Abstract
Testicular germ cell tumors (TGCT) are unique in their excellent response to DNA-damaging chemotherapy. Mutation of p53 is rare in both untreated and relapsed TGCTs, suggesting that p53 fails to respond effectively against malignant transformation in germ cells. Previous studies implicated the presence of a poorly defined TGCT-specific mechanism of p53 inactivation. Here we show that disruption of p53-mdm2 binding using the MDM2-specific inhibitor Nutlin activates p53 in TGCT cells and is sufficient to induce strong apoptosis. Knockdown of MDMX cooperates with Nutlin to activate p53. Surprisingly, we found that p53 activation induced a two-fold increase in MDMX mRNA and protein expression in TGCT cells. A p53-responsive promoter is identified in MDMX intron 1 that contains a functional p53-binding site, suggesting that MDMX also functions as a negative feedback regulator of p53 in a cell line-dependent fashion. These findings suggest that MDM2 and MDMX are responsible for the functional inactivation of p53 in TGCT. Furthermore, TGCT cells are unique in having a strong apoptosis response to p53. Direct activation of p53 by targeting MDM2 and MDMX may provide a backup approach for the treatment of TGCTs resistant to DNA-damaging drugs.
Keywords: p53, p21, PUMA, MDM2, MDMX, germ cell tumor
Introduction
Testicular germ cell tumors (TGCT) are the most frequent solid tumor in men between 20–40 years of age, accounting for ~60% of malignancies diagnosed at this age.1 The rapid growth and progression of TGCTs result in 25–60% of patients suffer from distant metastasis at the time of diagnosis. However, TGCT is also one of the most curable forms of solid tumors. Cisplatin-based combination chemotherapy cures more than 80% of metastatic TGCTs. Despite the high cure rate, TGCT is still the most frequent cause of death from solid tumors in men in the 20–40 year age group.2 Approximately 10–20% of patients with metastatic disease will not achieve a durable complete remission. These patients will benefit from the identification of novel agents effective against tumors resistant to traditional drugs.
The susceptibility of germ cells to apoptosis after DNA damage is likely responsible for the intrinsic sensitivity of TGCTs to chemotherapy drugs, since TGCTs develop from primordial germ cells and maintain many characteristics of their origin. The DNA damaging agents cisplatin, etoposide and bleomycin are mainly responsible for the successful treatment of testicular cancers.3 Unlike other types of solid tumors, TGCTs have low capacity in repairing DNA damage induced by cisplatin, which may account for drug sensitivity.1 Resistance to cisplatin in TGCTs can be due to reduced drug uptake, increased drug export or intracellular detoxification, and enhanced DNA repair capacity.4 Interestingly, p53 mutation does not seem to be a major cause of drug resistance in TGCTs.5 In one analysis, only 18% of TGCTs that failed treatment contain p53 mutation.6 Therefore, the presence of wild type p53 in the majority of treatment-resistant TGCTs provides an opportunity to use alternative treatments that target the p53 pathway, provided that these tumors still retain apoptosis capacity after p53 activation.
Because germ cell tumors rarely suffer p53 mutation in both untreated cases and after relapse,6–10 this unusual phenomenon led to the hypothesis that p53 in TGCT may be inactivated by a mechanism specific to this tumor type, much like the bypass of p53 mutation in HPV-positive cervical carcinomas by E6-mediated degradation of p53. A previous study showed that in the mouse teratocarcinoma carcinoma cell line F9, wild type p53 is present at high levels but in an inactive state until treated by cisplatin or by RA-induced differentiation.11 Deletion analysis of p53 suggests the presence of a repression domain aa 105–117 of p53 that is functional in several different cell types, although somewhat more efficient in TGCT cells.12,13 Therefore, whether there is a truly TGCT-specific mechanism of p53 inactivation is not clear.
In most tumors retaining wild type p53, MDM2 and MDMX play important roles in suppressing p53 activity. This is often caused by overexpression of MDM2 and MDMX, and silencing of the MDM2 regulator ARF.14–18 MDM2 functions as the principal E3 ligase in p53 degradation, whereas MDMX mainly inactivate p53 by complex formation.15 Genomic analysis of mutations in human glioblastomas showed that MDM2 or MDMX overexpression in combination with ARF deletion are important alternative mechanisms to p53 mutation.19 An analysis of the p53 pathway in retinoblastomas which rarely suffer p53 mutation suggests that MDMX amplification or overexpression is responsible for p53 functional inactivation.20 The significance of MDM2 and MDMX in TGCT has not been directly addressed in earlier studies. However, the fact that p53 level is inducible in TGCT cells by DNA damage suggests that MDM2 plays a key role in its functional regulation. Furthermore, the INK4a/ARF locus is frequently altered in TGCT,21 suggesting that hyperactivation of MDM2 plays a role in suppressing p53 function.
In this study, we tested the functional significance of MDM2 and MDMX in p53 regulation in TGCT cell lines. Our results show that disrupting MDM2-p53 binding in TGCT cells using the MDM2-specific inhibitor Nutlin leads to p53 stabilization and dramatic apoptosis. We also found that MDMX expression is inducible by p53 in TGCT cells, and that MDMX also contributes to p53 inactivation in TGCT. These findings indicate that MDM2 and MDMX are responsible for the functional inactivation of p53 in TGCT. Furthermore, TGCT cells have efficient apoptosis response to activated p53 that is unrelated to their sensitivity to cisplatin. Therefore, direct activation of p53 by targeting MDM2 and MDMX may be a useful alternative for the treatment of TGCTs resistant to DNA-damaging drugs.
Results
Inhibition of MDM2 activates p53 in TGCT cells
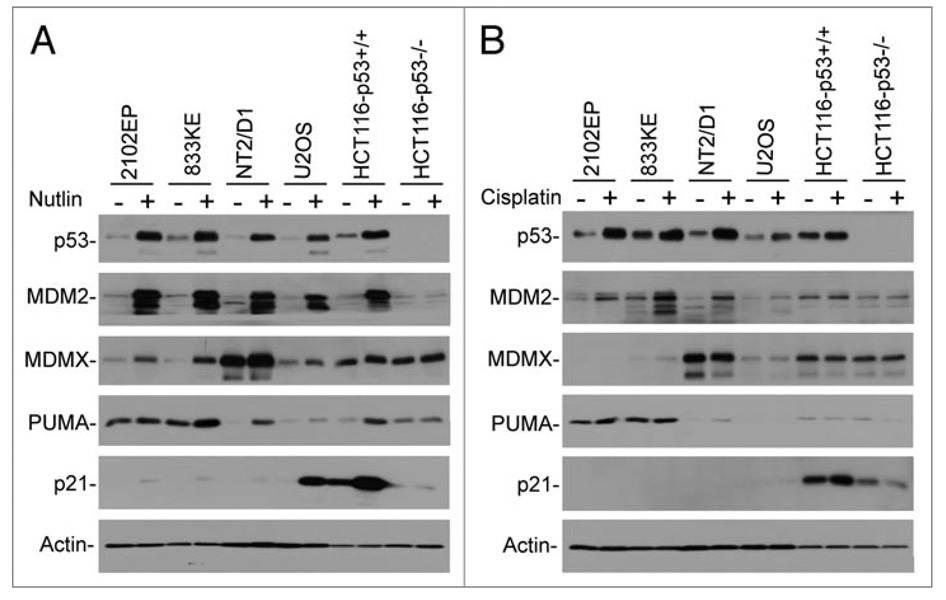
TGCT cells are reported to express high levels of wild type p53 that is functionally inactive, but can be reactivated by DNA damage or RA-induced differentiation. To test the functional significance of MDM2 in p53 inactivation in TGCT cells, three representative cell lines were treated with the MDM2-specific inhibitor Nutlin-3a.22 The results showed that Nutlin induced significant accumulation of p53 in TGCT cells similar to that in other solid tumor cell lines (Fig. 1A). p53 stabilization was associated with dramatic induction of MDM2, consistent with functional activation of p53 similar in magnitude to other tumor types after Nutlin treatment. Surprisingly, MDMX level was also significantly increased after p53 activation by Nutlin, whereas its induction was less obvious in U2OS and HCT116 cells (Fig. 1A). This suggests that MDMX expression may be induced by p53 in a cell line-dependent fashion (further investigated below).
Figure 1.
Inhibition of MDM2 activates p53 in TGCT cells. Cells treated with 5 µM Nutlin (A) or 4 µM cisplatin (B) for 18 hrs were analyzed by western blot for indicated markers.
Nutlin treatment also induced expression of the pro-apoptotic p53 target PUMA (Fig. 1A). Interestingly, TGCT cells express extremely low level of p21 compared to other tumor types, and p53 activation by Nutlin failed to induce p21 to a normal level. In contrast, two out of three TGCT cell lines express higher PUMA level compared to U2OS and HCT116 (Fig. 1A). The expression of Bax was not induced by Nutlin in these experiments (data not shown). Previous study revealed that p21 in TGCT cells is not inducible by cisplatin-mediated DNA damage.23 However, longer exposure of our western blot showed that the degree of p21 induction was significant, suggesting normal p53 binding and activation of the p21 promoter but less efficient transcription (discussed further below).
In comparison, when the TGCT cell lines were treated with the DNA damaging drug cisplatin, the magnitude of MDM2 and MDMX induction were less dramatic despite similar levels of p53 stabilization (Fig. 1B). This may be due to the fact that DNA damage can promote MDM2 and MDMX degradation that may reduce the steady state levels of these proteins. Similar to Nutlin, cisplatin did not induce much p21 expression (Fig. 1B). Overall, these results showed that p53 in TGCT cells can be activated to a similar level as in other tumor cell lines, despite some potentially cell line-specific difference in target gene expression profile.
MDMX also contributes to p53 inactivation in TGCT cells
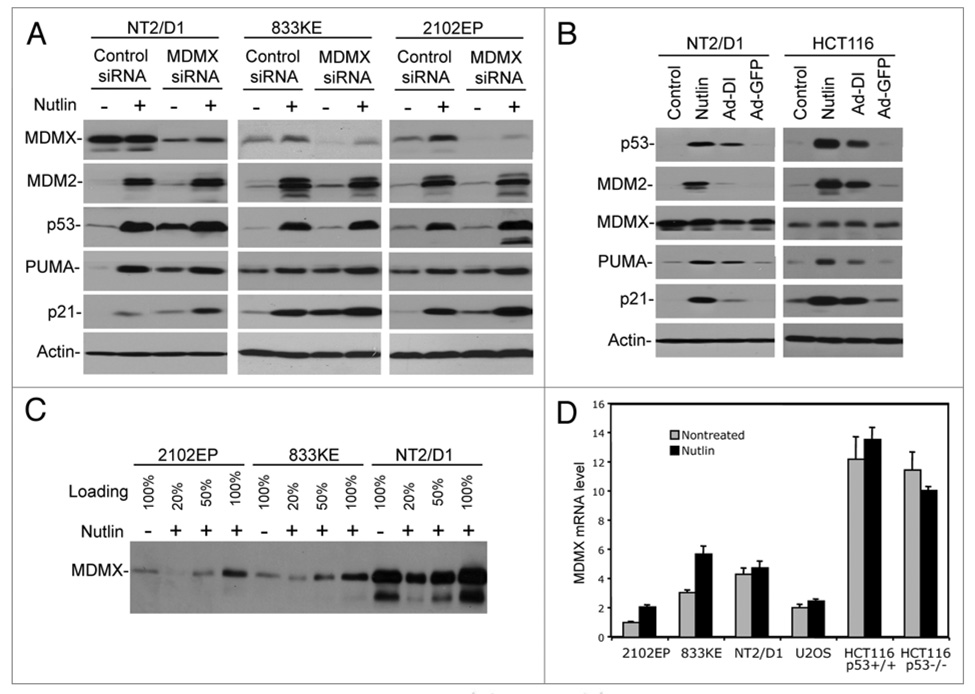
TGCT cell lines showed different levels of MDMX expression, with NT2/D1 having the highest MDMX level (Fig. 1A). Recent studies suggest that MDMX overexpression plays a role in inhibiting p53 in several tumor types.24,25 Nutlin is specific for MDM2 and does not inhibit MDMX-p53 binding under experimentally acceptable concentrations.26–28 To determine whether MDMX also plays a role in regulating p53 in TGCT cells, the cells were treated with MDMX siRNA. MDMX knockdown in all three TGCT cell lines resulted in increased p53 activity, as evidenced by higher p21 expression. Furthermore, MDMX siRNA cooperated with Nutlin to induce higher levels of p21 than Nutlin alone (Fig. 2A). MDMX knockdown also resulted in increased PUMA level, although the effect was marginal (Fig. 2A). Therefore, MDMX expression contributes to inactivation of wild type p53 in TGCT cells.
Figure 2.
MDMX contributes to p53 inactivation in TGCT cells. (A) Cells were transfected with MDMX siRNA or control siRNA for 48 hrs and then transfected again with the same amount of siRNA, and 19 hrs later cells were treated with 5 µM Nutlin for 8 hrs. Expression levels of indicated markers were analyzed by western blot. (B) Cells were infected with adenovirus Ad-DI or Ad-GFP (MOI = 300) and collected 48 hr post-infection. Expression levels of indicated markers were analyzed by western blot. (C) Cells treated with 5 µM Nutlin for 18 hrs were analyzed by western blot at different loading levels. (D) Cells treated with 5 µM Nutlin for 18 hrs were analyzed by quantitative RT-PCR to determine the level of MDMX mRNA.
To further test the role of MDM2 and MDMX in regulating p53 level and activity in TGCT cells, an adenovirus expressing thioredoxin fused to an optimized inhibitory peptide against MDM2 and MDMX (Ad-DI) was also used to disrupt MDM2-p53 and MDMX-p53 binding.29 Because of extremely poor efficiency of gene delivery by adenovirus in TGCT cells, the experiment using 833KE and 2102EP were uninformative (data not shown). NT2/D1 cells were infected by Ad-DI at a low level as judged by GFP expression, and showed p53 stabilization and induction of p21 and PUMA expression (Fig. 2B). Overall, these results support the notion that MDM2 and MDMX play an important role in inactivating p53 in TGCT cells.
MDMX is a p53-inducible gene in TGCT cells
As shown in Figure 1A, Nutlin treatment induced a significant increase of MDMX expression in TGCT cells in contrast to a marginal increase in U2OS and HCT116. A titration analysis showed that Nutlin induced MDMX by 2-fold in the protein level (Fig. 2C). Quantitative RT-PCR analysis also showed a 2-fold induction of MDMX mRNA by Nutlin in TGCT cells (Fig. 2D), suggesting that p53 activation induces MDMX transcription. Consistent with this observation, a previous ChIP-based global mapping of p53 binding sites in the human genome has identified a putative p53-binding site in intron 1 of the MDMX gene.30 Interestingly, only marginal increase of MDMX mRNA was detected in Nutlin-treated U2OS and HCT116 cells (Fig. 2D), suggesting that the ability of p53 to induce MDMX transcription is cell line-specific. These results reveal that contrary to widely accepted notion, MDMX is also a p53-responsive gene and may form a potential negative feedback loop to regulate p53 function.
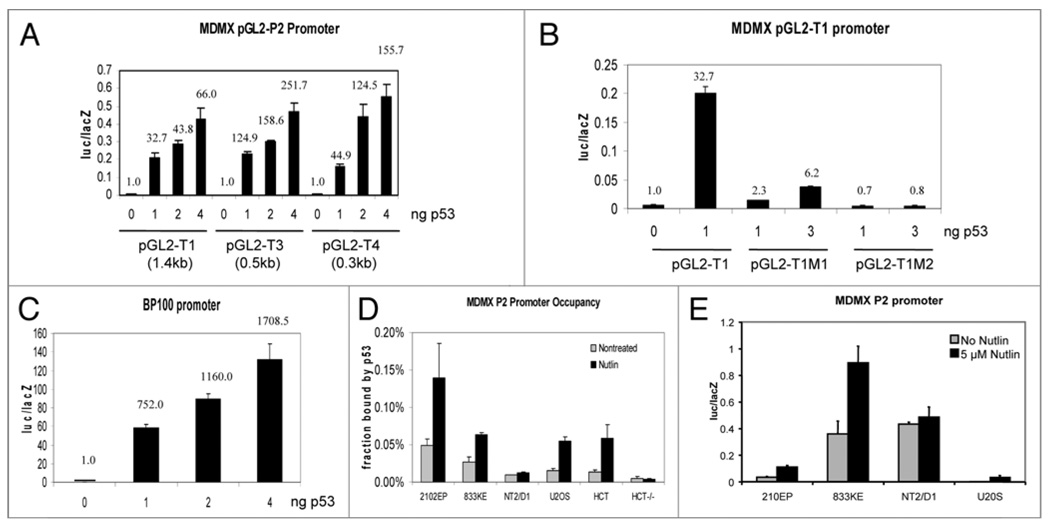
Previous study showed that MDMX intron 1 is bound by p53 during ChIP,30 but its functional significance has not been characterized. Analysis of human MDMX intron 1 sequence (8,974 bp) for p53 binding site revealed a low scoring site (4505-GGG GAT GTT AGT GCT TGT CT-4524, deviation from PuPuPuC(A/T)(T/A)GPyPyPy consensus underlined) located near the midpoint and a high scoring site (8537-AGA CAT GTT CCA ACA TGT TG-8556) near the boundary with exon 2 (Table 1). To test whether this region contains a functional p53-responsive promoter, a 1.4 kb fragment encompassing the high-scoring site was amplified by PCR and inserted into pGL2-basic luciferase reporter. Cotransfection of the reporter with p53 into p53-null H1299 cells resulted in significant induction of luciferase expression (Fig. 3A). To further test the role of the putative p53-binding element in mediating activation by p53, the 1.4 kb intron 1 promoter fragment was subjected to 5' truncations. The results showed that a 320 bp fragment containing the p53-binding site (Table 1) retained most of the response to p53 (Fig. 3A). Furthermore, point mutations introduced into the p53 binding site abrogated responsiveness to p53 (Fig. 3B). In comparison, the MDM2 intron 1 promoter construct BP100-luciferase was activated more significantly under the same conditions (Fig. 3C). The previously cloned 1.1 kb MDMX basal promoter (P1, upstream of exon 1) characterized by our laboratory was not activated by p53 (data not shown).31 Since the MDMX translation start codon is encoded by exon 2, these results showed that MDMX intron 1 contains a p53-responsive promoter with the potential to induce expression of full-length MDMX.
Table 1.
Summary of MDMX intron 1 P2 promoter
| MDMX intron 1 | 1–8974 |
|---|---|
| p53 consensus binding site | PuPuPuC(A/T)(T/A)GPyPyPy(N0–13) PuPuPuC(A/T)(T/A)GPyPyPy |
| Putative p53 binding site in MDMX intron 1 | 8537-AGACATGTTC CAACATGTTG-8556 (Deviation from consensus underlined) |
| MDMX P2M1 mutation | 8537-TCTCATGTTC CAACATGTTG-8556 |
| MDMX P2M2 mutation | 8537-AGAAATTTTC CAACATGTTG-8556 |
| MDMX P2 T1 construct | 7286–8725 (1,439 bp) |
| MDMX P2 T3 construct | 8185–8725 (540 bp) |
| MDMX P2 T4 construct | 8405–8725 (320 bp) |
Figure 3.
MDMX is a p53-inducible gene in TGCT cells. (A) p53 activates a promoter in MDMX intron 1. H1299 cells were cotransfected with reporter plasmids including pGL2-MDMX-P2, pGL2-MDMX-P2 truncations and p53. Twenty-four hours later, luciferase activity was determined and normalized with LacZ activity. The data is shown as mean +/− SD in three independent experiments. (B) Point mutations in the putative p53-binding site of MDMX P2 promoter abrogate the responsiveness to p53. The AGA sequence in the first quarter site was mutated to TCT in the M1 construct. And the first CWWG motif was mutated to AWWT in the M2 construct (refer to Table 1). (C) p53 activates MDM2 P2 promoter. The relative activation folds were marked in (A–C). (D) p53 binding to MDMX P2 promoter is increased in Nutlin-treated cells. Cells were treated with 5 µM Nutlin for 18 hrs and analyzed for p53 binding to MDMX P2 promoter by ChIP. Diluted input chromatin from each cell line was used during qPCR to help determine the percent of promoter DNA that were coprecipitated with p53 antibody (promoter occupancy). (E) Cells were transfected with MDMX P2 promoter-luciferase for 24 hrs and treated with 5 µM Nutlin for 18 hrs. Luciferase activity was determined and normalized to cotransfected CMV-lacZ expression level.
To further test whether Nutlin stimulates p53 binding to MDMX intron 1, p53 ChIP analysis was performed using primers flanking the high-scoring site. The results showed that Nutlin induced up to 3-fold increase in p53 binding to intron 1 in cells expressing p53, but not in HCT116-p53−/− cells (Fig. 3D). Overall, these results suggest the presence of a p53-responsive promoter (P2) in intron 1 of MDMX. However, the p53 response capability of the MDMX P2 promoter is weaker than the MDM2 P2 promoter. Furthermore, the impact of the P2 promoter on overall MDMX protein level during p53 activation is apparently cell line dependent, being most significant in TGCT cells 2102EP and 833KE but very limited in HCT116 and U2OS cells. ChIP assay indicated that p53 binding to MDMX intron 1 was significantly increased after Nutlin treatment in HCT116 and U2OS cells (Fig. 3D), but resulted in little increase in total MDMX level (Figs. 1A and 2D). Transient transfection of the MDMX P2 reporter into 833KE, 2102EP and U2OS cells showed that it was activated by Nutlin to the same degree in the three cell lines (2–5 fold, Fig. 3E). However, the absolute activity of MDMX P2 promoter in U2OS cells was much lower than 833KE and 2101EP (Fig. 3E). NT2/D1 cells showed high P2 promoter activity but no response to Nutlin, in agreement with ChIP and MDMX western blot results. Theses results suggest that additional factors may be needed to cooperate with p53 to achieve significant transcription from the MDMX P2 promoter. Cells such as U2OS and HCT116 may have insufficient level of these transcription factors, limiting the influence of P2 promoter on overall MDMX expression level during p53 activation.
Nutlin induces efficient apoptosis in TGCT cells
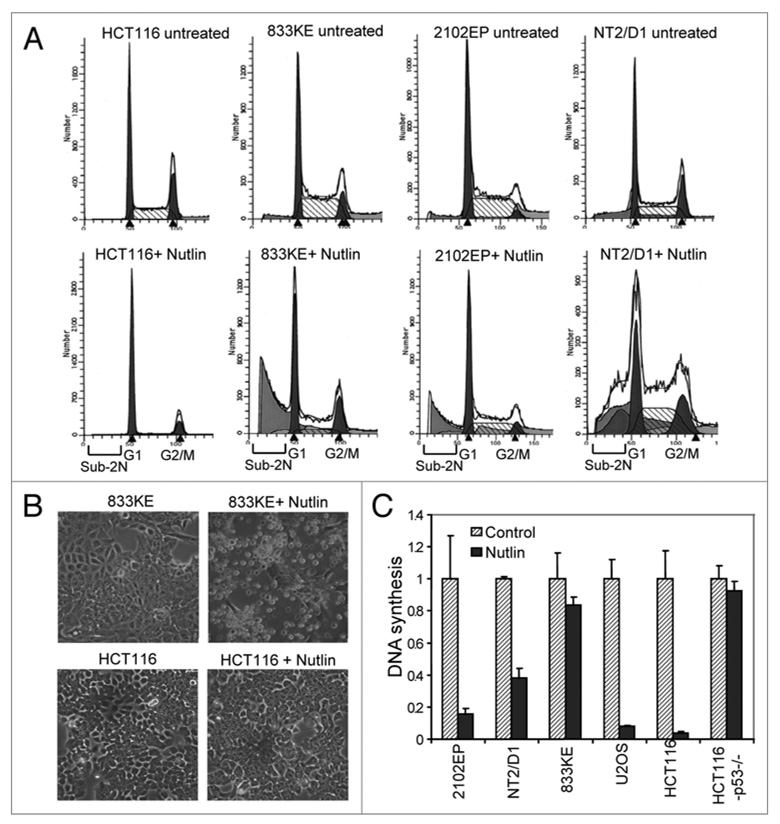
Nutlin is known to specifically activate p53 without causing DNA damage and p53 phosphorylation.32 Treatment of most tumor cell lines with Nutlin results in predominantly cell cycle arrest.33 As expected, we found that treatment of HCT116 with Nutlin resulted in p53-dependent cell cycle arrest with near complete depletion of S phase population (Fig. 4A). Similar result was observed in Nutlin-treated U2OS cells (data not shown). In contrast, TGCT cells treated with Nutlin showed dramatic increase in sub-2N apoptotic population, with little change in S phase population, suggesting that Nutlin induced strong apoptosis in TGCT cells (Fig. 4A and B). Therefore, unlike most tumor cells, TGCT cells respond to p53 activation with efficient apoptosis.
Figure 4.
Inhibition of MDM2 is sufficient to induce apoptosis in TGCT cells. (A) Cells were treated with 5 µM Nutlin for 18 hrs, fixed and stained with propidium iodide, and analyzed by FACS to detect the Sub-2N apoptotic cells. (B) Representative photographs of TGCT cells undergoing apoptosis after Nutlin treatment, compared to growth arrest response by HCT116 cells. (C) Cells were treated with 5 µM Nutlin for 12 hrs and labeled with methyl-3H-thymidine for 1 hr. The rate of DNA synthesis was determined by scintillation counting.
When DNA replication was measured by 3H-thymidine incorporation assay, the results showed that HCT116 and U2OS underwent strong growth arrest after Nutlin treatment in a p53-dependent fashion. In contrast, TGCT cells retained significant level of DNA synthesis in the presence of Nutlin (Fig. 4C). Therefore, TGCT cells do not undergo efficient growth arrest upon p53 activation, and activates the apoptotic response instead. The presence of high level PUMA and extremely low level p21 expression in TGCT cells are likely to play an important role in leading to the apoptotic response, since high level p21 expression is known to promote cell cycle arrest and inhibit apoptosis.34,35
MDMX siRNA cooperated moderately with Nutlin to induce p21 and PUMA in TGCT cells (Fig. 2A). MDMX knockdown also caused a 2-fold stimulation of apoptosis by Nutlin in NT2/D1 cells (data not shown). However, no effect of MDMX siRNA was observed on the apoptosis of 833KE and 2102EP cells after Nutlin treatment (data not shown). Therefore, the contribution of MDMX to p53 regulation appears to be moderate and cell line-dependent, whereas MDM2 is the major regulator of p53 in most cells.
Nutlin cooperates with DNA damage to induce apoptosis in TGCT cells
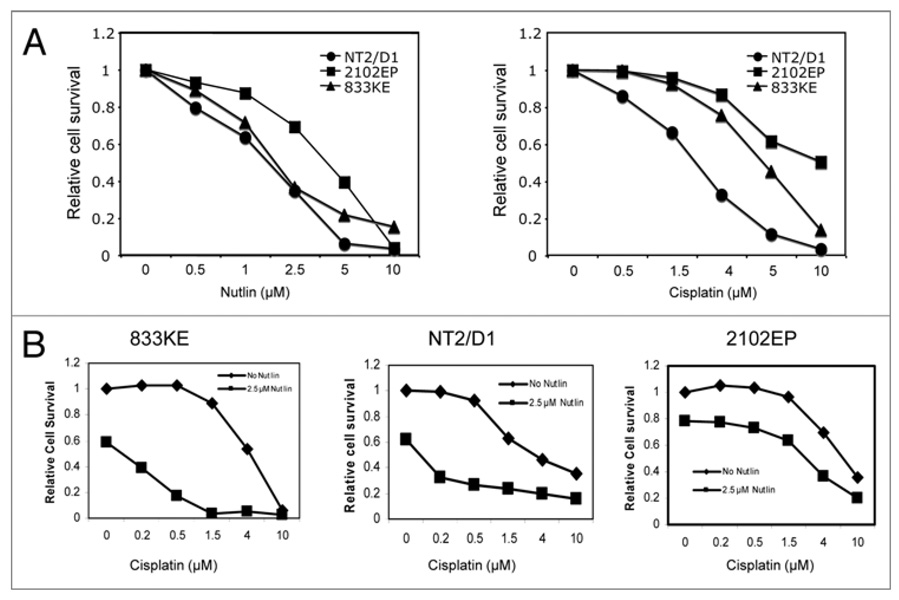
TGCTs are highly responsive to DNA damaging drugs. The presence of wt p53 is thought to contribute to favorable response to therapy, although other specific features of this cell type such as inability to repair damage induced by chemotherapy may be the main reason for its excellent response. However, drug-resistant TGCTs can develop and are responsible for treatment failures. As described above, optimal concentration of Nutlin (5–10 µM) alone was sufficient to induce near complete apoptosis in all TGCT cells in a 3-day MTS assay (Fig. 5A). In contrast, these cell lines showed clear difference in sensitivity to cisplatin (Fig. 5A). To test whether specific p53-activation can sensitize apoptotic response to cisplatin, the cells were treated with combinations of cisplatin and Nutlin. Low concentration of Nutlin (2.5 µM) alone induced partial cell death, but significantly increased sensitivity of 833KE and NT2/D1 to low doses of cisplatin, resulted in synergistic cell death (Fig. 5B). The 2102EP cells were less sensitive to both agents and an additive effect was observed in the combination treatment (Fig. 5B). These results suggest that specific MDM2 inhibitors such as Nutlin may be useful alone or as sensitizer to conventional drugs in treating TGCT.
Figure 5.
Nutlin cooperates with cisplatin to induce apoptosis in TGCT cells. (A and B) Cells were treated with different doses of cisplatin and/or Nutlin for 72 hrs. The number of viable cells was quantified by MTS assay. Relative cell survival was determined by comparing to untreated TGCT cells.
Effects of nutlin on cisplatin-resistant TGCT cell lines
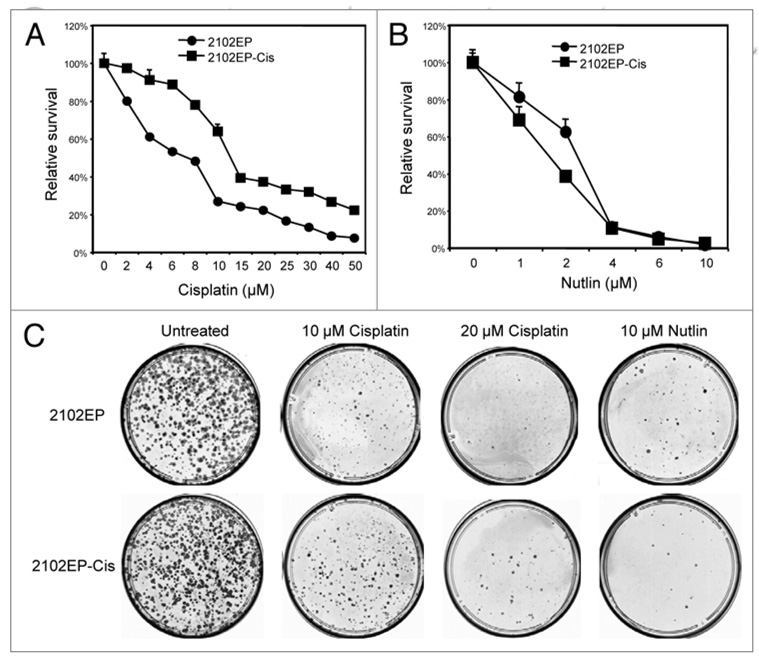
Drug-resistant TGCTs is a major cause of treatment failure and mortality. Therefore, it is interesting to determine whether Nutlin retains apoptotic activity against TGCT cells that have developed resistance to cisplatin. To obtain TGCT cells with reduced sensitivity to cisplatin, 2102EP, 833KE and NT2/D1 cells were treated with multiple cycles with low-dose cisplatin followed by recovery period. This resulted in the accumulation of cells (2102EP-Cis) that retained identical morphology but showed a moderate resistance to cisplatin (IC50 increased from 9 µM to 15 µM in MTS assay, Fig. 6A). When the cells were treated with Nutlin, the 2102EP-Cis cells showed no change or even slightly higher sensitivity (Fig. 6B). In a colony formation assay that measures long-term survival after short-term drug exposure, 2102EP-Cis cells also showed increased resistance to cisplatin but remained sensitive to Nutlin (Fig. 6C). Similar results were also observed using cisplatin-selected 833KE cells (not shown).
Figure 6.
Nutlin induces cell death in certain cisplatin-resistant TGCT cells. (A and B) 2102EP-Cis cells that have been selected for increased resistance to cisplatin in culture were compared to parental 2102EP cells for sensitivity to cisplatin and Nutlin using MTS assay after 72 hrs of drug treatments. (C) 2102EP-Cis and parental cells (5,000/plate) were treated with indicated drugs for 18 hrs and cultured in drug-free medium for 10 days. Colony formation efficiency was determined by crystal violet staining.
However, it is note-worthy that our drug selection scheme resulted in an NT2/D1-Cis cell line that was morphologically different (fibroblast-like) from parental NT2/D1 cells, suggesting that a differentiated subpopulation was enriched. This was accompanied by increased resistance to Cisplatin and strong induction of p21 after Nutlin treatment (not shown). Furthermore, the intrinsically cisplatin-resistant TGCT cell line 1411HP originally established from a patient with relapsed tumor after DNA damage treatment was also resistant to apoptosis by Nutlin (not shown).36,37 These results suggest that targeting MDM2 may be a useful alternative treatment against a subset but not all of cisplatin-resistant TGCT, dependent on the mechanism of drug resistance and differentiation state of the tumor.
Cell line-specific variation of p21 response to p53
The results described above showed that Nutlin did not induce significant p21 expression in TGCT cells, despite strong induction of MDM2 (Fig. 1A). We wondered whether it is due to a functional deficiency in p53 itself (unable to bind to p21 promoter), or due to intrinsic silencing of the p21 promoter. Therefore, the activities of cloned promoters of MDM2, p21 and PUMA were tested by transient transfection in TGCT cells and compared to U2OS and HCT116. The results indicated that the p21 promoter was highly active in TGCT cells when present in an episomal state, similar to MDM2 and PUMA promoters (data not shown).
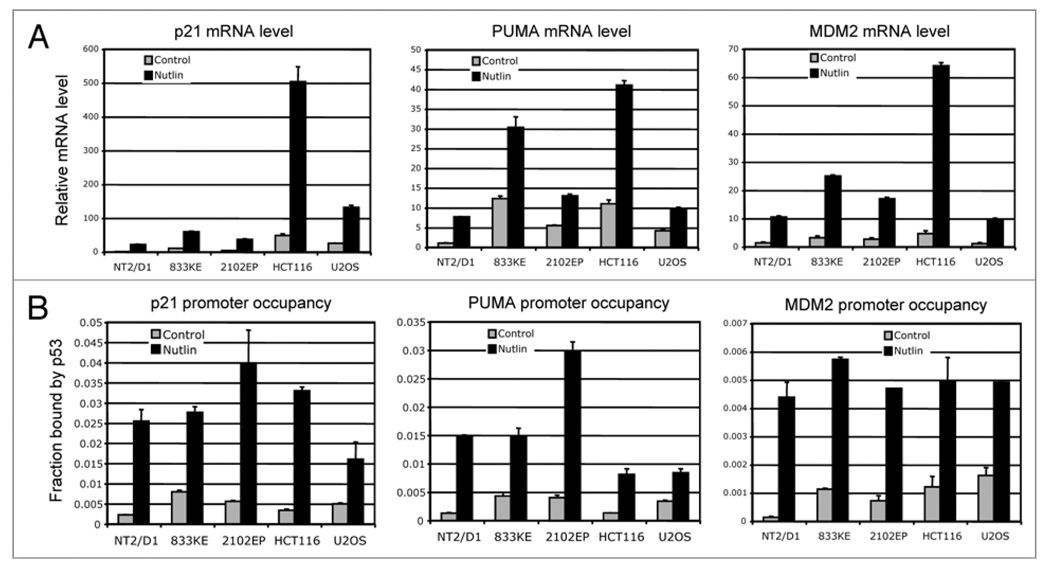
When the mRNA levels of p21, PUMA and MDM2 were analyzed by quantitative RT-PCR assay, p21 mRNA levels were very low in TGCT cells, in agreement with the low protein levels (Fig. 7A). However, comparing the mRNA level before and after treatment showed the same degree (~10-fold) of induction in Nutlin treated TGCT cells, similar to the 10-fold induction in U2OS and HCT116 cells (Fig. 7A). Therefore, the dynamics of p53-mediated p21 induction is normal in TGCT cells, suggesting that the p53 molecules are fully functional after activation by Nutlin.
Figure 7.
Cell type-specific variation of p21 response to p53. (A) Cells were treated with 5 µM Nutlin for 18 hrs and the mRNA levels of p53 target genes p21, PUMA and MDM2 were determined by quantitative RT-PCR and normalized to actin mRNA level. (B) Cells were treated with 5 µM Nutlin for 18 hrs and analyzed for p53 binding to p21, PUMA and MDM2 promoters by ChIP. Diluted input chromatin from each cell line was used during qPCR to help determine the percent of promoter DNA that were coprecipitated with p53 antibody (promoter occupancy).
Next, we examined p53 binding to the p21 promoter in TGCT cells. ChIP analysis using p53 antibody showed that the level of p53 binding to p21 promoter increased by 5–10 fold after Nutlin treatment, consistent with the magnitude of p53 accumulation and p21 mRNA induction. Furthermore, the occupancy of p53 on p21 promoter showed similar levels compared to U2OS and HCT116 cells (Fig. 7B). These results suggest that p53 in TGCT cells is competent in binding to the p21 promoter after stabilization by Nutlin. However, the p21 promoter in TGCT cells does not support high-level transcription after p53 binding, most likely due to the undifferentiated epigenetic state of the gene locus in these cells.
Discussion
The results presented above revealed that similar to other tumor types, wild type p53 in TGCT cells is kept at an inactive state by interactions with MDM2 and MDMX. Therefore, it is unlikely that a germ cell tumor-specific mechanism is responsible for the tolerance of wild type p53 in TGCT. Our finding is consistent with an emerging theme that tumors with infrequent p53 mutation achieve p53 functional inactivation using the MDM2 and MDMX proteins. This is often accomplished by gene amplification or overexpression of MDM2 and MDMX, or inactivation of the INK4a/ARF locus.
Our results showed that TGCT cells respond to p53 activation alone by undergoing complete apoptosis rather than G1 cell cycle arrest. This is unique among solid tumor cell lines which often undergo cell cycle arrest upon Nutlin activation of p53.33 Consistent with previous studies using cisplatin as a p53 activator, 23 we found that direct activation of p53 by Nutlin induced very low levels of p21 expression in TGCT cells compared to other tumor types. p21 can function as an antagonist of apoptosis and determine cell fate after p53 activation.38 Our ChIP results suggest that p53 occupancy on p21, PUMA and MDM2 promoters in TGCT and other tumor cell lines is similar. Therefore, the low p21 level in Nutlin-treated TGCT cells is not due to failure of p53 to bind the p21 promoter, but is likely due to the unique epigenetic state of the p21 locus or lack of proper cofactor that prevents high level transcription after p53 binding. In contrast, the pro-apoptotic p53 target PUMA is expressed at high level in TGCT cells after Nutlin treatment. The greatly distorted PUMA/p21 ratio may partially explain the selection of apoptosis over cell cycle arrest after p53 activation. However, it is unlikely that p21 expression alone determines the apoptosis response. Other factors such as high Bax/Bcl2 ratio may also play a role.39
Although DNA damaging drugs such as cisplatin and etoposide are highly successful in curing TGCT, a 10–20% failure rate still causes significant mortality in a relatively young age group. It will be important to develop novel therapeutics effective against these drug-resistant tumors. Furthermore, DNA-damaging drugs cause severe short-term toxicity, affects fertility and increase the risk for secondary malignancies and chronic diseases later in life. These long-term effects are particularly relevant for the young age of patients affected by TGCT. The retention of wild type p53 in drug-resistant TGCTs and the apoptotic response of a subset of these tumors to p53 activation make it an attractive target for the application of specific MDM2 inhibitors now in development.
Our study of TGCT cells led to the unexpected finding that MDMX is also a p53-responsive gene and may form a negative feedback loop similar to MDM2. Previous study indicated that DNA damage by UV did not induce MDMX mRNA expression. 40 However, Nutlin treatment of TGCT cells 833KE and 2102EP resulted in significant increase of MDMX expression at protein and mRNA levels. The ability of Nutlin to induce MDMX is clearly cell line-dependent, which may explain the negative reports in previous studies. Luciferase assay showed that p53 activates MDMX P2 promoter in a dose-dependent manner, although to a lesser degree than the MDM2 P2 promoter. Furthermore, mutagenesis of the putative p53-binding consensus sequence in MDMX P2 promoter completely abrogated p53-mediated activation. Reporter assays showed that the MDMX P2 promoter has very low-level activity in U2OS cells compared to TGCT cells despite similar fold response to p53 activation. Therefore, activation of the P2 promoter may have limited influence on overall MDMX expression in U2OS or similar cell lines, explaining their lack of MDMX induction by p53. The fact that MDMX response to p53 appears to be most obvious in germ cell tumors but not in more differentiated tumor cells suggest that MDMX may function as a dynamic regulator of p53 in stem cell maintenance. These results are consistent with the observation that MDMX knockout in mice caused severe proliferation defect during early embryogenesis,41 but has less effect in adult tissues,42,43 probably reflecting its important role in stem cells but not in terminally differentiated tissues.
It is noteworthy that compared to the large induction of MDM2 by p53, the magnitude of MDMX induction by p53 (~2-fold) appears quite moderate. However, recent observations of MDMX gene dosage effect using mouse model suggest that a 2-fold difference in MDMX expression level caused by heterozygous MDMX knockout can lead to significantly increased radiation sensitivity and altered tumor development timing.44 Furthermore, studies of MDM2 gene dosage effect and expression level due to SNP309 polymorphism also revealed an exquisite sensitivity of p53 function to small changes in MDM2 expression level.45,46 Therefore, the response of MDMX P2 promoter to p53 is likely to be an important part of the rheostat that controls p53 activity and should have significant biological effects under physiological condition. Further study of its function will require mutating the p53 responsive element in mouse model.
Materials and Methods
Cell lines and reagents
NT2/D1 cells were provided by Dr. Michael Spinella. 833KE and 2102EP cells were provided by Dr. Stuart Lutzker. Cells were maintained in DMEM medium with 10% fetal bovine serum. 1411HP cells were provided by Dr. Thomas Mueller and maintained in RPMI medium with 10% fetal bovine serum. Racemic Nutlin-3a was purchased from Cayman Chemical Company (Ann Arbor, Michigan). Cisplatin was purchased from Sigma.
Western blot
Cells were lysed in lysis buffer [50 mM Tris-HCl (pH 8.0), 5 mM EDTA, 150 mM NaCl, 0.5% NP40, 1 mM PMSF], centrifuged for 5 min at 10,000 g, and the insoluble debris was discarded. Cell lysate (20 µg protein) was fractionated by SDS-PAGE and transferred to Immobilon P filters (Millipore). The filter was blocked for 1 hr with phosphate-buffered saline (PBS) containing 5% non-fat dry milk, 0.1% Tween-20. MDM2, MDMX and p53 were detected using 3G9, 8C6 and DO1 antibodies respectively. p21 and PUMA were detected using antibodies purchased from Santa Cruz Biotechnology. The filter was developed using ECL-plus reagent (Amersham).
Inhibition of MDMX by RNA interference
To transiently inhibit MDMX expression, cells were transfected with 100 nM control siRNA (AAT TCT CCG AAC GTG TCA CGT) or MDMX siRNA (AGA TTC AGC TGG TTA TTA A) using Oligofectamine. Fourty-eight hours later, cells were transfected again with the same amount of siRNA. Nineteen hours after the second transfection, 5 µM Nutlin was added and cells were collected 8 hr later for western blot analysis.
Promoter cloning and luciferase assay
DNA fragments (~1.4, 0.5 or 0.3 kb) including the p53 consensus binding site in MDMX P2 promoter was amplified by PCR using primers T1 (5'-GCG GGT ACC ATC TCA CGG ATG GAG CA), T3 (5'-GCG GGT ACC TTG AAC CTG GGA AGC AGA), T4 (5'-GCG GGT ACC GTC CAT CTC GGT TTA ACC C) and 5'-GCG CTC GAG ACG CTC CAA CCT CCA ACT with H1299 genomic DNA as template. PCR fragments were cloned into pGL2-Basic vector using KpnI and Xho I sites. Mutagenesis was performed with Quikchange II site-directed mutagenesis Kit (Stratagene) using primer pairs M1: 5'-CTA TGG TCC TTT GTC ATC TCA TGT TCC AAC ATG TTG and 5'-CAA CAT GTT GGA ACA TGA GAT GAC AAA GGA CCA TAG or M2: 5'-ATG GTC CTT TGT CAA GAA ATT TTC CAA CAT GTT GTA GA and 5'-TCT ACA ACA TGT TGG AAA ATT TCT TGA CAA AGG ACC AT. To analyze promoter activity, ~50,000 H1299 cells were seeded in 24-well plates and transfected with 40 ng pGL2-MDMX-P2, pGL2-MDMX-P1 (1.1 kb), or pGL2-MDM2-P2 (BP100-luciferase) with 200 ng GFP, 5 ng CMV-lacZ and different amounts of p53 plasmid (0, 1, 2, 4 ng). Twenty-four hours later, cells were lysed and luciferase activity was determined. The results were normalized with the lacZ activity, and shown as the mean +/− SD of three independent experiments.
Quantitation of DNA synthesis
Cells cultured in 24-well plates were treated with 5 µM Nutlin for 12 hrs and 5 µCi of methyl-3H-thymidine (Amersham) was added for additional 1 hr. Cells were lysed in 0.5 ml lysis solution (2% SDS, 10 mM EDTA, pH 8.0), incubated at 75°C for 20 min, vortexed for 20 sec, and 170 µl lysate was applied to S/P glass fiber filters (Baxter). The filters were incubated in ice-cold 10% trichloroacetic acid for 5 min, washed with 10 ml of ice-cold 5% TCA and 5 ml 95% ethanol using a vacuum manifold. The filters were dried, suspended in 2 ml scintillation cocktail, and counted in a liquid scintillation counter.
Cell death and colony formation assay
Cell death was measured by MTS assay using the Cell Titer kit (Promega). Cells were cultured in 24-well plates and treated with compounds for 72 hrs. Culture medium was replaced with fresh medium containing the MTS reagent and the cells were cultured for 15–30 min. Conversion of MTS reagent into color-absorbing product by metabolically active cells were measured by determining OD at 490 nm. For colony formation assay, cells (5,000/10 cm plate) were cultured for 24 hrs and treated with indicated drugs for 18 hrs. Cells were refed with normal medium and cultured for 10 more days. Colonies were stained with 0.5% crystal violet. To analyze cell cycle and apoptosis by FACS, cells were treated with 5 µM Nutlin for 18 hrs, fixed in ethanol, stained with propidium iodide and analyzed by flow cytometry.
Quantitative real-time PCR
Cells were treated with 5 µM nutlin for 18 hrs and mRNA was extracted with RNeasy Mini kit (QIAGEN). Approximately 3 µg mRNA was used as template for reverse transcription by using SuperScript™ III First-Strand synthesis System (Invitrogen). The products were used for real-time PCR using the following primer pairs: MDMX 5'-GCC TTG AGG AAG GAT TGG TA and 5'-TCG ACA ATC AGG GAC ATC AT; p21 5'-CAG ACC AGC ATG ACA GAT TTC and 5'-TTA GGG CTT CCT CTT GGA GA; Puma 5'-AGA GGG AGG AGT CTG GGA GTG and 5'-GCA GCG CAT ATA CAG TAT CTT ACA GG; MDM2 5'-CCC TTA ATG CCA TTG AAC CT and 5'-CAT ACT GGG CAG GGC TTA TT; Actin 5'-GCT CGT CGT CGA CAA CGG CTC and 5'-CAA ACA TGA TCT GGG TCA TCT TCT C. The relative levels of target genes were determined using actin as an internal control.
Chromatin immunoprecipitation (ChIP)
CHIP assay was performed using standard procedure. p53 complexes were immunoprecipitated with a mixture of Pab1801 and DO-1 antibodies. Samples were subjected to SYBR Green real-time PCR analysis using forward and reverse primers for the p53 binding sites in the MDM2 promoter (5'-CGG GAG TTC AGG GTA AAG GT and 5'-CCT TTT ACT GCA GTT TCG), p21 promoter (5'-TGG CTC TGA TTG GCT TTC TG and 5'-TTC AGA GTA AGA GGC TAA GG), puma promoter (5'-CTG TGG CCT TGT GTC TGT GAG TAC and 5'-CCT AGC CCA AGG CAA GGA GGA C) and the MDMX P2 promoter (5'-GCC CAT CTT AGT CTC CCA AAC-3' and 5'-CAA GCC ACA TCA GGG AAA GG-3').
Acknowledgements
We would like to thank the Moffitt Molecular Biology Core for DNA sequencing and Flow Cytometry Core for FACS analyses. We also want to thank Dr. Michael Spinella for providing NT2/D1 cells, Dr. Stuart Lutzker for 833KE and 2102EP cells, Dr. Thomas Mueller and Dr. Hans-Joachim Schmoll for 1411HP cells. This work was supported in part by grants from the National Institutes of Health (CA109636, CA118210).
References
- 1.Masters JR, Koberle B. Curing metastatic cancer: lessons from testicular germ-cell tumours. Nat Rev Cancer. 2003;3:517–525. doi: 10.1038/nrc1120. [DOI] [PubMed] [Google Scholar]
- 2.di Pietro A, Vries EG, Gietema JA, Spierings DC, de Jong S. Testicular germ cell tumours: the paradigm of chemo-sensitive solid tumours. Int J Biochem Cell Biol. 2005;37:2437–2456. doi: 10.1016/j.biocel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Jones RH, Vasey PA. Part II: testicular cancer—management of advanced disease. Lancet Oncol. 2003;4:738–747. doi: 10.1016/s1470-2045(03)01279-8. [DOI] [PubMed] [Google Scholar]
- 4.Gosland M, Lum B, Schimmelpfennig J, Baker J, Doukas M. Insights into mechanisms of cisplatin resistance and potential for its clinical reversal. Pharmacotherapy. 1996;16:16–39. [PubMed] [Google Scholar]
- 5.Kersemaekers AM, Mayer F, Molier M, van Weeren PC, Oosterhuis JW, Bokemeyer C, et al. Role of p53 and MDM2 in treatment response of human germ cell tumors. J Clin Oncol. 2002;20:1551–1561. doi: 10.1200/JCO.2002.20.6.1551. [DOI] [PubMed] [Google Scholar]
- 6.Houldsworth J, Xiao H, Murty VV, Chen W, Ray B, Reuter VE, et al. Human male germ cell tumor resistance to cisplatin is linked to TP53 gene mutation. Oncogene. 1998;16:2345–2349. doi: 10.1038/sj.onc.1201770. [DOI] [PubMed] [Google Scholar]
- 7.Riou G, Barrois M, Prost S, Terrier MJ, Theodore C, Levine AJ. The p53 and mdm-2 genes in human testicular germ-cell tumors. Mol Carcinog. 1995;12:124–131. doi: 10.1002/mc.2940120303. [DOI] [PubMed] [Google Scholar]
- 8.Heimdal K, Lothe RA, Lystad S, Holm R, Fossa SD, Borresen AL. No germline TP53 mutations detected in familial and bilateral testicular cancer. Genes Chromosomes Cancer. 1993;6:92–97. doi: 10.1002/gcc.2870060205. [DOI] [PubMed] [Google Scholar]
- 9.Peng HQ, Hogg D, Malkin D, Bailey D, Gallie BL, Bulbul M, et al. Mutations of the p53 gene do not occur in testis cancer. Cancer Res. 1993;53:3574–3578. [PubMed] [Google Scholar]
- 10.Schenkman NS, Sesterhenn IA, Washington L, Tong YA, Weghorst CM, Buzard GS, et al. Increased p53 protein does not correlate to p53 gene mutations in microdissected human testicular germ cell tumors. J Urol. 1995;154:617–621. doi: 10.1097/00005392-199508000-00081. [DOI] [PubMed] [Google Scholar]
- 11.Lutzker SG, Levine AJ. A functionally inactive p53 protein in teratocarcinoma cells is activated by either DNA damage or cellular differentiation. Nat Med. 1996;2:804–810. doi: 10.1038/nm0796-804. [DOI] [PubMed] [Google Scholar]
- 12.Curtin JC, Dragnev KH, Sekula D, Christie AJ, Dmitrovsky E, Spinella MJ. Retinoic acid activates p53 in human embryonal carcinoma through retinoid receptor-dependent stimulation of p53 transactivation function. Oncogene. 2001;20:2559–2569. doi: 10.1038/sj.onc.1204370. [DOI] [PubMed] [Google Scholar]
- 13.Curtin JC, Spinella MJ. p53 in human embryonal carcinoma: identification of a transferable, transcriptional repression domain in the N-terminal region of p53. Oncogene. 2005;24:1481–1490. doi: 10.1038/sj.onc.1208130. [DOI] [PubMed] [Google Scholar]
- 14.Stott FJ, Bates S, James MC, McConnell BB, Starborg M, Brookes S, et al. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–5014. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marine JC, Jochemsen AG. Mdmx as an essential regulator of p53 activity. Biochem Biophys Res Commun. 2005;331:750–760. doi: 10.1016/j.bbrc.2005.03.151. [DOI] [PubMed] [Google Scholar]
- 16.Sherr CJ. Divorcing ARF and p53: an unsettled case. Nat Rev Cancer. 2006;6:663–673. doi: 10.1038/nrc1954. [DOI] [PubMed] [Google Scholar]
- 17.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 18.Oliner JD, Kinzler KW, Meltzer PS, George DL, Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358:80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 19.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurie NA, Donovan SL, Shih CS, Zhang J, Mills N, Fuller C, et al. Inactivation of the p53 pathway in retinoblastoma. Nature. 2006;444:61–66. doi: 10.1038/nature05194. [DOI] [PubMed] [Google Scholar]
- 21.Iwato M, Tachibana O, Tohma Y, Arakawa Y, Nitta H, Hasegawa M, et al. Alterations of the INK4a/ARF locus in human intracranial germ cell tumors. Cancer Res. 2000;60:2113–2115. [PubMed] [Google Scholar]
- 22.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 23.Spierings DC, de Vries EG, Stel AJ, te Rietstap N, Vellenga E, de Jong S. Low p21Waf1/Cip1 protein level sensitizes testicular germ cell tumor cells to Fas-mediated apoptosis. Oncogene. 2004;23:4862–4872. doi: 10.1038/sj.onc.1207617. [DOI] [PubMed] [Google Scholar]
- 24.Danovi D, Meulmeester E, Pasini D, Migliorini D, Capra M, Frenk R, et al. Amplification of Mdmx (or Mdm4) directly contributes to tumor formation by inhibiting p53 tumor suppressor activity. Mol Cell Biol. 2004;24:5835–5843. doi: 10.1128/MCB.24.13.5835-5843.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilkes DM, Chen L, Chen J. MDMX regulation of p53 response to ribosomal stress. EMBO J. 2006;25:5614–5625. doi: 10.1038/sj.emboj.7601424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu B, Gilkes DM, Farooqi B, Sebti SM, Chen J. MDMX overexpression prevents p53 activation by the MDM2 inhibitor Nutlin. J Biol Chem. 2006;281:33030–33035. doi: 10.1074/jbc.C600147200. [DOI] [PubMed] [Google Scholar]
- 27.Patton JT, Mayo LD, Singhi AD, Gudkov AV, Stark GR, Jackson MW. Levels of HdmX expression dictate the sensitivity of normal and transformed cells to Nutlin-3. Cancer Res. 2006;66:3169–3176. doi: 10.1158/0008-5472.CAN-05-3832. [DOI] [PubMed] [Google Scholar]
- 28.Wade M, Wong ET, Tang M, Stommel JM, Wahl GM. Hdmx modulates the outcome of p53 activation in human tumor cells. J Biol Chem. 2006;281:33036–33044. doi: 10.1074/jbc.M605405200. [DOI] [PubMed] [Google Scholar]
- 29.Hu B, Gilkes DM, Chen J. Efficient p53 Activation and Apoptosis by Simultaneous Disruption of Binding to MDM2 and MDMX. Cancer Res. 2007;67:8810–8817. doi: 10.1158/0008-5472.CAN-07-1140. [DOI] [PubMed] [Google Scholar]
- 30.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 31.Gilkes DM, Pan Y, Coppola D, Yeatman T, Reuther GW, Chen J. Regulation of MDMX expression by mitogenic signaling. Mol Cell Biol. 2008;28:1999–2010. doi: 10.1128/MCB.01633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson T, Tovar C, Yang H, Carvajal D, Vu BT, Xu Q, et al. Phosphorylation of p53 on key serines is dispensable for transcriptional activation and apoptosis. J Biol Chem. 2004;279:53015–53022. doi: 10.1074/jbc.M410233200. [DOI] [PubMed] [Google Scholar]
- 33.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci USA. 2006;103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suzuki A, Tsutomi Y, Akahane K, Araki T, Miura M. Resistance to Fas-mediated apoptosis: activation of caspase 3 is regulated by cell cycle regulator p21WAF1 and IAP gene family ILP. Oncogene. 1998;17:931–939. doi: 10.1038/sj.onc.1202021. [DOI] [PubMed] [Google Scholar]
- 35.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 36.Vogelzang NJ, Bronson D, Savino D, Vessella RL, Fraley EF. A human embryonal-yolk sac carcinoma model system in athymic mice. Cancer. 1985;55:2584–2593. doi: 10.1002/1097-0142(19850601)55:11<2584::aid-cncr2820551110>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 37.Mueller T, Voigt W, Simon H, Fruehauf A, Bulankin A, Grothey A, et al. Failure of activation of caspase-9 induces a higher threshold for apoptosis and cisplatin resistance in testicular cancer. Cancer Res. 2003;63:513–521. [PubMed] [Google Scholar]
- 38.Polyak K, Waldman T, He TC, Kinzler KW, Vogelstein B. Genetic determinants of p53-induced apoptosis and growth arrest. Genes Dev. 1996;10:1945–1952. doi: 10.1101/gad.10.15.1945. [DOI] [PubMed] [Google Scholar]
- 39.Chresta CM, Masters JR, Hickman JA. Hypersensitivity of human testicular tumors to etoposide-induced apoptosis is associated with functional p53 and a high Bax:Bcl-2 ratio. Cancer Res. 1996;56:1834–1841. [PubMed] [Google Scholar]
- 40.Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996;15:5349–5357. [PMC free article] [PubMed] [Google Scholar]
- 41.Parant J, Chavez-Reyes A, Little NA, Yan W, Reinke V, Jochemsen AG, et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonover-lapping pathway with MDM2 to regulate p53. Nat Genet. 2001;29:92–95. doi: 10.1038/ng714. [DOI] [PubMed] [Google Scholar]
- 42.Grier JD, Xiong S, Elizondo-Fraire AC, Parant JM, Lozano G. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol Cell Biol. 2006;26:192–198. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maetens M, Doumont G, Clercq SD, Francoz S, Froment P, Bellefroid E, et al. Distinct roles of Mdm2 and Mdm4 in red cell production. Blood. 2007;109:2630–2633. doi: 10.1182/blood-2006-03-013656. [DOI] [PubMed] [Google Scholar]
- 44.Terzian T, Wang Y, Van Pelt CS, Box NF, Travis EL, Lozano G. Haploinsufficiency of Mdm2 and Mdm4 in tumorigenesis and development. Mol Cell Biol. 2007;27:5479–5485. doi: 10.1128/MCB.00555-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bond GL, Menin C, Bertorelle R, Alhopuro P, Aaltonen LA, Levine AJ. MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet. 2006;43:950–952. doi: 10.1136/jmg.2006.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alt JR, Greiner TC, Cleveland JL, Eischen CM. Mdm2 haplo-insufficiency profoundly inhibits Myc-induced lymphomagenesis. EMBO J. 2003;22:1442–1450. doi: 10.1093/emboj/cdg133. [DOI] [PMC free article] [PubMed] [Google Scholar]