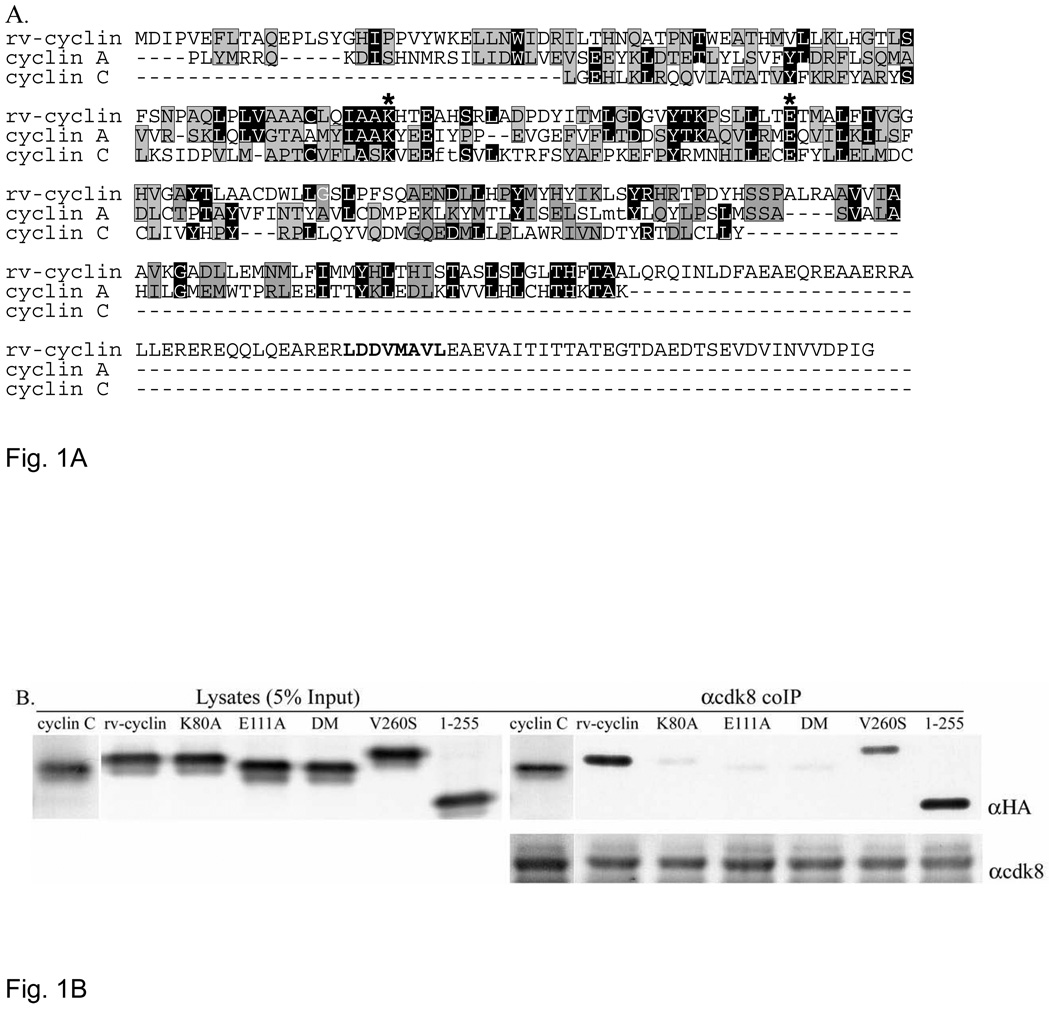
Fig. 1.
A. Phyre alignment of WDSV rv-cyclin with cyclin A (D. melanogaster)(E-value=4e−43, identity 19.2%) and human cyclin C (E-value=5e−06, identity 14.9%). The alignment was generated with 998 additional cyclins. Black positions are identical; gray are conserved residues. Asterisks indicate conserved cdk contacts and correspond to residues K80 and E111 in the rv-cyclin. The unaligned carboxy terminal region containing the TAF9 binding motif, LDDV260MAVL (bold), extends beyond the cyclin box of all of the aligned cyclins. Small letters, ft in cyclin C and mt in cyclin A, indicate deletions introduced by the alignment software. B. HA-tagged cyclin C, rv-cyclin, rv-cyclin point mutants, K80A, E111A, double mutant (DM; K80A/E111A), and V260S, and a.a.1–255 were transiently co-expressed with FLAG-tagged cdk8 in HeLa cells and whole cell lysates were immune precipitated with anti-cdk8 antibody. Lysates represent 5% of the co-IP input protein (5 µg) (left panel). Expressed and precipitated HA-tagged cyclins were detected on western blots with anti-HA antibody (αHA). The bottom panel shows precipitated cdk8 in each reaction after reprobe with anti-cdk8 antibody (αcdk8).