Abstract
Recent observations suggest that immune response is involved in the development of pancreatitis. However, the exact pathogenesis underlying this immune-mediated response is still under debate. TGF-β has been known to be an important regulating factor in maintaining immune homeostasis. To determine the role of TGF-β in the initiation or progression of pancreatitis, TGF-β signaling was inactivated in mouse pancreata by overexpressing a dominant-negative mutant form of TGF-β type II receptor in the pancreas, under control of the pS2 mouse trefoil peptide promoter. Transgenic mice showed marked increases in MHC class II molecules and matrix metalloproteinase expression in pancreatic acinar cells. These mice also showed increased susceptibility to cerulein-induced pancreatitis. This pancreatitis was characterized by severe pancreatic edema, inflammatory cell infiltration, T- and B-cell hyperactivation, IgG-type autoantibodies against pancreatic acinar cells, and IgM-type autoantibodies against pancreatic ductal epithelial cells. Therefore, TGF-β signaling seems to be essential either in maintaining the normal immune homeostasis and suppressing autoimmunity or in preserving the integrity of pancreatic acinar cells.
Introduction
Acute pancreatitis is characterized by edema, leukocyte infiltrations, hemorrhage, and cellular necrosis. Inflammatory events are believed to play an important role in its pathogenesis, but the exact mechanisms that trigger the inflammatory and necrotizing process are not completely understood (1). Despite the existence of several experimental models of acute pancreatitis and a variety of pathophysiological hypotheses, none of these have been able to explain convincingly the development of this disease (2–4). Recently, several studies reported the possible role of proinflammatory cytokines in mediating the different events in acute pancreatitis and its systemic complications (5–7). However, local mechanisms involving cytokines in pancreatitis are not yet well understood. As in other organs, the balance between proinflammatory and anti-inflammatory cytokines seems to be very important in maintaining the immune homeostasis in the pancreas (7–8). Because the inflammatory and necrotic processes in acute pancreatitis may be mediated by proinflammatory cytokines, anti-inflammatory cytokines such as TGF-β, IL-4, and IL-10 may be able to alter the course of the disease. IL-10 or IL-4 knockout (KO) mice showed only normal histology or enterocolitis alone, indicating that neither of these 2 cytokines is likely to play a major role in the pathogenesis of pancreatitis (9–10). Recently, however, IL-10 or IL-4 has been reported to prevent death or necrosis in murine experimental acute pancreatitis and limit the severity of acute pancreatitis (7, 8, 11).
TGF-β is a multifunctional cytokine with effects on nearly every tissue and cell type. Dysregulated expression of or response to TGF-β has been implicated in a variety of disease processes, including autoimmune disease, fibrosis and chronic inflammation, parasitic infection, neurodegenerative disease, and carcinogenesis (12–13). The predominant phenotype of the TGF-β1 knockout mouse suggests that the loss of this gene eliminates a critical regulator of immune and inflammatory responses (14). The phenotype is best characterized as an excessive inflammatory response consisting of a massive infiltration of leukocytes into numerous organs including the heart, lung, liver, salivary gland, pancreas, stomach, and intestine. This overwhelming tissue inflammation is associated with circulating autoantibodies, immune complex deposition, increased tissue expression of both classes of MHC antigens, and dysregulated myelopoiesis (15). This spontaneous initiation of autoimmunity in the TGF-β1 knockout mouse suggests that dysregulation of TGF-β signaling may potentially underlie a number of autoimmune disorders, including multiple sclerosis, experimental autoimmune encephalomyelitis, and inflammatory bowel disease. More detailed examination of the role of this cytokine in inflammation and inflammatory diseases suggests that it plays a critical role in the suppression of Th1-mediated colitis, induction of oral tolerance, inhibition of aberrant MHC class II antigen expression, and generation of Th2-type cytokines (16). Therefore, we hypothesized that loss of TGF-β signaling in epithelia of targeted organs might promote inflammatory disorders through the increased expression of MHC class II antigens and the initiation of autoimmunity in the epithelia of affected organs.
Because the germline-null mutation of TGF-β1 can cause embryonic lethality or widespread inflammation with a markedly shortened life span, we have used transgenic overexpression of a dominant-negative mutant form of the TGF-β receptor II (TGF-β dnRII) driven by tissue-specific promoters to study tissue-restricted functional inactivation of TGF-β RII. For an appropriate transcriptional control element, we selected the trefoil peptide promoter pS2. The pS2 promoter was expressed exclusively in the epithelia of the stomach, duodenum, and pancreas. The production of these trefoil peptides is locally upregulated at the site of injury in such conditions as gastritis, peptic ulceration, inflammatory bowel disease, breast cancer, and pancreatitis (17, 18).
Based on previous studies in our laboratory revealing significant pathological changes in the pancreata of TGF-β1 KO and dominant-negative TGF-β receptor II mutant mice (19), we hypothesized that the selective loss of TGF-β signaling in the pancreas can cause increased susceptibility to cerulein-induced pancreatitis. To functionally inactivate TGF-β signaling selectively in pancreatic acinar and ductal cells, we made dominant-negative TGF-β receptor II (TGF-β dnRII) mutant mice under the influence of the pS2 promoter.
Methods
Generation of dominant-negative mutant TGF-β RII mice.
The mouse pS2 fragment spans –2400 bp to +10 bp relative to the transcriptional start site. The human TGF-β RII fragment spans +1 to + 911 and contains both a hemagglutinin (HA) tag sequence (TATGATGTTCCTGATTATGCTAGCCTC) and a segment of the mouse protamine 1 gene (mP) tract that provides an intron and a polyadenylation site (20). The 3.8-kb fragment was microinjected into (FVB/N strain) fertilized eggs. Animal care was in accordance with our institutional guidelines (National Cancer Institute/National Institutes of Health). We obtained 4 lines of transgenic mice named pS2-1, pS2-2, pS2-3, and pS2-4. Genotyping was performed by Southern hybridization and PCR. For Southern hybridization, DNA obtained from the mouse tail was cut with HindIII and BglII overnight, electrophoresed on 1.0% agarose gel, and transferred onto a N+ membrane (Bio-Rad Laboratories Inc., Hercules, California, USA). After hybridization with dnRII probe, the presence of the transgene, 3.8 kb for pS2-dnRII, was confirmed. For PCR genotyping, the following set of primers was used: primer A, GGTGGTGATGTGGACAAAG; primer B, CTGCAGT-CGCTCATGCAGG. Two lines of pS2-dnRII, pS2-1 and pS2-3, showing high expressions of transgene, were used for this experiment.
RNA isolation and RT-PCR.
RNA was isolated from mouse tissue using Trizol (GIBCO BRL, Gaithersburg, Maryland, USA). RT-PCR was performed according to the manufacturer’s instructions using a Perkin-Elmer RT-PCR kit (Perkin-Elmer Corp., Branchburg, New Jersey, USA). The primers used were as follows: for TGF-β dnRII, 5′-ACGACATGATAGTCACTGACAACA-3′ and 5′-TTGGGGTCATGGCAAACTGTCTC-3′; for mITF, 5′-GAAGTTTGCGTGCTGCCATGGAG-3′ and 5′-CCGCAATTAGAACAGCCTTGTG-3′; for IL-2, 5′-TGGAGCAGCTGTTGATGGACCTAC-3′ and 5′-AGATGATGCTTTGACAGAAGGCTATC-3′; for IL-10, 5′-CTGGACAACATACTGCTAACCGAC-3′ and 5′-ATTCATTCATGGCCTTGTAGACACC-3′; for IL-1β, 5-TGACGGACCCCAAAAGATGAAG-3′ and 5′-CTGCTTGTGAGGTGCTGATGTA-3′; for TNF-α, 5—CCAGACCCTCACACTCAGAT-3′ and 5′-AACACCCATTCCCTTCACAG-3′; for IFN-γ, —TGGCTGTTTCTGGCTGTTACTG-3′ and 5′-AATCAGCAGCGACTCCTTTTCC-3′; and for β-actin, 5′-GTGGGCCGCTCTAGGCACCA-3′ and 5′-CGGTTGGCCTTAGGGTTCAGGGGGG-3′. The β-actin served as a positive control, and in each sample primers for each cytokine and β-actin were used. Samples were amplified for 25 cycles for 30 seconds at 94°C, 30 seconds at 60°C, and 30 seconds at 72°C in a GeneAmp 9000 PCR instrument (Perkin-Elmer Corp.) (21).
Immunoprecipitation and Western blotting.
Pancreata from either control mice or pS2-dnRII transgenic mice were homogenized with a Dounce homogenizer in lysis buffer. Immunoprecipitation using anti-HA antibody (Sigma Chemical Co., St. Louis, Missouri, USA) was carried out. For the subsequent anti–TGF-β RII Western blot, the immunoprecipitated proteins, separated by SDS-PAGE, were transferred onto membrane, incubated with anti–TGF-β RII antibody (Upstate Biotechnology Inc., Lake Placid, New York, USA), and antibody-bound proteins were revealed by chemiluminescence (Pierce Chemical Co., Rockford, Illinois, USA).
Immunohistochemical staining.
Tissues were fixed in 5% paraformaldehyde, mounted in paraffin, and stained with hematoxylin and eosin. Unstained 5-μm cut slides were used for immunohistochemical staining with HA tag, MHC class II (OX-3; Serotec, Raleigh, North Carolina, USA), and SP 3-1 (kind gift from I. Nishimori, Kochi Medical School, Japan) antibodies, respectively. Binding of the primary antibody was revealed by biotinylated secondary antibody, avidin, biotinylated horseradish peroxidase, and aminoethylcarbazole (AEC) as chromogen (Zymed Laboratories Inc. South San Francisco, California, USA). The slides were counterstained with Mayer’s hematoxylin.
Induction of acute pancreatitis and evaluation pancreatitis.
Mice were given 50 μg/kg cerulein (Sigma Chemical Co.) intraperitoneally 3 times, once per hour.
Edema index.
Pancreatic edema was evaluated by the ratio of pancreatic wet weight (milligrams) to body weight (grams) (22). Significance of the differences in edema index (EI) between wild-type and pS2-dnRII mice was shown at 3, 6, and 8 hours after cerulein injection (P < 0.01).
Histologic scoring.
The tissue samples fixed in formalin and routinely processed were assessed histologically using light microscopy, performed in a blinded fashion by 3 pathologists. Three variables were considered, each scored on a 0–4 scale in accordance with the severity of the induced damage, which were modified from the scoring system of Schmidt et al. (23): acinar cell necrosis, degree of pancreatic edema, and extent of inflammation.
Myeloperoxidase activities.
Myeloperoxidase (MPO) was extracted from homogenized pancreatic tissue by suspending the materials in 0.5% hexadecyltrimethylammonium bromide (Sigma Chemical Co.) in a 50 mM potassium phosphate buffer, pH 6.0, before sonication in an ice bath (24). The specimens were freeze-thawed 3 times. A supernatant (0.1 mL) was mixed with 2.9 mL of 50 mM phosphate buffer, pH 6.0, containing 0.167 mg/mL o-dianisidine peroxide (ICN Biomedicals, Costa Mesa, California, USA) and 0.005% hydrogen peroxide. The change in absorbance at 460 nm was measured with a Beckman DU (Beckman Coulter, Inc., Fullerton, California, USA) spectrophotometer. One unit of MPO activity was defined as the degradation of 1 μM peroxide per minute. All values are expressed as mean ± SE. Comparisons were made by SPSS software (SPSS Science, Chicago, Illinois, USA), using ANOVA to detect the statistical significance.
Autoantibodies against pancreatic acinar cells.
Autoantibodies were detected by indirect immunofluorescence methods using cryopreserved slides of monkey pancreatic acinar cells (Biochips; Euroimmune, Lubeck, Germany). For immunofluorescence, sera were diluted 1:20 in PBS containing 2% BSA. Twenty microliters of positive or negative control sera was placed on 2 of the 10 well-defined areas of each glass slide with monkey pancreas, and 20 μL diluted sera was applied on the remaining 8 areas. After incubating in a humid chamber for 30 minutes at room temperature and then for another 30 minutes with a 1:50 dilution of FITC–labeled Fab′(2) goat anti-mouse immunoglobulin antibodies (Sigma Chemical Co.), the slides were washed again in PBS for 5 minutes and then mounted.
Changes of pancreatic matrix metalloproteinase activities.
Pancreatic tissues were homogenized, mixed with sample buffer, and applied directly, without previous heating or reduction, to 4–16% acrylamide gels containing 1 mg/mL of gelatin. After removal of SDS from the gel by incubation in 2.5% (vol/vol) Triton X-100 for 1 hour, the gels were incubated at 37°C for 16 hours in 50 mM Tris-HCl, pH 7.6, containing 0.2 M NaCl, 5 mM CaCl2, and 0.02% (wt/vol) Brij-35. The gels were stained for 3 hours in 40% methanol/10% glacial acetic acid containing 0.2% Coomassie brilliant blue G-250 and destained in the same solution without dye. The genolytic activity of each matrix metalloproteinase (MMP) was evident as a clear band against the blue background of stained gelatin.
In situ hybridization.
The MMP-3 and MMP-9 were each synthesized by RT-PCR and inserted into pBluescript SK(–) vector. Each DNA probe was labeled with biotin using the BioNick Labeling System (GIBCO BRL). The in situ hybridization and detection system (GIBCO BRL) was used according to the manufacturer’s instructions. Briefly, the formalin-fixed tissue sections were treated with 0.2 mmol/L HCl, permeabilized with proteinase K (50 μg/mL), and fixed in 4% paraformaldehyde. After dehydration with ethanol, the tissue sections were hybridized with biotinylated probes. Thereafter, the hybridized probe was detected by incubating sections with the dye substrates for streptavidin-alkaline phosphatase, nitro blue tetrazolium, and 4-bromo-5-chloro-3-indolylphosphate. The sections were counterstained with fast nuclear red (Zymed). Localization of the hybridized probe was detected by formation of purple granular signals. As negative controls, the reaction was processed with the control DNA probe provided by the manufacturer.
Results
Generation of dominant-negative mutant mice of TGF-β receptor II using mouse pS2 promoter.
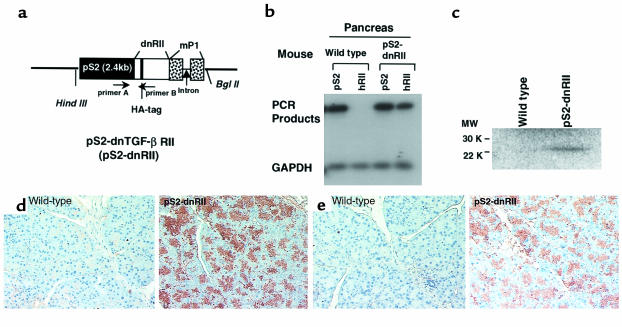
The dnRII transgenes encode the extracellular and transmembrane domains of TGF-β RII, and transgene expression was regulated by the pS2 promoter (Figure 1a). Because we inserted a human TGF-β RII fragment spanning bp +322 to bp +911, we could identify the presence of dnRII by RT-PCR using primers specific to human TGF-β RII (Figure 1b). DnRII protein was detected by Western blotting using antibodies against a HA tag and a TGF-β RII in the pancreata of pS2-dnRII transgenic mice (Figure 1c). We also performed immunohistochemical staining using a TGF-β RII antibody. In the pancreata of wild-type littermates, no significant expression of TGF-β RII was noted on pancreatic acinar and ductal cells, but in the pancreata of pS2-dnRII transgenic mice, TGF-β RII expression was markedly increased in acinar and ductal cells because of the expression of dnRII (Figure 1d). We also observed the distribution of positive staining of dnRII parallel with expression of HA tag antigens. Whereas staining of the HA tag was not detectable in wild-type littermates, the HA tag was strongly expressed in the pS2-dnRII transgenic mice (Figure 1e). The dnRII was also detected in pancreatic islet cells, stomach, and proximal duodenum (data not shown).
Figure 1.
Generation of pS2 dominant-negative mutant TGF-β RII mice. (a) A schematic representation of the transgene. The mpS2 fragment spans bp –2400 to bp +5. The 0.6-kb human TGF-β RII fragment spans +322 to + 911 and contains a HA tag sequence and a segment of the mP1 that provides an intron and a polyadenylation site. Transgenic mice were generated using inbred FVB/N zygotes. Of the 10 mice born, 4 were positive for pS2-dnRII (which was bred into lines) and designated pS2-1 though pS2-4. Pups from pS2-1 and pS2-2 were used for the current experiments. (b–e) Tissue distribution of pS2 and dnRII expression. (b) Using total RNAs isolated from the intestines of wild-type mice and pS2-dnRII transgenic mice, RT-PCR was performed using the specific primer sets for human TGF-β RII, mouse ITF, and mouse GAPDH. (c) Western blotting analysis of dnRII protein. (d) Immunohistochemical staining was performed for the presence of human dnRII using human anti–TGF-β RII (residues 1–28) antibody. (e) A HA tag was used for identifying the pS2-dnRII with immunohistochemical staining, which showed presence of the HA tag in the pancreata of transgenic mice alone. (d and e) × 200.
Comparison of severity of cerulein-induced pancreatitis between pS2-dnRII transgenic mice and wild-type littermates.
We generated 4 lines of transgenic mice, lines pS2-1, pS2-2, and pS2-3, with approximately 3 copies, and line pS2-4, with 1 copy per haploid genome, respectively, in the strain FVB/N. We could not find the evidence of spontaneous pancreatitis in younger pS2-dnRII mice, but some older mice do develop pancreatitis spontaneously, more frequently in mice with high expression of the transgene. Therefore, we have examined susceptibility to pancreatitis in younger, more homogenous mice. We treated pS2-1 and pS2-2 dnRII mice with synthetic cholecystokinin (cerulein) to observe susceptibility to chemically induced pancreatitis. Cerulein-induced pancreatitis is an established animal model of nonlethal pancreatitis that produces interstitial edema, spotty necrosis of acinar cells, infiltration of inflammatory cells into the pancreas, and oxidative injury (4). Supramaximal doses of cerulein (> 25 μg/kg) cause intracellular activation of trypsinogen, which activates other pancreatic enzymes such as elastase and phospholipase A2. Edematous pancreatitis can develop through infiltration of inflammatory cells and macrophages. Eighteen wild-type and 24 pS2-dnRII transgenic mice, all with body weight more than 25 g, were treated 3 times with intraperitoneal injections of cerulein at 1-hour intervals.
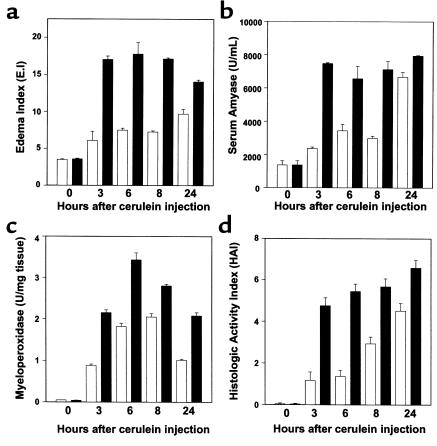
The pS2-dnRII mice showed marked susceptibility to cerulein-induced pancreatitis and developed earlier, more severe pancreatitis. Most of the pS2-dnRII mice showed severe pancreatic edema and pancreatic ascites. In contrast, the pancreata of the cerulein-treated wild-type mice developed only mild pancreatic edema with no pancreatic ascites or hemothorax noted. These changes in gross appearance were reflected by EI scoring. The EI, representing the degree of edematous pancreatitis, was significantly increased in pS2-dnRII mice compared with wild-type mice (Figure 2a). The changes in serum amylase levels, which were consistent with the changes in the EI (Figure 2b), were significantly higher in pS2-dnRII transgenic mice. We measured MPO activity, an index of neutrophil infiltrations in tissue, using pancreas homogenates, and found that pancreatic MPO activity of the pS2-dnRII mice was significantly elevated compared with that of the wild-type mice (Figure 2c). Microscopic examination revealed massive pancreatic acinar cell necrosis and inflammatory cell infiltration in the pS2-dnRII mice, whereas only mild pancreatic inflammation with edema was observed in the wild-type mice. Histopathologic scoring based on the degree of pancreatic acinar cell necrosis, pancreatic edema, and extent of inflammation (23) also showed significant differences between these 2 groups (Figure 2d).
Figure 2.
Increased susceptibility of cerulein-induced pancreatitis was noted in pS2-dnRII mice compared with wild-type littermates. (a) The EI was significantly higher in pS2-dnRII mice, which indicates that more severe edematous pancreatitis developed in pS2-dnRII mice than in wild-type littermates. (b) Serum amylase levels were significantly higher in pS2-dnRII mice (P < 0.001). (c) Changes in pancreatic MPO activities. PS2-dnRII mice showed significantly higher levels of pancreatic MPO activities than wild-type mice (P < 0.01). (d) Histologic activity index was also significantly higher in pS2-dnRII mice. Filled bar indicates pS2-dnRII transgenic mice from pS1 and pS2 lines; open bar indicates wild-type littermates.
Increased expression of MHC class II antigens and T- and B-cell hyperactivation occurring by loss of TGF-β signaling.
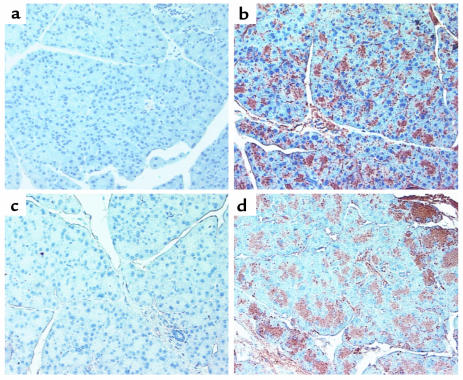
MHC class II molecules are normally expressed on limited cell types involved in presenting antigens to T-helper cells. These cell types include B lymphocytes, activated T lymphocytes and macrophages, dendritic cells, glial cells, and thymic epithelial cells (25). Because TGF-β–null mice exhibit enhanced expression of MHC class II antigens at or before the onset of the inflammatory process and because the characteristic inflammatory lesions do not develop in TGF-β–null mice crossed into the severe combined immunodeficiency background (16), we studied the expression of MHC class II antigens in the pancreata of wild-type and pS2-dnRII mice. As demonstrated by immunostaining with MHC class II antibody, prominent, increased MHC class II expression was noted only in the pancreatic acini (Figure 3b) of transgenic mice, whereas little or no staining was observed in the pancreatic acinar cells (Figure 3a) of the wild-type mice. The elevated expression of MHC class II antigens was seen before any evidence of inflammatory infiltrates before the induction of pancreatitis, and MHC class II expression became more prominent and neutrophil infiltration was observed after inducing pancreatitis in the transgenic mice (Figure 3, d and f) as compared with the wild-type littermates (Figure 3, c and e).
Figure 3.
Increased expression of MHC class II in the pancreata of pS2-dnRII mice. MHC class II expression was not observed in acinar cells of the pancreas (a) of wild-type FVB/N mice, but increased immunohistochemical intensity of MHC class II molecules was observed in pancreatic acinar cells (b) of pS2-dnRII mice. After the induction of pancreatitis through cerulein injection, MHC class II antigen expression was more markedly increased in transgenic mice (d) than in wild-type littermates (c).
Cytokine expression.
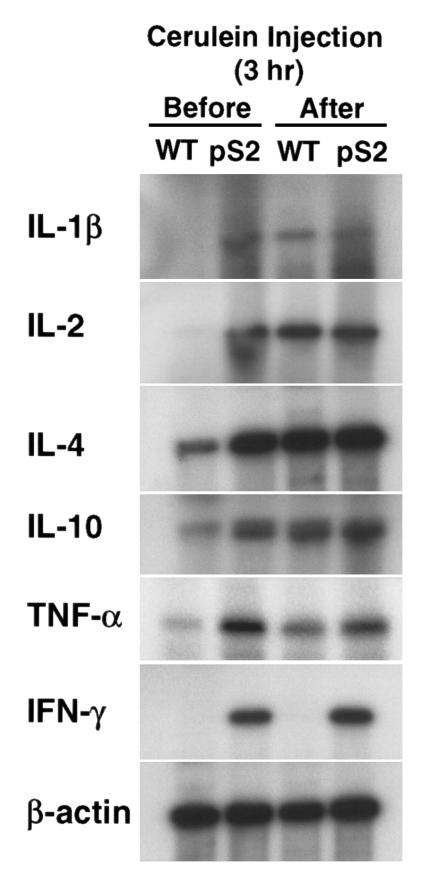
Significant increases in the expressions of various inflammatory cytokines such as IL-1β, IL-2, TNF-α, IFN−γ, and anti-inflammatory cytokines such as IL-4 and IL-10, were noted in mRNA extracted from the pancreata of pS2-dnRII mice compared with that of wild-type littermates (Figure 4). Densitometric analysis of the data shows that expression of IL-4 and IL-10 in the pancreata of transgenic mice was 5- and 3-fold higher than that of wild-type, respectively. Induction of pancreatitis enhanced IL-4 and IL-10 expression 5- and 4-fold in wild-type mice, respectively, whereas in the pancreata of transgenic mice IL-4 and IL-10 mRNAs are only slightly induced after cerulein treatment. Neither IL-1β, IFN−γ, nor IL-2 mRNA was expressed in the pancreata of wild-type mice. However, induction of pancreatitis in wild-type mice enhanced expression of these cytokines. We also examined expression of TNF-α and IFN-γ and found that expression of these cytokines was markedly increased even before the induction of pancreatitis in the pS2-dnRII transgenic mice. Interestingly, cerulein treatment slightly induced expression of TNF-α but not IFN-γ in wild-type mice, and it did not enhance expression of these cytokines in transgenic mice.
Figure 4.
Changes of cytokines. Increased IL-1β was observed in transgenic mice, whereas IL-1β was not detected in wild-type littermates. IL-2, IFN-γ, and IL-10 were all expressed at higher levels in the pancreata of pS2-dnRII transgenic mice compared with those of wild-type littermates. Expression of TNF-α was increased after the induction of pancreatitis.
IgG autoantibodies against pancreatic acinar cells and IgM antibodies against pancreatic ductal cells.
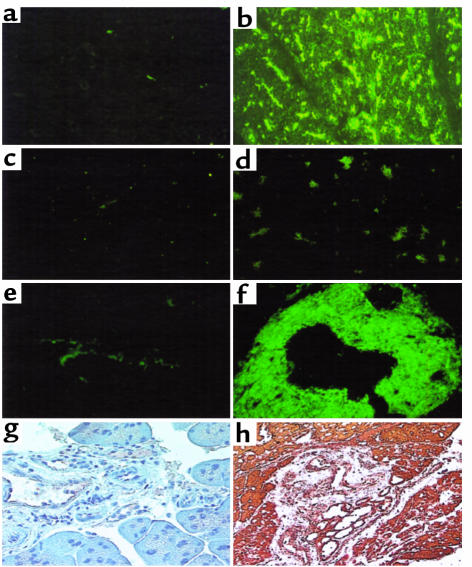
Further evidence for an autoimmune mechanism to explain the observation that loss of TGF-β signaling in our dominant-negative TGF-β RII transgenic mice resulted in increased susceptibility to pancreatitis is supplied by the presence of autoantibodies in the animal sera. Indirect immunofluorescence methods were used to detect autoantibodies against pancreatic acinar cells. We identified the presence of autoantibodies against pancreatic acinar cells of pS2-dnRII mice (Figure 5b) in the sera from 85% of the pS2-dnRII mice (17 positive out of 20 tested), whereas none was detected in the wild-type mice (Figure 5, a and c). When the sera from pS2-dnRII mice were reacted with pancreatic acinar cell antibodies isolated from sera of Crohn’s enteritis (Euroimmune) before applying to monkey pancreatic tissue, the positive immunofluorescence noted in Figure 5b disappeared (Figure 5d). This observation suggests that pS2-dnRII mice possess the IgG-type autoantibody in the sera and are subject to autoimmune response that causes pancreatitis. Moreover, IgA-type autoantibodies against pancreatic ductal cells were also observed in the sera from the pS2-dnRII mice (Figure 5f). One of the known IgA-type autoantigens of the pancreatic duct, SP 3-1 (26, 27), was studied, and SP 3-1 expression was significantly increased in pS2-dnRII mice compared with wild-type littermates (Figure 5h).
Figure 5.
Autoantibodies in serum of pS2-dnRII transgenic mice before cerulein treatment. IgG-type autoantibodies are identified by indirect immunofluorescence using monkey pancreatic sections and an FITC-coupled rat anti-mouse immunoglobulin polyclonal antibody. (a) The absence of autoantibody in the sera of wild-type littermates is shown. (b) The presence of antipancreatic acinar cell antibodies in the serum of pS2-dnRII mice is shown. Positive sera containing IgG-autoantibodies showed blurry, droplike staining in pancreatic acinar cells. When the sera from pS2-dnRII transgenic mice were reacted with proteins extracted from the pancreata of pS2-dnRII mice, the positive staining seen in b disappeared (d). Result of staining using sera from the wild-type mice reacted with protiens from the pancreata of wild-type mice is shown (c). IgA-type autoantibody against the pancreatic duct was noted in the sera of pS2-dnRII transgenic mice (f), whereas such antibody was absent in the sera of wild-type littermates (e). Expressions of SP3-1, an autoantigen of pancreatic ductal cells, were also markedly increased in the pancreatic duct of pS2-dnRII mice (h) compared with that of wild-type littermates (g).
Increased MMP activities in the pancreata of pS2-dnRII transgenic mice compared with that of wild-type littermates.
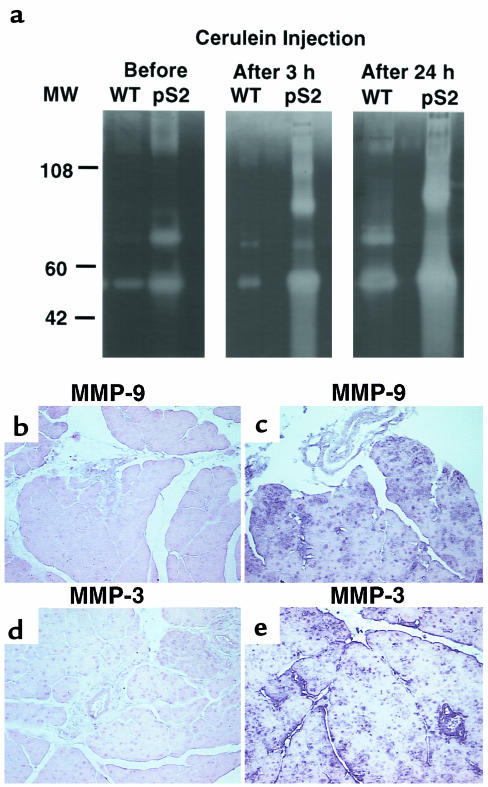
Because TGF-β is known to be an important regulator of MMP and a tissue inhibitor of metalloproteinase, we studied the changes of MMP activity in the pancreata of pS2-dnRII transgenic mice. Even before inducing pancreatitis, MMP-3 and MMP-9 were elevated in tissue homogenates from pS2-dnRII mice compared with those from wild-type littermates, and MMP activities were significantly elevated after the induction of pancreatitis (Figure 6a). In situ hybridization showed that MMP-3 and MMP-9 mRNA were significantly increased in the pancreata of pS2-dnRII mice (Figure 6b, II and IV) compared to that of wild-type littermates (Figure 6b, I and III).
Figure 6.
MMP activities in pancreatic homogenates of wild-type and pS2-dnRII transgenic mice. (a) Increased MMP activities. Even before the induction of pancreatitis, the pancreatic extracts of transgenic mice showed increased 52-kDa, 72-kDa, and 92-kDa MMP activities, which became more prominent after the induction of pancreatitis. Even using only one tenth the amounts of protein compared with the wild-type littermates, MMP activities were significantly increased in the pancreata of pS2-dnRII mice. (b–e) In situ hybridization of MMP-3 and MMP-9 mRNAs. MMP-3 and MMP-9 mRNAs were significantly increased in the pancreata of pS2-dnRII mice (c and e) compared with that of wild-type littermates (b and d).
Discussion
TGF-β1–null mice showed multifocal inflammatory infiltrations in multiple organs, including the pancreas. However, because of the short life span and severe inflammatory infiltrations in most organs, we cannot study the effect on individual organs for susceptibility to disease. Furthermore, for the pancreatitis seen in TGF-β1–null mice, we do not know the relative contribution of lack of TGF-β1 in the pancreatic acinar cells versus infiltrating lymphocytes. Our study allows us to investigate loss of TGF-β signaling specifically within pancreatic acinar cells while the animals retain an intact immune response.
One of the most frequently used models for pancreatitis involves the administration of high doses of cerulein. A widely accepted hypothesis is that cerulein hyperstimulation leads to intracellular digestive enzyme activation, which, in turn, causes pancreatitis. In our study wild-type mice showed increasing severity of pancreatitis throughout the course of 24 hours based on the majority of the examined parameters. Only myeloperoxidase measurements had started to decrease by the last time point. The transgenic mice tended to show an increased severity of disease at earlier time points compared with the wild-type, and they also generally showed greater maxima for these parameters. For the serum amylase levels, however, the maxima for both transgenic and wild-type mice were the same, raising the possibility that the effect of the transgene is to hasten the onset of the disease rather than increase its severity.
MHC class II molecules are normally expressed on limited cell types involved in presenting antigens to T-helper cells. These cell types include B lymphocytes, activated T lymphocytes and macrophages, dendritic cells, glial cells, and thymic epithelial cells. Yet in certain autoimmune diseases, immune activity appears to be directed against cells that inappropriately express MHC class II molecules, such as β cells in the diabetic pancreas and thyroid cells in Grave’s disease (28–30). TGF-β–null mice exhibit enhanced expression of MHC class II antigens at or before the onset of the inflammatory process, but the characteristic inflammatory lesions do not develop in TGF-β–null mice crossed into the severe combined immunodeficiency background (14). Prominently increased MHC class II expression was noted in the pancreatic acinar cells of transgenic mice. The elevated expression of MHC class II antigens was seen before any evidence of inflammatory infiltrates, and its expression became more prominent after inducing pancreatitis in the transgenic mice. The aberrant MHC class II expression in the targeted organ might provide a Th-mediated inflammatory milieu that quickly exacerbates the damage.
The inflammatory response of autoimmune disease is thought to be mediated by release of many cytokines, including IL-1, TNF-α, and IFN-γ. These cytokines are normally released from activated immune cells in response to foreign antigens. In autoimmune disease, activation of immune cells is frequently elicited by the presentation of self antigens in the context of cell-surface MHC molecules by cells typically uninvolved in antigen presentation. Our transgenic mice typically showed increased expression of these cytokines even before the induction of pancreatitis, which suggests that loss of TGF-β signaling leads to the presentation of autoantigens by these aberrantly expressed MHC class II molecules, thereby initiating the increased production of this array of cytokines and indicating Th lymphocyte hyperactivation. Norman et al. (31) evaluated tissue-specific cytokine production during cerulein-induced acute pancreatitis. There was no cytokine mRNA or protein detectable before induction of pancreatitis. TNF-α and IL-1β mRNA and protein were detected within the pancreas early in the course of pancreatitis, coinciding with the development of hyperamylasemia (32). Interestingly, TNF-α and IFN-γ expression was markedly increased in the pS2-dnRII transgenic mice even before induction of inflammation. Increased expression of TNF-α and IFN-γ might be related to increased susceptibility to inflammatory response and autoimmune response. Conversely, levels of mRNA for IFN-γ were nearly identical in TGF-β1–null and –heterozygous mice (15). It is possible that expression of IFN-γ mRNA is unchanged in TGF-β1–null mice because the presence of TGF-β2 and TGF-β3 still act to suppress IFN-γ expression. IL-4 and IL-10 expression was also significantly increased in the pS2-dnRII transgenic mice even before the induction of pancreatitis. Cerulein treatment induced expression of IL-4 and IL-10 in the wild-type mice, but only slightly increased expression of these cytokines in the transgenic mice. Enhanced expression of IL-4 and IL-10 in the pS2-dnRII transgenic mice may be a compensatory response to prevent inflammation.
Pancreatitis is occasionally associated with certain autoimmune diseases, such as systemic lupus erythematosus, primary biliary cirrhosis, and Sjogren’s syndrome (33). Recently, Haspel et al. (34) proposed the concept of “multiple organ autoimmunity,” based on the existence of monoclonal multiple organ–reactive autoantibodies or autoantibodies with a common antigenic determinant on different molecules in multiple organs. Although the term “autoimmune pancreatitis” is controversial (35, 36), our current observation suggests that immune derangement with autoimmune characterization seems to be the important pathogenic factor in pancreatitis. In this study, we identified the presence of the autoantibodies in the sera from pS2-dnRII mice, whereas none were detected in wild-type mice. We have also demonstrated that SP3-1 expression is markedly increased in the pancreatic ducts of our pS2-dnRII transgenic mice. SP3-1 mAb recognizes the antigen in duct cells of various exocrine organs and has been reported to be the autoantibody to a pancreatic antigen in patients with idiopathic chronic pancreatitis and Sjogren’s syndrome (37). Mice null for the TGF-β1 gene show that loss of this gene generates circulating systemic lupus erythematosis–like IgG antibodies to nuclear antigens and pathogenic glomerular IgG deposits. However, presence of pancreas-specific autoantibodies has not been demonstrated.
MMPs are secreted and activated extracellularly and can degrade all classes of extracellular matrix (ECM) (38). TGF-β has been known to upregulate tissue inhibitor of metalloproteinases (TIMP) mRNA and downregulate MMP, and, likewise, IL-4 and IL-10 downregulate MMP production and prevent mucosal damage in the gut through TGF-β. In situ hybridization showed interstitial collagenase and stromelysin-1 mRNA in granulation tissue, which is associated with Crohn’s disease, ulcerative colitis, gastric ulcers, and pancreatitis (39). Our transgenic mice show the markedly increased expression of MMPs. The extensive acinar cell destruction seen in the pS2-dnRII mice may have resulted at least in part from markedly increased MMP activities, implicating TGF-β signaling in maintenance of the integrity of pancreatic acinar cells.
Recently, Böttinger et al. (19) have generated transgenic mice of a dominant-negative mutant of TGF-β RII under the control of a mouse metallothionein promoter. They found increased cell proliferation in pancreatic acinar cells and perturbed acinar differentiation. In pS2-dnRII mice, the pancreatic acinar cells also showed increased proliferation. However, some of phenotypic changes observed in the pancreata of the MT1-dnRII were not seen in our mice. The current experiment was performed using 3–4-month-old mice. We are planning to examine older mice to see whether these mice develop increased proliferation in acinar cells, fibrosis, neoangiogenesis, and macrophage infiltration.
In conclusion, TGF-β signaling seems to be a critical determinant in maintaining immune homeostasis in the pancreas. Disturbances in TGF-β signaling through the overexpression of a dominant-negative mutant of TGF-β RII impaired normal immune homeostasis, which resulted in the dysregulation of the immune system and production of autoantibodies against target cells, from which point pathological inflammation might be initiated and aggravated. Although scattered cases showing the autoimmune background as the plausible pathogenesis of pancreatitis have been reported (40–42), current observation suggests that an autoimmune mechanism might be one of the important pathogenic events in pancreatitis.
Acknowledgments
We thank M.C. Rio for mouse pS2 gene, I. Nishimore for SP 3-1 antibody, and A.B. Roberts for critically reviewing the manuscript.
Footnotes
K.-B. Hahm’s present address is: Department of Gastroenterology, Ajou University School of Medicine, San 5, Wonchon-dong, Paldal-ku, Suwon, 442-749, Korea.
References
- 1.Saluja AK, Steer ML. Pathophysiology of pancreatitis. Digestion. 1999;60:27–33. doi: 10.1159/000051450. [DOI] [PubMed] [Google Scholar]
- 2.Lerch MM, et al. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opposum. Gastroenterology. 1993;104:853–861. doi: 10.1016/0016-5085(93)91022-a. [DOI] [PubMed] [Google Scholar]
- 3.Lombardi B, Estes LW, Longnecker DW. Acute hemorrhagic pancreatitis (massive necrosis) with fat necrosis induced in mice by dl-ethionine fed with a choline-deficient diet. Am J Pathol. 1975;79:465–480. [PMC free article] [PubMed] [Google Scholar]
- 4.Lampel M, Kern J. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch. 1977;373:97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- 5.Norman JG. New approaches to acute pancreatitis: role of inflammatory mediators. Digestion. 1999;60:57–60. doi: 10.1159/000051455. [DOI] [PubMed] [Google Scholar]
- 6.Gloor B, Todd KE, Lane JS, Rigberg DA, Reber HA. Mechanism of increased lung injury after acute pancreatitis in IL-10 knockout mice. J Surg Res. 1998;80:110–114. doi: 10.1006/jsre.1997.5289. [DOI] [PubMed] [Google Scholar]
- 7.Berney T, et al. Serum profile of interleukin-6, interleukin-8, and interleukin-10 in patients with severe and mild acute pancreatitis. Pancreas. 1999;18:371–377. doi: 10.1097/00006676-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Van Laethem JL, et al. Multisystemic production of interleukin 10 limits the severity of acute pancreatitis in mice. Gut. 1998;43:408–413. doi: 10.1136/gut.43.3.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 11.Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284–288. doi: 10.1016/s0039-6060(96)80299-6. [DOI] [PubMed] [Google Scholar]
- 12.Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor-β (TGF-β) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- 13.Markowitz SD, Roberts AB. Tumor suppressor activity of the TGF-β pathway in human cancers. Cytokine Growth Factor Rev. 1996;7:93–102. doi: 10.1016/1359-6101(96)00001-9. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni AB, et al. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geiser AG, et al. Transforming growth factor β1 (TGF-β1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-β1 null mice phenotype. Proc Natl Acad Sci USA. 1993;90:9944–9948. doi: 10.1073/pnas.90.21.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letterio JJ, et al. Autoimmunity associated with TGF-β1-deficiency in mice is dependent on MHC class II antigen expression. J Clin Invest. 1996;98:2109–2119. doi: 10.1172/JCI119017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefebvre O, et al. Gastric mucosa abnormalities and tumorigenesis in mice lacking the pS2 trefoil protein. Science. 1996;274:259–262. doi: 10.1126/science.274.5285.259. [DOI] [PubMed] [Google Scholar]
- 18.Ebert PPA, et al. Induction of TFF1 gene expression in pancreas overexpressing transforming growth factor α. Gut. 1999;45:105–111. doi: 10.1136/gut.45.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Böttinger EP, et al. Expression of a dominant negative mutant TGF-β type II receptor in transgenic mice reveals essential role for TGF-β in regulation of growth and differentiation in the exocrine pancreas. EMBO J. 1997;16:2621–2633. doi: 10.1093/emboj/16.10.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercer EH, Hoyle GW, Kapur RP, Brinster RL, Palmiter RD. The dopamine beta-hydroxylase gene promoter directs expression of E. coli lacZ to sympathetic and other neurons in adult transgenic mice. Neuron. 1991;7:703–716. doi: 10.1016/0896-6273(91)90274-4. [DOI] [PubMed] [Google Scholar]
- 21.Zhou N, et al. A competitive RT-PCR method for the quantitative analysis of cytokine mRNAs in mouse tissues. Cytokine. 1997;9:212–218. doi: 10.1006/cyto.1996.0156. [DOI] [PubMed] [Google Scholar]
- 22.Abe R, et al. Lipopolysaccharide-induced desensitization to pancreatic edema formation in rat cerulein pancreatitis. Pancreas. 1998;16:539–544. doi: 10.1097/00006676-199805000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt J, et al. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44–56. doi: 10.1097/00000658-199201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krawisz JE, Sharon R, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity–assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 25.Castellino F, Zhong G, Germain RN. Antigen presentation by MHC class II molecules: invariant chain function, protein trafficking, and the molecular basis of diverse determinant capture. Hum Immunol. 1997;54:159–169. doi: 10.1016/s0198-8859(97)00078-5. [DOI] [PubMed] [Google Scholar]
- 26.Onodera M, Okazaki K, Morita M, Nishimori I, Yamamoto Y. Immune complex specific for the pancreatic duct antigen in patients with idiopathic chronic pancreatitis and Sjogren syndrome. Autoimmunity. 1994;19:23–29. doi: 10.3109/08916939409008005. [DOI] [PubMed] [Google Scholar]
- 27.Kino-Ohsaki J, et al. Serum antibodies to carbonic anhydrase I and II in patients with idiopathic chronic pancreatitis and Sjogren’s syndrome. Gastroenterology. 1996;110:1579–1586. doi: 10.1053/gast.1996.v110.pm8613065. [DOI] [PubMed] [Google Scholar]
- 28.Jalleh RP, Gilbertson JA, Williamson RCN, Slater SD, Foster CS. Expression of major histocompatibility antigens in human chronic pancreatitis. Gut. 1993;34:1452–1457. doi: 10.1136/gut.34.10.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bedossa P, Bacci J, Lemaigre G, Martin E. Lymphocyte subsets and HLA-DR expression in normal pancreas and chronic pancreatitis. Pancreas. 1990;5:415–420. doi: 10.1097/00006676-199007000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Ogami Y, Otsuki M. Exocrine pancreatic physiology: overview. Pancreas. 1998;16:265–272. doi: 10.1097/00006676-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Norman JG, et al. Tissue-specific cytokine production during experimental acute pancreatitis. A probable mechanism for distant organ dysfunction. Dig Dis Sci. 1997;42:1783–1788. doi: 10.1023/a:1018886120711. [DOI] [PubMed] [Google Scholar]
- 32.Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76–83. doi: 10.1016/s0002-9610(97)00240-7. [DOI] [PubMed] [Google Scholar]
- 33.Nishimori I, et al. Identification of autoantibodies to a pancreatic antigen in patients with idiopathic chronic pancreatitis and Sjogren’s syndrome. Pancreas. 1994;9:374–381. doi: 10.1097/00006676-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Haspel MV, et al. Multiple organ-reactive monoclonal autoantibodies. Nature. 1983;304:73–76. doi: 10.1038/304073a0. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida K, et al. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]
- 36.Horiuchi A, et al. Autoimmune chronic pancreatitis simulating pancreatic lymphoma. Am J Gastroenterol. 1996;91:2607–2609. [PubMed] [Google Scholar]
- 37.Nishimori I, et al. Specific cellular immune response to pancreatic antigen in chronic pancreatitis and Sjogren’s syndrome. J Clin Immunol. 1993;13:265–271. doi: 10.1007/BF00919385. [DOI] [PubMed] [Google Scholar]
- 38.Birkedal-Hansen H, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- 39.Delany AM, Brinckerhoff CE. Post-transcriptional regulation of collagenase and stromelysin gene expression by epidermal growth factor and dexamethasone in cultured human fibroblasts. J Cell Biochem. 1992;50:400–410. doi: 10.1002/jcb.240500409. [DOI] [PubMed] [Google Scholar]
- 40.Nishimori I, Fujikawa-Adachi K, Onishi S, Hollingsworth MA. Carbonic anhydrase in human pancreas: hypotheses for the pathophysiological roles of CA isozymes. Ann NY Acad Sci. 1999;880:5–16. doi: 10.1111/j.1749-6632.1999.tb09505.x. [DOI] [PubMed] [Google Scholar]
- 41.Frulloni L, et al. Salivary gland involvement in patients with chronic pancreatitis. Pancreas. 1999;19:33–38. doi: 10.1097/00006676-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida K, et al. Chronic pancreatitis caused by an autoimmune abnormality; proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561–1568. doi: 10.1007/BF02285209. [DOI] [PubMed] [Google Scholar]