Abstract
Cholesterol is an important lipid of mammalian cells. Its unique physicochemical properties modulate membrane behavior and it serves as the precursor for steroid hormones, oxysterols and vitamin D. Cholesterol is effluxed from the late endosomes/lysosomes via the concerted action of at least two distinct proteins: Niemann-Pick C1 and Niemann-Pick C2. Mutations in these two proteins manifest as Niemann-Pick type C disease – a very rare, usually fatal, autosomal, recessive, neurovisceral, lysosomal storage disorder. In this review we discuss the possible mechanisms of action for NPC1 and NPC2 in mediating cholesterol efflux, as well as the different therapeutic approaches being pursued for the treatment of this lipid storage disorder.
Keywords: cyclodextrin, miglustat, acid sphingomyelinase, lysosomal storage organelle, lysosomal acid lipase, bis(monoacylglycerol)phosphate
Overview
Cholesterol is a critical lipid of mammalian cells. Its unique physicochemical properties modulate membrane behavior and it serves as the precursor for steroid hormones, oxysterols and vitamin D and its deregulation affects numerous pathological processes. In the peripheral tissues of mammals there are two sources of cholesterol. Cholesterol can be supplied exogenously, being delivered to the cells as part of receptor-mediated lipoprotein uptake. Alternatively, cholesterol can be generated via de novo synthesis in the endoplasmic reticulum (ER). In the central nervous system (CNS), the latter pathway is utilized exclusively due to the inability of LDL particles to cross the blood-brain barrier. Free cholesterol synthesized in the glia and complexed with apolipoprotein E (ApoE) is delivered to neurons to supplement their endogenous sterol production (Dietschy & Turley 2004). Cholesterol homeostasis is integrated at the cellular level by the tight regulation via the SCAP/INSIG/SREBP sterol sensing system. In the peripheral tissues, after lipoprotein delivery to late endosomes/lysosomes (LE/LY), cholesterol is liberated from cholesteryl esters by lysosomal acid lipase (LAL, EC 3.1.1.3), an acidic hydrolase that also cleaves triacylglycerides and is part of the lipase family that includes gastric and lingual lipases (Anderson & Sando 1991). LAL is produced as a proenzyme that is targeted to the LE/LY via mannose-6-phosphate modification (Sando & Henke 1982), and its deficiency results in either Wolman disease or its milder variant, Cholesteryl Ester Storage Disease (CESD) (Sloan & Fredrickson 1972, Assmann 1995). The difference in the severity of the phenotype between CESD and Wolman disease originates primarily from the levels of residual enzymatic activity of LAL, depending on the molecular nature of the mutation (Anderson et al. 1999). Enzyme replacement therapy has been pursued as a treatment option for Wolman/CESD (Du et al. 2001). In addition, LAL has been suggested to play a significant role in atherosclerosis (Du & Grabowski 2004, Du et al. 2004, Grosheva et al. 2009, Haka et al. 2009), thus generating further interest in elucidating its function in cells and obtaining tools (e.g., specific small molecule inhibitors) for such studies (Rosenbaum et al. 2010a).
Cholesterol is effluxed from the LE/LY via the concerted action of at least two distinct proteins: Niemann-Pick C1 (NPC1) (Carstea et al. 1997) and Niemann-Pick C2 (NPC2) (Naureckiene et al. 2000). In normal cells, cholesterol is exported from the LE/LY and delivered to other organelles, including the plasma membrane, the endocytic recycling compartment and the endoplasmic reticulum (Mesmin & Maxfield 2009). The exact nature of intracellular cholesterol transport is an area of active investigation (Mesmin & Maxfield 2009), with both vesicular and non-vesicular transport mechanisms playing a role (Maxfield & Wustner 2002, Hao et al. 2002). Elevated levels of cholesterol in the ER lead to increased esterification by acyl Co-A:cholesterol acyl transferase (ACAT) (Goldstein et al. 1974). Cholesterol esters formed by ACAT are stored as lipid droplets that are hydrolyzed by cytoplasmic neutral cholesterol ester hydrolase (Okazaki et al. 2008, Small et al. 1989). Cellular cholesterol can also be exported to extracellular acceptors, such as high density lipoproteins, in a process mediated by ATP-binding cassette transporters such as ABCA1 (Tall 2003).
Mutations in the two NPC proteins manifest as NPC disease – a rare, usually fatal, autosomal, recessive, lysosomal storage disorder. In both types of NPC disease cholesterol, bis(monoacylglycerol)phosphate (BMP), and various sphingolipids accumulate in specialized compartments, lysosomal storage organelles (LSOs), leading to altered protein and lipid trafficking (Pipalia et al. 2007, Choudhury et al. 2004). While most of the evidence points to both LDL-derived (Pentchev et al. 1985) and non-LDL-derived (Pipalia et al. 2007) cholesterol as the primary storage metabolite in NPC disease, especially in the peripheral tissues, other possibilities include glycosphingolipids, sphingomyelin, and sphingosine (Lloyd-Evans & Platt 2010). In the CNS, the GM2 and GM3 gangliosidoses are particularly prominent, while cholesterol accumulations is present, albeit to a lesser degree than what is observed in the peripheral tissues (Zervas et al. 2001).
Because of this defect in cholesterol trafficking, re-esterification of lipoprotein-derived cholesterol by ACAT is reduced (Lin et al. 2003, Pentchev et al. 1985). In addition, cholesterol levels in the plasma membrane of NPC-deficient cells may be low, even though total cellular cholesterol is increased (Sokol et al. 1988). Clinical manifestations of NPC disease include severe neuronal degeneration, hepatosplenomegaly, and effects in other organs (Patterson et al. 2001).
Possible mechanism for NPC1 and NPC2 role in cholesterol efflux
NPC2 has been shown to transfer cholesterol in vitro between model membranes (Cheruku et al. 2006) and from membranes to NPC1-N-terminal domain (NPC1(NTD)) (Infante et al. 2008b). The crystal structure of NPC2 was solved in both the apo form (Friedland et al. 2003) and with cholesterol sulfate bound (Xu et al. 2007). From these structures it appears that there are no major conformational changes outside of the binding pocket of NPC2 associated with ligand binding. The structure of the NPC2/cholesterol sulfate complex has revealed that the ligand was positioned in the binding cavity with the iso-octyl side chain facing inwards and the sulfate moiety facing outwards to the solvent. Assuming that cholesterol sulfate accurately represents cholesterol in this binding interaction, this orientation presents a topological problem for cholesterol extraction from and delivery to lipid membranes since cholesterol is positioned with the β-OH facing towards the hydrophilic solvent and with the iso-octyl side chain facing into the hydrophobic bilayer (Nakatani et al. 1996). Consequently, for NPC2 to bind cholesterol directly from or deliver to membranes in the orientation observed for cholesterol sulfate in the crystal structure, cholesterol would need to reverse its orientation as it transfers to or from the bilayer for proper positioning in the binding pocket of NPC2.
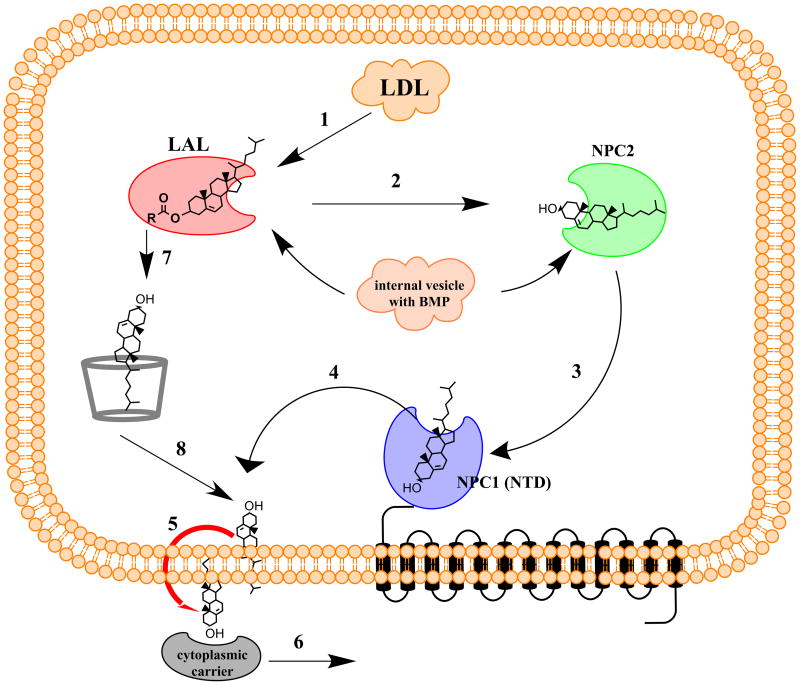
Alternatively, if NPC2 acts to transfer cholesterol from a protein donor or to a protein acceptor, which binds cholesterol in a complementarily reverse orientation (i.e., with the iso-octyl side chain facing towards the solvent and with the β-OH facing inwards to a binding pocket) then no flipping of cholesterol would be required and NPC2 could shuttle cholesterol within the intralysosomal lumen and thus facilitate its efflux from that compartment. Precisely this complementary orientation has been observed in the recently elucidated crystal structure of the NPC1(NTD) (Kwon et al. 2009) and is supported by substantial biochemical evidence (Infante et al. 2008a). In one possible model of NPC1/2 function, upon cholesteryl ester hydrolysis by LAL, in the peripheral tissues, liberated cholesterol is transferred directly from LAL to NPC2 and then from NPC2 to NPC1(NTD) and then out of the lysosomal compartment (Figure 1). The structure of LAL is yet to be elucidated, however biochemical evidence suggests that LAL has significant preference for the length, number and the saturation of the fatty acid acyl chains, and only a weak preference for the nature of the esterified alcohol (Imanaka et al. 1984). This is consistent with LAL’s ability to hydrolyze both cholesteryl esters and triacylglycerides. This preference points to the possibility that cholesteryl esters might be accommodated in the LAL binding pocket with an orientation that would facilitate the transfer of cholesterol to NPC2. Interestingly, both NPC2 (Cheruku et al. 2006) and LAL (Makino et al. 2006) activity in vitro are stimulated significantly by the presence of BMP, a specialized lipid of lysosomal membranes. BMP levels correlate with cholesterol levels in LE/LY (Chevallier et al. 2008), and BMP has been shown to accumulate in the LSOs of NPC cells (Kobayashi et al. 1999), possibly as a homeostatic response to increased cholesterol levels. It is unclear what role LAL might play in this model as it applies to the CNS as it is the free cholesterol/ApoE complex that mediates cholesterol intercellular transport in the CNS (Dietschy & Turley 2004).
Figure 1.
Model for NPC1, NPC2 and cyclodextrin action in cholesterol efflux from LE/LY. In normal cells, cholesteryl esters are hydrolyzed by LAL to produce free cholesterol and fatty acid (1), then free cholesterol is transferred to NPC2 (2), and subsequently to NPC1(NTD) (3). Free cholesterol is then delivered to the luminal leaflet of the limiting membrane of LE/LY (4), where it can spontaneously flip to the cytosolic side (5) and then be carried to its various destinations in the cell by cytoplasmic carriers (6). In the absence of functional NPC2 or NPC1, cyclodextrins can replace their functions in promoting cholesterol efflux (7, 8), bypassing steps 2–4. BMP stimulates the activities of LAL and NPC2. In the CNS, free cholesterol is shuttled from the glia to neurons via an ApoE complex, and no plasma LDL is believed to permeate the blood-brain barrier. Thus, all the CNS cholesterol is supplied through endogenous synthesis.
While substantial biochemical characterization of the role of NPC1 and NPC2 in efflux of cholesterol from the LE/LY has been underway, precise elucidation of their molecular mechanism of action has been elusive. One major obstacle in establishing this mechanism has been inability of observing the proposed interaction of NPC1 and NPC2, although potential interaction surfaces of these proteins have been identified (Wang et al. 2010). Another obstacle has been, until the recent advances in the Brown and Goldstein group (Infante et al. 2008a), the availability of purified NPC1 protein for biochemical studies.
Potential NPC disease therapeutics
Substrate reduction strategies
Several strategies have been attempted for treatment of NPC disease. Substrate reduction approaches including statins, low-cholesterol diet and ezetimibe treatments, while reducing peripheral cholesterol burden failed in affecting NPC disease neuropathology and clinical outcomes (Patterson & Platt 2004, Liu et al. 2009). An alternative substrate reduction strategy for the remedy of the visceral phenotype would be to inhibit cholesterol production via the hydrolysis of cholesteryl esters by LAL. This approach has been shown to cause significant reduction in cholesterol storage in human NPC-defective cells (Rosenbaum et al. 2009), albeit accumulation of neutral lipids as the result of LAL inhibition might lead to Wolman disease-like complications at high levels of LAL inhibition and would not affect delivery of free cholesterol mediated by ApoE in the CNS. These substrate reduction approaches are not expected to correct insufficient steroidogenesis, which has been suggested to be an important part of NPC neuropathology (Griffin et al. 2004) or to affect delivery of free cholesterol mediated by ApoE in the CNS.
Miglustat and Acid Sphingomyelinase treatments
Miglustat (N-butyl-deoxynojirimycin, Zavesca®) has been shown to reduce disease progression in NPC patients in clinical trials (Pineda et al. 2009, Wraith et al., Patterson et al. 2007). Miglustat was originally developed as a therapeutic for Gaucher disease, a β-glucosidase deficiency (Pastores & Barnett 2003). Miglustat reduces glucosylceramide accumulation by inhibiting glucosylceramide synthase. Thus, miglustat leads to a reduction of glucosylceramide and an increase of ceramide levels. Chemical potential of sterols in membranes has been proposed to be a key regulatory factor in establishing cholesterol levels in different cellular compartments (Maxfield & Menon 2006, Mesmin & Maxfield 2009). Thus, alterations in membrane composition could lead to changes in the chemical activity coefficient of cholesterol and consequently changes in the distribution of cholesterol among membranes. Ceramide shares some chemical similarity with cholesterol in terms of having a relatively small polar headgroup (a hydroxyl in both cases) and it has been shown to displace cholesterol in lipid domains in model membranes (Megha & London 2004). Consequently, ceramide could alter the chemical activity coefficient of cholesterol in biological membranes, thus facilitating sterol efflux from the LSOs. Similarly, a reduction in sphingolipid (glucosylceramide itself and the complex gangliosides, for which it serves as a metabolic precursor) levels could lead to a similar increase in sterol chemical activity coefficient and increased cholesterol efflux.
Recent investigation of the interplay of sphingolipids and cholesterol in NPC disease has focused on acid sphingomyelinase (ASM) deficiency associated with NPC disease (Devlin et al. 2010). This study demonstrated that despite a normal ASM gene, NPC-deficient cells have much lower levels of ASM activity. Restoration of ASM activity levels via either transfection or exogenous addition of recombinant ASM reverted both cholesterol and BMP accumulation in both NPC1 and NPC2 mutant cells, and increased ASM activity restored normal protein trafficking (e.g., transferrin recycling). This beneficial outcome of the correction of a secondary defect indicates that NPC disease is a complex, multifaceted, disorder whose pathogenesis may include positive feedback loops that exacerbate the observed phenotype. This finding opens up new, previously unconsidered, pathways for treatment strategies, and it encourages the use of parallel therapeutic approaches that target multiple aspects of NPC pathology, and in particular the storage of glycosphingolipids associated with the neuropathology of NPC disease.
Cyclodextrins
An important part of the NPC disease pathology involves insufficient delivery of cholesterol to mitochondria for steroid synthesis. Neurosteroid allopregnenolone (ALLO) has been shown deficient in NPC disease. Treatments of npc1−/− mice with ALLO, solubilized with 20 % hydroxypropyl-β-cyclodextrin (HPβCD), demonstrated significant improvements in murine viability and neuropathology (Griffin et al. 2004). However, subsequent studies by multiple investigators (Liu et al. 2009, Liu et al. 2010, Davidson et al. 2009) have shown that HPβCD injections alone were responsible for most of the effect of HPβCD+ALLO. These investigations have demonstrated that single injections into npc1−/− mice at P7 significantly reduced the NPC-phenotype in the brain, liver and other organs and resulted in increased viability. Single injections administered on later days were significantly less effective. Repeated injections (Davidson et al. 2009, Ramirez et al. in press) were more effective in further prolongation of lifespan due to rapid elimination of cyclodextrins (CDs) in the urine (Liu et al. 2010). The decrease in efficacy in animals treated at later days could be attributed to at least two factors: closure of the blood-brain barrier and the progressive nature of NPC disease neuropathology.
CDs are membrane-impermeant cyclic oligosaccharides, which have been used extensively to modulate cholesterol and other lipids’ composition of model and biological membranes (Zidovetzki & Levitan 2007) as well as to solubilize various pharmaceuticals (Davis & Brewster 2004). Despite their inability to cross membranes, CDs have been shown to be retained by cells (Kilsdonk et al. 1995). CDs can be delivered via pinocytosis to LE/LY, where they can replace the function of NPC1 and NPC2 proteins (Rosenbaum et al. 2010b) and promote cholesterol esterification by acetyl CoA:acyl transferase (Abi-Mosleh et al. 2009). The exact nature of CD’s mechanism of action to replace NPC1 and NPC2 function in LE/LY is yet to be determined. One possibility would be that CDs could just solubilize cholesterol and thus promote its efflux via vesicular transport. Data with 70 kDa CD-dextran polymers (Rosenbaum et al. 2010b) that have been shown to be competent in reducing cholesterol storage and that colocalize with filipin staining of LSOs argue against this possibility since large dextran polymers have been shown to remain in LE/LY for extended periods of time (Mukherjee et al. 1997). Our preferred explanation for CDs action in this process is that they can act catalytically to shuttle cholesterol from LAL directly to the limiting membrane of the lysosome, bypassing the potential requirement for NPC2-NPC1 interaction (Figure 1). Once delivered to the lumenal leaflet of the limiting membrane, cholesterol can then spontaneously flip to the cytosolic leaflet and be carried away to various destinations in the cell, via still poorly characterized transport processes (Maxfield & Menon 2006).
From the clinical treatment perspective, further optimization of the pharmacological properties of CD-based NPC therapeutics would be necessary to enhance CD delivery to the lysosomal compartment, enhance transcytosis across the blood-brain barrier, and decrease CD elimination through urine.
Chemical Chaperones
Proteostasis modulation with chemical chaperones has been proposed as a therapy for a series of folding diseases, including lysosomal storage disorders (Balch et al. 2008). Enhancement of folding of the I1061T mutant of NPC1 protein with non-specific chemical chaperones that caused better trafficking of NPC1 to LE/LY and reduced NPC phenotype has been recently demonstrated in cultured cells (Gelsthorpe et al. 2008). This finding encourages further development of patient-specific chemical chaperone therapy for NPC disease. Further work would be needed in identifying the specific mutations that would be susceptible to such treatment as well as other chemical chaperones that might be more specific to NPC disease.
Summary
While there are still remaining questions about the precise molecular mechanism of NPC1 and NPC2-mediated cholesterol efflux from LE/LY, our understanding of this critical step in cellular cholesterol homeostasis is rapidly improving. In addition, the rapid pace of the current development of novel therapies for NPC disease, less than two decades after the identification of the proteins involved, offers renewed hope for the NPC patients that suffer from this severe disorder. Moreover, recent reports of the role for NPC1 in Alzheimer’s disease (Kosicek et al. 2010, Malnar et al. 2010, Kagedal et al. 2010) and human immunodeficiency virus infection (Tang et al. 2009) make NPC disease research relevant to much broader audiences.
Acknowledgments
The authors thank the members of the Maxfield laboratory for helpful discussions and the Ara Parseghian Medical Research Foundation and NIH (grant R37-DK27083) for financial support.
Abbreviations
- ALLO
allopregnenolone
- ASM
acid sphingomyelinase
- ApoE
apolipoprotein E
- BMP
bis(monoacylglycerol)phosphate
- CNS
central nervous system
- CD
cyclodextrin
- HPβCD
hydroxypropyl-β-cyclodextrin
- LAL
lysosomal acid lipase
- LE/LY
late endosomes/lysosomes
- LSO
lysosomal storage organelle
- NPC
Niemann-Pick type C disease
- NPC1(NTD)
NPC1-N-terminal domain
Footnotes
The authors declare no conflict of interest.
References
- Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc Natl Acad Sci U S A. 2009;106:19316–19321. doi: 10.1073/pnas.0910916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Bryson GM, Parks JS. Lysosomal acid lipase mutations that determine phenotype in Wolman and cholesterol ester storage disease. Mol Genet Metab. 1999;68:333–345. doi: 10.1006/mgme.1999.2904. [DOI] [PubMed] [Google Scholar]
- Anderson RA, Sando GN. Cloning and expression of cDNA encoding human lysosomal acid lipase/cholesteryl ester hydrolase. Similarities to gastric and lingual lipases. J Biol Chem. 1991;266:22479–22484. [PubMed] [Google Scholar]
- Assmann G, Seedorf U. Acid Lipase Deficiency: Wolman Disease and Cholesteryl Ester Storage Disease. In: Valle B, Vogelstein, Kinzler, Antonarakis, Ballabio, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 1995. [Google Scholar]
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, et al. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281:31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- Chevallier J, Chamoun Z, Jiang G, Prestwich G, Sakai N, Matile S, Parton RG, Gruenberg J. Lysobisphosphatidic acid controls endosomal cholesterol levels. J Biol Chem. 2008;283:27871–27880. doi: 10.1074/jbc.M801463200. [DOI] [PubMed] [Google Scholar]
- Choudhury A, Sharma DK, Marks DL, Pagano RE. Elevated endosomal cholesterol levels in Niemann-Pick cells inhibit rab4 and perturb membrane recycling. Mol Biol Cell. 2004;15:4500–4511. doi: 10.1091/mbc.E04-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson CD, Ali NF, Micsenyi MC, Stephney G, Renault S, Dobrenis K, Ory DS, Vanier MT, Walkley SU. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS One. 2009;4:e6951. doi: 10.1371/journal.pone.0006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov. 2004;3:1023–1035. doi: 10.1038/nrd1576. [DOI] [PubMed] [Google Scholar]
- Devlin C, Pipalia NH, Liao X, Schuchman EH, Maxfield FR, Tabas I. Improvement in lipid and protein trafficking in Niemann-Pick C1 cells by correction of a secondary enzyme defect. Traffic. 2010;11:601–615. doi: 10.1111/j.1600-0854.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietschy JM, Turley SD. Thematic review series: brain Lipids. Cholesterol metabolism in the central nervous system during early development and in the mature animal. J Lipid Res. 2004;45:1375–1397. doi: 10.1194/jlr.R400004-JLR200. [DOI] [PubMed] [Google Scholar]
- Du H, Grabowski GA. Lysosomal acid lipase and atherosclerosis. Curr Opin Lipidol. 2004;15:539–544. doi: 10.1097/00041433-200410000-00007. [DOI] [PubMed] [Google Scholar]
- Du H, Schiavi S, Levine M, Mishra J, Heur M, Grabowski GA. Enzyme therapy for lysosomal acid lipase deficiency in the mouse. Hum Mol Genet. 2001;10:1639–1648. doi: 10.1093/hmg/10.16.1639. [DOI] [PubMed] [Google Scholar]
- Du H, Schiavi S, Wan N, Levine M, Witte DP, Grabowski GA. Reduction of atherosclerotic plaques by lysosomal acid lipase supplementation. Arterioscler Thromb Vasc Biol. 2004;24:147–154. doi: 10.1161/01.ATV.0000107030.22053.1e. [DOI] [PubMed] [Google Scholar]
- Friedland N, Liou HL, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc Natl Acad Sci U S A. 2003;100:2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelsthorpe ME, Baumann N, Millard E, Gale SE, Langmade SJ, Schaffer JE, Ory DS. Niemann-Pick type C1 I1061T mutant encodes a functional protein that is selected for endoplasmic reticulum-associated degradation due to protein misfolding. J Biol Chem. 2008;283:8229–8236. doi: 10.1074/jbc.M708735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Dana SE, Brown MS. Esterification of low density lipoprotein cholesterol in human fibroblasts and its absence in homozygous familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1974;71:4288–4292. doi: 10.1073/pnas.71.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- Grosheva I, Haka AS, Qin C, Pierini LM, Maxfield FR. Aggregated LDL in contact with macrophages induces local increases in free cholesterol levels that regulate local actin polymerization. Arterioscler Thromb Vasc Biol. 2009;29:1615–1621. doi: 10.1161/ATVBAHA.109.191882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haka AS, Grosheva I, Chiang E, Buxbaum AR, Baird BA, Pierini LM, Maxfield FR. Macrophages create an acidic extracellular hydrolytic compartment to digest aggregated lipoproteins. Mol Biol Cell. 2009;20:4932–4940. doi: 10.1091/mbc.E09-07-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M, Lin SX, Karylowski OJ, Wustner D, McGraw TE, Maxfield FR. Vesicular and non-vesicular sterol transport in living cells. The endocytic recycling compartment is a major sterol storage organelle. J Biol Chem. 2002;277:609–617. doi: 10.1074/jbc.M108861200. [DOI] [PubMed] [Google Scholar]
- Imanaka T, Amanuma-Muto K, Ohkuma S, Takano T. Characterization of lysosomal acid lipase purified from rabbit liver. J Biochem. 1984;96:1089–1101. doi: 10.1093/oxfordjournals.jbchem.a134926. [DOI] [PubMed] [Google Scholar]
- Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC1 protein. I. Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008a;283:1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci U S A. 2008b;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagedal K, Kim WS, Appelqvist H, et al. Increased expression of the lysosomal cholesterol transporter NPC1 in Alzheimer’s disease. Biochim Biophys Acta. 2010;1801:831–838. doi: 10.1016/j.bbalip.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem. 1995;270:17250–17256. doi: 10.1074/jbc.270.29.17250. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- Kosicek M, Malnar M, Goate A, Hecimovic S. Cholesterol accumulation in Niemann Pick type C (NPC) model cells causes a shift in APP localization to lipid rafts. Biochem Biophys Res Commun. 2010;393:404–409. doi: 10.1016/j.bbrc.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Lu X, Chang CC, Chang TY. Human acyl-coenzyme A:cholesterol acyltransferase expressed in chinese hamster ovary cells: membrane topology and active site location. Mol Biol Cell. 2003;14:2447–2460. doi: 10.1091/mbc.E02-11-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Ramirez CM, Miller AM, Repa JJ, Turley SD, Dietschy JM. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J Lipid Res. 2010;51:933–944. doi: 10.1194/jlr.M000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Turley SD, Burns DK, Miller AM, Repa JJ, Dietschy JM. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc Natl Acad Sci U S A. 2009;106:2377–2382. doi: 10.1073/pnas.0810895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E, Platt FM. Lipids on trial: the search for the offending metabolite in Niemann-Pick type C disease. Traffic. 2010;11:419–428. doi: 10.1111/j.1600-0854.2010.01032.x. [DOI] [PubMed] [Google Scholar]
- Makino A, Ishii K, Murate M, et al. D-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol alters cellular cholesterol homeostasis by modulating the endosome lipid domains. Biochemistry. 2006;45:4530–4541. doi: 10.1021/bi052104y. [DOI] [PubMed] [Google Scholar]
- Malnar M, Kosicek M, Mitterreiter S, Omerbasic D, Lichtenthaler SF, Goate A, Hecimovic S. Niemann-Pick type C cells show cholesterol dependent decrease of APP expression at the cell surface and its increased processing through the beta-secretase pathway. Biochim Biophys Acta. 2010;1802:682–691. doi: 10.1016/j.bbadis.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, Menon AK. Intracellular sterol transport and distribution. Curr Opin Cell Biol. 2006;18:379–385. doi: 10.1016/j.ceb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Maxfield FR, Wustner D. Intracellular cholesterol transport. J Clin Invest. 2002;110:891–898. doi: 10.1172/JCI16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megha, London E. Ceramide selectively displaces cholesterol from ordered lipid domains (rafts): implications for lipid raft structure and function. J Biol Chem. 2004;279:9997–10004. doi: 10.1074/jbc.M309992200. [DOI] [PubMed] [Google Scholar]
- Mesmin B, Maxfield FR. Intracellular sterol dynamics. Biochim Biophys Acta. 2009;1791:636–645. doi: 10.1016/j.bbalip.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Yamamoto M, Diyizou Y, Warnock W, Dolle V, Hahn W, Milon A, Ourisson G. Studies on the topography of biomembranes: Regioselective photolabelling in vesicles with the tandem use of cholesterol and a photoactivable transmembrane phospholipidic probe. Chemistry-a European Journal. 1996;2:129–138. [Google Scholar]
- Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- Okazaki H, Igarashi M, Nishi M, et al. Identification of neutral cholesterol ester hydrolase, a key enzyme removing cholesterol from macrophages. J Biol Chem. 2008;283:33357–33364. doi: 10.1074/jbc.M802686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastores GM, Barnett NL. Substrate reduction therapy: miglustat as a remedy for symptomatic patients with Gaucher disease type 1. Expert Opin Investig Drugs. 2003;12:273–281. doi: 10.1517/13543784.12.2.273. [DOI] [PubMed] [Google Scholar]
- Patterson MC, Platt F. Therapy of Niemann-Pick disease, type C. Biochim Biophys Acta. 2004;1685:77–82. doi: 10.1016/j.bbalip.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Patterson MC, Vanier MT, Suzuki K, Morris JA, Carstea E, Neufeld EB, Blanchette-Mackie EJ, Pentchev PG. Niemann-Pick Disease TypeC: A lipid Trafficking Disorder. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. Vol. 3. McGraw-Hill; New York: 2001. pp. 3611–3633. [Google Scholar]
- Patterson MC, Vecchio D, Prady H, Abel L, Wraith JE. Miglustat for treatment of Niemann-Pick C disease: a randomised controlled study. Lancet Neurol. 2007;6:765–772. doi: 10.1016/S1474-4422(07)70194-1. [DOI] [PubMed] [Google Scholar]
- Pentchev PG, Comly ME, Kruth HS, Vanier MT, Wenger DA, Patel S, Brady RO. A defect in cholesterol esterification in Niemann-Pick disease (type C) patients. Proc Natl Acad Sci U S A. 1985;82:8247–8251. doi: 10.1073/pnas.82.23.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda M, Wraith JE, Mengel E, et al. Miglustat in patients with Niemann-Pick disease Type C (NP-C): a multicenter observational retrospective cohort study. Mol Genet Metab. 2009;98:243–249. doi: 10.1016/j.ymgme.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Pipalia NH, Hao M, Mukherjee S, Maxfield FR. Sterol, protein and lipid trafficking in Chinese hamster ovary cells with Niemann-Pick type C1 defect. Traffic. 2007;8:130–141. doi: 10.1111/j.1600-0854.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- Ramirez CM, Liu B, Taylor AM, Repa JJ, Burns DK, Weinberg AG, Turley SD, Dietschy JM. Weekly Cyclodextrin Administration Normalizes Cholesterol Metabolism in Nearly Every Organ of the Niemann-Pick Type C1 Mouse and Markedly Prolongs Life. Pediatr Res. doi: 10.1203/PDR.0b013e3181ee4dd2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum AI, Cosner CC, Mariani CJ, Maxfield FR, Wiest O, Helquist P. Thiadiazole Carbamates: Potent Inhibitors of Lysosomal Acid Lipase and Potential Niemann-Pick Type C Disease Therapeutics. J Med Chem. 2010a;53:5281–5289. doi: 10.1021/jm100499s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum AI, Rujoi M, Huang AY, Du H, Grabowski GA, Maxfield FR. Chemical screen to reduce sterol accumulation in Niemann-Pick C disease cells identifies novel lysosomal acid lipase inhibitors. Biochim Biophys Acta. 2009;1791:1155–1165. doi: 10.1016/j.bbalip.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum AI, Zhang G, Warren JD, Maxfield FR. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc Natl Acad Sci U S A. 2010b;107:5477–5482. doi: 10.1073/pnas.0914309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando GN, Henke VL. Recognition and receptor-mediated endocytosis of the lysosomal acid lipase secreted by cultured human fibroblasts. J Lipid Res. 1982;23:114–123. [PubMed] [Google Scholar]
- Sloan HR, Fredrickson DS. Enzyme deficiency in cholesteryl ester storage idisease. J Clin Invest. 1972;51:1923–1926. doi: 10.1172/JCI106997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small CA, Goodacre JA, Yeaman SJ. Hormone-sensitive lipase is responsible for the neutral cholesterol ester hydrolase activity in macrophages. FEBS Lett. 1989;247:205–208. doi: 10.1016/0014-5793(89)81335-3. [DOI] [PubMed] [Google Scholar]
- Sokol J, Blanchette-Mackie J, Kruth HS, et al. Type C Niemann-Pick disease. Lysosomal accumulation and defective intracellular mobilization of low density lipoprotein cholesterol. J Biol Chem. 1988;263:3411–3417. [PubMed] [Google Scholar]
- Tall AR. Role of ABCA1 in cellular cholesterol efflux and reverse cholesterol transport. Arterioscler Thromb Vasc Biol. 2003;23:710–711. doi: 10.1161/01.ATV.0000068683.51375.59. [DOI] [PubMed] [Google Scholar]
- Tang Y, Leao IC, Coleman EM, Broughton RS, Hildreth JE. Deficiency of niemann-pick type C-1 protein impairs release of human immunodeficiency virus type 1 and results in Gag accumulation in late endosomal/lysosomal compartments. J Virol. 2009;83:7982–7995. doi: 10.1128/JVI.00259-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ML, Motamed M, Infante RE, Abi-Mosleh L, Kwon HJ, Brown MS, Goldstein JL. Identification of surface residues on niemann-pick C2 essential for hydrophobic Handoff of cholesterol to NPC1 in lysosomes. Cell Metab. 2010;12:166–173. doi: 10.1016/j.cmet.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wraith JE, Vecchio D, Jacklin E, Abel L, Chadha-Boreham H, Luzy C, Giorgino R, Patterson MC. Miglustat in adult and juvenile patients with Niemann-Pick disease type C: long-term data from a clinical trial. Mol Genet Metab. 99:351–357. doi: 10.1016/j.ymgme.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J Biol Chem. 2007;282:23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas M, Dobrenis K, Walkley SU. Neurons in Niemann-Pick disease type C accumulate gangliosides as well as unesterified cholesterol and undergo dendritic and axonal alterations. J Neuropathol Exp Neurol. 2001;60:49–64. doi: 10.1093/jnen/60.1.49. [DOI] [PubMed] [Google Scholar]
- Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]