostaining of basidia (arrows), indicated by the sterigmata (the two slender projections), sterile cells (arrowheads), and the subhymenium (CLSM superimposed on DIC). Sterigmata are the slender projections from the basidia that bear the basidiospores, which are no longer present in these sections. Sterile cells are the cells in the hymenium that, in Amanita, resemble basidia but do not bear spores. (F) Same section as in panel E viewed with DIC alone. (G) Antiamanitin immunostaining of hymenium and subhymenium showing basidia (arrows) with sterigmata and sterile cells containing amanitin (CLSM superimposed on DIC). (H) Same section as in panel G observed by DIC. (I) Basidia (arrows) showing absence of amanitin and adjacent sterile cells (arrowheads) showing presence of amanitin (CLSM superimposed on DIC).
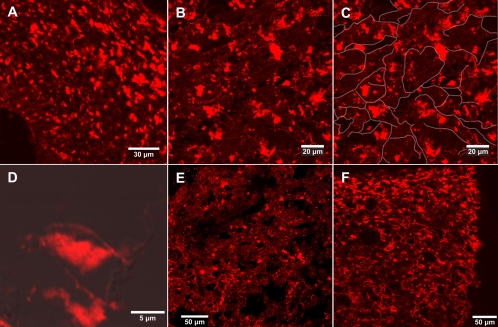
The pileus (cap) of A. bisporigera also gave strong signals with the antiamanitin antibody. Figure 3A shows a cross-section of the pileus of a young specimen of A. bisporigera, and Fig. 3B shows a mature pileus. The individual areas of signal were irregular in shape, and their locations did not correspond to cellular boundaries (Fig. 3B and C). The areas giving an amanitin signal were consistently smaller than the cells. In Fig. 3C, the cellular boundaries of the section in Fig. 3B have been outlined in white in order to better illustrate this point. The apparent subcellular localization of amanitin was also visible at higher magnification, where the outlines of individual cells could be seen (Fig. 3D). A similar irregular and apparent subcellular pattern of amanitin distribution was seen in the stipe (Fig. 3E) and in the universal veil (Fig. 3F).
Fig. 3.
Amanitin immunostaining of different tissues of A. bisporigera by LSM. (A) Section of the pileus of the immature basidiocarp shown in Fig. 1B. (B) Section of a mature pileus. (C) Same view as in panel B with white lines manually drawn to highlight outlines and boundaries of the cells. (D) Higher magnification of an individual hypha of a mature pileus (CLSM superimposed on DIC). (E) Section of stipe (stem) of a mature basidiocarp. (F) Section of a universal veil (volva) of a mature basidiocarp (Fig. 1A).
Mushrooms of the amanitin-nonproducing species A. citrina and A. muscaria were fixed, sectioned, and treated identically with the antiamanitin antibody. No signals were seen in any sections (data not shown).
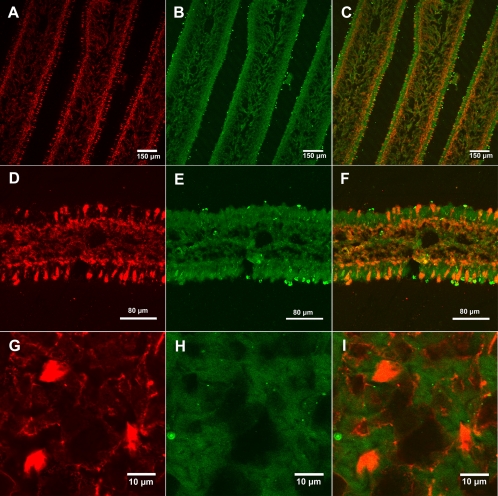
In order to improve visualization of background cells not containing amanitin, a universal antiactin antibody was used as a general indicator of all cells. Figure 4A to C show immunostaining of lamellae with antiamanitin antibody, antiactin antibody, and a merge of the two. Figure 4D to F show higher magnification of a single lamella. Amanitin was present in the trama, the subhymenium, and a subset of cells in the hymenium (Fig. 4C and F). Compared to the amanitin distribution, the antiactin antibody produced an even staining throughout the lamellae, as expected for the distribution of a housekeeping protein, with a somewhat stronger signal in the hymenium (Fig. 4B and E).
Fig. 4.
Dual immunostaining of A. bisporigera lamellae using antiamanitin and antiactin antibodies. (A to C) Lamella cross-sections, showing amanitin staining, actin staining, and the merge of the two, respectively. (D to F) Higher-magnification views of a cross-section of a lamella (same staining order as for panels A to C). (G to I) Higher-magnification views of a cross-section of a mature pileus.
Identification of a candidate amatoxin-processing peptidase in A. bisporigera.
It is of particular interest to understand the relationship between the sites of amanitin accumulation and amanitin biosynthesis. To date, no genes involved in amatoxin biosynthesis except those encoding the toxins themselves have been identified (10, 18) in A. bisporigera. A prolyl oligopeptidase (POP) from the phalloidin-producing mushroom Conocybe apala cleaves a synthetic phalloidin proprotein to release the linear heptapeptide corresponding to mature phalloidin, consistent with this enzyme being responsible for the initial cleavage of the toxin propeptides (18). Using the C. apala POP protein (GenBank accession no. ACQ65797) and the human POP protein (BAA86936) as queries, we identified partial sequences for two POP genes, AbPOPA and AbPOPB, in the genome survey sequence of A. bisporigera. Based on an analysis of a partial genome sequence of Galerina marginata, which makes amatoxins (but not phallotoxins), this mushroom also has two POP genes (reference 22 and our unpublished results). In contrast, all other basidiomycetes whose full genomes were available in GenBank or at the DOE Joint Genome Institute as of May 2010 have a single POP gene (10). Highly similar orthologs of AbPOPA and AbPOPB are present in plants, bacteria, and animals. No evidence was found for any POP genes in ascomycetes, except perhaps weakly similar orthologs in Nectria haematococca (EEU39197) and Phaeosphaeria nodorum (XP_001801532).
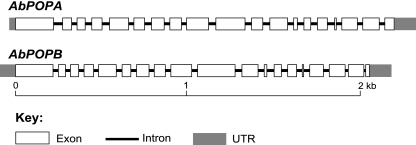
The two predicted POP genes of A. bisporigera and their corresponding cDNA copies were cloned and sequenced. AbPOPA and AbPOPB each have 18 introns (Fig. 5). The amino acid sequences of the predicted translational products of AbPOPA (761 amino acids) and AbPOPB (730 amino acids) were 57% identical to each other and 62% and 57% identical, respectively, to the phalloidin-processing POP (733 amino acids) of C. apala (18).
Fig. 5.
Gene structures of AbPOPA and AbPOPB of A. bisporigera.
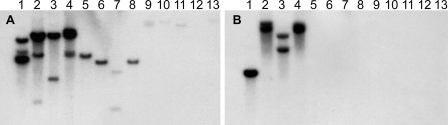
Within the genus Amanita, the genes encoding α-amanitin and phallacidin (AMA1 and PHA1, respectively) were found by DNA blotting to be present only in species that make these compounds (10). We probed genomic DNA blots of 13 species in the genus Amanita, representing all five sections, with AbPOPA and AbPOPB. DNAs from all of the tested species showed hybridization to an AbPOPA probe, whereas only DNAs from species in section Phalloideae, which contains all of the amatoxin- and phallotoxin-producing species, showed hybridization to an AbPOPB probe (Fig. 6). Due to taxonomic divergence and variation in loading, some of the signals with AbPOPA were weak (Fig. 6A). However, most significantly, DNAs from none of the species in section Validae, the sister group to section Phalloideae, showed hybridization to AbPOPB even with prolonged exposure (Fig. 6B, lanes 5 to 8). DNAs from these same specimens showed the same pattern of hybridization or nonhybridization with AMA1 and PHA1 probes (10).
Fig. 6.
DNA blotting of 13 Amanita species probed with AbPOPA (A) or AbPOPB (B). Amanita species: lanes 1, A. aff. suballiacea; lanes 2, A. bisporigera; lanes 3, A. phalloides; lanes 4, A. ocreata; lanes 5, A. novinupta; lanes 6, A. flavoconia; lanes 7, A. porphyria; lanes 8, A. franchetii; lanes 9, A. muscaria; lanes 10, A. gemmata; lanes 11, A. hemibapha; lanes 12, A. velosa; lanes 13, unidentified species of Amanita section Vaginatae. Mushrooms represent sections Phalloideae (lanes 1 to 4), Validae (lanes 5 to 8), Amanita (lanes 9 and 10), Caesareae (lane 11), and Vaginatae (lanes 12 and 13). Approximately the same amount of DNA was loaded in each lane (10).
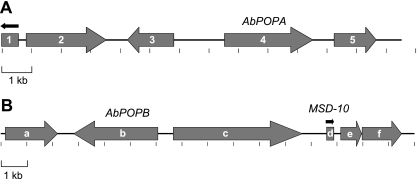
Because the genes of a particular secondary metabolite biosynthetic pathway tend to be clustered in fungi, clustering of AbPOPA or AbPOPB with other genes involved in amatoxin biosynthesis would suggest a role for one or both of these genes in amatoxin biosynthesis (1, 27, 35). To test this, we screened a lambda phage clone library with AbPOPA and AbPOPB, and two hybridizing clones were sequenced completely. FGENESH (Softberry Inc., Mt. Kisco, NY) was used to predict proteins ab initio using the Coprinopsis cinerea model. Each predicted protein was then used to search GenBank nr using BLASTP. Five genes were predicted in the 13.5-kb AbPOPA-containing clone (Fig. 7). Gene 4 was AbPOPA. The predicted products of genes 1, 2, 3, and 5 had strong hits (score of <1e−62) to hypothetical proteins in other basidiomycetes. Genes 3 and 5 were predicted to encode mitochondrial transporters (pfam 00153) with orthologs in other basidiomycetes.
Fig. 7.
Gene maps of lambda phage clones containing AbPOPA (A) or AbPOPB (B). Arrows indicate transcriptional direction. Genes were predicted using FGENESH (Softberry Inc.).
Six genes were predicted by FGENESH in the 16.0-kb lambda clone containing AbPOPB. None had expect scores of less than 1e−26 against any protein in GenBank nr. The best hits of genes a, c, e, and f were to hypothetical proteins in the ectomycorrhizal basidiomycete Laccaria bicolor. Gene b was AbPOPB. Gene d was a member of the MSDIN family previously identified in the genome survey sequence of A. bisporigera (MSD-10; GenBank accession no. EU196153). A full-length cDNA copy of MSD-10 was obtained using 5′- and 3′-RACE, indicating that this gene is expressed and contains three introns, like AMA1 and PHA1. The predicted amino acid sequence of the hypervariable putative toxin region of MSD-10 is GAYPPVPMP, and therefore MSD-10 is predicted to encode a cyclic nonapeptide containing four Pro residues and lacking tryptathionine.
Immunolocalization of A. bisporigera prolyl oligopeptidase B.
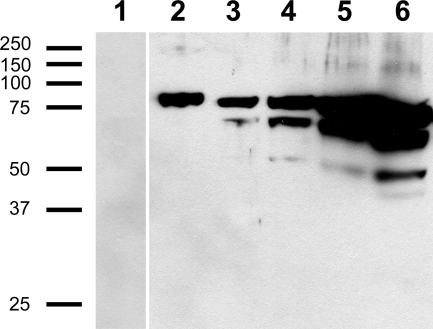
Based on these several lines of evidence supporting a role for AbPOPB in the biosynthesis of amatoxins in A. bisporigera, antibodies were raised against AbPOPB in order to determine the relationship between cellular amatoxin accumulation and biosynthesis. Because neither AbPOPA nor AbPOPB could be expressed in an enzymatically active form in either E. coli or Pichia pastoris (unpublished results), we isolated AbPOPB from inclusion bodies of E. coli (Fig. 8). The resulting anti-AbPOPB antibody did not recognize other proteins in total extracts of A. bisporigera (Fig. 8). Like other fungal POPs (18), AbPOPB was very sensitive to degradation during extraction. Full-length AbPOPB could not be detected without the addition of high levels of protease inhibitors in the extraction buffer. Even then, degradation usually occurred, as could be seen with high protein loadings (Fig. 8). Although antibodies against AbPOPA were successfully raised, the antibodies failed to produce a clear and specific signal when tested on whole mushroom extracts (data not shown). We therefore used only the anti-AbPOPB antibody for immunohistochemical studies.
Fig. 8.
Immunoblotting of E. coli inclusion bodies and total extracts of A. bisporigera with anti-AbPOPB antibody. Lanes: 1, BL21 empty vector (PET21) control; 2, inclusion body (1 μl = 0.05 μg) of an E. coli BL21 line expressing AbPOPB; 3 through 6, total soluble protein from A. bisporigera loaded at 24 μg, 47 μg, 94 μg, and 141 μg, respectively. All lanes were from the same gel, but some lanes between lanes 1 and 2 were removed. Size indicators (left) are in kDa.
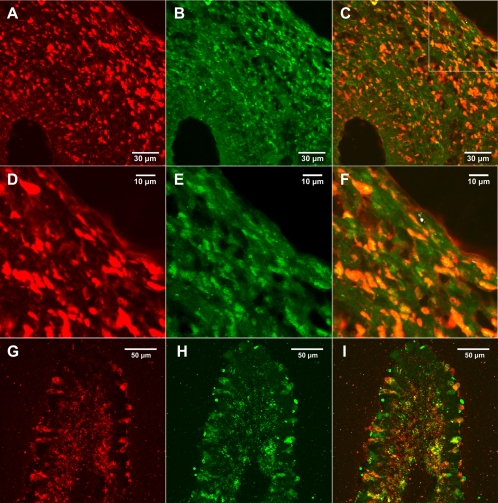
Dual labeling of pileus (cap) tissue from an immature mushroom indicated strong colocalization of amanitin and AbPOPB, as indicated by the orange to yellow color (Fig. 9). Figure 9D to F show higher-magnification views of the area indicated by the white box in Fig. 9C. Calculation of the overlap coefficients showed 52.0% of AbPOPB colocalizing with amanitin and 99.9% of amanitin colocalizing with AbPOPB. Colocalization of amanitin and AbPOPB was also examined in lamellae (Fig. 9G to I). The merge between the two (i.e., orange to yellow color) showed significant overlap of the two signals (Fig. 9I). Amanitin and AbPOPB were both present in certain hymenial cells and not others (Fig. 9G to I). Several areas were analyzed, and the colocalization was calculated to be greater than 75%.
Fig. 9.
Colocalization of amanitin and AbPOPB. (A to C) Colocalization in the pileus. The stipe is on the left. (A) Antiamanitin; (B) anti-AbPOPB; (C) merge of panels A and B. (D to F) Higher magnification of a portion of the pileus in panels A to C, respectively. (G to I) Colocalization in a single lamella, in the same order as panels A to C.
DISCUSSION
Although amanitins have been known to be distributed throughout the tissue of Amanita mushrooms, their cellular localization has not previously been determined (8). Here we show that the distribution of amanitin is not uniform among cells of the basidiocarp, nor are they sequestered in a morphologically distinct cell type, unlike the anticollembola toxins of Russula bella or the antinematode compounds of C. apala (12, 23). Selective distribution of amatoxins was particularly apparent in the organized cells of the hymenium and subhymenium as opposed to the interwoven ground hyphae of the pileus and stipe. Furthermore, our results indicate that amanitin is not evenly distributed within the cells. Rather, amanitin appears to be compartmentalized at the subcellular level. Although we did not resolve the structures containing amanitin, they are most likely vacuoles, such as are known to be the sites of accumulation of secondary metabolites in other organisms (16). Aflatoxins and some their biosynthetic intermediates accumulate in vacuoles and vesicles in Aspergillus parasiticus, even though other steps in aflatoxin biosynthesis occur in peroxisomes or the cytoplasm (26). However, a fundamental difference between amanitins and aflatoxins (and most other ascomycete secondary metabolites) is that whereas aflatoxins are ultimately secreted into the medium, amanitins are not secreted and are therefore stored inside cells in a way that sequesters them from primary metabolism (37).
A particular question that this paper begins to address is the relationship between amanitin accumulation and amanitin biosynthesis. At this point we know little about the enzymology of the posttranslational processing steps of amanitin biosynthesis, which include cleavage of the proprotein, cyclization, hydroxylation, formation of the cross bridge between Trp and Cys, and, in the case of the phallotoxins, epimerization of l-Thr or l-Asp to the corresponding d isomer.
Several lines of evidence implicate prolyl oligopeptidase (POP) in cleavage of the proprotein, which is the predicted first step of posttranslational maturation of the amatoxins and phallotoxins (18). First, the products of all members of the MSDIN gene family, which includes the genes encoding α-amanitin, β-amanitin, phallacidin, phalloidin, and a cyclic decapeptide related to antamanide, have invariant Pro residues as the amino acid immediately upstream of the hypervariable toxin region that contains the peptide sequence of the mature toxin and as the last amino acid in the toxin region (10, 18, 36). This indicates that posttranslational cleavage of the toxin proproteins is probably catalyzed by a Pro-specific peptidase (6, 29). Second, an enzyme capable of cleaving a phalloidin precursor protein at both Pro residues, resulting in release of the cognate linear heptapeptide, was identified as a POP in the phalloidin-producing mushroom C. apala (18).
In this paper we provide additional evidence to support the in vivo role of POP in amanitin biosynthesis. First, AbPOPB, but not AbPOPA, is restricted taxonomically to those species in the genus Amanita that make amatoxins. A plausible explanation for why toxic Amanita species (and the amanitin-producing species Galerina marginata [our unpublished data]) have two POP genes whereas other basidiomycetes have only one is that one of the POPs (AbPOPA in the case of A. bisporigera) is a housekeeping POP that is functionally equivalent to the single POP found in other basidiomycetes, whereas the second POP (AbPOPB) has a dedicated function in toxin biosynthesis. Although POPs are widespread in nature, having been described in plants, animals, trypanosomes, bacteria, and other basidiomycetes, little is known about their native substrates except in mammals (2). Human POP cleaves several peptide hormones in vitro and in vivo and has been implicated in human pathologies, including dementia, amnesia, multiple sclerosis, and sleeping sickness (24). The native substrates of POPs in other organisms have been little studied. Our results point to the proproteins of the MSDIN family as the probable in vivo substrates of a particular class of POPs in certain basidiomycetes.
A third line of evidence in support of a role of AbPOPB in amatoxin processing is genetic linkage between AbPOPB and an expressed member of the MSDIN family (MSD-10). Clustering of genes involved in a common pathway is the usual situation in fungal secondary metabolism (32). Although there has been less work done on the genetics of secondary metabolism in basidiomycetes than on that in ascomycetes, this appears to be the emerging consensus for this group of organisms as well (1, 27, 35).
Taken together, the evidence strongly implicates AbPOPB in the biosynthesis of the amatoxins and phallotoxins, and therefore we raised antibodies against AbPOPB to use as a marker to localize the site of amanitin biosynthesis. In fact, there was a high degree of colocalization between amanitin and AbPOPB, which is consistent with synthesis and accumulation occurring in the same cells.
Natural products serve as the major source of pharmaceutical agents against a wide variety of old and emerging human diseases. However, most of our medicines are based on only a few fundamental molecular scaffolds, which have been heavily exploited by generations of tailoring (31). Historically, toxins have shown great value in drug development and pharmaceutical use, especially the highly potent ones that have specific targets. Amanita toxins are highly potent and highly specific, which has led to their widespread use as research reagents. In several ways, the peptide toxins of Amanita are chemically and biochemically unique among known natural products; e.g., they are the only ones that contain the Cys-Trp cross bridge (tryptathionine), and they are the smallest known ribosomally synthesized peptides. They may therefore provide a novel scaffold on which to design new pharmaceutical agents (9, 19, 20, 25). Identification of the genes and enzymes involved in the biosynthesis of the Amanita toxins may make it possible to produce a large number of new compounds based on the naturally occurring toxins.
Compared to the other main groups of microorganisms that make secondary metabolites (namely, bacteria and ascomycetes), relatively little is known about the chemistry, biochemistry, or biology of secondary metabolites made by higher basidiomycetes, commonly known as mushrooms (27). Compared to ascomycetes and bacteria, mushrooms have more complex anatomies and consequently more cell specialization. The results presented in this paper illustrate this cellular specialization for both toxin accumulation and biosynthesis.
ACKNOWLEDGMENTS
We thank Heniz Faulstich (Heidelberg, Germany) for the generous gift of antiamanitin antibodies, Melinda Frame (Michigan State University [MSU] Microscopy Facility) and Markus Günl (MSU) for scientific advice, and Federica Brandizzi (MSU) for comments on the manuscript.
This work was funded by grant DE-FG02-91ER200021 from the U.S. Department of Energy Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Biosciences.
Footnotes
Published ahead of print on 1 October 2010.
REFERENCES
- 1.Agger S., Lopez-Gallego F., Schmidt-Dannert C. 2009. Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus. Mol. Microbiol. 72:1181–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bastos I. M., Motta F. N., Charneau S., Santana J. M., Dubost L., Augustyns K., Grellier P. 2010. Prolyl oligopeptidase of Trypanosoma brucei hydrolyzes native collagen, peptide hormones and is active in the plasma of infected mice. Microbes Infect. 12:457–466 [DOI] [PubMed] [Google Scholar]
- 3.Bresinsky A., Besl H. 1990. A colour atlas of poisonous fungi: a handbook for pharmacists, doctors and biologists, p. 295 Wolfe, Würzburg, Germany [Google Scholar]
- 4.Bushnell D. A., Cramer P., Kornberg R. D. 2002. Structural basis of transcription: alpha-amanitin-RNA polymerase II cocrystal at 2.8 A resolution. Proc. Natl. Acad. Sci. U. S. A. 99:1218–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clémençon H. 2004. Cytology and plectology of the Hymenomycetes, p. 24–50 J. Cramer, Berlin, Germany [Google Scholar]
- 6.Cunningham D. F., O'Connor B. 1997. Proline specific peptidases. Biochim. Biophys. Acta 1343:160–186 [DOI] [PubMed] [Google Scholar]
- 7.Enjalbert F., Gallion C., Jehl F., Monteil H., Faulstich H. 1992. Simultaneous assay for amatoxins and phallotoxins in Amanita phalloides Fr. by high-performance liquid chromatography. J. Chromatogr. 598:227–236 [DOI] [PubMed] [Google Scholar]
- 8.Enjalbert F., Cassanas G., Salhi S. L., Guinchard C., Chaumont J.-P. 1999. Distribution of the amatoxins and phallotoxins in Amanita phalloides. Influence of the tissues and the collection site. C. R. Hebd. Seances Acad. Sci. III 322:855–862 [DOI] [PubMed] [Google Scholar]
- 9.Fischbach M. A., Walsh C. T. 2009. Antibiotics for emerging pathogens. Science 325:1089–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallen H. E., Luo H., Scott-Craig J. S., Walton J. D. 2007. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc. Natl. Acad. Sci. U. S. A. 104:19097–19101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallen H. E., Watling R., Adams G. C. 2003. Taxonomy and toxicity of Conocybe lactea and related species. Mycol. Res. 107:969–979 [DOI] [PubMed] [Google Scholar]
- 12.Hutchison L. J., Madzia S. E., Barron G. L. 1996. The presence and antifeedant function of toxin-producing secretory cells on hyphae of the lawn-inhabiting agaric Conocybe lactea. Can. J. Bot. 74:431–434 [Google Scholar]
- 13.Jenkinson T. S., Celio G. J., Padamsee M., Dentinger B. T. M., Meyer M. L., McLaughlin D. J. 2008. Conservation of cytoplasmic organization in the cystidia of Suillus species. Mycologia 100:539–547 [DOI] [PubMed] [Google Scholar]
- 14.Kirchner K., Faulstich H. 1986. Purification of amatoxin-specific antibodies from rabbit sera by affinity chromatography, their characterization and use in toxicological studies. Toxicon 24:273–283 [DOI] [PubMed] [Google Scholar]
- 15.Kuo M. 2006. Using a microscope: basidia and cystidia. http://www.mushroomexpert.com/microscope_cystidia.html
- 16.Kutchan T. M. 2005. A role for intra- and intercellular translocation in natural product biosynthesis. Curr. Opin. Plant Biol. 8:292–300 [DOI] [PubMed] [Google Scholar]
- 17.Largent D. L., Johnson D. 1977. How to identify mushrooms to genus. III. Microscopic features, p. 71–88 Mad River Press, Eureka, CA [Google Scholar]
- 18.Luo H., Hallen-Adams H. E., Walton J. D. 2009. Processing of the phalloidin proprotein by prolyl oligopeptidase from the mushroom Conocybe albipes. J. Biol. Chem. 284:18070–18077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May J. P., Perrin D. M. 2007. Tryptathionine bridges in peptide synthesis. Pept. Sci. 88:714–724 [DOI] [PubMed] [Google Scholar]
- 20.McIntosh J. A., Donia M. S., Schmidt E. W. 2009. Ribosomal peptide natural products: bridging the ribosomal and nonribosomal worlds. Nat. Prod. Rep. 26:537–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKnight T. A., McKnight K. B., Skeels M. C. 2010. Amatoxin and phallotoxin concentration in Amanita bisporigera spores. Mycologia 102:763–765 [DOI] [PubMed] [Google Scholar]
- 22.Nagendran S., Hallen-Adams H. E., Paper J. M., Aslam N., Walton J. D. 2009. Reduced genomic potential for secreted plant cell-wall-degrading enzymes in the ectomycorrhizal fungus Amanita bisporigera, based on the secretome of Trichoderma reesei. Fung. Genet. Biol. 46:427–435 [DOI] [PubMed] [Google Scholar]
- 23.Nakamori T., Suzuki A. 2007. Defensive role of cystidia against Collembola in thebasidiomycetes Russula bella and Strobilurus ohshimae. Mycol. Res. 111:1345–1351 [DOI] [PubMed] [Google Scholar]
- 24.Nolte W. M., Tagore D. M., Lane W. S., Saghatelian A. 2009. Peptidomics of prolyl endopeptidase in the central nervous system. Biochemistry 48:11971–11981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oman T. J., van der Donk W. A. 2010. Follow the leader: the use of leader peptides to guide natural product biosynthesis. Nat. Chem. Biol. 6:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roze L. V., Chanda A., Linz J. E. Compartmentalization and molecular traffic in secondary metabolism: a new understanding of established cellular processes. Fungal Genet. Biol., in press. doi:10.1016/j.fgb.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider P., Bouhired S., Hoffmeister D. 2008. Characterization of the atromentin biosynthesis genes and enzymes in the homobasidiomycete Tapinella panuoides. Fungal Genet. Biol. 45:1487–1496 [DOI] [PubMed] [Google Scholar]
- 28.Scott-Craig J. S., Panaccione D. G., Cervone F., Walton J. D. 1990. Endopolygalacturonase is not required for pathogenicity of Cochliobolus carbonum on maize. Plant Cell 2:1191–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szeltner Z., Polgár L. 2008. Structure, function and biological relevance of prolyl oligopeptidase. Curr. Protein Pept. Sci. 9:96–107 [DOI] [PubMed] [Google Scholar]
- 30.Truong B.-N., Okazaki K., Fukiharu T., Takeuchi Y., Futai K., Le X.-T., Suzuki A. 2007. Characterization of the nematocidal toxocyst in Pleurotus subgen. Coremiopleurotus. Mycoscience 48:222–230 [Google Scholar]
- 31.Walsh C. T., Fischbach M. A. 2010. Natural products version 2.0: connecting genes to molecules. J. Am. Chem. Soc. 132:2469–2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walton J. D. 2000. Horizontal gene transfer and the evolution of secondary metabolite gene clusters in fungi: an hypothesis. Fungal Genet. Biol. 30:167–171 [DOI] [PubMed] [Google Scholar]
- 33.Walton J. D., Panaccione D. G., Hallen H. E. 2004. Peptide synthesis without ribosomes, p. 127–162 InTkacz J., Lange L. (ed.), Advances in fungal biotechnology for industry, agriculture, and medicine. Kluwer Academic, New York, NY [Google Scholar]
- 34.Watkins M., Hillyard D. R., Olivera B. M. 2006. Genes expressed in a turrid venom duct: divergence and similarity to conotoxins. J. Mol. Evol. 62:247–256 [DOI] [PubMed] [Google Scholar]
- 35.Welzel K., Eisfeld K., Antelo L., Anke T., Anke H. 2005. Characterization of the ferrichrome A biosynthetic gene cluster in the homobasidiomycete Omphalotus olearius. FEMS Microbiol. Lett. 249:157–163 [DOI] [PubMed] [Google Scholar]
- 36.Wieland T. 1986. Peptides of poisonous Amanita mushrooms, p. 181–206 Springer, New York, NY [Google Scholar]
- 37.Zhang P., Chen Z., Hu J., Ei B., Zhang Z., Hu W. 2005. Production and characterization of amanitin toxins from a pure culture of Amanita exitialis. FEMS Microbiol. Lett. 252:223–228 [DOI] [PubMed] [Google Scholar]