Abstract
Purpose
Mesenchymal stem cells (MSCs) hold promise for the treatment of renal disease. While MSCs have been shown to accelerate recovery and prevent acute renal failure in multiple disease models, the effect of MSC therapy on chronic obstruction-induced renal fibrosis has not previously been evaluated.
Materials and Methods
Male Sprague-Dawley rats underwent renal artery injection of vehicle or fluorescent-labeled human bone marrow-derived MSCs immediately prior to sham operation or induction of left ureteral obstruction (UUO). One or 4 weeks later, the kidneys were harvested and the renal cortex analyzed for evidence of stem cell infiltration, epithelial-mesenchymal transition (EMT) as evidenced by E-cadherin/α-smooth muscle actin (α-SMA) expression and fibroblast specific protein (FSP+) staining, renal fibrosis (collagen content, Masson’s trichrome staining), and cytokine and growth factor activity (ELISA and real time RT-PCR).
Results
Fluorescent-labeled MSCs were detected in the interstitium of the kidney up to 4 weeks post-obstruction. Arterially delivered MSCs significantly reduced obstruction-induced α-SMA expression, FSP+ cell accumulation, total collagen content, and tubulointerstitial fibrosis, while simultaneously preserving E-cadherin expression, suggesting that MSCs prevent obstruction-induced EMT and renal fibrosis. Exogenous MSCs reduced obstruction-induced tumor necrosis factor-α (TNF-α) levels, but did not alter transforming growth factor-β1 (TGF-β1), vascular endothelial growth factor (VEGF), interleukin-10 (IL-10), fibroblast growth factor (FGF), or hepatocyte growth factor (HGF) expression.
Conclusions
Human bone marrow-derived MSCs remain viable several weeks after delivery into the kidney and provide protection against obstruction-induced EMT and chronic renal fibrosis. While the mechanism of MSCs-induced renal protection during obstruction remains unclear, our results demonstrate that alterations in TNF-α production may be involved.
Keywords: mesenchymal stem cell, obstruction, epithelial-mesenchymal transition, fibrosis, kidney
INTRODUCTION
Obstructive nephropathy is a major cause of renal failure and end-stage renal disease in adults and children characterized by the development of tubulointerstitial fibrosis. Interstitial fibrosis is a complex pathophysiological process involving inflammatory cell infiltration, fibroblast proliferation, and an imbalance in extracellular matrix (ECM) synthesis and degradation.1 Activated fibroblasts are the principal effector cells responsible for extracellular matrix deposition and the development of tubulointersitital fibrosis, and growing evidence suggests that growth factors, such as TGF-β1, can induce renal tubular epithelial cells to undergo phenotypic transformation into matrix producing myofibroblasts (EMT) under pathological conditions.2, 3 Renal cortical TGF-β1 levels increase significantly in response to obstruction,4 and evidence indicates that TGF-β1 is a major regulator of fibrosis via stimulation of EMT, fibroblast proliferation5, 6 and extracellular matrix synthesis.1, 4, 6 TNF-α also has a role in fibrotic renal injury, stimulating ECM accumulation and the upregulation of a number of cytokines and transcription factors involved in tubulointerstitial fibrosis, including TGF-β1.7
MSCs are undifferentiated non-embryonic stem cells of mesodermal origin that have the capacity to differentiate into cells of connective tissue lineage.8 While MSCs have the capacity to differentiate, recent studies suggest that a more significant component of their protective and reparative function resides in their paracrine activity. MSCs can secrete a number of growth factors and cytokines that are important for angiogenesis and cytoprotection, including VEGF, TGF-β1, FGF, and HGF.9, 10 VEGF is involved in matrix remodeling, monocyte chemotaxis, and adhesion molecule expression, and stimulates the proliferation of peritubular capillaries that are essential for tubular regeneration.11 FGF-2 is intimately involved in angiogenesis and may be a more potent angiogenic factor than VEGF.12 HGF improves cell growth, reduces cell apoptosis, and exerts a beneficial effect on neovascularization and tissue remodeling.13 While a number of studies in pathological models of kidney disease have shown that exogenous MSCs can home to injured kidneys and facilitate repair, the effect of MSCs therapy on chronic obstruction-induced renal fibrosis has not previously been evaluated. This study therefore evaluated 1) the localization of arterially injected MSCs during renal obstruction, 2) the effect of exogenous MSCs on obstruction-induced EMT and renal fibrosis, and 3) the effect of exogenous MSCs on obstruction-induced renal cytokine and growth factor activity in rat model of UUO.
MATERIALS AND METHODS
MSC Labeling
Human MSCs were purchased from Cambrex Bio Science Walkersville, Inc. (Cambrex, Walkersville, MD), and cultured in MSC Basal Medium (Lonza, Walkersville, MD) containing 10% fetal bovine serum for 14 days prior to injection. According to the manufacturer, cells are positive for CD105, CD166, CD29, and CD44, and negative for CD14, CD34, and CD45. MSCs were labeled with the PKH26 red fluorescence cell linker kit (Sigma-Aldrich, Saint Louis, MO) according to the manufacturer’s protocol just prior to injection.
Animals, Experimental Groups and Operative Techniques
The animal protocol was reviewed and accepted by the animal care and research committee at Indiana University School of Medicine. Adult male Sprague-Dawley rats weighing 250 to 300 gm were acclimated and maintained on a standard pellet diet for one week before experiment initiation. Rats were anesthetized using isoflurane inhalation. Following the induction of anesthesia, rats underwent renal arterial injection of either vehicle or MSCs (1 × 106 per rat). The left ureter was subsequently ligated to induce obstruction. Sham treated animals underwent an identical surgical procedure without ureteral ligation. Animals (6 per group) underwent renal arterial injection of fluorescently tagged MSCs vs. vehicle immediately prior to UUO and were subjected to 1 week or 4 weeks of obstruction vs. sham operation. The kidneys were subsequently removed, snap frozen in liquid nitrogen, and analyzed for MSC localization and markers of renal fibrosis. The animals were divided into 3 experimental groups (6 animals per group): 1-week sham operation (Sham), 1-week UUO plus vehicle (OB), and 1-week UUO plus MSCs (OB + SC).
MSC localization
In order to examine MSC localization following arterial delivery, kidney samples were prepared from each time point (1 week, 4 weeks) with a cryostat and fixed in 4% methanol-free formaldehyde for 15 minutes. MSCs were labeled with PKH26 as described above. The slides were then washed 3 times in PBS for 5 minutes and the nuclei counterstained with bis-Benzimide for 30 seconds. The slides were then washed and mounted with ProLong Antifade (Molecular Probes; Eugene, OR), and the sections photographed. Non-specific fluorescence was determined from vehicle treated sections and digitally subtracted from MSC treated sections and samples were then photographed using a fluorescent microscope (400X; Leica DM IRB; Wetzlar, Germany).
Tissue Homogenization
A portion of the renal cortex from each kidney was homogenized following dilution in 5 volumes of homogenate buffer composed of 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM egtazic acid, 1 mM dithiothreitol and 0.5 mM phenylmethylsulfonyl fluoride, using a VirTishear tissue homogenizer (VirTis, Gardiner, NY). Renal homogenates were centrifuged at 3,000 g for 15 minutes at 4°C. The supernatants were subsequently stored at −80°C until further testing could be performed.
Western Blot Analysis
Protein extracts (30 μg per lane) from homogenized samples were electrophoresed onto a tris-glycine gel (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene fluoride membrane. Immunoblotting was performed by incubating each membrane in 5% dry milk for 45 minutes at room temperature, followed by incubation with an anti-rat E-cadherin (1:500; BD Biosciences, San Jose, CA) or an anti-rat α-SMA (1:500; Sigma-Aldrich, Saint Louis, MO) antibody overnight at 4°C. After being washed 3 times in tris-PBS each membrane was incubated for one hour with a peroxidase-conjugated secondary antibody (1:2,000 for E-cadherin, 1:2,000 for α-SMA) at room temperature. The membranes were then developed using enhanced chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ). Equivalent protein loading for each lane was confirmed by stripping and reblotting each membrane for GAPDH (1:20,000 for 30 minutes at room temperature, secondary 1:20,000 for 30 minutes at room temperature; Biodesign International, Saco, ME). The analysis was repeated in triplicate to ensure the reproducibility of results. The density of each band was determined using NIH image analysis software and expressed as a percent of GAPDH density.
Fibroblast (FSP-1) Accumulation
Renal tissue sections were analyzed for the presence of transformed fibroblasts (EMT) utilizing an S100-A4 antibody (The S100-A4 antigen is also known as FSP-1).2, 14 Transverse 4 μm renal tissue sections were deparaffinized and dehydrated with xylene and alcohol. Antigen was retrieved by incubating the cells with proteinase K for 20 minutes in an oven. The tissues were then blocked with 1% bovine serum albumin and incubated with a polyclonal rabbit antibody directed against S100-A4 (FSP-1; 1:200, DakoCytomation, Carpinteria, CA) for one hour at 37°C. Sections were washed and incubated with a secondary antibody (goat anti-rabbit; 1:50) for 30 minutes. Peroxidase-stained sections were then developed with 3,3″-diaminobenzidine and counterstained with hematoxylin (Sigma-Aldrich, Saint Louis, MO). Sections incubated without primary antibody exhibited no staining. Spindle shaped FSP-1 positive (FSP-1+) cells were counted in 10 high powered fields (400 ×) in a blinded fashion and averaged.
Tissue Collagen
The total soluble collagen concentration within each renal cortical tissue sample was measured with the Sircol collagen assay kit (Accurate Chemical and Scientific, Westbury, NY) according to the manufacturer’s protocol. Tissue samples were dissolved in 0.5 M acetic acid and heated for 120 minutes at 60°C. Tissue suspensions were centrifuged, supernatants collected, and the total collagen concentration measured at 540 nm.
Masson’s Trichrome Staining
Tissue sections were deparaffinized and rehydrated with alcohol. The slides were then washed in distilled water and stained in Weigert’s iron hematoxylin working solution for 10 minutes. The slides were washed and stained in Biebrich scarlet-acid fuchsin solution for 15 minutes. The slides were then rewashed and differentiated in phosphomolybdic-phosphotungstic acid solution for 15 minutes. The tissue sections were then transferred directly to aniline blue solution for 5 to 10 minutes, rinsed, and differentiated in 1% acetic acid solution for 2 to 5 minutes. The slides were dehydrated, cleared in xylene, and mounted.
Renal Cytokine and Growth Factor Protein Levels
Renal cortical homogenate TNF-α, TGF-β1, VEGF, and IL-10 protein levels were determined using ELISA. Each ELISA was performed by adding 100 μl of each sample to wells in a 96-well plate of a rat ELISA kit (TNF-α: BD Biosciences, San Diego, CA; TGF-β1, VEGF and IL-10: R&D Systems, Minneapolis, MN). For TGF-β1, latent TGF-β1 in each homogenized sample was activated by adding 1N HCl to each sample at a 1:25 dilution for 60 minutes at 4°C. The samples were then neutralized with 1N NaOH and immediately tested. Samples were tested in duplicate. Each ELISA was performed according to manufacturer instructions. Final results were expressed as pg of TNF-α, TGF-β1, VEGF, or IL-10 per mg protein.
Renal Cytokine and Growth Factor Real Time PCR
Total RNA was extracted from the renal cortical tissue by homogenization in Trizol (Gibco BRL, Gaithersburg, MD), then isolated by precipitation with chloroform and isopropanol. Total RNA (0.5 μg) was subjected to cDNA synthesis using iScript (Bio-Rad, Hercules, CA). cDNA from each sample was analyzed for TNF-α (Rn99999017_m1), TGF-β1 (Rn00572010_m1), VEGF (Rn00582935_m1), IL-10 (Rn00563409_m1), FGF (Rn01269940_m1), and HGF (Rn00566673_m1) using a TaqMan gene expression assay (RT-PCR; Applied Biosystems, Foster City, CA). FAM Dye/MGB labeled probes for rat β-actin (Applied Biosystems, Foster City, CA) served as endogenous controls.
Statistical Analysis
Data were presented as mean ± standard error of the mean. Differences at the 95% confidence intervals were considered significant. The experimental groups were compared using ANOVA with the post-hoc Bonferroni-Dunn (JMP 5.0.1).
RESULTS
Localization of arterially injected MSCs
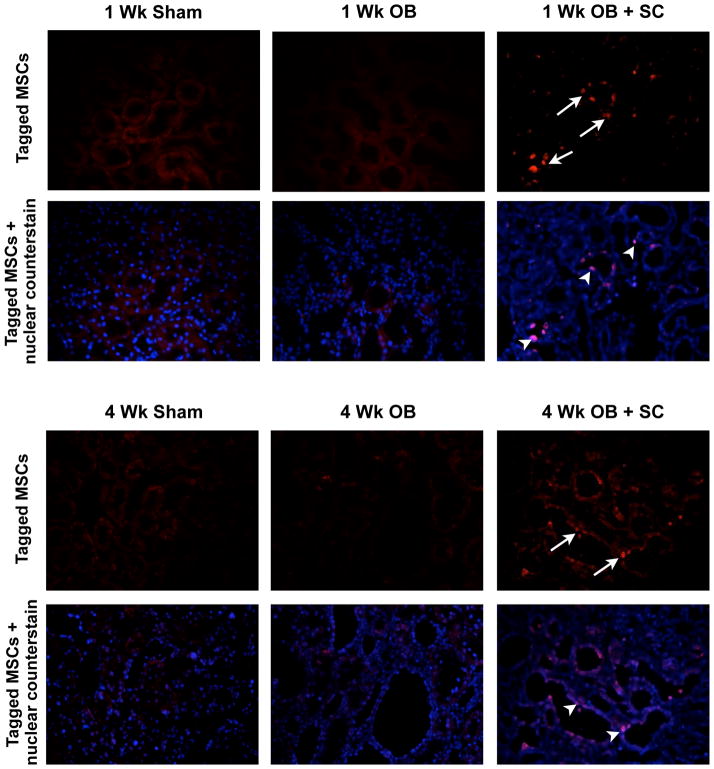
Fluorescently-labeled MSCs were identified within the renal cortex up to 4 weeks after arterial injection into obstructed kidneys, and localized primarily to renal tubules and the interstitial compartment of the kidney (Figure 1). No staining was detected in sham kidneys or obstructed kidneys in the absence of arterially delivered MSCs.
Figure 1. Photographs (400 ×) of renal tissue sections following arterial delivery of fluorescent-labeled MSCs.
Fluorescent-labeled MSCs (red stained cells; arrows) are localized to the renal tubules and the interstitium of the kidney 1 week and 4 weeks post-obstruction (OB + SC). In sham-treated (Sham) or vehicle-treated obstructed animals (OB) all red fluorescent staining represents background, while in the OB + SC group the nuclear counterstain confirms MSCs migration to tubules and the interstitial compartment (purple nuclei; arrow heads).
E-cadherin and α-SMA expression
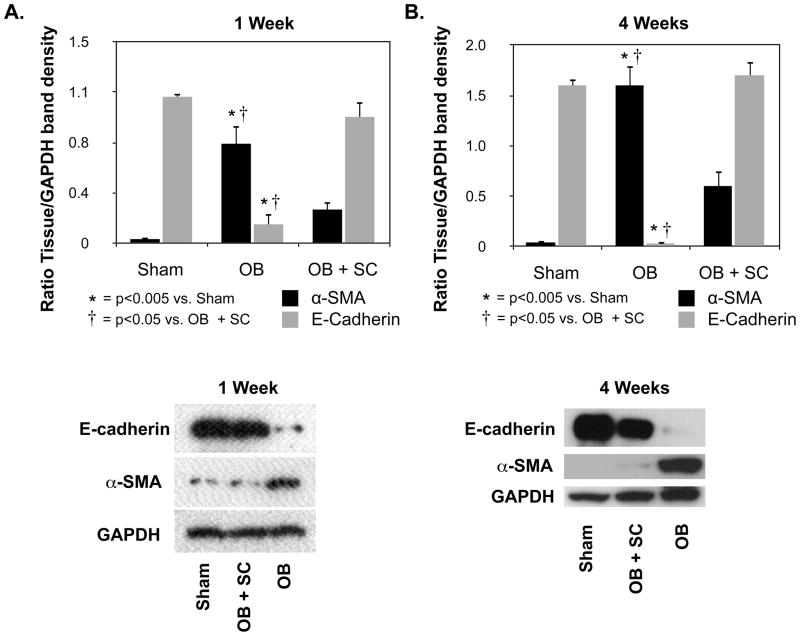
In order to evaluate the effect of arterially delivered MSCs on obstruction-induced EMT, renal cortical tissue samples were analyzed for E-cadherin and α-SMA expression (Figure 2A and 2B). E-cadherin expression, a marker of normal epithelium, was significantly reduced in rats exposed to one or 4 weeks of obstruction as compared to sham-treated animals (1 wk: WT OB = 0.14 ± 0.08 vs. sham = 1.08 ± 0.03, p<0.0001; 4 wk: WT OB = 0.03 ± 0.01 vs. sham = 1.6 ± 0.05, p<0.0001), but preserved in obstructed kidneys in the presence of exogenous MSCs (1 wk: WT OB = 0.14 ± 0.08 vs. SC OB = 0.93 ± 0.11, p<0.001; 4 wk: WT OB = 0.03 ± 0.01 vs. SC OB = 1.72 ± 0.13, p<0.0001). Conversely, α-SMA expression, a marker of myofibroblasts, was significantly increased in animals exposed to one or 4 weeks obstruction as compared to sham-treated animals (1 wk: WT OB = 0.74 ± 0.15 vs. sham = 0.03 ± 0.02, p<0.005; 4 wk: WT OB = 1.64 ± 0.22 vs. sham = 0.04 ± 0.003, p<0.0005). Obstruction-induced α-SMA expression was significantly reduced; however, in the presence of exogenous MSCs (1 wk: WT OB = 0.74 ± 0.15 vs. SC OB = 0.25 ± 0.06, p<0.05; 4 wk: WT OB = 1.64 ± 0.22 vs. SC OB = 0.61 ± 0.16, p<0.05).
Figure 2. Renal cortical E-cadherin and α-SMA expression following UUO.
A. Densitometric analysis and gel photograph of E-cadherin and α-SMA expression as a percentage of GAPDH in animals exposed to sham operation (Sham) or 1 week of UUO in the presence of vehicle (OB) or exogenous MSC (OB + SC). B. Densitometric analysis and gel photograph of E-cadherin and α-SMA expression as a percentage of GAPDH in animals exposed to sham operation (Sham) or 4 weeks of UUO in the presence of vehicle (OB) or exogenous MSC (OB + SC).
Fibroblast (FSP-1) Accumulation
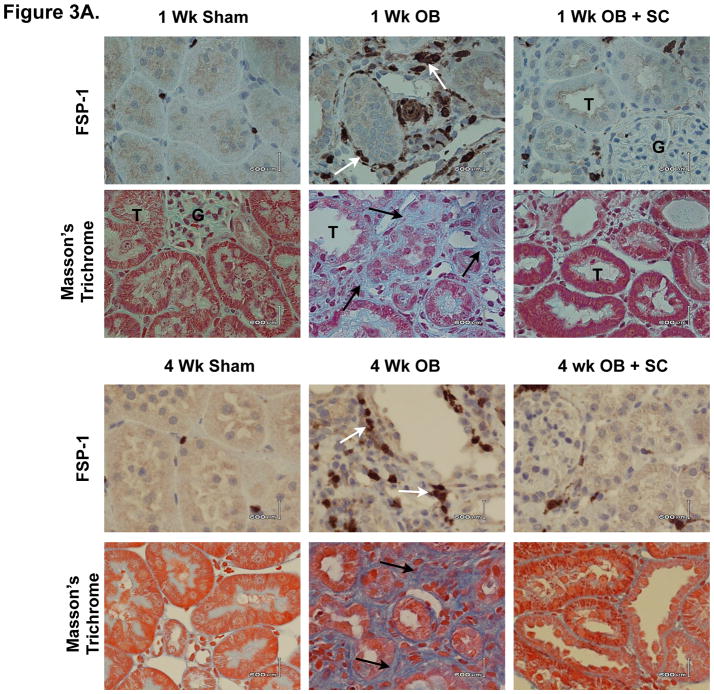
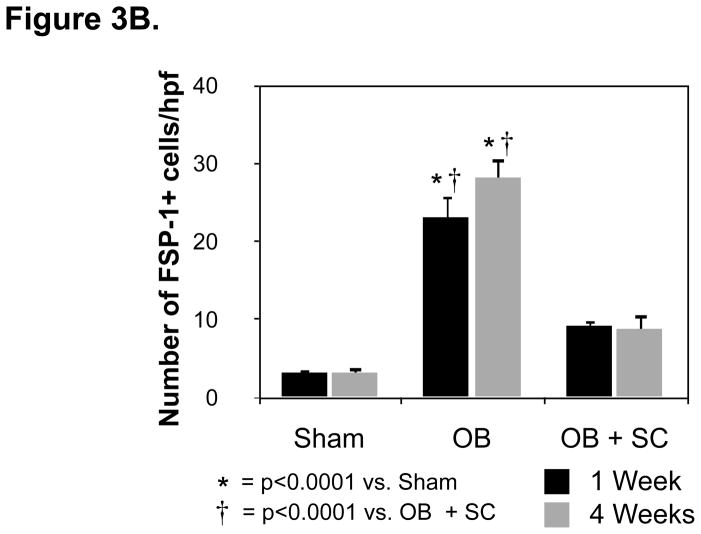
The effect of arterially delivered MSCs on obstruction-induced renal tubular cell EMT was further evaluated by counting the number of FSP-1+ cells in renal cortical tissue sections (Figure 3A and 3B). While the number of FSP-1+ fibroblasts was significantly increased in rats exposed to one or 4 weeks of renal obstruction (1 wk: WT OB = 23 ± 2.8 vs. sham = 3.2 ± 0.4, p<0.0001; 4 wk: WT OB = 28 ± 2.6 vs. sham = 3.2 ± 0.8, p<0.0001), a marked reduction in FSP-1+ fibroblasts was detected in obstructed animals exposed to exogenous MSCs (1 wk: WT OB = 23 ± 2.8 vs. SC OB = 9.2 ± 0.8, p<0.0001; 4 wk: WT OB = 28 ± 2.6 vs. SC OB = 9 ± 1.8, p<0.0001). These findings provide evidence that arterially delivered MSCs prevent the early stages of EMT and fibroblast accumulation during renal obstruction.
Figure 3. Renal cortical FSP-1+ fibroblast accumulation and Masson’s trichrome staining following UUO.
A. Photographs (magnification 400 ×) depicting renal cortical FSP-1+ fibroblasts (brown stain; white arrows) and tubulointerstitial collagen deposition (blue stain; black arrows) in rats exposed to sham operation (Sham), or one or 4 weeks of UUO in the presence of vehicle (OB) or exogenous MSC (OB + SC). B. Graph depicting the number of FSP-1+ fibroblasts in cortical tissue exposed to sham operation (Sham), or one or 4 weeks of UUO in the presence of vehicle (OB) or exogenous MSC (OB + SC).
Collagen Deposition
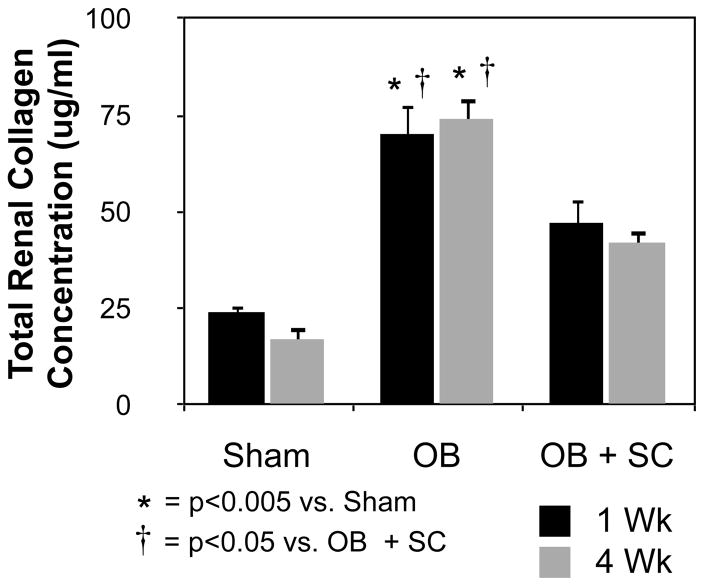
Renal tissue sections were analyzed for collagen deposition and total soluble collagen concentrations as a measure of tubulointerstitial fibrosis. Masson’s trichrome staining revealed minimal collagen deposition in sham treated samples, but a significant increase in tubulointerstitial collagen deposition in response to obstruction (Figure 3A). Obstruction-induced collagen deposition; however, was markedly reduced in the presence of exogenous MSCs. Similarly, while total renal collagen concentrations were significantly increased in rats exposed to one or 4 weeks of obstruction (Figure 4; 1 wk: WT OB = 71 ± 7.5 vs. sham = 24 ± 1.7, p<0.0001; 4 wk: WT OB = 74 ± 5.5 vs. sham = 17 ± 3.4, p<0.0001), collagen concentrations were markedly reduced in obstructed kidneys in the presence of exogenous MSCs (1 wk: WT OB = 71 ± 7.5 vs. SC OB = 47 ± 6.5, p<0.05; 4 wk: WT OB = 74 ± 5.5 vs. SC OB = 42 ± 3.6, p<0.005). These results suggest that arterially delivered MSCs ameliorate obstruction-induced tubulointerstitial fibrosis.
Figure 4. Total renal collagen concentration following UUO.
Graph depicting total soluble collagen concentration in renal tissue sections obtained from rats exposed to sham operation (Sham), or one or 4 weeks of UUO in the presence of vehicle (OB) or exogenous MSC (OB + SC).
Cytokine and Growth Factor Activity
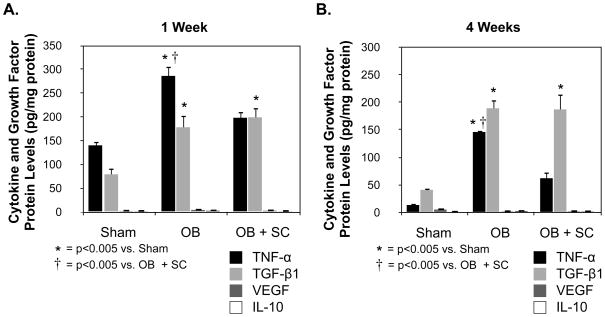
In order to evaluate the potential mechanisms of MSC-induced renal protection, the protein and gene expression levels of an array of cytokines and growth factors were evaluated (Figure 5 and 6). While renal VEGF (1 Wk: Sham = 2 ± 0.2; WT OB = 4 ± 0.9; SC OB = 2.5 ± 0.2; 4 Wk: Sham = 5 ± 0.5; WT OB = 2 ± 0.2; SC OB = 2.0 ± 0.4) and IL-10 protein levels (1 Wk: Sham = 1.4 ± 0.6; WT OB = 2.3 ± 0.4; SC OB = 1.3 ± 0.2; 4 Wk: Sham = 0.8 ± 0.05; WT OB = 2.1 ± 0.1; SC OB = 1.4 ± 0.1) remained very low in all treatment groups, TNF-α (1 Wk: WT OB = 286 ± 21 vs. sham = 140 ± 21; p<0.0001; 4 Wk: WT OB = 146 ± 3 vs. sham = 13 ± 1.4, p<0.0001) and TGF-β1 (1 Wk: WT OB = 178 ± 24 vs. sham = 79 ± 13; p<0.005; 4 Wk: WT OB = 189 ± 15 vs. sham = 41 ± 3, p<0.0001) protein levels were significantly increased in response to one and 4 weeks of obstruction as compared to sham-treated animals (Figure 5). In the presence of exogenous MSCs, obstruction-induced TNF-α levels were significantly reduced (1 Wk: WT OB = 286 ± 21 vs. SC OB = 198 ± 13; p<0.005; 4 Wk: WT OB = 146 ± 3 vs. SC OB = 62 ± 11, p<0.0001), while TGF-β1 levels remained unchanged (1 Wk: SC OB = 199 ± 21; 4 Wk: WT OB = 187 ± 28).
Figure 5. Renal cortical cytokine and growth factor levels following UUO.
A. TNF-α, TGF-β1, VEGF, and IL-10 protein levels in renal cortical tissue samples obtained from rats exposed to sham operation (Sham) or 1 week of UUO in the presence of vehicle (OB) or exogenous MSC (OB + SC). B. TNF-α, TGF-β1, VEGF, and IL-10 protein levels in renal cortical tissue samples obtained from rats exposed to sham operation (Sham) or 4 weeks of UUO in the presence of vehicle (OB) or exogenous MSC (OB + SC).
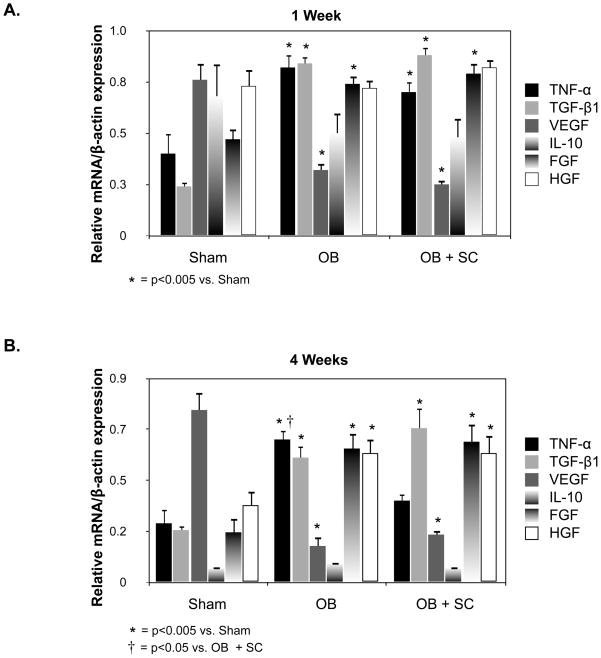
Figure 6. Renal cortical cytokine and growth factor gene expression following UUO.
A. Quantitative renal cortical TNF-α, TGF-β1, VEGF, IL-10, FGF, and HGF mRNA expression represented as a percentage of β-actin in animals exposed to sham operation (Sham) or 1-week of UUO in the presence of vehicle (OB) or exogenous MSC (OB + SC). B. Quantitative renal cortical TNF-α, TGF-β1, VEGF, IL-10, FGF, and HGF mRNA expression represented as a percentage of β-actin in animals exposed to sham operation (Sham) or 4 weeks of UUO in the presence of vehicle (OB) or exogenous MSC (OB + SC).
Renal cortical TNF-α (1 Wk: Sham = 0.4 ± 0.1; WT OB = 0.8 ± 0.06; SC OB = 0.7 ± 0.05; 4 Wk: Sham = 0.3 ± 0.06; WT OB = 0.6 ± 0.04; SC OB = 0.4 ± 0.03), TGF-β1 (1 Wk: Sham = 0.2 ± 0.02; WT OB = 0.8 ± 0.03; SC OB = 0.9 ± 0.04; 4 Wk: Sham = 0.2 ± 0.02; WT OB = 0.55 ± 0.05; SC OB = 0.68 ± 0.09), and FGF (1 Wk: Sham = 0.5 ± 0.05; WT OB = 0.7 ± 0.04; SC OB = 0.8 ± 0.05; 4 Wk: Sham = 0.2 ± 0.06; WT OB = 0.6 ± 0.07; SC OB = 0.6 ± 0.08) mRNA expression were significantly increased, and VEGF (1 Wk: Sham = 0.76 ± 0.08; WT OB = 0.3 ± 0.03; SC OB = 0.25 ± 0.02; 4 Wk: Sham = 0.76 ± 0.08; WT OB = 0.16 ± 0.04; SC OB = 0.2 ± 0.02) mRNA expression was significantly decreased in response to one or 4 weeks of UUO (Figure 6). HGF gene expression was significantly increased in response to 4 weeks of UUO (1 Wk: Sham = 0.7 ± 0.07; WT OB = 0.7 ± 0.04; SC OB = 0.8 ± 0.04; 4 Wk: Sham = 0.34 ± 0.06; WT OB = 0.57 ± 0.06; SC OB = 0.57 ± 0.08). A significant reduction in obstruction-induced TNF-α gene expression was detected in response to exogenous MSCs after 4 weeks of UUO, but no alteration in TGF-β1, VEGF, IL-10, FGF, or HGF gene expression was detected in response to exogenous MSCs. These results suggest that the mechanism of MSC-induced renal protection may involve alterations in TNF-α production.
DISCUSSION
MSC therapy is becoming an attractive strategy for renal repair because both nephrons and collecting ducts are of mesenchymal orgin, MSCs can be isolated from a variety of tissues, have low immunogenicity,15 and do not transdifferentiate into a malignant phenotype when the cells are implanted in vivo.8 While the impact of MSCs on various forms of acute renal injury has been examined at length,16–19 this study constitutes the initial demonstration that arterially delivered MSCs prevent chronic obstruction-induced renal fibrosis and EMT.
One characteristic of MSCs is their ability to home to sites of tissue damage or inflammation.20 Intravenously injected-MSCs have been shown to migrate to glomeruli, interstitium, peritubular capillaries, and tubuli in both acute and chronic kidney injury models.16, 21, 22 This is presumably related to increased chemokine concentrations at the site of inflammation, as MSCs have been demonstrated to express several chemokine receptors.23 In order to evaluate MSC incorporation and retention during renal obstruction, MSCs were fluorescently tagged immediately prior to arterial injection and renal tissue sections were subsequently evaluated 1 week and 4 weeks following UUO. Our results demonstrate that human MSCs were well incorporated into obstructed kidneys, localizing primarily to the interstitial space and renal tubules, and were retained for up to one month following injection. Human bone marrow-derived MSCs were selected for these experiments as they are readily commercially available, and their survival (secondary to an innate ability to evade rejection) and contribution to the functional recovery of injured organs has previously been demonstrated in non-immunodeficient xenogeneic models.24
While MSCs have been shown to accelerate recovery and prevent renal failure during acute injury, the therapeutic potential of MSCs in the treatment of chronic renal disease remains unclear. Ninichuk et al. demonstrated that weekly injections of MSCs prevented a loss in peritubular capillaries and reduced markers of renal fibrosis in a mouse model of Alport’s disease, but unfortunately, these findings were not associated with an improvement in renal function or animal survival.21 The impact of MSCs on chronic renal failure induced by 5/6 nephrectomy has recently been reported.25, 26 Caldas et al. demonstrated that progression of chronic renal failure is retarded by the presence of exogenous MSCs, resulting in an almost fourfold slower decline in creatinine clearance.25 Choi et al. similarly observed less proteinuria and greater weight gain following injection of MSCs 26; however, neither group demonstrated a significant histological improvement in response to MSC injection.
This is the first study to evaluate the impact of exogenous MSCs on obstruction-induced EMT and renal fibrosis. Our results demonstrate that arterially delivered MSCs significantly reduce α-SMA expression and FSP-1+ fibroblast accumulation, while simultaneously preserving E-cadherin expression, in response to one and 4 weeks of renal obstruction. This suggests that MSCs prevent obstruction-induced EMT, which is thought to contribute greatly to renal fibrosis, as a large proportion of interstitial fibroblasts originate from tubular epithelium during obstruction.27 Our results further demonstrate that arterially delivered MSCs significantly reduce obstruction-induced collagen deposition and tubulointerstitial fibrosis as determined by total tissue collagen concentrations and Masson’s trichrome staining.
Although the mechanism of MSC-induced renal protection remains unclear, it was initially hypothesized that immature stem cells differentiate into the phenotype of injured tissue and/or fuse with existing host cells in order to repopulate the diseased organ with healthy cells and improve organ function. Several recent studies; however, suggest that MSCs may mediate their beneficial effects, in part, through paracrine and/or endocrine activity.19, 28 The protective effects of MSCs are consistently observed within 24 to 48 hours of injection,17 well before there is sufficient time for cell growth, division, and differentiation, and in vitro and in vivo studies have revealed that much of the functional improvement and attenuation of injury afforded by stem cells can be replicated by cell-free, conditioned media derived from stem cells.28 MSCs secrete a broad array of factors, including growth factors, prostaglandins, and cytokines.9, 10 While a specific signaling mechanism is yet to be identified, the elaboration these various growth factors and cytokines appears to be an important component of MSC-induced renoprotection.
In order to elucidate a potential mechanism of MSC-induced renoprotection during UUO, the renal cortical expression of a variety of growth factors and cytokines was evaluated. Renal VEGF and IL-10 protein levels remained very low in all treatment groups, while TNF-α and TGF-β1 protein levels were significantly increased in response to obstruction. In the presence of exogenous MSCs; however, obstruction-induced TNF-α levels were significantly reduced, while TGF-β1 levels remained unchanged. As expected, renal cortical TNF-α, TGF-β1, FGF, and HGF gene expression were significantly increased, and VEGF gene expression was significantly reduced in response to obstruction. TNF-α gene expression was significantly reduced in response to exogenous MSCs after 4 weeks of obstruction, while the expression of the remaining mediators was unchanged in response to exogenous MSCs. These results suggest that the mechanism of MSC-induced renal protection may involve alterations in TNF-α production. TNF-α is a potent proinflammatory cytokine that has been implicated in the pathophysiology of a wide variety of renal and glomerular diseases. TNF-α is produced predominantly by tubular epithelial cells, not infiltrating macrophage, in response to renal obstruction and has previously been shown to have a significant role in obstruction-induced renal injury.29 TNF-α directly stimulates obstruction-induced renal tubular cell apoptosis, α-SMA expression and deposition, collagen deposition, and a deterioration in renal function.7, 30 While altered TNF-α production may be a mechanism of MSC-induced renoprotection during UUO, TNF-α’s specific role in MSC-induced renoprotection and the signaling mechanisms involved in MSC-induced alterations in TNF-α production remain to be determined.
CONCLUSIONS
Human bone marrow-derived MSCs remain viable several weeks after delivery into the kidney and provide protection against obstruction-induced EMT and renal fibrosis. While the mechanism of MSCs-induced renal protection during obstruction remains unclear, our results demonstrate that alterations in TNF-α production may be involved. An improved understanding of MSC-mediated paracrine activity and an elucidation of the mechanisms of stem cell activation may allow earlier and more effective clinical therapies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eddy AA. Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol. 1996;7:2495. doi: 10.1681/ASN.V7122495. [DOI] [PubMed] [Google Scholar]
- 2.Strutz F, Okada H, Lo CW, et al. Identification and characterization of a fibroblast marker: FSP1. J Cell Biol. 1995;130:393. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeisberg M, Strutz F, Muller GA. Renal fibrosis: an update. Curr Opin Nephrol Hypertens. 2001;10:315. doi: 10.1097/00041552-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Miyajima A, Chen J, Lawrence C, et al. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000;58:2301. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- 5.Rastaldi M. Epithelial-mesenchymal transition and its implications for the development of renal tubulointerstitial fibrosis. J Nephrol. 2006;19:407. [PubMed] [Google Scholar]
- 6.Roberts AB, McCune BK, Sporn MB. TGF-beta: regulation of extracellular matrix. Kidney Int. 1992;41:557. doi: 10.1038/ki.1992.81. [DOI] [PubMed] [Google Scholar]
- 7.Meldrum KK, Misseri R, Metcalfe P, et al. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1456. doi: 10.1152/ajpregu.00620.2005. [DOI] [PubMed] [Google Scholar]
- 8.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 10.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 12.Kano MR, Morishita Y, Iwata C, et al. VEGF-A and FGF-2 synergistically promote neoangiogenesis through enhancement of endogenous PDGF-B-PDGFRbeta signaling. J Cell Sci. 2005;118:3759. doi: 10.1242/jcs.02483. [DOI] [PubMed] [Google Scholar]
- 13.Rehman J, Traktuev D, Li J, et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 14.Barraclough R. Calcium-binding protein S100A4 in health and disease. Biochim Biophys Acta. 1998;1448:190. doi: 10.1016/s0167-4889(98)00143-8. [DOI] [PubMed] [Google Scholar]
- 15.Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485. doi: 10.1080/14653240310003611. [DOI] [PubMed] [Google Scholar]
- 16.Herrera MB, Bussolati B, Bruno S, et al. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 17.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 18.Morigi M, Introna M, Imberti B, et al. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26:2075. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- 19.Togel F, Cohen A, Zhang P, et al. Autologous and allogeneic marrow stromal cells are safe and effective for the treatment of acute kidney injury. Stem Cells Dev. 2009;18:475. doi: 10.1089/scd.2008.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devine SM, Bartholomew AM, Mahmud N, et al. Mesenchymal stem cells are capable of homing to the bone marrow of non-human primates following systemic infusion. Exp Hematol. 2001;29:244. doi: 10.1016/s0301-472x(00)00635-4. [DOI] [PubMed] [Google Scholar]
- 21.Ninichuk V, Gross O, Segerer S, et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70:121. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 22.Wong CY, Cheong SK, Mok PL, et al. Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model. Pathology. 2008;40:52. doi: 10.1080/00313020701716367. [DOI] [PubMed] [Google Scholar]
- 23.Fox JM, Chamberlain G, Ashton BA, et al. Recent advances into the understanding of mesenchymal stem cell trafficking. Br J Haematol. 2007;137:491. doi: 10.1111/j.1365-2141.2007.06610.x. [DOI] [PubMed] [Google Scholar]
- 24.Wairiuko GM, Crisostomo PR, Wang M, et al. Stem cells improve right ventricular functional recovery after acute pressure overload and ischemia reperfusion injury. J Surg Res. 2007;141:241. doi: 10.1016/j.jss.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Caldas HC, Fernandes IM, Gerbi F, et al. Effect of whole bone marrow cell infusion in the progression of experimental chronic renal failure. Transplant Proc. 2008;40:853. doi: 10.1016/j.transproceed.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Choi S, Park M, Kim J, et al. The role of mesenchymal stem cells in the functional improvement of the chronic renal failure. Stem Cells Dev. 2009;18:521. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 27.Iwano M, Plieth D, Danoff TM, et al. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bi B, Schmitt R, Israilova M, et al. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007;18:2486. doi: 10.1681/ASN.2007020140. [DOI] [PubMed] [Google Scholar]
- 29.Misseri R, Meldrum DR, Dagher P, et al. Unilateral ureteral obstruction induces renal tubular cell production of tumor necrosis factor-alpha independent of inflammatory cell infiltration. J Urol. 2004;172:1595. doi: 10.1097/01.ju.0000138902.57626.70. [DOI] [PubMed] [Google Scholar]
- 30.Bani-Hani AH, Campbell MT, Meldrum DR, et al. Cytokines in epithelial-mesenchymal transition: a new insight into obstructive nephropathy. J Urol. 2008;180:461. doi: 10.1016/j.juro.2008.04.001. [DOI] [PubMed] [Google Scholar]