Abstract
Objective
This study sought to determine whether chronic social stress can desensitize leukocytes to normal physiologic regulation by endogenous glucocorticoids.
Methods
We analyzed the longitudinal relationship between plasma cortisol levels and peripheral blood lymphocyte counts over 16 monthly assessments in 18 rhesus macaques randomized to recurrent social encounters with a stable set of conspecifics or continually varying social partners (unstable socialization).
Results
Animals socialized under Stable conditions showed the expected inverse relationship between plasma cortisol concentrations and circulating lymphocyte frequencies. That relationship was significantly attenuated in animals subject to Unstable social conditions. Differences in leukocyte redistributional sensitivity to endogenous glucocorticoids emerged within the first week of differential socialization, persisted throughout the 60-week study period, and were correlated with other measures of glucocorticoid desensitization (blunted HPA axis response to acute stress and redistributional response to dexamethasone challenge). Effects of Unstable social conditions on leukocyte sensitivity to cortisol regulation were not related to physical aggression.
Conclusion
Chronic social stress can impair normal physiologic regulation of leukocyte function by the HPA axis in ways that may contribute to the increased physical health risks associated with social adversity.
Keywords: social stress, glucocorticoid resistance, hypothalamic-pituitary-adrenal axis, psychoneuroimmunology, immune system, rhesus macaque
INTRODUCTION
Social stress is a well-established risk factor for specific diseases (1–9) and for all-cause mortality (10–14). Understanding the biological basis for these effects has been complicated by the paradoxical role of neuroendocrine mediators in relationships between social conditions and the inflammatory drivers of disease pathogenesis (15–17). Inflammation contributes to the initiation and progression of several diseases that are aggravated by social stress, including cardiovascular disease, viral infections, and certain types of cancer (18–21). Hypothalamic-pituitary-adrenal (HPA) axis release of glucocorticoids constitutes a primary physiologic regulator of inflammation (22–26), implying that decreased glucocorticoid signaling might contribute to the increased health risks associated with social stress. However, stressful social conditions are generally associated with stable or increasing levels of circulating glucocorticoids (15, 27–29), rather than the decreased levels that would explain pathological inflammation. In light of glucocorticoids’ anti-inflammatory actions, how can their relative abundance during social stress accommodate a simultaneous increase in the risk of inflammation-driven disease?
One potential explanation for this paradox has emerged from analyses showing that impaired signal transduction by glucocorticoid receptor (GR) can render inflammatory cells insensitive to glucocorticoid regulation even in the presence of high ambient glucocorticoid hormone levels (24). Correlational studies of chronically stressed human beings show several indications of leukocyte desensitization to glucocorticoid signaling, including impaired glucocorticoid inhibition of LPS-induced cytokine responses ex vivo (16, 17), blunted glucocorticod regulation of leukocyte trafficking in vivo (30), and reductions in glucocorticoid-mediated gene transcription in the presence of normal circulating glucocorticoid levels (15, 31). However, it remains unclear whether these correlational observations reflect a causal effect of social factors on leukocyte sensitivity to glucocorticoid regulation. Decisive experiments are difficult to perform in humans for ethical reasons, but one line of experimental research in rodents has shown that repeated social conflict can induce glucocorticoid resistance in leukocytes (32–34). It is unclear if those effects are pertinent to human social stress, however, because the development of glucocorticoid resistance in the murine social disruption model depends on aggression-related tissue wounding (33, 35) and consequent activation of the pro-inflammatory cytokine, IL-1β (36). Several human experimental studies have shown that acute social stress can transiently alter sensitivity of the circulating leukocyte pool to glucocorticoid inhibition (16, 37), although such effects last for a short period of time and vary as a function of sex, reproductive hormones, and major depression (16, 37). It is not known whether purely psychological stress might induce a persistent impact on leukocyte sensitivity to regulation by endogenous glucocorticoids.
In the present study, we examined the effects of chronic social stress on leukocyte sensitivity to glucocorticoid regulation in a rhesus macaque model that is analogous to human chronic stress in the lack of socially mediated tissue wounding. Previous studies have shown that daily social contact with a varying complement of peers activates behavioral and neuroendocrine indicators of stress in adult male rhesus macaques (1, 38). To determine whether such unstable social conditions can decrease leukocyte sensitivity to regulation by endogenous glucocorticoids, we utilized a hematological probe of GR-mediated signal transduction based on cortisol regulation of leukocyte subset trafficking (30). Under normal conditions, rising glucocorticoid levels lead to an increase in the number of circulating neutrophils and a decrease in the number of circulating lymphocytes and monocytes (39–44). These effects are driven by altered trafficking of leukocyte subsets between circulating blood and extravascular compartments such as the bone marrow and spleen (34, 41, 45, 46). Because this process is mediated specifically by GR signaling (42, 47), the strength of association between circulating cortisol levels and leukocyte subset redistribution in circulating blood provides an in vivo hematological measure of immune cell sensitivity to regulation by endogenous glucocorticoids (30). The present study utilized that hematological indicator to determine whether chronic social stress can desensitize leukocytes to normal physiologic regulation by endogenous glucocorticoids, and whether those effects are associated with other indications of glucocorticoid desensitization such as blunted HPA response to stress or reduced leukocyte redistributional sensitivity to pharmacologic glucocorticoid challenge.
METHODS
Study sample
Eighteen healthy adult male rhesus macaques (Macaca mulatta) aged 5–8 years were selected based on good physical health, intermediate social status in their natal cages, no prior participation in invasive research, and seronegativity for Simian Retrovirus Type D, Simian Immunodeficiency Virus (SIV), and Simian T-Lymphotropic Virus. These animals served as uninfected controls for a parallel condition analyzing social influences on progression of SIV infection (1, 48), and each received a 1 ml i.v. saline injection as a control for SIV inoculation. Injections took place after 3 exposures to the experimental social conditions described below. All procedures were conducted under the supervision of Institutional Animal Care and Use Committee (IACUC) at the University of California at Davis, and all efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques.
Social stress
Animals were relocated from outdoor field cages to indoor individual housing for approximately one year prior to the initiation of experimental procedures. As previously described (1), animals were randomized to Stable or Unstable socialization conditions which were implemented by transferring each animal to a 5.6 m2 socialization cage containing one to three other study macaques from the same experimental condition for 100 min per day on three to five days each week. Under Stable conditions, the same three animals met on each occasion. Under Unstable conditions, the size and composition of the group varied daily (sets of two to four animals differing in composition on each occasion). Socialization conditions were maintained over the entire 16 month study period (60 weeks) and were continuously monitored for aggressive physical contact as previously described (1). Unstable social conditions induce continual re-establishment of social dominance hierarchies, resulting in behavioral and neuroendocrine indications of stress (1). However, aggression-related wounding or injury virtually never occurs (as verified by continuous observation of social encounters and IACUC-mandated intervention to prevent aggression-related wounding, and confirmed by daily veterinary inspections for signs of injury or illness, which did not differ across groups). When adult male macaques form relationships in the absence of social complexities introduced by females and young animals, role differentiation occurs quickly and with virtually no contact aggression (49). In the Stable social condition of this study, animals were able to establish dominance hierarchies within one socialization cycle (1). To the extent that aggression contributed to stress under Unstable social conditions, it is the threat of harm, rather than actual wounding, that would account for such effects.
Blood sampling
Arm pulls were employed to draw resting blood samples from the antecubital vein of conscious animals between 1500 and 1530 hr on days in which no socialization occurred. All samples were obtained within 5 min of initial animal contact (i.e., draw time), with draw times lasting < 3 min in 90% of cases. Samples were drawn from animals in their individual residential cages, and the duration of time between staff entry into the residence room and initial contact with a given animal (time to contact) (50) ranged from 0–25 min (mean = 8.7, SD = 5.7), depending on the number of animals whose blood was drawn on a given day. Plasma was obtained by centrifugation of 1.5 ml EDTA-anticoagulated aliquots at 3,000 rpm for 20 min, followed by storage at −70°C. Blood samples were obtained 4 and 2 months prior to the initiation of experimental social conditions, after 3 socialization cycles, and at 4-week intervals thereafter for 60 weeks, generating a total of 18 blood samples for each animal (2 baseline, 16 socialization).
Plasma cortisol
Plasma cortisol concentrations were assessed using a commercial radioimmunoassay (Diagnostic Products, Los Angeles CA) with 9.6% inter-assay coefficient of variation and 8.5% intra-assay coefficient of variation in this sample (1). Data are available from 324 measurements (18 animals × 18 time points).
Hematological parameters
Frequencies of circulating lymphocytes, monocytes, and banded neutrophils were determined by automated hematologic analysis using a Serono Baker Diagnostic System (Allentown, PA) followed by a manual differential, as previously described (39, 50). Variation in replicate assays of the same sample was < 5%. Two hematology assessments were not obtained due to technical difficulties, for a total of 322 available values.
HPA reactivity assessment
During weeks 15–21 of differential socialization, each animal was tested for stress-induced HPA axis response to 2h of restraint stress, and for HPA axis feedback inhibition by dexamethasone suppression test (DST), both as previously described (1). Briefly, each individual animal received an i.m. injection of 50 μg/kg dexamethasone or an equivalent volume of saline (150 ul) at 0700 hr. 6 h later, the animal was restrained in a primate chair (Primate Products, Redwood City CA) for 2 hrs as a model of acute stress. Plasma cortisol and lymphocyte frequencies were quantified as described above in 5 ml of antecubital venous blood drawn immediately upon seating and 120 min later. This procedure was repeated a second time at least ten days after the initial session, with the order of pre-treatments (dexamethasone vs. saline) counterbalanced. HPA axis and hematological response to stress was gauged by the change in circulating cortisol and leukocyte subset numbers from baseline to 120 min in the saline-treated condition. HPA axis sensitivity to dexamethasone inhibition was gauged by the difference in baseline cortisol levels obtained 6 hrs after dexamethasone injection vs. saline injection (i.e., immediately after seating).
Statistical analyses
Exploratory data analyses found plasma cortisol levels to be positively correlated with both time to animal contact (Spearman rank correlation rS = .20, p < .001) and blood draw time (rS = .14, p = .011) (SAS PROC CORR; SAS Institute, Cary NC), and these procedural parameters were thus included as covariates in all subsequent analyses. To identify the optimal hematological indicator of glucocorticoid regulation, nonparametric Spearman correlation analyses assessed relationships between plasma cortisol levels and circulating leukocyte subset frequencies while controlling for time to contact and blood draw time. Mixed effect linear models (51) were used to analyze leukocyte subset distributions as a function of plasma cortisol levels while controlling for blood draw procedural parameters. Models were fit using SAS PROC MIXED, specifying longitudinal measurements from the same animal as a repeated measure (51) and rank-transforming lymphocyte numbers at each time point to minimize the effect of outliers (52). In mixed model analyses, glucocorticoid sensitivity was quantified by the parameter relating leukocyte subset frequencies to cortisol levels, and the differential strength of that relationship under Stable vs. Unstable social conditions was quantified by a Cortisol × Social Stability interaction term (in a model including main effects of Cortisol and Social Stability). Change in the magnitude of group differences from baseline to the differential socialization period was tested by a Period × Cortisol × Social Stability 3-way interaction. Residual plots verified that results met the distributional assumptions of general linear models (51, 53). Effects of experimental condition on cortisol and hematological responses to Dexamethasone and physical restraint stress were analyzed by repeated measures analysis of variance (PROC GLM). All available data were analyzed, and reported p-values represent two-sided significance levels.
RESULTS
Cortisol regulation of leukocyte subset trafficking
To identify the optimal hematologic indicator of immune cell sensitivity to glucocorticoid-mediated trafficking, preliminary analyses examined relationships between plasma cortisol levels and circulating numbers of lymphocytes, monocytes, and neutrophils in 322 available observations from 18 repeated measurements on each of 18 animals (9 each in Stable and Unstable social conditions). Consistent with the established effects of glucocorticoids on leukocyte subset trafficking in humans (30, 40–42, 45), results showed the expected negative relationship between plasma cortisol concentrations and circulating lymphocyte numbers (rS = −.20, p < .001). Contrary to previous observations in humans (30), however, plasma cortisol levels were not associated with either lower monocyte counts (rS = −.03, p = .66) or higher neutrophil counts (rS = .07, p = .24).
Experimental verification of the hematological indicator
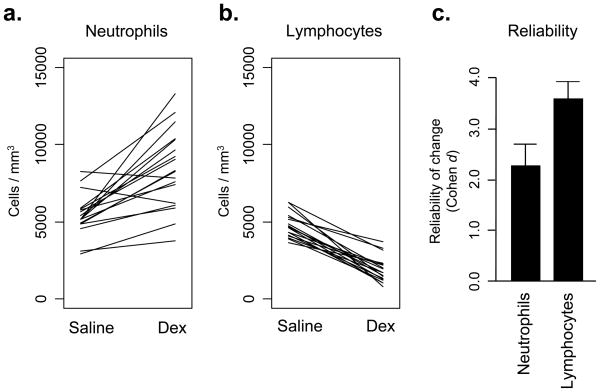
To experimentally confirm the reliability of circulating leukocyte numbers as a hematological indicator of glucocorticoid regulation, each animal was injected with dexamethasone or saline and assessed for circulating neutrophil and lymphocyte frequencies 6 hrs later. Dexamethasone altered circulating numbers of each leukocyte subset in the predicted direction (average +3,017 ± 547 neutrophils/μl and −2,898 ± 244 lymphocytes/μl; both F(1,16) > 20.00, p < .001). Consistent with lymphocytes’ greater cross-sectional correlation with endogenous cortisol variation (above), experimental glucocorticoid administration also affected lymphocyte counts more reliably than neutrophil counts (Figure 1; difference in standardized effect size coefficients, z = 2.96, p = .005).
FIGURE 1.
Effect of dexamethasone administration on (a.) circulating neutrophil numbers, and (b.) circulating lymphocyte numbers. (c.) Reliability of leukocyte subset responses to dexamethasone injection, expressed as change relative to baseline standard deviation (Cohen’s d).
Effects of social stress on glucocorticoid regulation of lymphocyte distributions
This study’s primary analyses sought to determine whether chronic social stress might reduce lymphocyte sensitivity to redistribution by endogenous glucocorticoids. In 2 baseline blood samples collected 2 months apart, animals that were subsequently randomized to Stable vs. Unstable social conditions showed no difference in circulating lymphocyte numbers or plasma cortisol concentrations (Table 1). Mixed effect linear model analyses also confirmed that the groups did not differ in lymphocyte redistributional sensitivity to cortisol (F(1,12) = 1.14, p = .30). The entire sample showed the expected inverse relationship between circulating lymphocyte numbers and plasma cortisol levels (rS = −.30, p = .09).
TABLE 1.
Basal hematologic and endocrine parameters by group.
| Parameter | Stable | Unstable | Difference |
|---|---|---|---|
| Cortisol (μg/dl) | |||
| Baselinea | 13.2c (1.9) | 14.9 (1.3) | p = .37 |
| Socializationb | 13.6 (1.6) | 13.6 (1.2) | p = .53 |
| Month 1 | 15.9 (1.4) | 17.5 (1.3) | p = .42 |
| Month 16 | 15.4 (1.4) | 15.6 (1.0) | p = .93 |
| Lymphocytes (cells/μl) | |||
| Baseline | 4,088 (275) | 4,340 (251) | p = .65 |
| Socialization | 4,124 (399) | 4,129 (495) | p = .62 |
| Month 1 | 3,938 (359) | 3,830 (334) | p = .83 |
| Month 16 | 4,398 (398) | 4,532 (375) | p = .81 |
| Lymphocytes (% WBC) | |||
| Baseline | 53.8 (2.0) | 53.0 (2.6) | p = .95 |
| Socialization | 43.3 (3.0) | 45.2 (3.5) | p = .30 |
| Month 1 | 39.1 (3.8) | 40.5 (4.0) | p = .82 |
| Month 16 | 44.6 (2.0) | 45.7 (2.5) | p = .72 |
Average of 2 baseline observations 4 and 8 weeks prior to socialization
Average of 16 socialization observations over 60 wks
Mean (SE)
During 16 subsequent months of differential socialization, animals randomized to interact with a stable set of partners continued to show an inverse relationship between plasma cortisol levels and circulating lymphocyte numbers (mean rS = −.21, p = .006) (Figure 2). However, that relationship was substantially attenuated in animals subject to Unstable social conditions (mean rS = −.03, p = .78). Statistical significance of differential change in cortisol sensitivity was verified by a Period (Baseline vs. Socialization) × Group (Stable vs. Unstable) × Cortisol 3-way interaction term in a mixed effect linear model analysis of circulating lymphocyte numbers (F(1,296) = 7.06, p = .008). Social conditions did not affect average levels of plasma cortisol or circulating lymphocyte numbers or percentages (Table 1); only the correlation between cortisol and circulating lymphocyte numbers was affected by Unstable socialization.
FIGURE 2.
Sensitivity of circulating lymphocyte frequencies to endogenous cortisol levels in Stable vs. Unstable social conditions. Magnitude of cortisol sensitivity is quantified by the Spearman rank correlation between plasma cortisol concentrations and circulating lymphocyte numbers at 2 and 4 months prior to study entry, and monthly afterward during 16 months of differential socialization.
We next asked whether the magnitude of lymphocyte desensitization to cortisol regulation might progressively increase or decrease over the 16-month socialization period. Analysis of the three-way interaction term in a Month × Cortisol × Social Condition mixed effect linear model analysis showed no significant time trend in the magnitude of differential sensitivity across groups (F(1,260) = 0.67, p = .41).
The role of aggression
Aggressive physical contact (bite, hit, slap) was uncommon (mean 0.153 ± 0.038 instances per hr of social contact), but did occur more often in Unstable social conditions (mean 0.301 ± 0.074) than in Stable conditions (mean 0.005 ± 0.005; difference p = .003). Analyses controlling for rates of physical aggression continued to show reductions in lymphocyte sensitivity to cortisol redistribution under Unstable social conditions (Period × Group × Cortisol 3-way interaction: F(1,295) = 6.67, p = .01).
Relationship to HPA axis glucocorticoid response
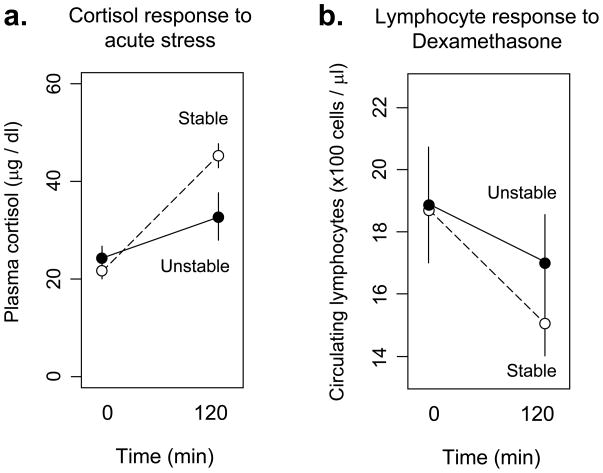
To determine whether blunted hematological sensitivity to glucocorticoids paralleled other physiologic alterations in glucocorticoid regulation, we assessed HPA axis response to acute stress during weeks 15–21 of differential socialization (Figure 3a). Animals in the Unstable social condition showed a blunted cortisol response to physical restraint stress compared to animals in the Stable condition (F(1,16) = 15.21, p = .001). Inter-individual variations in the magnitude of stress-induced cortisol response also showed a significant positive association with the magnitude of lymphocyte redistributional sensitivity to endogenous cortisol variation (F(1,16) = 3.03, p = .008).
FIGURE 3.
(a.) Effect of acute restraint stress on plasma cortisol levels in macaques subject to Stable (dashed line) vs. Unstable social conditions (solid line). (b.) Effect of pharmacologic dexamethasone challenge on circulating lymphocyte numbers in macaques subject to Stable (dashed line) vs. Unstable social conditions (solid line).
Consistent with blunted glucocorticoid regulation of leukocyte trafficking in response to endogenous cortisol, animals in the Unstable condition also showed a 52% reduction in sensitivity to dexamethasone-induced suppression of circulating lymphocyte numbers relative to animals socialized under Stable conditions (Figure 3b). Although the group difference failed to reach statistical significance (F(1,16) = 0.22, p = .64), the sample-wide correlation between lymphocyte redistributional response to dexamethasone and redistributional sensitivity to endogenous cortisol variations was highly significant (F(1,16) = 10.23, p = .006).
DISCUSSION
The present results show that chronic social stress can persistently desensitize cells of the immune system to normal physiologic regulation by endogenous glucocorticoids. Rhesus macaques that socialized daily with a stable set of peers showed the well-established inverse relationship between plasma cortisol levels and circulating lymphocyte numbers (30, 40–45, 47). However, this regulatory dynamic was significantly attenuated in macaques that were randomized to more stressful daily socialization with a continually varying set of peers. Group differences in leukocyte redistributional sensitivity to endogenous cortisol emerged rapidly and did not significantly diminish over 60 weeks of continuing socialization. Consistent with previous observational studies of chronically stressed humans (15, 30, 54–56) and non-human primates (39, 57), these regulatory alterations emerged in the absence of increased circulating cortisol levels and were accompanied by parallel reductions in HPA response to acute stress. Also consistent with previous studies in humans, but in contrast to rodent models of socially induced glucocorticoid resistance (33, 35), these alterations emerged in the absence of tissue wounding and were independent of aggressive social interaction. Thus, social-psychological dynamics alone appear to be sufficient to desensitize lymphocytes to physiologic regulation by the HPA axis in primates.
The present experimental findings parallel previous observational studies in suggesting that chronic social stress can inhibit glucocortiocid signaling pathways in ways that might ultimately contribute to increased risk of inflammation-mediated disease (15–17, 30, 31). These results also extend previous studies showing that acute laboratory stress can transiently alter glucocorticoid regulatory dynamics in the circulating leukocyte pool (16, 37) by documenting more persistent decreases in immune cell sensitivity to glucocorticoid regulation in vivo (i.e., measured at least 24 h after differential social activity, and persisting for at least 60 wks). We interpret changes in leukocyte redistribution sensitivity as a functional marker of GR signaling dynamics that may also impact other glucocorticoid-regulated processes more directly relevant to disease (e.g., inflammatory gene expression and cytokine response) (15, 24, 32–34, 58–60). However, some have argued that stress-induced alterations in leukocyte trafficking may be health-relevant in their own right (47). Clear health implications of GR desensitization have been observed in mouse models of inflammatory and infectious diseases (61–63), but it has been unclear how the wounding-related biology of those models might apply to contemporary human conditions. The present results indicate that purely social stress can also alter regulatory interactions between the HPA axis and immune system, and they establish the macaque social stability paradigm as a useful experimental model for defining the health consequences of such dynamics.
The fact that GR desensitization emerged independently of physical aggression suggests that more subtle cognitive and affective responses to unfamiliar others may play a key role in the biological effects observed here. As Charles Horton Cooley noted more than a century ago (64), “even sitting in the same room with unfamiliar people can be harassing and exhausting.” “The mere presence of people… can cause a vague discomfort, doubt, and tension.” The present findings suggest that chronic exposure to such social uncertainty can impact immunoregulatory dynamics, and may also illuminate previous results showing increased viral pathogenesis in individuals facing chronic social uncertainty (1, 65). In holding constant the quantity of social opportunity, these results emphasize the critical role of the quality of social contact in shaping its immunobiological consequences (38).
Findings from this study’s in vivo hematological probe of glucocorticoid sensitivity are consistent with data from human observational studies (30) and analyses of genome-wide transcriptional profiling of circulating leukocytes (15, 31) in suggesting that stressful social conditions can alter basal immunoregulatory relationships (i.e., in the absence of any exogenous immunological challenge). Previous experimental studies have shown that glucocorticoid-induced leukocyte redistribution is specifically attributable to GR signaling (42), and represents a causal effect of glucocorticoids (i.e., glucocorticoid manipulation induces hematological redistribution (40–42, 45, 47), but hematological manipulations do not directly influence glucocorticoid levels (66, 67)). The experimental dexamethasone challenge examined here confirms that glucocorticoids causally regulate leukocyte trafficking in rhesus macaques, and identifies the lymphopenic response as the most sensitive indicator of that dynamic in this non-human primate model. This study’s ancillary results also verify that desensitization of the in vivo hematological redistribution probe is correlated with blunting of the HPA response to acute stress, and with blunted lymphocyte redistribution in response to dexamethasone. Thus, chronic social stress may induce coordinated alterations in glucocorticoid responsiveness across multiple tissue systems (57). However, the physiologic generality of these results should be considered provisional until future studies assess the relationship between the in vivo hematological probe used here and other measures of GR desensitization based on ex vivo cytokine inhibition assays (16, 17, 32–34) or genome-wide transcriptional profiles (15, 31).
Several limitations need to be considered when interpreting the results of this study. Given the diurnal variation in circulating cortisol levels and hematologic parameters (68), greater density of sampling over the course of a day would be desirable to more precisely quantify the magnitude lymphocyte redistributional sensitivity. In addition, this study’s finding that GR desensitization can emerge within three cycles of differential socialization (i.e., < 1 week) and persist for up to 60 weeks should be considered provisional until it is replicated in larger samples with additional measures of GR sensitivity. The rapid onset of GR desensitization observed here is remarkable, but it is consistent with previous observations from rodent models showing altered endocrine, hematological, and functional parameters within two cycles of differential social exposure (35, 69). Because glucocorticoids induce leukocyte redistribution over several hours (40), the present analysis of concurrent plasma cortisol and hematological parameters may not provide an optimal measure of GR sensitivity (30). However, the present results do show that the expected relationships can be identified in concurrent measures if enough longitudinal data are available to average over occasion-specific noise (e.g., 16 repeated measurements per individual over 60 weeks). The sub-optimal measurement format of the present study likely attenuated the observed relationships between cortisol and hematological parameters, but that effect applied to all samples similarly and would not explain group differences in GR sensitivity as a function of differential socialization. Finally, no direct measures of GR number or functional alteration (e.g., phosphorylation) are available in the present study, so future analyses will be required to identify the specific molecular mechanism of social stress-induced GR desensitization.
The emergence of glucocorticoid desensitization in the absence of increased plasma cortisol concentrations underscores the need to assess HPA axis regulation of immune cell function using post-receptor measures that integrate the effects of hormone concentrations with variations in receptor-mediated signaling response (70). Several other studies have also shown that social stress can alter glucocorticoid regulation of cellular function in the absence of chronic glucocorticoid elevations (15, 17, 30, 31, 56). From a physiologic perspective, the relative abundance of glucocorticoids and the GR’s sensitivity to glucocorticoid ligation are functionally separate parameters (70). Several non-glucocorticoid molecular signaling pathways have been found to regulate GR sensitivity, including the pro-inflammatory cytokine IL-1β (36) and GR phosphorylation by serine/threonine and tyrosine kinase signaling cascades (24, 70). It is conceivable that the increased levels of sympathetic nervous system activity documented in other studies of the macaque unstable socialization paradigm (38, 71, 72) could potentially alter GR signal transduction efficiency via catecholamine induction of GR phosphorylation by PKA (70). Identification of the specific molecular pathways mediating stress-induced desensitization of glucocorticoid immunoregulation in the present model is an important topic for future research.
In addition to coordinating a wide variety of physiologic responses to stress, the HPA axis also plays a key physiologic role in controlling systemic inflammation via glucocorticoid inhibition of the pro-inflammatory transcription factor NF-κB (23–26). To the extent that social stress undermines that immunoregulatory relationship by inhibiting glucocorticoid signal transduction, the consequent increase in pro-inflammatory gene expression might contribute to the variations in inflammation-related disease observed in human social epidemiology (2–4, 6–15). The non-human primate paradigm analyzed here provides an ecologically valid experimental model for dissecting the psychological and biological mechanisms of social stress-induced GR desensitization, and defining its teleologic role in relationships between social behavior and immunity (15, 38, 71, 73–76).
Acknowledgments
This research was supported by the U.S. National Institutes of Health
Data collection was supported by grants from the National Institute of Mental Health (MH49033 to JPC) and the National Center for Research Resources (RR000169 to JPC), and analysis was supported by grants from the National Institute of Drug Abuse (DA024441 to JPC) and the National Cancer Institute (CA116778 to SWC).
References
- 1.Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proceedings of the National Academy of Sciences, USA. 1998;95:4714–4719. doi: 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caspi A, Harrington H, Moffitt TE, Milne BJ, Poulton R. Socially isolated children 20 years later: risk of cardiovascular disease. Arch Pediatr Adolesc Med. 2006;160:805–11. doi: 10.1001/archpedi.160.8.805. [DOI] [PubMed] [Google Scholar]
- 3.Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. Journal of the American Medical Association. 1997;227:1940–1944. [PubMed] [Google Scholar]
- 4.Cole SW, Kemeny ME, Fahey JL, Zack JA, Naliboff BD. Psychological risk factors for HIV pathogenesis: Mediation by the autonomic nervous system. Biological Psychiatry. 2003;54:1444–1456. doi: 10.1016/s0006-3223(02)01888-7. [DOI] [PubMed] [Google Scholar]
- 5.Eng PM, Rimm EB, Fitzmaurice G, Kawachi I. Social ties and change in social ties in relation to subsequent total and cause-specific mortality and coronary heart disease incidence in men. Am J Epidemiol. 2002;155:700–9. doi: 10.1093/aje/155.8.700. [DOI] [PubMed] [Google Scholar]
- 6.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24:1105–11. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 7.Krongrad A, Lai H, Burke MA, Goodkin K, Lai S. Marriage and mortality in prostate cancer. J Urol. 1996;156:1696–70. [PubMed] [Google Scholar]
- 8.Reynolds P, Kaplan GA. Social connections and risk for cancer: prospective evidence from the Alameda County Study. Behav Med. 1990;16:101–10. doi: 10.1080/08964289.1990.9934597. [DOI] [PubMed] [Google Scholar]
- 9.Soler-Villa H, Kasl SV, Jones BA. Prognostic significance of psychosocial factors in African-American and White breast cancer patients. Cancer. 2003;98:1299–1308. doi: 10.1002/cncr.11670. [DOI] [PubMed] [Google Scholar]
- 10.Berkman LF. Social Networks, Host Resistance and Mortality: A Follow-Up Study of Alameda County Residents. University of California; Berkeley: 1977. [Google Scholar]
- 11.Berkman LF, Kawachi I. Social Epidemiology. New York: Oxford University Press; 2000. [Google Scholar]
- 12.Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46:S39–52. [PubMed] [Google Scholar]
- 13.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 14.Seeman TE. Social ties and health: the benefits of social integration. Ann Epidemiol. 1996;6:442–51. doi: 10.1016/s1047-2797(96)00095-6. [DOI] [PubMed] [Google Scholar]
- 15.Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biol. 2007;8:R189. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679–87. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- 17.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21:531–41. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 18.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 20.Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 21.Naugler WE, Karin M. The wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;6:6. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Munck A, Guyre PM. Glucocorticoid physiology, pharmacology and stress. Advances in Expermental Medicine and Biology. 1986;196:81–96. doi: 10.1007/978-1-4684-5101-6_6. [DOI] [PubMed] [Google Scholar]
- 23.Onard JL, Schoenveld M, Cidlowski JA. Glucocorticoids and Immunity: Mechanisms of Regulation. In: Ader R, editor. Psychoneuroimmunology. 4. Vol. 1. Burlington, MA: Elsevier Academic Press; 2007. pp. 45–62. [Google Scholar]
- 24.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: Relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. Epub 2006 Oct 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 26.Ruzek MC, Pearce BD, Miller AH, Biron CA. Endogenous glucocorticoids protect against cytokine-mediated lethality during viral infection. J Immunol. 1999;162:3527–33. [PubMed] [Google Scholar]
- 27.Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–91. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- 28.Sapolsky RM. Why zebras don’t get ulcers: A guide to stress, stress-related diseases, and coping. New York: Freeman; 1994. [Google Scholar]
- 29.Weiner H. Perturbing the Organism: The Biology of Stressful Experience. Chicago: University of Chicago Press; 1992. [Google Scholar]
- 30.Cole SW. Social regulation of leukocyte homeostasis: The role of glucocorticoid sensitivity. Brain Behav Immun. 2008;22:1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–72. doi: 10.1016/j.biopsych.2008.03.017. Epub 2008 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stark J, Avitsur R, Padgett DA, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. American J Physiol Reg Int Comp Physiol. 2001;280:R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- 33.Bailey MT, Avitsur R, Engler H, Padgett DA, Sheridan JF. Physical defeat reduces the sensitivity of murine splenocytes to the suppressive effects of corticosterone. Brain Behav Immun. 2004;18:416–24. doi: 10.1016/j.bbi.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Engler H, Bailey MT, Engler A, Sheridan JF. Effects of repeated social stress on leukocyte distribution in bone marrow, peripheral blood and spleen. J Neuroimmunol. 2004;148:106–15. doi: 10.1016/j.jneuroim.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 35.Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39:247–57. doi: 10.1006/hbeh.2001.1653. [DOI] [PubMed] [Google Scholar]
- 36.Engler H, Bailey MT, Engler A, Stiner-Jones LM, Quan N, Sheridan JF. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–17. doi: 10.1016/j.psyneuen.2007.10.007. Epub 2007 Nov 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohleder N, Wolf JM, Kirschbaum C. Glucocorticoid sensitivity in humans-interindividual differences and acute stress effects. Stress. 2003;6:207–22. doi: 10.1080/1025389031000153658. [DOI] [PubMed] [Google Scholar]
- 38.Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27:8857–65. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capitanio JP, Mendoza SP, Lerche NW. Individual differences in peripheral blood immunological and hormonal measures in adult male rhesus macaques (Macaca mulatta): evidence for temporal and situational consistency. Am J Primatol. 1998;44:29–41. doi: 10.1002/(SICI)1098-2345(1998)44:1<29::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 40.Dale DC, Fauci AS, Guerry DI, Wolff SM. Comparison of agents producing a neutrophilic leukocytosis in man. Hydrocortisone, prednisone, endotoxin, and etiocholanolone. J Clin Invest. 1975;56:808–13. doi: 10.1172/JCI108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–15. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- 42.Miller AH, Spencer RL, hassett J, Kim C, Rhee R, Ciurea D, Dhabhar F, McEwen B, Stein M. Effects of selective type I and II adrenal steroid agonists on immune cell distribution. Endocrinology. 1994;135:1934–44. doi: 10.1210/endo.135.5.7956914. [DOI] [PubMed] [Google Scholar]
- 43.Kim CY, Han JS, Suzuki T, Han SS. Indirect indicator of transport stress in hematological values in newly acquired cynomolgus monkeys. J Med Primatol. 2005;34:188–92. doi: 10.1111/j.1600-0684.2005.00116.x. [DOI] [PubMed] [Google Scholar]
- 44.Morrow-Tesch JL, McGlone JJ, Norman RL. Consequences of restraint stress on natural killer cell activity, behavior, and hormone levels in rhesus macaques (Macaca mulatta) Psychoneuroendocrinology. 1993;18:383–95. doi: 10.1016/0306-4530(93)90013-b. [DOI] [PubMed] [Google Scholar]
- 45.Fauci AS, Dale DC. The effect of Hydrocortisone on the kinetics of normal human lymphocytes. Blood. 1975;46:235–43. [PubMed] [Google Scholar]
- 46.Stefanski V. Social stress in laboratory rats: hormonal responses and immune cell distribution. Psychoneuroendocrinology. 2000;25:389–406. doi: 10.1016/s0306-4530(99)00066-9. [DOI] [PubMed] [Google Scholar]
- 47.Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol. 1996;157:1638–44. [PubMed] [Google Scholar]
- 48.Capitanio JP, Mendoza SP, Baroncelli S. The relationship of personality dimensions in adult male rhesus macaques to progression of simian immunodeficiency virus disease. Brain, Behavior, and Immunity. 1999;13:138–154. doi: 10.1006/brbi.1998.0540. [DOI] [PubMed] [Google Scholar]
- 49.Mendoza SP. Social conflict on first encounters. In: Mason WA, Mendoza SP, editors. Primate Social Conflict. Albany, NY: SUNY Press; 1993. pp. 85–110. [Google Scholar]
- 50.Capitanio JP, Mendoza SP, McChesney M. Influences of blood sampling procedures on basal hypothalamic-pituitary-adrenal hormone levels and leukocyte values in rhesus macaques (Macaca mulatta) J Med Primatol. 1996;25:26–33. doi: 10.1111/j.1600-0684.1996.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan LM, Dukes KA, Losina E. Tutorial in biostatistics. An introduction to hierarchical linear modelling. Stat Med. 1999;18:855–88. doi: 10.1002/(sici)1097-0258(19990415)18:7<855::aid-sim117>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 52.Barnett V, Lewis T. Outliers in Statistical Data. New York: John Wiley & Sons; 1994. [Google Scholar]
- 53.Miller RG. Beyond ANOVA: Basics of applied statistics. New York: Wiley; 1986. [Google Scholar]
- 54.Yehuda R, Kahana B, Binder-Brynes K, Southwick SM, Mason JW, Giller EL. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry. 1995;152:982–6. doi: 10.1176/ajp.152.7.982. [DOI] [PubMed] [Google Scholar]
- 55.Bremner JD, Vythilingam M, Anderson G, Vermetten E, McGlashan T, Heninger G, Rasmusson A, Southwick SM, Charney DS. Assessment of the hypothalamic-pituitary-adrenal axis over a 24-hour diurnal period and in response to neuroendocrine challenges in women with and without childhood sexual abuse and posttraumatic stress disorder. Biol Psychiatry. 2003;54:710–8. doi: 10.1016/s0006-3223(02)01912-1. [DOI] [PubMed] [Google Scholar]
- 56.Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-Inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol Psychiatry. 2004;55:745–51. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 57.Mendoza SP, Capitanio JP, Mason WA. Chronic social stress: Studies in non-human primates. In: Moberg GP, Mench JA, editors. Biology of animal stress: Basic principles and implications for animal welfare. New York: CABI Publishing; 2000. pp. 227–247. [Google Scholar]
- 58.Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav Immun. 2005;19:311–7. doi: 10.1016/j.bbi.2004.09.005. Epub 2004 Nov 21. [DOI] [PubMed] [Google Scholar]
- 59.Avitsur R, Stark JL, Dhabhar FS, Sheridan JF. Social stress alters splenocyte phenotype and function. J Neuroimmunol. 2002;132:66–71. doi: 10.1016/s0165-5728(02)00310-7. [DOI] [PubMed] [Google Scholar]
- 60.Quan N, Avitsur R, Stark JL, He L, Lai W, Dhabhar F, Sheridan JF. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137:51–8. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 61.Padgett DA, Sheridan JF, Dorne J, Berntson GG, Candelora J, Glaser R. Social stress and the reactivation of latent herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1998;95:7231–5. doi: 10.1073/pnas.95.12.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheridan JF, Stark JL, Avitsur R, Padgett DA. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann N Y Acad Sci. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- 63.Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PT, Sheridan JF. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- 64.Cooley CH. Human nature and the social order. New Brunswick, NJ: Transaction Books; 1983/1902. [Google Scholar]
- 65.Cole SW, Kemeny ME, Taylor SE. Social identity and physical health: Accelerated HIV progression in rejection-sensitive gay men. Journal of Personality and Social Psychology. 1997;72:320–336. doi: 10.1037//0022-3514.72.2.320. [DOI] [PubMed] [Google Scholar]
- 66.Schuld A, Mullington J, Hermann D, Hinze-Selch D, Fenzel T, Holsboer F, Pollmacher T. Effects of granulocyte colony-stimulating factor on night sleep in humans. Am J Physiol. 1999;276:R1149–55. doi: 10.1152/ajpregu.1999.276.4.R1149. [DOI] [PubMed] [Google Scholar]
- 67.Rovelli F, Barni S, Tancini G, Ardizzoia A, Lissoni P. Endocrine effects of granulocyte colony-stimulating factor in cancer patients. Tumori. 1995;81:438–9. doi: 10.1177/030089169508100611. [DOI] [PubMed] [Google Scholar]
- 68.Winkel P, Statland BE, Saunders AM, Osborn H, Kupperman H. Within-day physiologic variation of leukocyte types in healthy subjects as assayed by two automated leukocyte differential analyzers. Am J Clin Pathol. 1981;75:693–700. doi: 10.1093/ajcp/75.5.693. [DOI] [PubMed] [Google Scholar]
- 69.Engler H, Engler A, Bailey MT, Sheridan JF. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J Neuroimmunol. 2005;163:110–9. doi: 10.1016/j.jneuroim.2005.03.002. Epub 2005 Apr 20. [DOI] [PubMed] [Google Scholar]
- 70.Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245–61. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- 71.Sloan EK, Capitanio JP, Cole SW. Stress-induced remodeling of lymphoid innervation. Brain Behav Immun. 2008;22:15–21. doi: 10.1016/j.bbi.2007.06.011. Epub 2007 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sloan EK, Capitanio JP, Tarara RP, Cole SW. Social temperament and lymph node innervation. Brain Behav Immun. 2007;6:6. doi: 10.1016/j.bbi.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amdam GV, Aase AL, Seehuus SC, Kim Fondrk M, Norberg K, Hartfelder K. Social reversal of immunosenescence in honey bee workers. Exp Gerontol. 2005;40:939–47. doi: 10.1016/j.exger.2005.08.004. Epub 2005 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cole SW. The complexity of dynamic host networks. In: Deisboeck TS, Kresh JY, editors. Complex Systems Science in BioMedicine. New York: Kluwer Academic - Plenum Publishers; 2005. pp. 605–629. [Google Scholar]
- 75.McDade TW. Life history theory and the immune system: steps toward a human ecological immunology. Am J Phys Anthropol. 2003;(Suppl):100–25. doi: 10.1002/ajpa.10398. [DOI] [PubMed] [Google Scholar]
- 76.Stow A, Beattie A. Chemical and genetic defenses against disease in insect societies. Brain Behav Immun. 2008;22:1009–13. doi: 10.1016/j.bbi.2008.03.008. Epub 2008 May 8. [DOI] [PubMed] [Google Scholar]