Abstract
The oxidative conversion of LDL into an atherogenic form is considered a pivotal event in the development of cardiovascular disease. Recent studies have identified reactive nitrogen species generated by monocytes by way of the myeloperoxidase-hydrogen peroxide-nitrite (MPO-H2O2-NO2–) system as a novel mechanism for converting LDL into a high-uptake form (NO2-LDL) for macrophages. We now identify the scavenger receptor CD36 as the major receptor responsible for high-affinity and saturable cellular recognition of NO2-LDL by murine and human macrophages. Using cells stably transfected with CD36, CD36-specific blocking mAbs, and CD36-null macrophages, we demonstrated CD36-dependent binding, cholesterol loading, and macrophage foam cell formation after exposure to NO2-LDL. Modification of LDL by the MPO-H2O2-NO2– system in the presence of up to 80% lipoprotein-deficient serum (LPDS) still resulted in the conversion of the lipoprotein into a high-uptake form for macrophages, whereas addition of less than 5% LPDS totally blocked Cu2+-catalyzed LDL oxidation and conversion into a ligand for CD36. Competition studies demonstrated that lipid oxidation products derived from 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine can serve as essential moieties on NO2-LDL recognized by CD36. Collectively, these results suggest that MPO-dependent conversion of LDL into a ligand for CD36 is a likely pathway for generating foam cells in vivo. MPO secreted from activated phagocytes may also tag phospholipid-containing targets for removal by CD36-positive cells.
Introduction
Cholesterol is an essential component of cellular membranes and is required for normal growth and survival. However, an elevated level of LDL, the major carrier of cholesterol in blood, is a primary risk factor for the development of atherosclerotic vascular disease. The mechanism(s) through which arterial macrophages take up and accumulate LDL cholesterol leading to foam cell formation, the first cellular hallmark of the atherosclerotic process, has accordingly been the focus of intense research interest (1–6).
Cells such as macrophages are normally protected from the toxic effects of excess cholesterol by multiple mechanisms, including the downregulation of surface LDL receptor molecules in response to replete intracellular cholesterol stores (1). In contrast, LDL, which is first oxidized or chemically modified, may be taken up by alternate “scavenger” or “oxidized LDL” receptors whose surface expression is not diminished upon exposure to excess cholesterol (2–6). Free metal ions readily catalyze the oxidation of LDL in vitro and convert the lipoprotein into a form that is rapidly taken up and degraded by macrophages, leading to cholesterol accumulation (7). Indeed, many studies have examined the pathogenic properties of metal ion–catalyzed LDL preparations and the cellular scavenger receptors mediating their recognition (2–10). However, because of the multiple redundant mechanisms for chelating redox-active free metal ions in vivo, the role of free transition metal ions in converting LDL into a high-uptake form in vivo has been questioned (11–13). For example, no significant enrichment of metal ion–generated protein oxidation products was observed during mass spectrometric analyses of early atherosclerotic lesions in humans (13), though in a similar study significant increases were observed in advanced lesions (14).
We described recently an alternative pathway for converting LDL into a form capable of promoting macrophage foam cell formation that involves oxidative modification of LDL by myeloperoxidase-generated (MPO-generated) reactive nitrogen species (15). MPO is an abundant constituent of monocytes, neutrophils, and certain subpopulations of tissue macrophages (16). This heme protein uses hydrogen peroxide (H2O2) as cosubstrate to generate a variety of reactive oxidants and diffusible radical species (17–22). Immunohistochemical and mass spectrometric studies demonstrate that MPO and multiple distinct oxidation products formed by MPO are enriched in human atheroma (23–26). Moreover, nitrating oxidants derived from nitric oxide (nitrogen monoxide, NO), a long-lived free radical generated by arterial wall cells, are formed at sites of vascular disease (25, 27). Exposure of LDL to either monocyte- (28) or MPO-generated (15) reactive nitrogen species results in nitration of apo B-100 tyrosyl residues and initiation of LDL lipid peroxidation. Similar biochemical characteristics (i.e., enhancement in nitrotyrosine and products of lipid peroxidation) have been reported for LDL recovered from human atherosclerotic lesions (25, 26, 29, 30). Modification of LDL by monocytes activated in the presence of physiological levels of nitrite (NO2–), a major end product of NO metabolism, results in MPO-dependent conversion of the particle into a form (NO2-LDL) that binds to murine and human macrophages with high affinity in a concentration-dependent and saturable manner (15). These binding characteristics are consistent with a receptor-mediated process; however, neither the macrophage receptor(s) nor the moiety(ies) on NO2-LDL responsible for macrophage binding and foam cell formation have been established.
A variety of scavenger receptors expressed on macrophages have been described. Among these, the scavenger receptor class A (SR-A) and CD36 are thought to be major receptors expressed on tissue-differentiated macrophages (8, 10). A contributory role for SR-A in atherogenesis has been supported recently by studies with SR-A–receptor knockout mice; however, the reductions in lesions noted were modest, suggesting that alternative scavenger receptors are also likely involved (31, 32). CD36 is a 88-kDa glycoprotein that binds copper-oxidized LDL (Cu2+-oxLDL) (33, 34), fatty acids (34), anionic phospholipids (35–37), and apoptotic cells (38) and serves as an adhesion receptor for collagen (39), thrombospondin (40, 41), Plasmodium falciparum–infected erythrocytes (42), and shed photoreceptor outer segments (43). CD36 also mediates the antiangiogenic effects of thrombospondin 1 and 2 on microvascular endothelial cells (41). Recent studies with CD36-null mice demonstrated that this scavenger receptor is intimately involved in lipoprotein and fatty acid metabolism in vivo (42). In addition, functional roles for CD36 in processing apoptotic cells by dendritic cells, differentiation of monocytes, accumulation of lipid in macrophages, fatty acid transport, and the induction of nuclear receptors such as the peroxisome proliferator–activated receptor γ (PPARγ) have been suggested (38, 43–46). Recent studies have also shown that lipoprotein lipids can upregulate the expression of the CD36 gene and protein (47, 48). Although a role for CD36 in atherogenesis in humans has not yet been established, multiple lines of evidence suggest that it plays a role in lipid accumulation and macrophage foam cell formation in vitro and in vivo (44, 49, 50).
Here we use multiple approaches, including cells transfected with either the SR-A type I (SR-AI) or the scavenger receptor CD36, macrophages from mice homozygous for a null mutation in the allele for CD36, and CD36-specific blocking mAbs, to identify the receptor(s) that recognizes the novel, high-uptake form of nitrated and oxidized LDL, NO2-LDL, in human and murine macrophages.
Methods
Materials.
Tissue culture media and additives were purchased from Life Technologies (Rockville, Maryland, USA). Na[125I] and [14C]oleate were supplied by ICN Pharmaceuticals, Inc. (Costa Mesa, California, USA). C57BL/6 mice (16–20 weeks of age) were purchased from the Trudeau Institute (Saranac Lake, New York, USA). 1-Hexadecanoyl-2-eicosatetra-5′,8′,11′,14′-enoyl-sn-glycero-3-phosphocholine (PAPC), 1-hexadecanoyl-2-octadec-9′-enoyl-sn-glycero-3-phosphocholine (POPC), and 1-hexadecanoyl-2-octadecadi-9′,12′-enoyl-sn-glycero-3-phosphocholine (PLPC) were purchased from Avanti Polar Lipids (Alabaster, Alabama, USA). All other reagents were obtained from Sigma Chemical Co. (St. Louis, Missouri, USA) unless specified otherwise.
General procedures.
Human myeloperoxidase (donor: hydrogen peroxide, oxidoreductase, EC 1.11.1.7) and LDL were isolated and quantified as described (15). LDL concentrations are expressed as milligrams of protein per milliliter. All buffers were treated with Chelex-100 resin (Bio-Rad Laboratories Inc., Hercules, California, USA) and supplemented with diethylenetriamine pentaacetic acid (DTPA) to remove trace levels of transition metal ions that might catalyze LDL oxidation during incubations. LDL was labeled with Na[125I] to a specific activity between 100 and 250 dpm/ng protein as described (51). Agarose gel electrophoresis, quantifying remaining lysine groups, LDL acetylation, and cholesteryl [14C]oleate synthesis by cells incubated with the indicated lipoproteins (75 μg/mL), were determined as described (15). Human peripheral blood monocytes used for LDL oxidation were isolated by elutriation (52). The contents of nitrotyrosine and total 9-H(P)ETE (9-hydroxy-eicosatetraenoic acid + 9-hydroperoxyeicosatetraenoic acid) or 9-H(P)ODE (9-hydroxy-10,12-octadecadienoic acid + 9-hydroperoxy-10,12-octadecadienoic acid) were determined by gas chromatography mass spectrometry (GC/MS) (53) and reverse-phase HPLC with on-line tandem mass spectrometry (LC/MS/MS) (28), respectively.
Lipoprotein modification.
LDL modified by MPO-generated nitrating intermediates (NO2-LDL) was typically formed by incubating LDL (0.2 mg protein/mL) at 37°C in 50 mM sodium phosphate, pH 7.0, 100 μM DTPA, 30 nM MPO, 100 μg/mL glucose, 20 ng/mL glucose oxidase, and 0.5 mM NaNO2 for 8 hours unless otherwise specified. Under these conditions, a constant flux of H2O2 (10 μM/h) is generated by the glucose/glucose oxidase (GGOx) system, as determined by the oxidation of Fe(II) and formation of Fe(III)-thiocyanate complex (21). Oxidation reactions were terminated by addition of 40 μM butylated hydroxytoluene (BHT) and 300 nM catalase to the reaction mixture.
LDL modification by monocytes was performed by incubating monocytes (106/mL) with LDL (0.2 mg/mL) in HBSS (magnesium, calcium, phenol, and bicarbonate-free, pH 7.20) supplemented with DTPA (100 μM) for 8 hours at 37°C in the presence of the additions or deletions indicated. PMA (200 nM final concentration) was added to monocytes as an agonist to initiate a respiratory burst and to promote MPO secretion. Reactions were terminated by addition of BHT (20 μM) and pelleting of cells at 0°C. Oxidation of LDL (0.2 mg/mL) by Cu2+ was performed by dialysis versus 5 μM CuSO4 in PBS for 24 hours at 37°C unless otherwise indicated. Oxidation was terminated by addition of BHT (40 μM) and DTPA (100 μM) and dialysis against PBS containing DTPA (100 μM).
Cells.
Thioglycolate-elicited mouse peritoneal macrophages (MPM) from wild-type (C57BL/6) and CD36-null mice (42) were isolated and cultured as described (15). Resident MPM were similarly harvested from mice not subjected to thioglycolate stimulation. Human foreskin fibroblasts were cultured as described previously (54). Chinese hamster ovary (CHO) cells expressing mouse SR-AI (CHO-mSR-AI) and control vector–transfected, parental LDL receptor–negative CHO cells were a generous gift of M. Krieger (MIT, Boston, Massachusetts, USA) (55). Experiments with CHO-mSR-AI were performed on confluent cell monolayers in Ham’s F-12 medium containing 3% lipoprotein-deficient fetal calf serum, BHT (20 μM), DTPA (100 μM), and catalase (300 nM). Embryonic kidney epithelial cells transformed with adenovirus, 293 cells, were obtained from ATCC (Rockville, Maryland, USA) and maintained in DMEM with 5% FCS. The human CD36 cDNA was cloned into the pcDNA1/Neo expression vector (Invitrogen Corp., Carlsbad, California, USA) as described previously (56). The 293-transfected cells were grown in the presence of G418 (500 μg/mL), clones were isolated, and expression of CD36 was confirmed by FACS analysis using the mAb FA6-152 (Immunotech, Westbrook, Maine, USA). Human monocyte-derived macrophage (hMDM) culturing (10–14 days), and lipoprotein uptake, degradation, and binding by cultured cells, was performed as described (15). Experiments with 293 cells and hMDM were performed on confluent cell monolayers in the appropriate culturing media containing 200 μg/mL LDL, BHT (20 μM), DTPA (100 μM), and catalase (300 nM). Flow cytometry studies demonstrated that no significant SR-AI was expressed on the surface of 293 cells and fibroblasts. Likewise, no significant surface expression of CD36 was noted on the CHO cells transfected with mSR-AI or fibroblasts.
Vesicle and liposome preparation.
Stock solutions (2 mg/mL) of small unilamellar vesicles comprised of PAPC, PLPC, or POPC were prepared in argon-sparged sodium phosphate buffer by extrusion (10 times) through a 0.1-μm polycarbonate filter using an Avanti Mini-Extruder Set (Avanti Polar Lipids Inc.) at 37°C. Vesicles were diluted to a final concentration of 0.2 mg lipid/mL and incubated with MPO (30 nM), glucose (100 μM), and glucose oxidase (100 ng/mL) in the presence or absence of NaNO2 (0.5 mM) at 37°C under air as described in the figure legends. Under these conditions, a constant flux of H2O2 (0.80 μM/min) was generated by the GGOx system. Reactions were stopped by addition of BHT (50 μM) and catalase (300 nM). Liposomes containing BSA were similarly prepared using lipids supplemented with BSA (2 mg/mL) before extrusion. In some experiments, hydroperoxide-free PAPC was used. Trace levels of any potential contaminating hydroperoxides in commercial PAPC preparations were removed by reduction with 1 mM SnCl2, reisolation of PAPC under argon atmosphere, and then immediately used in vesicle formation as above.
Liposomes from lipid extracts of native LDL and NO2-LDL were prepared by a modification of the method described by Terpstra and colleagues for Cu2+-oxLDL (57). Briefly, lipids were extracted 3 sequential times by the method of Bligh and Dyer (58) from native LDL and NO2-LDL preparations immediately after saturating amounts of solid NaCl were added (to enhance lipid extraction). The combined extracts were evaporated under nitrogen, hydrated in argon-sparged buffer, vortexed, and extruded through polycarbonate filters under argon atmosphere as described above. Delipidated apo B-100 from modified lipoproteins was solubilized in n-octylglucoside microemulsions (59).
Statistics.
Data represent the mean plus or minus SD of the indicated number of samples. Statistical analyses were made using a paired Student’s t test. For all hypotheses the significance level was 0.05. When multiple comparisons were made, a Bonferroni correction to the significance criterion for each test was made.
Results
The scavenger receptor CD36 recognizes LDL modified by activated human monocytes.
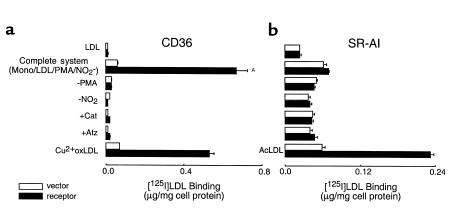
[125]LDL incubated in the presence of activated human monocytes in media containing NO2– was readily converted into a form that bound to 293 cells expressing CD36, but not to control vector–transfected 293 cells (Figure 1a, complete system). In contrast, LDL modified by the complete system of monocytes failed to bind to CHO cells expressing murine SR-AI more than their control vector–transfected counterparts (Figure 1b). Surface expression of CD36 and SR-AI on their respective cells was confirmed by FACS analyses (data not shown) and by demonstrating that Cu2+-oxidized [125I]LDL (Cu2+-oxLDL) and acetylated LDL (AcLDL), prototypic ligands for CD36 and SR-AI, respectively, bound to CD36- and SR-AI–transfected cells, but not their control vector–transfected counterparts (Figure 1). Neither native LDL nor LDL modified by activated human monocytes in the absence of NO2– bound significantly to cells transfected with either CD36 or SR-AI (Figure 1). Monocyte-dependent conversion of LDL into a ligand for CD36 required cell activation and was inhibited by either the H2O2 scavenger catalase or by peroxidase inhibitors such as 3-aminotriazole (Figure 1). These results suggest that monocytes employ the MPO-H2O2-NO2– system for conversion of LDL into a ligand for CD36, but not SR-AI.
Figure 1.
Binding of [125I]LDL by CD36- and SR-AI–transfected cells after modification by activated human monocytes. [125I]LDL was incubated with NO2– and phorbol ester-stimulated human monocytes at 37°C for 8 hours (complete system) as described in Methods. Additions or deletions to the complete system were as indicated. Reactions were stopped by addition of BHT (40 μM) and catalase (300 nM), the cells were pelleted, and then lipoproteins (5 μg/mL) were incubated with either (a) CD36- or vector-transfected 293 cells or (b) murine SR-AI– or vector-transfected CHO cells at 37°C for 5 hours in the appropriate media containing additional catalase (300 nM) and BHT (20 μM). Cellular binding of lipoproteins at 4°C was subsequently determined as described in Methods. The final concentrations of additions to the complete system were catalase (300 nM) and 3-aminotriazole (1 mM). Data represent the mean ± SD of triplicate determinations of a representative experiment performed 3 times. AP < 0.001 for complete system versus complete system–NO2–. Atz, 3-aminotriazole; Mono, human peripheral blood monocytes.
Reactive nitrogen species formed by the MPO-H2O2-NO2– system convert LDL into a ligand for CD36.
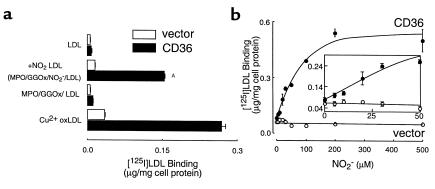
Because monocyte-dependent conversion of LDL into a ligand for CD36 demonstrated a requirement for NO2– in media (Figure 1), the results suggest that either NO2Cl (60) or the MPO-H2O2-NO2– system (15, 21, 22, 28) was responsible for lipoprotein conversion into a ligand for CD36. They also demonstrate that under the conditions employed, monocyte-generated ONOO– and HOCl are not sufficient to convert the lipoprotein into a stable ligand for either the scavenger receptor CD36 or SR-AI. To more fully explore the mechanism(s) through which monocytes transform LDL into a ligand for CD36, we modified LDL using a model system comprised of purified MPO and an H2O2-generating system. [125I]LDL exposed to MPO isolated from human leukocytes, a H2O2-generating system (GGOx), and NO2– was transformed into a form (NO2-LDL) that readily bound to CD36-transfected 293 cells, but not to their control vector–transfected counterparts (Figure 2a). Conversion of LDL into a ligand for CD36 by isolated MPO had an absolute requirement for NO2– and occurred in both the presence (not shown) and absence of chloride in buffer (Figure 2a). Examination of the NO2– concentration dependence for MPO-dependent conversion of LDL into a ligand for CD36 (in the presence of plasma levels of chloride) demonstrated that levels of NO2– that approximate those commonly observed in normal plasma and inflammatory tissues and fluids (up to 50 μM) converted the lipoprotein into a ligand for the scavenger receptor (Figure 2b and inset). Collectively, these results suggest that under conditions in which MPO-generated oxidants are formed by a physiological flux of H2O2, the reactive nitrogen species formed by the MPO-H2O2-NO2– system are sufficient to convert LDL into a ligand for CD36. Because MPO-generated HOCl can modify lipid and protein components of lipoproteins (26, 61–63), but is not required for MPO-dependent conversion of LDL into a ligand for the scavenger receptor CD36, subsequent studies characterizing the biological consequences of NO2-LDL engagement of CD36 and the structural nature of the ligand(s) responsible for CD36 recognition were performed on LDL modified under chloride-free conditions, unless otherwise indicated.
Figure 2.
Binding of [125I]LDL by CD36-transfected cells after modification by MPO-generated nitrating intermediates. (a) [125I]LDL was incubated with isolated human MPO (30 nM), glucose (100 μg/mL), glucose oxidase (20 ng/mL), and NO2– (0.5 mM) in sodium phosphate buffer supplemented with DTPA (100 μM) for 8 hours at 37°C (complete system, NO2-LDL) as described in Methods. Reactions were stopped by addition of BHT (40 μM) and catalase (300 nM), and then lipoproteins (5 μg/mL) were incubated with CD36- or vector-transfected 293 cells at 4°C for 3 hours in the appropriate media containing additional catalase (300 nM) and BHT (20 μM). LDL modified by dialysis against copper for 5 hours (Cu2+ oxLDL, 5 μg/mL) was used as a positive control. Cellular binding of lipoproteins was subsequently determined as described in Methods. AP < 0.001 for comparison versus LDL modified in the presence of MPO- and a H2O2-generating system (MPO/GGOx/LDL). (b and inset) [125I]LDL (0.2 mg/mL) was incubated with isolated human MPO (30 nM), glucose (100 μM), glucose oxidase (20 nM), and the indicated concentrations of NO2– in sodium phosphate buffer supplemented with DTPA (100 μM) and NaCl (100 mM) overnight at 37°C. Cellular binding of lipoproteins (10 μg/mL) was subsequently determined as described in Methods. Data represent the mean ± SD of triplicate determinations of a representative experiment performed 3 times.
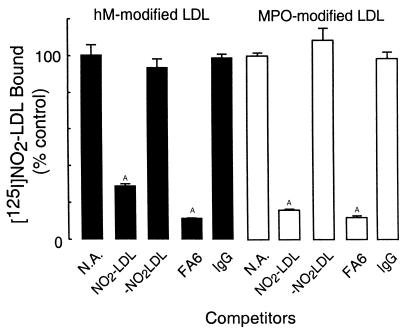
Additional evidence for the specificity of CD36-mediated recognition of NO2-LDL was obtained from competition experiments. Excess amounts of unlabeled NO2-LDL effectively competed with [125I]NO2-LDL formed by either activated human monocytes or isolated MPO for binding to CD36-transfected 293 cells (Figure 3). In contrast, a 40-fold molar excess of nonlabeled LDL modified by activated monocytes or MPO in the absence of NO2– (–NO2LDL, Figure 3) failed to block binding of monocyte- and MPO-generated [125I]NO2-LDL to CD36-transfected 293 cells. Similarly, a large molar excess of native LDL did not attenuate NO2-LDL binding to CD36-transfected cells (data not shown). Finally, the anti-CD36 blocking mAb, FA6 (64), significantly inhibited binding of monocyte- and MPO-generated NO2-LDL to CD36-transfected cells, whereas isotype-matched nonimmune antibody had no effect (Figure 3).
Figure 3.
Effect of CD36-blocking mAbs on the binding of LDL modified by monocyte- or MPO-generated reactive nitrogen species to CD 36-transfected cells. [125I]LDL was modified by reactive nitrogen species generated by (solid bars) activated human monocytes (as in complete system, Figure 1) or by (open bars) isolated human MPO (as in complete system, Figure 2a). Reactions were stopped by addition of BHT (40 μM) and catalase (300 nM), and then lipoproteins (5 μg/mL) were incubated with CD36-transfected 293 cells for 3 hours at 4°C either in the absence or presence of the indicated competitors. Cellular binding of lipoproteins was subsequently determined as described in Methods. Data represent the mean ± SD of triplicate determinations of a representative experiment performed 3 times. AP < 0.001 for comparison versus control (NA, no addition). Final concentrations of competitors are 200 μg/mL for LDL and 10 μg/mL for immunoglobulins.
Time course for acquisition of scavenger receptor recognition, and loss of LDL receptor recognition, of LDL modified by the MPO/GGOx/NO2– system.
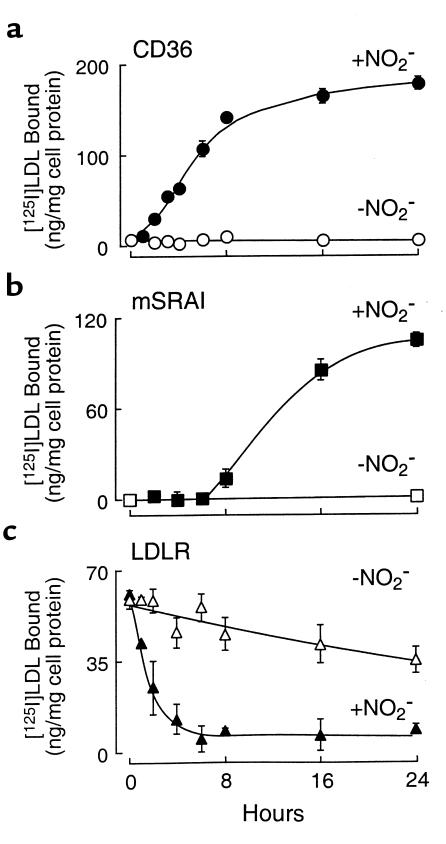
We have demonstrated previously that with increasing exposure time of LDL to the MPO-H2O2-NO2– system, the degree of LDL modification increases (15). We therefore examined the time course of LDL conversion into a high-uptake form after exposure to MPO, NO2–, and a low, steady flux (10 μM/h) of H2O2 by assessing the extent of lipoprotein binding to cells expressing CD36, SR-AI, or the LDL receptor. Incubation of LDL with the complete MPO-H2O2-NO2– system resulted in conversion of LDL into a ligand for CD36 after only a brief (1–2 hour) period (Figure 4a). Recognition of the modified lipoprotein by CD36-transfected cells progressively increased with the time of incubation, reaching 80% of maximal binding by 8 hours. No increase in CD36-dependent binding was observed in LDL incubated with MPO and H2O2 in the absence of NO2– for up to 24 hours of modification (Figure 4a). These results suggest that the ligand for CD36 on NO2-LDL is formed rapidly upon exposure of LDL to MPO-generated reactive nitrogen species.
Figure 4.
Time dependence of LDL-ligand properties after exposure to the MPO-H2O2-NO2– system. [125I]LDL (0.2 mg/mL) was incubated with isolated human MPO (30 nM), glucose (100 μM), and glucose oxidase (20 ng/mL) in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) for the indicated times in either the presence (+ NO2–, filled symbols) or absence (– NO2–, open symbols) of NO2– as described in Methods. Binding of the modified lipoprotein by (a) CD36-transfected 293 cells; (b) mSRA-I–expressing CHO cells, and (c) human foreskin fibroblasts was then determined as described in Methods. Note that the time course for the acquisition of scavenger receptor CD36 recognition and loss of LDLR recognition are early events during lipoprotein modification, whereas acquisition of mSR-AI recognition requires more prolonged lipoprotein oxidation. Data represent the mean ± SD for triplicate determinations of a representative experiment performed at least 3 times. The REM of lipoprotein preparations exposed to the MPO-H2O2-NO2– system for 0, 1, 2, 4, 8, 16, and 24 hours were 1.0, 1.02, 1.13, 1.26, 1.44, 2.01, and 2.22, respectively. The lysine content (percent of control) of LDL exposed to the MPO-H2O2-NO2– system for 0, 1, 2, 4, 8, 16, and 24 hours was 100, 93.4, 90.3, 86.4, 83.7, 75.9, and 68.8, respectively.
Neutralization of Nε-amino groups of lysine on apo B-100 of LDL is regarded as critical for recognition of modified forms of LDL by SR-A (65). After exposure to the MPO-H2O2-NO2– system, however, the content of free amino groups on apo B-100 in LDL demonstrates only modest decreases with time (∼13.6 % reduction at t = 4 hours; Figure 4 legend). Consistent with these findings, no SR-AI–mediated binding of NO2-LDL was observed for up to 6–8 hours of LDL modification (Figure 4b). Recognition of NO2-LDL by SR-AI rapidly increased after prolonged (> 8 hours) modification by the MPO-H2O2-NO2– system. The increase in NO2-LDL recognition was accompanied by an increase in relative electrophoretic mobility (REM) (> 1.44 beyond 8 hours’ incubation; Figure 4 legend) and a decrease in free Nε-amino groups of lysine on LDL apo B-100 (> 20% reduction beyond 8 hours’ incubation). No increase in SR-AI–dependent binding was observed in LDL incubated for 24 hours with MPO and H2O2 in the absence of NO2– (Figure 4b). The more prolonged exposure required before conversion of LDL into a ligand for SR-AI suggests that this receptor will play a role in NO2-LDL recognition only after more extensive oxidative modification.
Because the LDL receptor (LDLR) is expressed on hMDMs (66), we also assessed the ability of NO2-LDL to be recognized by this receptor. Exposure of LDL to MPO, NO2–, and an H2O2-generating system, resulted in rapid loss of lipoprotein recognition by human foreskin fibroblasts that had been preincubated for 24 hours in media containing lipoprotein-deficient serum (LPDS) to upregulate the LDL receptor (Figure 4c). Thus, the binding site for LDLR on apo B is very sensitive to oxidation by the MPO-H2O2-NO2– system; furthermore, the LDLR does not contribute to the binding and uptake of NO2-LDL in MPMs and hMDMs. Collectively, these results suggest that brief exposure of LDL to the MPO-H2O2-NO2– system of monocytes will likely result in the prolongation of lipoprotein half-life by rapid loss of LDLR recognition.
LDL is converted into a ligand for CD36 in the presence of serum by MPO-generated reactive nitrogen species.
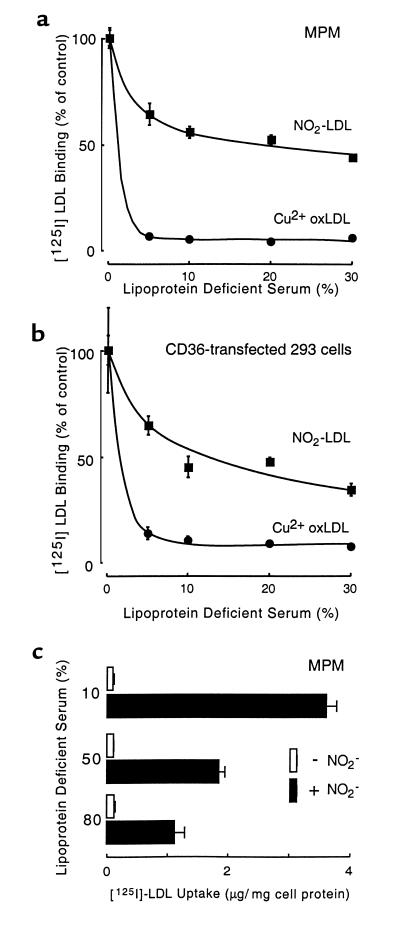
To assess the potential physiological relevance of LDL conversion into a ligand for CD36 by the MPO-H2O2-NO2– system of monocytes, we compared the influence of serum constituents on lipoprotein modification by isolated MPO versus Cu2+-catalyzed oxidation. Addition of 30% LPDS to LDL in buffer containing plasma levels of chloride, isolated human MPO, a H2O2-generating system and NO2– only partially attenuated conversion of the lipoprotein into a high-uptake form for MPM (Figure 5a) and the scavenger receptor CD36 (Figure 5b). Similar results were observed in reactions where LDL was oxidized by MPO in buffer free of plasma levels of chloride (data not shown), consistent with the critical role of the MPO-H2O2-NO2– system (and not HOCl or NO2Cl) in rendering LDL a ligand for CD36. In a parallel experiment, addition of up to 80% LPDS to reaction mixtures containing LDL, human MPO, and the H2O2-generating system, failed to block NO2–-dependent conversion of the lipoprotein into a high uptake form for MPM (Figure 5c). In stark contrast, addition of only 5% LPDS to LDL dialyzed against Cu2+ totally prevented conversion of the lipoprotein into a form recognized by either MPM or CD36-transfected cells (Figure 5, a and b). These results suggest that the MPO-H2O2-NO2– system is a physiologically plausible mechanism for converting LDL into a ligand for the scavenger receptor CD36.
Figure 5.
Binding and uptake of [125I]LDL by MPM and CD36-transfected cells after modification by MPO-generated nitrating intermediates or by Cu2+ in the presence of plasma proteins. [125I]LDL was modified in the presence of the indicated concentrations of human LPDS by (filled squares) incubation with isolated human MPO, a H2O2-generating system (GGOx), and NO2– in PBS supplemented with DTPA or by (filled circles) dialysis against Cu2+ as described in Methods. Binding of modified lipoproteins by (a) MPM and (b) CD36-transfected 293 cells was then determined as described in Methods. (c) [125I]LDL was modified in the presence of the indicated concentrations of human LPDS by incubation with isolated human MPO and a H2O2-generating system (GGOx) either in the presence (filled bars) or absence (open bars) of NO2– in 50 mM phosphate buffer (pH 7.40) supplemented with 100 mM NaCl and 100 μM DTPA overnight at 37°C, and then the extent of lipoprotein uptake by MPM was determined as described in Methods. Each experimental point represents the mean ± SD for triplicate determinations of a representative experiment performed at least 3 times.
CD36 plays a major role in the recognition of NO2-LDL by MPM.
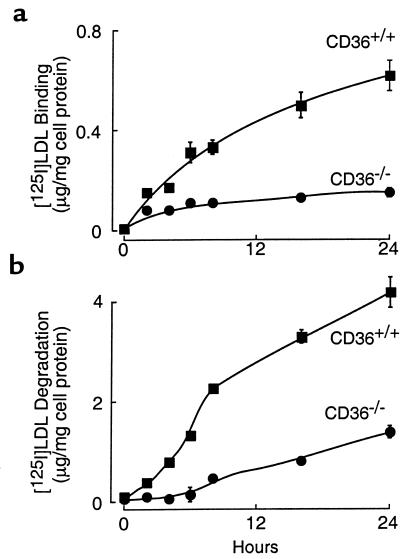
Several scavenger receptors for modified lipoproteins are present on macrophages including SR-AI, CD36, and others (8, 10). The relative contribution of these receptors to macrophage recognition of NO2-LDL depends on both their relative affinities for the modified lipoprotein and on the extent of their surface expression. To define the relative contribution of CD36 to the recognition of NO2-LDL by macrophages, we compared the binding (4°C), uptake (37°C), and degradation (37°C) of the modified lipoprotein using MPM recovered from mice homozygous for a null mutation in CD36 (CD36–/–) and from wild-type mice possessing isogenetic backgrounds (CD36+/+) (42). Binding (Figure 6a) and degradation (Figure 6b) of LDL modified by only a brief exposure to the MPO-H2O2-NO2– system were significantly greater with CD36+/+ MPM versus CD36–/– MPM. Both binding and degradation of the MPO-modified lipoprotein by CD36+/+ MPM continued to increase for up to 24 hours, with kinetics similar to that observed for NO2-LDL binding to CD36-transfected cells (Figures 4a and Figure 6). In contrast, binding, uptake, and degradation by CD36–/– cells was negligible for the first 8 hours of modification by the MPO-H2O2-NO2– system, and only modest increases were noted after LDL modification for longer periods (Figure 6). This likely reflects a nominal contribution of SR-AI to NO2-LDL recognition by both CD36–/– and CD36+/+ MPM after prolonged modification of the lipoprotein. Modification of LDL by MPO and the H2O2-generating system in the absence of NO2– resulted in negligible binding, uptake, and degradation by either CD36+/+ or CD36–/– MPM (data not shown). To rule out the possibility that eliciting MPM with thioglycolate affected the function of the macrophages, separate studies were performed using nonelicited peritoneal macrophages. Resident peritoneal macrophages were obtained from CD36–/– and CD36+/+ mice, and the binding and degradation of LDL modified for 8 hours by the MPO-H2O2-NO2– system were determined. Again, CD36 played a major role in both binding (0.31 ± 0.11 vs. 0.13 ± 0.06 μg/mg cell protein for CD36+/+ vs. CD36–/– MPM, respectively; mean ± SD, n = 6 each group) and degradation (7.67 + 0.20 vs. 3.45 + 0.03 μg/mg cell protein for CD36+/+ and CD36–/– MPM, respectively; mean ± SD, n = 6 each group) of the modified lipoprotein. Collectively, these results indicate that CD36 plays a major role in the recognition of NO2-LDL by resident and elicited MPM.
Figure 6.
Time dependence of LDL recognition by macrophages from wild-type mice and CD36-null mice after exposure to the MPO-H2O2-NO2– system. [125I]LDL was incubated with isolated human MPO, a H2O2-generating system (GGOx), and NO2– in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) for the indicated times as described in Methods. Binding (a) at 4°C and degradation (b) at 37°C of the modified lipoprotein by macrophages from wild-type mice (CD36+/+, filled squares) and CD36-null mice (CD36–/–, filled circles) were then determined as described in Methods. Data represent the mean ± SD for triplicate determinations of a representative experiment performed at least 3 times.
CD36-mediated recognition of NO2-LDL by macrophages induces cholesteryl ester synthesis and foam cell formation.
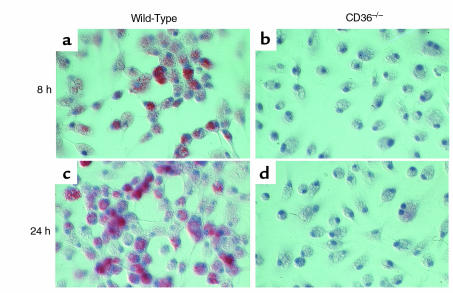
To determine whether CD36-mediated uptake of NO2-LDL might promote cholesterol accumulation in macrophages, we first compared the ability of NO2-LDL to stimulate cholesteryl ester (CE) biosynthesis in CD36+/+ and CD36–/– MPM. NO2-LDL markedly stimulated [14C]oleate incorporation into cellular CE pools in CD36+/+ MPM, whereas in CD36–/– cells, incorporation of [14C]oleate was comparable to that of native LDL (Table 1). In a parallel set of experiments, we examined whether stimulation of CE biosynthesis in macrophages mediated by CD36-dependent recognition of NO2-LDL resulted in the formation of lipid-laden foam cells, an early cellular event in the atherosclerotic process. MPMs from CD36+/+ and CD36–/– mice were incubated for 72 hours with either native LDL (negative control), acetylated LDL (positive control), NO2-LDL, or LDL exposed to MPO and H2O2 in the absence of NO2–, and then examined microscopically after neutral lipid staining with oil red-O. LDL modified by the MPO-H2O2-NO2– system promoted cholesterol deposition and foam cell formation in CD36+/+ MPM (Figure 7, a and c). However, foam cell formation in similarly treated CD36–/– MPM was almost completely inhibited (Figure 7, b and d). As anticipated, LDL modified by MPO and H2O2 in the absence of NO2– showed no ability to stimulate neutral lipid accumulation in MPMs (Table 1) and failed to promote macrophage foam cell formation (data not shown). CD36–/– MPMs exposed to acetylated LDL still formed foam cells (not shown), further demonstrating that NO2-LDL–dependent formation of macrophage foam cells was specifically mediated by the macrophage scavenger receptor CD36.
Table 1.
[14C]oleate incorporation into cholesteryl esters of macrophages from wild-type and CD36-knockout mice.
Figure 7.
LDL modified by the MPO-H2O2-NO2– system induces lipid loading of macrophages from wild-type mice but not from CD36-null mice. Thioglycollate-elicited MPM from wild-type mice (a and c) and from CD36-null mice (b and d) were grown for 24 hours in RPMI-1640 containing 10% FBS. Cells were then incubated for 72 hours in the same media containing catalase (100 nM), BHT (20 μM), and vitamin E (20 μM) in the presence of the following lipoprotein preparations (75 μg/mL): LDL incubated with isolated human MPO, a H2O2-generating system (GGOx), and NO2– in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA (100 μM) at 37°C for either 8 hours (a and b) or 24 hours (c and d) as described in Methods. Cells were fixed with 4% formaldehyde and stained with hematoxylin and oil red-O. ×300.
CD36 plays a significant role in the recognition of NO2-LDL in hMDMs.
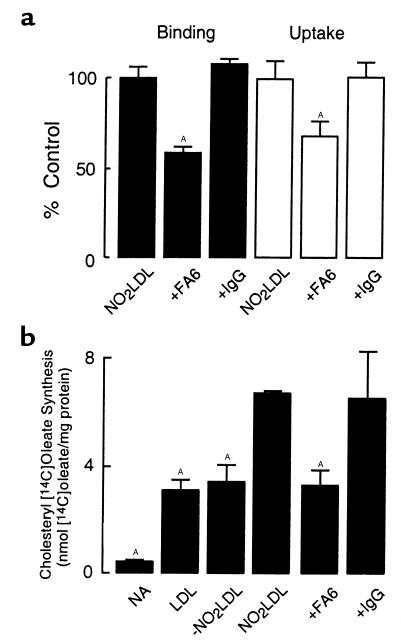
To determine the relative contribution of the macrophage scavenger receptor CD36 in the recognition of NO2-LDL by hMDMs, we initially examined NO2-LDL binding and uptake to cells in the presence and absence of the anti-CD36 blocking mAb FA6 (64). Incubation of NO2-LDL with hMDMs in the presence of FA6 resulted in a 41% (± 2%) reduction in binding and 32% (± 3%) reduction in uptake of NO2-LDL, whereas isotype-specific nonimmune antibody had no effect (Figure 8a). Addition of a 40-fold excess of –NO2LDL failed to block NO2-LDL binding or uptake by hMDM (data not shown). To further confirm the biological significance of CD36-mediated uptake of NO2-LDL in hMDMs, we assessed the effect of FA6 on the rate of CE accumulation, as measured by the incorporation of [14C]oleate into the cellular CE pool. Addition of NO2-LDL, but not -NO2LDL, significantly stimulated cholesteryl[14C]oleate synthesis in hMDMs over levels observed with native LDL. Addition of FA6, but not isotype-specific nonimmune control antibody, to media containing NO2-LDL inhibited the increase in [14C]oleate incorporation by 51% (± 3%) (Figure 8b)—similar to the reduction observed in [125I]NO2-LDL binding (Figure 8a). Similar results were observed when analyzing the effect of FA6 on NO2-LDL binding, uptake, degradation, and [14C]oleate incorporation into the CE pool of human monocytic cell lines (THP-1 and U937; data not shown). Taken together, these results demonstrate that the scavenger receptor CD36 is a major pathway for LDL recognition by murine and human macrophages after modification by monocyte- and MPO-generated reactive nitrogen species.
Figure 8.
Effect of anti-CD36 blocking mAb on (a) binding and uptake of NO2-LDL and (b) cholesteryl oleate synthesis in hMDM exposed to NO2-LDL. (a) [125I]LDL or (b) unlabeled LDL was incubated with isolated human MPO, a H2O2-generating system (GGOx), and NO2– in sodium phosphate buffer (50 mM, pH 7.0) supplemented with DTPA as described in Methods. Either (a) [125I]NO2-LDL or (b) unlabeled NO2-LDL was then incubated with hMDM alone (a, NO2LDL; and b, no addition), or in the presence of the indicated additions. (a) Binding (filled bars) at 4°C and uptake (open bars) at 37°C of modified lipoproteins were then determined as described in Methods. (b) Lipoproteins were incubated overnight with hMDM in the presence of [14C]oleate and the indicated additions, and cholesteryl [14C]oleate formation was determined as described in Methods. Data represent the mean ± SD of triplicate determinations of a representative experiment performed 3 times. AP < 0.05 for comparison versus NO2-LDL. NA, No addition.
Identification of potential ligands for CD36 on NO2-LDL.
In a final series of experiments, we sought to gain insights into the chemical nature of the ligand(s) for CD36 that was formed during even brief exposure of LDL to the MPO-H2O2-NO2– system. Both monocyte- (28) and MPO-generated (15) reactive nitrogen species oxidatively modify multiple protein and lipid components on LDL. We therefore reasoned that the component(s) in NO2-LDL recognized by CD36 could be a modified lipid, a protein oxidation product, or a lipid-protein adduct. To distinguish among these potential ligands, we initially performed competition experiments with [125I]NO2-LDL binding to CD36-transfected 293 cells as the screening assay. As expected, neither native LDL (Figure 9a) nor LDL modified by the MPO-H2O2 system in the absence of NO2– (data not shown) effectively blocked [125I]NO2-LDL binding to CD36-transfected cells. In contrast, a 40-fold molar excess of unlabeled NO2-LDL was an effective competitive ligand, inhibiting binding of [125I]NO2-LDL to the level of binding observed in vector-transfected cells (Figure 9a). Liposomes generated from lipid extracts of NO2-LDL were also potent competitive ligands for [125I]NO2-LDL binding to CD36-transfected 293 cells (Figure 9a), in contrast to liposomes generated from either native LDL (Figure 9a) or LDL modified by the MPO-H2O2 system in the absence of NO2– (data not shown). Thus, at least 1 ligand for CD36 formed in NO2-LDL is an oxidized lipid moiety. The concentration dependence for inhibition of CD36 recognition of [125I]NO2-LDL by intact NO2-LDL and liposomes derived from lipid extracts of NO2-LDL are shown in Figure 9b.
Figure 9.
Effect of various competitors on the binding of NO2-LDL to CD36- transfected cells. [125I]LDL was modified by isolated MPO, a H2O2-generating system, and NO2– as described for the complete system in Figure 2a. [125I]-labeled lipoproteins ([125I]NO2-LDL, 5 μg/mL) were then incubated with vector-transfected cells (hatched bar) (a) or CD36-expressing 293 cells (filled bars) (a) and (b), for 3 hours at 4°C either in the absence (NA) or presence of the indicated competitors. Cellular binding of lipoproteins was subsequently determined as described in Methods. Liposomes generated from lipid extracts of native LDL (LDL lipids) and NO2-LDL (NO2-LDL lipids) were prepared as described in Methods. NO2-LDL protein was prepared as described in Methods. BSA was exposed to the same MPO-H2O2-NO2– system as LDL (NO2-BSA) and used as a competitive ligand as well. (a) The final concentrations of competitors used were 200 μg protein/mL for LDL, NO2-LDL, NO2-LDL protein, BSA, and NO2-BSA. The concentration of LDL lipids and NO2-LDL lipids used was equivalent to that present in 200 μg LDL protein/mL. Synthetic tri-peptides were used at 200 μM final concentration. (b) The concentration of intact LDL and LDL lipids used was equivalent to the amount present in the indicated amount of LDL (μg protein/mL). Data represent the mean ± SD of triplicate determinations (a) or means of triplicate determinations (b) of a representative experiment that was performed 3 times. AP < 0.001 for comparison versus control (NA).
To determine whether an oxidation product of a protein or a protein-lipid adduct was also a potential ligand for CD36 on NO2-LDL, we examined the ability of apo B-100 from delipidated NO2-LDL to either bind to CD36-transfected cells (using [125I]apo B-100) or to block [125I]NO2-LDL binding to CD36-transfected cells. This approach has been used successfully when looking for potential ligands on Cu2+-oxLDL for hMDM (57, 59, 67). We were able to resolubilize delipidated apo B-100 from NO2-LDL only after exposure to the MPO-H2O2-NO2– system for approximately 24 hours (i.e., extensive oxidation). Apo B-100 from NO2-LDL failed to block the binding of [125I]NO2-LDL (Figure 9a), and [125I]NO2-apo B-100 recovered from 24-hour modified [125I]NO2-LDL failed to bind to the CD36-expressing cells (data not shown). Because of the potential that detergent in the microemulsions of resolubilized apo B-100 might interfere with the binding assay, we also studied model proteins exposed to the MPO-H2O2-NO2– system and synthetic peptides containing nitrotyrosine as potential competitive ligands. When BSA was modified by MPO-H2O2 in the presence of NO2–, extensive nitration of tyrosine residues was observed, as determined by stable isotope dilution GC/MS analyses (nitrotyrosine/tyrosine = 34 ± 2 mmol/mol)—nearly a 100-fold higher level of tyrosine nitration than that observed with NO2-LDL under similar conditions (15). However, a 500-fold molar excess of “nitrated BSA” had no effect on the binding of NO2-LDL to CD36 (Figure 9a). Similar results were obtained using a vast molar excess of a synthetic tri-peptide consisting of glycine-nitrotyrosine-alanine, a potent ligand for antinitrotyrosine antibodies (68). In separate binding experiments, we found that nitration of several irrelevant proteins ([125I]BSA, [125I]ovalbumin, and [125I]ribonuclease A) by the MPO-H2O2-NO2– system, did not convert them into ligands for either CD36-transfected cells or MPM (data not shown).
We next sought to characterize the nature of lipid oxidation products that potentially contribute to CD36 recognition using a model vesicle system. Small unilamellar vesicles comprised of synthetic homogeneous phosphatidylcholine molecular species were exposed to the MPO-H2O2 system (± NO2–), and then tested for their ability to compete for [125I]NO2-LDL binding to CD36-transfected 293 cells. PAPC vesicles that were modified by the complete MPO-H2O2-NO2– system (NO2-PAPC) completely inhibited binding of [125I]NO2-LDL (Figure 10a). Significant inhibition was observed at concentrations of less than 2 μg lipid/mL, with half-maximal inhibition at less than 5 μg/mL (Figure 10b). Similar results (inhibition of [125I]NO2-LDL binding) were observed in control experiments where CD36-transfected cells were first preincubated with NO2-PAPC for 2 hours at 4°C, washed, and then [125I] NO2-LDL binding was determined (data not shown). Thus, NO2-PAPC likely inhibits NO2-LDL recognition by CD36-transfected cells by direct binding to CD36 and not through interaction with [125I]NO2-LDL. Moreover, the inhibitory effect of NO2-PAPC was specific for CD36 because NO2-PAPC failed to inhibit the binding of either [125I]AcLDL or LDL modified by the MPO-H2O2-NO2– system for 24 hours to mSR-AI–transfected CHO cells, as well as the binding of LDL to LDLR on human foreskin fibroblasts (data not shown). The possibility that trace levels of preexisting hydroperoxides are required for conversion of PAPC into a ligand for CD36 was excluded by demonstrating that identical results were observed with hydroperoxide-free preparations of parent PAPC vesicles (see Methods) oxidized by MPO-generated reactive nitrogen species (Figure 10b). PLPC vesicles oxidized in the presence of NO2– demonstrated an intermediate inhibitory activity (Figure 10a), and modified POPC vesicles failed to compete for [125I]NO2-LDL recognition by CD36-positive cells (Figure 10). None of the vesicles (including PAPC) modified in the absence of NO2– were able to inhibit [125I]NO2-LDL binding to CD36-transfected cells (Figure 10). The ability of vesicles to block binding of [125I]NO2-LDL paralleled their extent of lipid peroxidation, as assessed by LC/MS/MS quantification of the content of esterified 9-H(P)ETE or 9-H(P)ODE formed (data not shown). Finally, oxidation of vesicles in the presence of BSA (to assess the possible participation of putative lipid-protein adducts in the recognition by CD36) had no effect on the observed results (Figure 10a). Collectively, these results support a role for oxidized lipids as the major moiety in NO2-LDL that confers CD36-dependent receptor recognition.
Figure 10.
Effect of lipid competitors on the binding of NO2-LDL to CD36-transfected cells. [125I]LDL was modified as described for the complete system in Figure 2a. [125I]-NO2LDL (5 μg/mL) was then incubated with CD36-expressing 293 cells for 3 hours at 4°C in the presence of (a) 20 μg lipid/mL or (b) the indicated concentrations (μg lipid/mL) of competitors. PAPC, PAPC(SnCl2), PLPC, and POPC unilamellar vesicles were oxidized for 8 hours at 37°C as described for the complete system in Figure 2 in the presence (+NO2–, filled symbols) or absence (–NO2–, open symbols) of NO2–. Where indicated, BSA (0.2 mg protein/mL final concentration) was also included during liposome preparation as described in Methods (hatched bars). PAPC (SnCl2), hydroperoxide-free PAPC generated by reduction of PAPC with SnCl2, and then reisolation of PAPC under argon atmosphere before use were used as described in Methods. Data represent the mean ± SD of triplicate determinations (a) or means of triplicate determinations (b) of a representative experiment performed 3 times. AP < 0.001 for comparison versus control (no competitor).
Finally, we evaluated the ability of NO2-PAPC vesicles to block NO2-LDL binding to MPM and hMDM because it appears to be a specific and potent ligand for CD36-dependent recognition (Figure 11). We found that NO2-PAPC inhibited the binding of [125I]NO2-LDL to MPM by at least 80% and to hMDM by 50%. In contrast, large molar excess of –NO2 PAPC failed to inhibit MPM and hMDM recognition of [125I]NO2-LDL. Finally, addition of FA6 to NO2-PAPC failed to further suppress [125I]NO2-LDL binding to hMDM (Figure 11b). These results are in agreement with our previous data with the CD36-specific blocking antibody FA6 (Figures 3 and 8) and CD36–/– MPM (Figures 5 and 6). These findings confirm that CD36 is a major macrophage scavenger receptor responsible for the recognition of NO2-LDL in murine and human macrophages.
Figure 11.
Effect of oxidized PAPC vesicles on the binding of NO2-LDL to hMDM and MPM. [125I]LDL was modified as described for the complete system in Figure 2. [125I]NO2-LDL (5 μg/mL) was then incubated with (a) MPM or with (b) hMDM for 3 hours at 4°C either in the absence (NA) or presence of the indicated additions. Cellular binding of lipoproteins was subsequently determined as described in Methods. The concentrations of competitors used were 200 μg protein/mL for lipoproteins, 20 μg lipid/mL for vesicles, and 20 μg/mL for antibody. NO2-LDL, LDL modified by the MPO-H2O2-NO2– system as in the complete system of Figure 2a; –NO2LDL, LDL modified by the MPO-H2O2 system but in the absence of NO2–; NO2-PAPC, PAPC vesicles modified by the complete MPO-H2O2-NO2– system; –NO2 PAPC, PAPC modified by the MPO-H2O2 system but in the absence of NO2–. Data represent the mean ± SD of triplicate determinations of a representative experiment performed at least 3 times. AP < 0.001 for comparison versus control (NA).
Discussion
We demonstrated recently that modification of LDL by monocyte- and MPO-generated reactive nitrogen species converts the lipoprotein into a high-uptake form (NO2-LDL) that promotes macrophage lipid loading and foam cell formation (15). The results of this study extend these observations by: (a) identifying the scavenger receptor CD36 as the major pathway for murine and human macrophage recognition of NO2-LDL and foam cell formation; (b) demonstrating that the MPO-H2O2-NO2– system of monocytes readily converts LDL into a ligand for CD36 in complex biological fluids such as serum and might thus be a physiologically plausible mechanism for foam cell formation in vivo; (c) demonstrating that conversion of LDL into a ligand for CD36 and loss of LDLR recognition are early events after LDL modification by MPO-generated reactive nitrogen species; and (d) demonstrating that an oxidation product(s) of PAPC serves as a potent ligand for CD36-dependent recognition. Taken together, these results suggest that CD36-dependent recognition of NO2-LDL may play a role in lipid accumulation and foam cell formation in human macrophages in vivo.
Interactions between CD36 and NO2-LDL were examined in 293 cells that had been stably transfected with the human CD36 receptor. The specificity of binding was confirmed by demonstrating: (a) that no increase in binding was present in vector-transfected control cells; and (b) that a CD36-specific mAb inhibited binding of NO2-LDL to CD36-transfected 293 cells by approximately 90%. The potential significance of this pathway for LDL recognition in human macrophages was illustrated with an anti–CD36-specific mAb that blocked approximately 50% of the specific binding, uptake, degradation, and [14C]oleate incorporation into CE pools of hMDM exposed to NO2-LDL. It is interesting to note that others have reported that the accumulation of CE mass in hMDM exposed to LDL oxidized by free copper is similarly inhibited (40%) in individuals with CD36 deficiency (44). Moreover, a dramatic decrease in the binding and degradation of NO2-LDL to both thioglycolate-elicited and resident MPM from mice lacking functional CD36 was observed. Finally, the biological significance of CD36-mediated uptake of NO2-LDL by MPM was demonstrated by the lack of lipid accumulation and foam cell formation in thioglycolate-elicited MPM from CD36–/– mice.
The oxidation pathways responsible for converting LDL into a high-uptake form in vivo have not yet been established. Cu2+-oxLDL has been used widely as a prototype of LDL modification believed to exist in vivo. Although products of lipid peroxidation formed in Cu2+-oxLDL have been observed in human atheroma (29, 69), the role of free transition metal ions in converting LDL into a high-uptake form in vivo has been questioned (11–13). Biological matrices such as serum, plasma, and interstitial fluid possess high levels of proteins that chelate redox-active free metal ions, rendering them redox inactive (11, 12, 70). Furthermore, recent mass spectrometric studies were unable to demonstrate significant increases in levels of metal ion–dependent protein oxidation products in early atherosclerotic lesions (13). In vitro studies demonstrate that trace levels of serum or plasma constituents will inhibit LDL oxidation by free copper (11). Similarly, we observed that in the presence of 5% LPDS, Cu2+-mediated conversion of LDL into a ligand for CD36-transfected cells and a high-uptake form for hMDM was totally ablated (Figure 5). In contrast, even at LPDS concentrations as high as 80% (vol/vol), the MPO-H2O2 system used NO2– to convert the lipoprotein into a high-uptake form for macrophages (Figure 5c). These results are consistent with recent LC/MS/MS studies that demonstrate that MPO-generated reactive nitrogen species can initiate lipid peroxidation and form bioactive oxidized lipids in serum (71). MPO is enriched in human lesions (23) and mass spectrometric studies quantifying chlorotyrosine, a specific marker for MPO, demonstrate that the enzyme participates in oxidative damage of vascular tissues early in the disease process (26). Isolated MPO (15, 21), neutrophils (22), and monocytes (28) can promote aromatic nitration reactions. Furthermore, both immunohistochemical (27) and mass spectrometric studies (25) demonstrate that nitrotyrosine, a global marker of protein damage by reactive nitrogen species, is enriched in human atheroma. The present results further suggest that MPO-generated nitrating intermediates may be one pathway for promoting oxidative modification of lipoproteins and other biological targets at sites of inflammation and vascular disease. As far as we are aware, the present studies represent the first reported mechanism for oxidatively converting LDL into a high-uptake form in the presence of serum or plasma constituents.
Whereas our studies show that LDL modification by reactive nitrogen species formed by either activated monocytes or MPO readily transforms the lipoprotein into a ligand for the scavenger receptor CD36, the biological significance of this pathway in vivo is unknown. The ability of modified forms of LDL to promote accumulation of cellular CEs and form foam cells is widely accepted as an index of the atherogenic potential of a lipoprotein. However, foam-cell and fatty-streak formation are not necessarily pathogenic. Indeed, both foam cells and fatty streaks are commonly observed in vascular tissues of infants and young children (72, 73), and the critical transition from normal to disease pathology is thought to lie in the mechanisms that permit fatty streaks to progress into more advanced lesions (74). In fact, the presence of foam cells is not unique to either hyperlipidemia or vascular disease. For example, macrophage foam cell formation is observed in a variety of tissues during chronic inflammatory conditions, particularly where granuloma formation is prominent (75–78). It is therefore conceivable that oxidative modification of lipoproteins and cell membranes by the MPO-H2O2-NO2– pathway may participate in foam cell formation in a variety of conditions through a CD36-dependent pathway. It is also possible that MPO-generated reactive nitrogen species may play a protective role under some conditions—such as in the targeting of trapped or senescent LDL or damaged cells in the subendothelial space for removal by CD36-positive phagocytes.
The biochemical alterations in an LDL particle that occur during oxidation are complex, and the structural changes that promote scavenger receptor recognition are incompletely understood. Multiple lipid and protein components of LDL are subjected to oxidation during modification by the MPO-H2O2-NO2– system (15). The question arises as to which of those components renders NO2-LDL a ligand for CD36. A priori, it is possible that modified lipids, modified proteins, or lipid-protein adducts in NO2-LDL could serve as ligands for macrophage scavenger receptor(s). For Cu2+-oxLDL, it has been shown that all 3 components of modified LDL serve as a ligands for macrophages (57, 59, 67). Previous studies demonstrated that CD36-dependent binding of Cu2+-oxLDL was inhibited by high concentrations (100 μM) of free fatty acids such as oleic acid (34). Moreover, the ability of CD36 to recognize phospholipid membranes rich in anionic lipids such as phosphatidyl serine has led to the suggestion that the receptor may also function in the recognition of apoptotic particles and cells (35, 36, 38). Unlike other forms of modified LDL, NO2-LDL contains unique structural determinants such as nitrated amino acids (15, 28) and nitrated lipids (D. Schmitt and S. Hazen, unpublished data). The inability of multiple nitrated proteins and synthetic peptides either to bind directly to CD36 or compete with NO2-LDL suggests that protein oxidation products may not play a significant role in CD36-dependent recognition by macrophages. Similarly, the lack of inhibition observed with resolubilized apo B-100 from delipidated NO2-LDL suggests that putative lipid-protein adducts are either not formed in NO2-LDL or are not recognized by CD36. However, it should be noted that the difficulty in resolubilizing apo B-100 after extraction of lipids makes it difficult to totally exclude a potential role for a modified protein or protein-lipid adduct on apo B-100 as a ligand for CD36. The ability of lipid extracts derived from NO2-LDL to effectively compete for [125I]NO2-LDL binding to CD36-transfected cells identifies lipid oxidation products as at least one class of ligand for CD36 on NO2-LDL. Our demonstration that small unilamellar vesicles generated from PAPC are readily converted into potent ligands for CD36-containing cells confirms this hypothesis. It is therefore tempting to speculate that MPO-catalyzed lipid peroxidation may play a more general role in the tagging of phospholipid-containing targets for removal by CD36 positive cells in vivo.
Acknowledgments
We thank M. Krieger for generously providing CHO cells expressing mSR-AI and their vector-transfected counterparts. This work was supported in part by the American Heart Association and by National Institutes of Health grants HL-62526 and HL-61878 (to S.L. Hazen), HL-58559 (to M. Febbraio), HL-42540 (to R.L. Silverstein), HL-56987 (to D.P. Hajjar), CA-65872 (to W.A. Frazier), and HL-53315 (to H.F. Hoff). M. Febbraio is the recipient of a Silberman Foundation Fellowship.
References
- 1.Brown M, Goldstein J. A receptor mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 2.Steinbrecher UP, Zhang HF, Lougheed M. Role of the oxidatively modified LDL in atherosclerosis. Free Radic Biol Med. 1990;9:155–168. doi: 10.1016/0891-5849(90)90119-4. [DOI] [PubMed] [Google Scholar]
- 3.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88:1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berliner JA, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–2496. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg D. Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 6.Chisolm GM, III, Hazen SL, Fox PL, Cathcart MK. The oxidation of lipoproteins by monocytes-macrophages. Biochemical and biological mechanisms. J Biol Chem. 1999;274:25959–25962. doi: 10.1074/jbc.274.37.25959. [DOI] [PubMed] [Google Scholar]
- 7.Steinbrecher UP, Witztum JL, Parthasarathy S, Steinberg D. Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis. 1987;7:135–143. doi: 10.1161/01.atv.7.2.135. [DOI] [PubMed] [Google Scholar]
- 8.Steinbrecher UP. Receptors for oxidized low density lipoprotein. Biochim Biophys Acta. 1999;1436:279–298. doi: 10.1016/s0005-2760(98)00127-1. [DOI] [PubMed] [Google Scholar]
- 9.Holvoet P, Collen D. Oxidized lipoproteins in atherosclerosis and thrombosis. FASEB J. 1994;8:1279–1284. doi: 10.1096/fasebj.8.15.8001740. [DOI] [PubMed] [Google Scholar]
- 10.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: Macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–637. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 11.Dabbagh AJ, Frei B. Human suction blister interstitial fluid prevents metal ion-dependent oxidation of low density lipoprotein by macrophages and in cell-free systems. J Clin Invest. 1995;96:1958–1966. doi: 10.1172/JCI118242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas CE. The influence of medium components in copper-dependent oxidation of low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985;38:341–350. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- 13.Leeuwenburgh C, et al. Mass spectrometric quantification of markers for protein oxidation by tyrosyl radical, copper, and hydroxyl radical in low density lipoprotein isolated from human atherosclerotic plaques. J Biol Chem. 1997;272:3520–3526. doi: 10.1074/jbc.272.6.3520. [DOI] [PubMed] [Google Scholar]
- 14.Fu S, Davies MJ, Stocker R, Dean RT. Evidence for roles of radicals in protein oxidation in advanced human atherosclerotic plaque. Biochem J. 1998;333:519–525. doi: 10.1042/bj3330519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klebanoff, S.J., and Clark, R.A. 1978. The neutrophil: function and clinical disorders. In The neutrophil: function and clinical disorders. Elsevier/North Holland Biomedical Press. Amsterdam, The Netherlands. 447–451.
- 17.Agner, K. 1972. Structure and function of oxidation-reduction enzyme. In Structure and function of oxidation-reduction enzymes. A. Akeson and A. Ehrenberg, editors. Pergamon Press Inc. Tarrytown, NY. 329–335.
- 18.Krinsky NI. Singlet excited oxygen as a mediator of the antibacterial action of leukocytes. Science. 1974;186:363–365. doi: 10.1126/science.186.4161.363. [DOI] [PubMed] [Google Scholar]
- 19.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 20.Hazen SL, Hsu FF, Mueller DM, Crowley JR, Heinecke JW. Human neutrophils employ chlorine gas as an oxidant during phagocytosis. J Clin Invest. 1996;98:1283–1289. doi: 10.1172/JCI118914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Vleit A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite. A potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- 22.Eiserich JP, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazell LJ, et al. Presence of hypochlorite-modified proteins in human atherosclerotic lesions. J Clin Invest. 1996;97:1535–1544. doi: 10.1172/JCI118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leeuwenburgh C, et al. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J Biol Chem. 1997;272:1433–1436. doi: 10.1074/jbc.272.3.1433. [DOI] [PubMed] [Google Scholar]
- 26.Hazen SL, Heinecke JW. 3-chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beckman JS, et al. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 28.Hazen SL, et al. Formation of nitric oxide-derived oxidants by myeloperoxidase in monocytes: pathways for monocyte-mediated protein nitration and lipid peroxidation in vivo. Circ Res. 1999;85:950–958. doi: 10.1161/01.res.85.10.950. [DOI] [PubMed] [Google Scholar]
- 29.Ylä-Herttuala S. Macrophages and oxidized low density lipoproteins in the pathogenesis of atherosclerosis. Ann Med. 1991;23:561–567. doi: 10.3109/07853899109150518. [DOI] [PubMed] [Google Scholar]
- 30.Suarna C, Dean RT, May J, Stocker R. Human atherosclerotic plaque contains both oxidized lipids and relatively large amounts of alpha-tocopherol and ascorbate. Arterioscler Thromb Vasc Biol. 1995;15:1616–1624. doi: 10.1161/01.atv.15.10.1616. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki H, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 32.Sakaguchi H, et al. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest. 1998;78:423–434. [PubMed] [Google Scholar]
- 33.Endemann G, et al. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 34.Nicholson AC, Frieda S, Pearce A, Silverstein RL. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines: evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler Thromb. 1995;15:269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- 35.Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 36.Tait JF, Smith C. Phosphatidylserine receptors: role of CD36 in binding of anionic phospholipid vesicles to monocytic cells. J Biol Chem. 1999;274:3048–3054. doi: 10.1074/jbc.274.5.3048. [DOI] [PubMed] [Google Scholar]
- 37.Ryeom SW, Silverstein RL, Scotto A, Sparrow JR. Binding of anionic phospholipids to retinal pigment epithelium may be mediated by the scavenger receptor CD36. J Biol Chem. 1996;271:20536–20539. doi: 10.1074/jbc.271.34.20536. [DOI] [PubMed] [Google Scholar]
- 38.Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tandon NN, Kralisz U, Jamieson GA. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989;264:7576–7583. [PubMed] [Google Scholar]
- 40.Silverstein RL, Asch AS, Nachman RL. Glycoprotein IV mediates thrombospondin-dependent platelet-monocyte and platelet-U937 cell adhesion. J Clin Invest. 1989;84:546–552. doi: 10.1172/JCI114197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dawson DW, et al. CD36 mediates the in vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Febbraio M, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 43.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nozaki S, et al. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 46.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 47.Han J, Hajjar DP, Febbraio M, Nicholson AC. Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36. J Biol Chem. 1997;272:21654–21659. doi: 10.1074/jbc.272.34.21654. [DOI] [PubMed] [Google Scholar]
- 48.Han J, Hajjar DP, Tauras JM, Nicholson AC. Cellular cholesterol regulates expression of the macrophage type B scavenger receptor, CD36. J Lipid Res. 1999;40:830–838. [PubMed] [Google Scholar]
- 49.Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- 50.Nakata A, et al. CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol. 1999;19:1333–1339. doi: 10.1161/01.atv.19.5.1333. [DOI] [PubMed] [Google Scholar]
- 51.Hoppe G, O’Neil J, Hoff HF. Inactivation of lysosomal proteases by oxidized low density lipoprotein is partially responsible for its poor degradation by mouse peritoneal macrophages. J Clin Invest. 1994;94:1506–1512. doi: 10.1172/JCI117490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Czerniecki BJ, et al. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of activated dendritic cells. J Immunol. 1997;159:3823–3837. [PubMed] [Google Scholar]
- 53.Crowley JR, Yarasheski K, Leeuwenburgh C, Turk J, Heinecke JW. Isotope dilution mass spectrometric quantification of 3-nitrotyrosine in proteins and tissues is facilitated by reduction to 3-aminotyrosine. Anal Biochem. 1998;259:127–135. doi: 10.1006/abio.1998.2635. [DOI] [PubMed] [Google Scholar]
- 54.Morel DW, Hessler JR, Chisolm GM. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983;24:1070–1076. [PubMed] [Google Scholar]
- 55.Ashkenas J, et al. Structures and high and low affinity ligand binding properties of murine type I and type II macrophage scavenger receptors. J Lipid Res. 1993;34:983–1000. [PubMed] [Google Scholar]
- 56.Sheibani N, Frazier WA. Thrombospondin 1 expression in transformed endothelial cells restores a normal phenotype and suppresses their tumorigenesis. Proc Natl Acad Sci USA. 1995;92:6788–6792. doi: 10.1073/pnas.92.15.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terpstra V, Bird DA, Steinberg D. Evidence that the lipid moiety of oxidized low density lipoprotein plays a role in its interaction with macrophage receptors. Proc Natl Acad Sci USA. 1998;95:18006–18011. doi: 10.1073/pnas.95.4.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bligh EJ, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 59.Parthasarathy S, Fong LG, Otero D, Steinberg D. Recognition of solubilized apoproteins form delipidated, oxidized low density lipoprotein (LDL) by the acetyl-LDL receptor. Proc Natl Acad Sci USA. 1987;84:537–540. doi: 10.1073/pnas.84.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eiserich JP, Cross CE, Jones AD, Halliwell B, van der Vliet A. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J Biol Chem. 1996;271:19199–19208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- 61.Hazell LJ, van den Berg JJ, Stocker R. Oxidation of low-density lipoprotein by hypochlorite causes aggregation that is mediated by modification of lysine residues rather than lipid oxidation. Biochem J. 1994;302:297–304. doi: 10.1042/bj3020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hazell LJ, Davies MJ, Stocker R. Secondary radicals derived from chloramines of apolipoprotein B-100 contribute to HOCl-induced lipid peroxidation of low-density lipoproteins. Biochem J. 1999;339:489–495. [PMC free article] [PubMed] [Google Scholar]
- 63.Hazen SL, Hsu FF, Gaut JP, Crowley JR, Heinecke JW. Modification of proteins and lipids by myeloperoxidase. Methods Enzymol. 1999;300:88–105. doi: 10.1016/s0076-6879(99)00117-2. [DOI] [PubMed] [Google Scholar]
- 64.Puente Navazo MD, Daviet L, Ninio E, McGregor JL. Identification on human CD36 of a domain (155–183) implicated in binding oxidized low-density lipoproteins (Ox-LDL) Arterioscler Thromb Vasc Biol. 1996;16:1033–1039. doi: 10.1161/01.atv.16.8.1033. [DOI] [PubMed] [Google Scholar]
- 65.Haberland ME, Olch CL, Folgelman AM. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984;259:11305–11311. [PubMed] [Google Scholar]
- 66.Traber MG, Defendi V, Kayden HJ. Receptor activities for low-density lipoprotein and acetylated low-density lipoprotein in a mouse macrophage cell line (IC21) and in human monocyte-derived macrophages. J Exp Med. 1981;154:1852–1867. doi: 10.1084/jem.154.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bird DA, et al. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: Implications with respect to macrophage recognition of apoptotic cells. Proc Natl Acad Sci USA. 1999;96:6347–6352. doi: 10.1073/pnas.96.11.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beckman JS, Chen J, Ischiropoulos H, Crow JP. Oxidative chemistry of peroxinitrite. Methods Enzymol. 1994;233:229–240. doi: 10.1016/s0076-6879(94)33026-3. [DOI] [PubMed] [Google Scholar]
- 69.Niu X, Zammit V, Upston JM, Dean RT, Stocker R. Coexistence of oxidized lipids and alpha-tocopherol in all lipoprotein density fractions isolated from advanced human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 1999;19:1708–1718. doi: 10.1161/01.atv.19.7.1708. [DOI] [PubMed] [Google Scholar]
- 70.Aasa R, Malmstrom BG, Saltman P, Vangard T. The specific binding of Fe(III) and Cu(II) to transferrin and conalbumin. Biochim Biophys Acta. 1963;75:203–222. doi: 10.1016/0006-3002(63)90599-7. [DOI] [PubMed] [Google Scholar]
- 71.Schmitt D, et al. Leukocytes utilize myeloperoxidase-generated nitrating intermediates as physiological catalysts for the generation of biologically active oxidized lipids and sterols in serum. Biochemistry. 1999;38:16904–16915. doi: 10.1021/bi991623w. [DOI] [PubMed] [Google Scholar]
- 72.Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Natural history of aortic and coronary atherosclerotic lesions in youth: findings from the PDAY study. Arterioscler Thromb. 1993;13:1291–1298. doi: 10.1161/01.atv.13.9.1291. [DOI] [PubMed] [Google Scholar]
- 73.Napoli C, et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 1997;100:2680–2690. doi: 10.1172/JCI119813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 75.Iwata M, Colby TV, Kitaichi M. Diffuse panbronchiolitis: diagnosis and distinction from various pulmonary diseases with centrilobular interstitial foam cell accumulations. Hum Pathol. 1994;25:357–363. doi: 10.1016/0046-8177(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 76.Cozzutto C, Carbone A. The xanthogranulomatous process. Xanthogranulomatous inflammation. Pathol Res Pract. 1988;183:395–402. doi: 10.1016/S0344-0338(88)80085-2. [DOI] [PubMed] [Google Scholar]
- 77.Nakashiro H, et al. Xanthogranulomatous cholecystis. Cell composition and a possible pathogenetic role of cell-mediated immunity. Pathol Res Pract. 1995;191:1078–1086. [PubMed] [Google Scholar]
- 78.Furue M. Colocalization of scavenger receptor in CD68 positive foam cells in verruciform xanthoma. J Dermatol Sci. 1995;10:213–219. doi: 10.1016/0923-1811(95)00406-i. [DOI] [PubMed] [Google Scholar]