Abstract
Background and Aim
This study aimed to analyze the mechanical properties of the esophagus in eosinophilic esophagitis (EoE) using the functional luminal imaging probe (EndoFLIP®, Crospon Medical Devices, Galway, Ireland).
Methods
35 EoE patients (24M, 23-67y) and 15 controls (6M, 21–68y) were included. Subjects were evaluated during endoscopy with the EndoFLIP® probe comprised of a compliant cylindrical bag (maximal diameter 25mm) with 16 impedance planimetry segments. Stepwise bag distensions from 2 to 40mL were conducted and the associated intra-bag pressure and intra-luminal geometry were analyzed.
Results
The EndoFLIP® clearly displayed the tubular esophageal geometry and detected esophageal narrowing and localized strictures. Stepwise distension progressively opened the esophageal lumen until a distension plateau was reached such that the narrowest cross-sectional area (CSA) of the esophagus maximized despite further increases in intra-bag pressure. The esophageal distensibility (CSA vs. pressure) was reduced in EoE patients (p=0.02) with the distension plateau of EoE patients substantially lower than that of controls (median CSA 267 vs. 438 mm2, p<0.01). Neither mucosal eosinophil count, age, gender, nor current PPI treatment predicted this limiting caliber of the esophagus (p≥0.20).
Conclusion
Esophageal distensibility, defined by the change in the narrowest measurable CSA within the distal esophagus vs. intra-luminal pressure, was significantly reduced in EoE patients compared to controls. Measuring esophageal distensibility may be an important adjunct to the management of EoE as it is capable of providing an objective means to measure the outcome of medical or dilation therapy.
Keywords: Eosinophilic esophagitis, esophageal distensibility, esophageal compliance
Introduction
Eosinophilic esophagitis (EoE) is a disease of increasing prevalence possibly related to a higher incidence of allergic diseases and greater awareness in specific patient populations.1-3 Patients present with a spectrum of esophageal symptoms; however, the dominant symptoms in adults are dysphagia, food impaction and, less commonly, chest pain.4 EoE pathogenesis entails an allergic immune response that involves T cell mediated hypersensitivity leading to eosinophil activation and the consequences of this cytokine cascade.5 Thus, the main diagnostic feature and marker of EoE is eosinophilic infiltration of the esophageal epithelium.
Although much of the emphasis on studying the pathogenesis of EoE is focused on delineating the causal antigens and the reactive cascade of cytokine mediators induced by this interaction, an important role of tissue remodeling and fibrosis has been described.5 Studies of eosinophilic mediated tissue remodeling and its effect on mechanical properties of the esophagus have been limited. This is partly due to the lack of a validated outcome measure that is an objective representation of the end-result of tissue remodeling and fibrosis. To date, studies have relied on radiographic and endoscopic surrogates to qualitatively assess degree of fibrosis and compliance of the esophagus.6-9 Unfortunately, these tools are subject to significant observer variability and they lack the ability to test distensile properties of the esophageal wall as the pressure-geometry relationship of the esophageal lumen cannot be measured.
Recently, our group has assessed the feasibility of a new technique that uses high-resolution impedance planimetry to investigate the pressure-geometry relationship of the esophagogastric junction (EGJ) in both gastroesophageal reflux disease (GERD) and post-fundoplication patients with encouraging results suggesting increased EGJ distensibility as a potentially causal abnormality in GERD and reduced distensibility after fundoplication.10,11 The device provides a measure of cross-sectional area (CSA) at detection sites extending over a 6.4 - 12 cm axial length while measuring intra-luminal pressure during controlled volume distensions of a infinitely compliant bag that can be also positioned within the lumen of the tubular esophagus. Hence, we hypothesized that this tool can be leveraged to study the distensile properties of the esophageal wall in EoE patients to provide an objective outcome measure of tissue remodeling and fibrosis. Thus, the goal of this study was to measure and compare esophageal distensibility in EoE patients and asymptomatic controls. We also sought to determine whether esophageal distensibility was correlated with the degree of eosinophilic infiltration or characteristic endoscopic features associated with EoE.
Materials and Methods
Subjects
15 control subjects (6M, 21 - 68 y) and 33 patients with EoE (22 M, 19 – 67 y) were studied. The control subjects were recruited from a pool of asymptomatic volunteers with no gastrointestinal symptoms, prior gastrointestinal surgery, or current use of medications known to affect gastrointestinal function. The patients were recruited from the Gastroenterology Clinic at Northwestern Memorial Faculty Foundation based on previous clinical documentation of esophageal symptoms suggestive of EoE and current endoscopic biopsies with histopathology confirming EoE (≥15 eos/hpf; magnification 0.196 mm2).12 Patient subjects were also stratified by PPI status to analyze the potential effect of acid suppression on eosinophilic esophigitis activity and distensibility. None of the subjects had a history of prior gastrointestinal surgery or significant medical disease. All subjects gave written informed consent. The study protocol was approved by the Northwestern University Institutional Review Board.
Endoscopic Functional Luminal Imaging Probe (EndoFLIP®) System
Esophageal body and EGJ distensibility were measured using a commercially developed functional luminal imaging probe (EndoFLIP®), based on the concepts described previously.13 In brief, the EndoFLIP® utilizes impedance planimetry to determine multiple adjacent cross-sectional areas (CSAs) within a cylindrical bag placed in a tubular organ during volumetric distension. The additional measure of the corresponding intra-bag pressure during distension allows assessment of the CSA-pressure response (distensibility) of the distended area.
The EndoFLIP® assembly was 240 cm long with a 3-mm outer diameter. An infinitely compliant bag (up to a volume limit of 40 mL) mounted on the distal 14 cm of the probe was fabricated to assume a 10-cm long cylindrical shape between tapering ends sealed at the assembly. The minimal-to-maximal range of CSA measureable by the device was 10 – 491 mm2. An 8-cm segment within the bag comprised of seventeen ring electrodes spaced 5-mm apart was designed for impedance planimetry measurement. Excitation electrodes situated proximal and distal to these seventeen ring electrodes established a constant small electrical current within the bag. Thus, as the bag was filled with a specially formulated conductive solution, the impedance between each of the sixteen adjacent pairs of ring electrodes was inversely proportional to the CSA of the bag at that locus. The probe also contained a solid-state pressure transducer that provided measurements of distensile pressure.
Measurements from the sixteen electrode pairs and pressure transducers were sampled at 10 Hz with the data acquisition system and transmitted to the recording unit which displayed them in real time as an 8-cm long cylinder of varying diameter along its length reflective of the 16 measured intra-luminal diameters. Both the probe and the pressure transducers were pre-calibrated by the manufacturer and required no additional calibration prior to use (CSA resolution 0.8 mm2, accuracy ±0.8 mm2; intra-bag pressure resolution 0.1 mm Hg, accuracy ±0.8 mm Hg). The only required preparation prior to use of the disposable probe assembly was to remove all air using an automated purge sequence controlled by the recording unit. Subsequent establishment of pressure baseline and infusion of the conductive solution into the balloon were controlled using the touch-screen on the recording unit.
Endoscopy
Subjects underwent esophagogastroduodenoscopy (EGD) in the left lateral decubitus position to evaluate for EoE findings (rings, furrows, exudates, stricture), anatomical landmarks (EGJ location) and to help place the EndoFLIP® across the EGJ for distensibility measurements. A diagnostic gastroscope of 9.9 mm outer diameter was used (Olympus® GIF type H180J, Olympus Corporation, Tokyo, Japan). Moderate sedation with 5-13 mg midazolam and 20-220 μg fentanyl was administered during the procedure. Still images taken during endoscopy were graded for EoE structural features by two blinded investigators (IH, JEP). The rings were defined as circumferential mucosal plications that persist during maximal insufflation oriented in the perpendicular plane, furrows as an indentation or a groove in the mucosa that was oriented parallel to the endoscope and strictures as a fixed abnormal narrowing over a focal length of the esophagus. Exudates were defined as white plaques or punctate lesions adhering to the esophageal mucosa.
EndoFLIP® Protocol
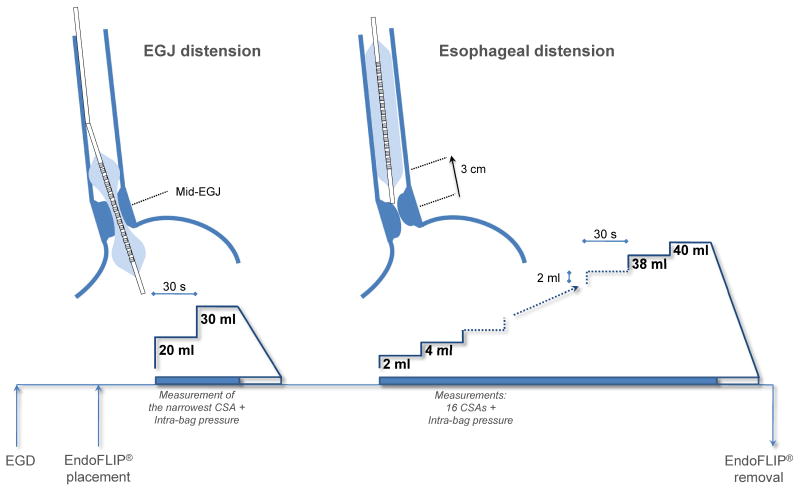
The schematic of the protocol is shown in Figure 1. At the end of the endoscopy, the EndoFLIP® was placed trans-orally until the center of the bag was positioned at the level of the SCJ measurement made during endoscopy. We have shown previously, that the EndoFLIP® bag assumes an hourglass shape when distended straddling the EGJ with the central constriction at the diaphragmatic hiatus.11 If the EGJ could not be confidently localized, the endoscope was reintroduced to confirm its position. The EndoFLIP® measures of EGJ CSAs and the corresponding distension pressure were made with the bag filled to 20 and 30 mL, volumes previously shown to optimally measure EGJ distensibility.11 In instances that measurements were interrupted by esophageal peristalsis, they were repeated. EndoFLIP® measurements were monitored in real-time to assure proper bag placement using the display of CSAs on the recording unit. If bag migration was detected, the bag was repositioned and the measurement repeated. After completing the EGJ measures, the EndoFLIP® bag was deflated and repositioned into the esophageal body by withdrawing the bag to a position such that the distal aspect of the recording segment was 3 cm proximal to the EGJ. This position provided a measurement of the esophageal body that extended to 11cm above the EGJ and could overlap with the initial EGJ measures. The CSAs along the esophageal lumen were measured with 2-mL stepwise distension starting with a bag volume of 2 mL up to a maximum of 40 mL. To prevent undo dilation of a stiff or poorly compliant esophagus, the recording unit was set to halt infusion and display an alarm message if the intra-bag pressure exceeded 60 mm Hg before proceeding further with the distension protocol.
Figure 1.
Schematic of the EndoFLIP® distensibility protocol. See Materials and Methods for details.
The EndoFLIP® bag was deflated and removed to facilitate completion of the endoscopy biopsy protocol. At least four mucosal biopsies were taken from the distal (3-5 cm from the squamocolumnar junction) and proximal esophagus (10 cm proximal to the distal biopsies).12
Data Analysis
EGJ and esophageal body CSAs and intra-bag pressures were assessed at each EndoFLIP® bag volume by quantifying the 50th percentile of each measure during each test 30-s recording.
EGJ and esophageal body distensibility (CSA vs. pressure) were based on the narrowest CSA and the corresponding intra-bag pressure. In the absence of any focal narrowing in the esophageal body detectable by the EndoFLIP®, the CSA measures from the sensors at the center of the bag were used. The EGJ and esophageal distensibility index at each distension volume was calculated as: (narrowest CSA in mm2/intra-bag pressure in mm Hg). Unlike the EGJ and esophageal distensibility measures that are restricted to a narrowed area, a bag distended within the tubular esophagus lends itself to additional measure of esophageal compliance. Esophageal compliance (volume vs. pressure) was estimated by summing the fifteen frustum volumes 5-mm high between the adjacent pairs of sixteen CSAs (mL). Esophageal compliance expressed as delta V/delta P for the entire 8-cm span of the impedance planimetry segment, was illustrated as volume-pressure curves.
In an ex-vivo bench-top experiment using volume distensions up to 40 mL, the EndoFLIP® bag expanded uniformly across the sixteen CSAs. The CSA vs. pressure relationship was characterized by a sigmoid curve with minimal-to-maximal limits of 10 – 491 mm2 for the CSA and 0 – 4.5 mm Hg for the intra-bag pressure. This confirmed infinite bag compliance within the volume range of 0 – 40 mL.
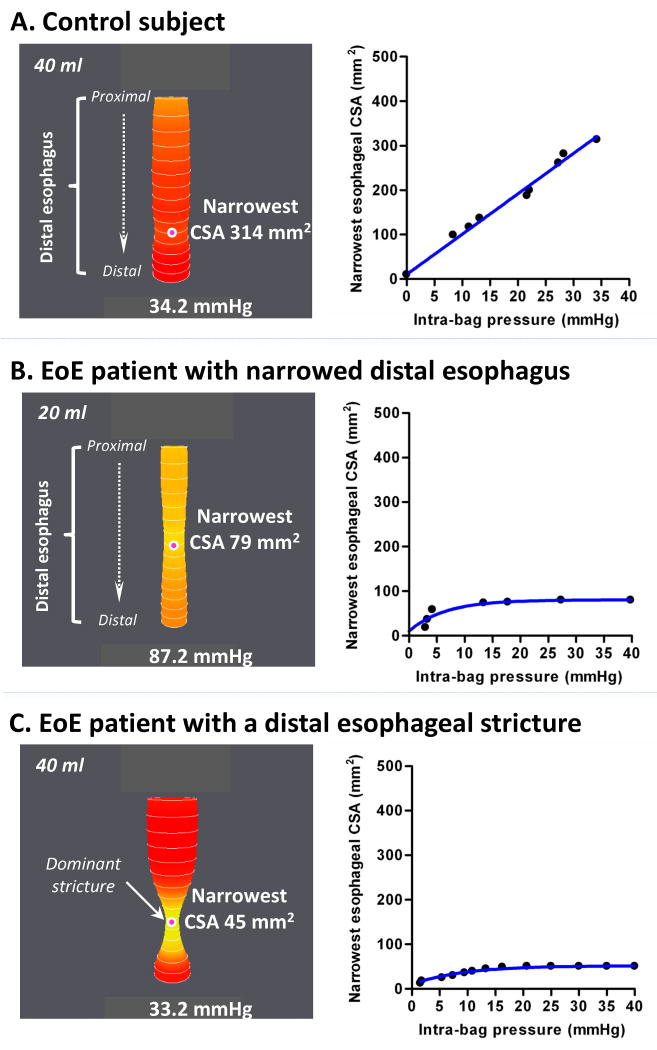
The dynamics of the esophageal distensibility/compliance were also assessed through regression analysis of the narrowest CSA/esophageal volume vs. intra-bag pressure data for each individual subject. The modeling of the data was performed using a function that best summarized the initial increase in esophageal distensibility/compliance which was followed by a distension plateau at which the dimension of CSA narrowing/esophageal volume remain the same despite concurrent increases in intra-bag pressure. Hence, the individual esophageal distensibility was modeled using a one phase exponential association function given as: narrowest CSADP = CSAmin + “distension plateau” × (1 − e (-κ × DP), where CSAmin is the minimal detectable CSA by EndoFLIP® of 10 mm2, “distension plateau” is the second phase of the curve where the function becomes constant (mm2), κ is a rate constant that modulates the initial phase of the curve (mm Hg-1) and DP is the distension pressure (mm Hg) (see examples in Figure 2).
Figure 2.
Examples of EndoFLIP® distensions in a control subject (A), an EoE patient with a diffusely narrowed distal esophagus (B) and an EoE patient with a dominant distal esophageal stricture (C). In each panel on the left, esophageal distension is illustrated as a cylinder of varying diameter corresponding to the 16 cross-sectional areas (CSAs) measured by impedance planimetry within the EndoFLIP® bag along with the location of the narrowest CSA (indicated by the pink dot) and corresponding intra-bag pressure. The corresponding CSA vs. distension pressure graphs are to the right. Note that in the example of a control subject (A) the distension plateau is not reached unlike in the examples of EoE patients (B-C). This occurred in 20% of control subjects.
Statistical Analysis
Data among subjects were expressed as median values and percentiles (5th – 95th percentile). Statistical comparisons used Wilcoxon matched pairs and Kruskal-Wallis tests with significance set at P < .05.
The modeled esophageal distensibility and compliance curves were compared with two-way repeated measures ANOVA with “Subject” (e.g. Controls vs. EoE patients) and “Distension Pressure” as fixed factors, and significance set at p < 0.05. Post-hoc analysis was performed using the Bonferroni/Dunn test with a significance level adjusted for multiple comparisons at P < .0167.
Three different statistical models were used to assess the relationship between the distension plateau and potential modulating variables: i) Pearson correlation (rs) for eosinophil count and age, ii) Mann-Whitney for gender, and iii) Kruskal-Wallis test for the bivariate relationship between the type of treatment (categorical variable) and esophageal distension plateau (continuous variable). Significance was set at P <.05. Post-hoc analysis for the Kruskal-Wallis test was performed using Bonferroni/Dunn test with a significance level adjusted for multiple comparisons at P < .0083.
The predictive values of mucosal eosinophil count, age, gender and type of treatment were tested with multivariate regression analysis using a significance level of P < 0.05.
Results
Demographic Data
Prior to the endoscopy with the EndoFLIP® protocol, all EoE patients had previously documented food impaction (14/33) and/or symptoms of dysphagia (32/33), chest pain (7/33) or heartburn (2/33). Twenty EoE patients were taking a proton pump inhibitor (PPI) and thirteen subjects were on no treatment at the time of the EndoFLIP® procedure.
The most common endoscopic features were rings (33/33) and furrows (27/33) with a stricture present in 27% of the patients (9/33). More than one of these features was present in 30/33 patients. Exudate was visible in more than half of the EoE group (17/33). There were no consistent differences in endoscopic features between EoE patients subgrouped into those taking or not taking PPI therapy (Table 1). No EoE features were present in control subjects, although esophagitis (Los Angeles grade A) was seen in 3/15.
Table 1.
Demographics of EoE patients grouped according to PPI therapy at the time of the EndoFLIP® procedure and asymptomatic control subjects.
| EoE patients | Control subjects (n = 15) | ||
|---|---|---|---|
| No PPI therapy (n = 13) | On PPI therapy (n = 20) | ||
| Age range (y) | 19 - 53 | 28 - 67 | 21 - 68 |
| Gender (male : female) | 9 : 4 | 13 : 7 | 6 : 9 |
| Esophageal eosinophila (range, eos/hpf) | |||
| Proximal esophagus | 5 – 100 | 0 – 189 | N/A |
| Distal esophagus | 15 – 110 | 5 – 189 | N/A |
| Endoscopic features (no. of subjects) | |||
| Rings ± furrows + stricture/s | 3 | 5 | 0 |
| Rings ± furrows | 10 | 15 | 0 |
The median eosinophil counts were 50 (1 – 130)/hpf for the biopsies collected from the proximal esophagus and 47 (15 – 115)/hpf for biopsies from the distal esophagus. Likewise as for endoscopic presentation, there were no consistent differences in eosinophil counts between EoE patients subgrouped into those taking or not taking PPI therapy (Table 1).
Feasibility
The EndoFLIP® distension protocol was well tolerated. No bleeding or mucosal tears were observed during endoscopy immediately afterward done to obtain esophageal biopsies. The distension protocol added approximately 10-15 minutes to the procedure.
The EndoFLIP® bag assumed a tubular geometry of the esophageal lumen in controls (as exemplified in Figure 2A), and clearly defined esophageal narrowing (Figure 2B) or localized strictures in patients (Figure 2C) at low distension volumes. Real-time monitoring of the EndoFLIP® measurements on the recording unit display allowed distinguishing peristaltic contractions from fixed mechanical constriction in the esophageal body.
Distensibility of the Esophageal Body
Individual esophageal distensibility curves were characterized by an initial increase in the dimensions of the narrowest CSA followed by a plateau phase in 12/15 control and 26/33 patient subjects (see examples in Figure 2B and C).
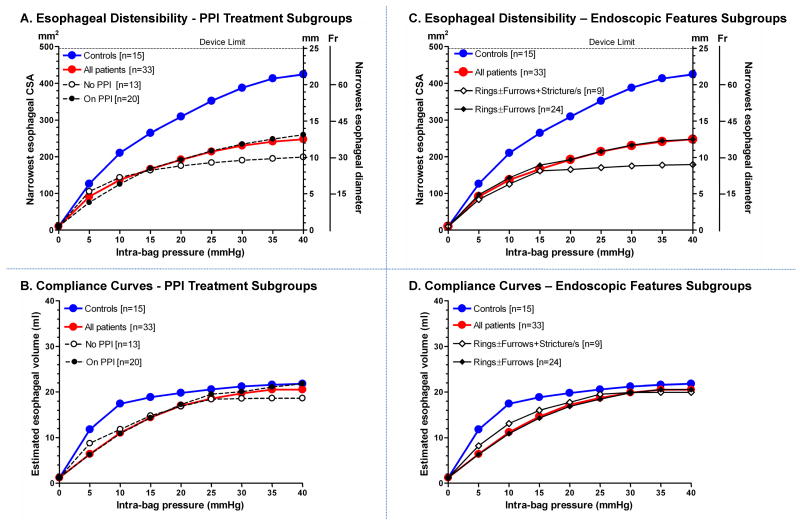
When modeled over a range of pressures, the esophageal diameter increased with greater distension pressures in both control and EoE subjects (P < .0001, Figure 3A and C). However, there was an appreciable initial diversion in the distensibility curves of the two subject groups (P < .01) beginning at the distension pressures of 5 - 30 mm Hg and progressively increasing with higher pressures. Hence, the overall extent of esophageal distensibility was significantly reduced in EoE patients when compared to controls (P = .02, Figures 3A and C). Sub-categorizing EoE patients into those taking (20/30) or not taking (13/33) PPI therapy at the time of the study revealed no significant differences in distensibility (P = 0.4) (Figure 3A). Likewise, there was no significant difference in distensibility between patients with endoscopically visible rings ± furrows (24/33) and those with an additional stricture (9/33) (P = 0.8) (Figure 3C).
Figure 3.
Esophageal distensibility (A & C) and compliance curves (B & D) in control subjects (blue) and EoE patients (red). Distensibility curves were computed from the fit to the one phase exponential association model defined by the distension plateau (Table 2) and κ. EoE patients exhibited diminished distensibility first evident in the 5-30 mm Hg pressure range and continuing for the remainder of the curves (P = .02). Esophageal compliance curves were significantly divergent, most evident at distension pressure 10 mmHg above which they seeming merge at 25 - 40 mm Hg (P = .02). There were no differences in either the distensibility or compliance curves between EoE patients subgrouped by PPI therapy at the time of evaluation (A & B) or the presence of a stricture in addition to rings ± furrows (C & D). Data shown as medians.
Distensibility differences were for the most part modulated by the narrowest CSA which increased progressively with distending volumes in both subject groups (p < 0.01), albeit to a significantly lesser degree in the EoE patients (P = .02), while the corresponding rise in pressure was similar between groups (P > .05). The distension plateau parameter of the one phase exponential association model (Table 2) also corroborated the data in Figure 3A and C. Although variability in the distension plateau parameter was observed in both groups, 24/33 (73 %) EoE patients exhibited a distension plateau of < 300 mm2, while 10/15 (67 %) control subjects had a distension plateau ≥ 400 mm2.
Table 2.
Distension plateau values of distensibility curves were computed from the averaged individual coefficients of the one phase exponential association model. The esophageal distension plateau describes the modeled limiting CSA despite increasing pressure.
| Distension Plateau (mm2) | |
|---|---|
| Control Subjects (n = 15) | 438 (227 – 491) |
| EoE Patients (n = 33) | 259 (78 – 491)* |
| No PPI therapy (n = 13) | 226 (95 – 373)* |
| On PPI therapy (n = 20) | 277 (80 – 491)* |
| Rings ± furrows (n = 24) | 267 (117 – 491)* |
| Rings ± furrows + stricture (n = 9) | 171 (70 – 387)* |
Median (5th - 95th percentile);
P < 0.01 vs. controls
The esophageal compliance curves (pressure vs. volume) also showed an initial increase with distension pressure before reaching a plateau (P < .01, Figure 3B and D). The distinct initial trajectory difference between the esophageal volumes for control and EoE subjects over the distension pressures of 5 - 20 mm Hg accounted for the overall significant difference between the subject groups (P = .02), despite merging of the curves at distension pressures of 25 – 40 mm Hg. The difference in the compliance curves along the pressure range of 5 to 20 mm Hg between EoE patients and controls is indicative of a difference in the compliant property of the esophageal wall (Phase 1) before the restrictive diameter associated with the distensibility plateau is reached. There were no differences in esophageal compliance between the EoE subgroups based on PPI treatment (P = 0.80) or endoscopic features (P = 0.70) in either the low or higher pressure range (Figure 3B and D).
Distensibility of the EGJ
The EndoFLIP® bag assumed an hourglass shape when distended straddling the EGJ with the central constriction at the diaphragmatic hiatus in both control and EoE subjects. The hiatal CSA, depicted as the narrowest CSA at the EGJ, increased with distending volumes (P ≤ .02), albeit to a lesser degree in EoE patients than in controls (P = .02, Table 3). However, the resultant intra-bag pressure was augmented similarly in both subject groups (P = 0.27, Table 3). Hence, the EGJ distensibility in EoE patients was slightly diminished compared to that of controls. The EGJ distensibility index, calculated from data in Table 3, was lower at the 20-mL (controls 0.9 (0.3 – 1.4) mm2/Hg vs. EoE 0.5 (0.2 – 2.5) mm2/Hg, P = .03) and 30-mL (controls 0.8 (0.4 - 2.8) mm2/Hg vs. EoE 0.5 (0.1 – 2.7) mm2/Hg, P = .01) distension volumes in EoE patients.
Table 3.
CSA at the diaphragmatic hiatus (narrowest EGJ CSA measured by the EndoFLIP®) and the pressure within the EndoFLIP® bag during volume distensions. The minimal detectable CSA was about 10 mm2.
| Hiatal CSA (mm2) | Intra-bag Pressure (mm Hg) | |||
|---|---|---|---|---|
| EndoFLIP® bag volume (mL) | Control Subjects (n = 15) | EoE Patients (n = 33) | Control Subjects (n = 15) | EoE Patients (n = 33) |
| 20 | 15 (9 – 23) | 13 (4 – 28)* | 19 (13 – 33) | 21 (7 – 30) |
| 30 | 22 (9 – 62) | 14 (4 – 54)* | 24 (18 – 39) | 29 (14 – 37) |
Median (5th - 95th percentile);
P < 0.05 vs. controls
Predictors of Reduced Distensibility
The esophageal distension plateau values in EoE patients had a poor correlation with mucosal eosinophil counts from the proximal (rs = -0.10, P = .58) or distal (rs = 0.01, P = .89) biopsies. Patient age (rs = 0.23, P = 0.2), gender (P = 0.73), and PPI treatment at the time of the EndoFLIP® protocol (P = .34) were also unrelated to the plateau measure. Hence, multivariate regression analysis showed no predictive values for EoE patient distension plateau of eosinophil count, age, gender or the PPI treatment (Table 4).
Table 4.
Regression analysis summary for EoE patient variables predicting the distal esophageal distension plateau measured by the EndoFLIP®. No tested variable, including current PPI treatment, was significantly correlated with distensibility.
| Variable | β coefficient† | P-value |
|---|---|---|
| Eosinophil count | ||
| Proximal esophagus | -0.52 | 0.18 |
| Distal esophagus | 0.44 | 0.27 |
| Age | 0.20 | 0.29 |
| Gender | -0.09 | 0.65 |
| Treatment at EndoFLIP® protocol | 0.24 | 0.21 |
measure of how predictor variable influences distension plateau
Discussion
This study quantified the distensile properties of the esophagus in patients with eosinophilic esophagitis (EoE) using the EndoFLIP®, a novel device that utilizes impedance planimetry technology. Luminal diameters of the distal esophagus along with concurrent intra-luminal pressure were measured during stepwise distension. The major findings were that: 1) EndoFLIP® provided technically successful measurements in all cases, 2) the esophageal distensibility, defined by the change in the narrowest measurable CSA within the distal esophagus as a function of intra-luminal pressure, was significantly reduced in EoE patients compared to controls, 3) likewise, esophageal compliance, defined by the change in esophageal volume as a function of intra-luminal pressure, was also lower in the patient group than in controls and 4) neither eosinophil count, age, gender nor current PPI treatment predicted the limiting caliber of the esophagus, as defined by the distension plateau.
Although the exact pathogenesis of EoE is incompletely understood, it is by definition associated with increased eosinophilic infiltration of the esophageal squamous epithelium. Current understanding is that this is associated with an allergic response to environmental antigens and most studies of EoE have focused on the pathways of eosinophil activation, reactive cascades of cytokine mediators that lead to their activation, or inflammatory features associated with esophageal eosinophilia.14-16 There has also been interest in studying factors responsible for tissue remodeling and fibrosis, as these most likely define the clinical presentation in adults which is dominated by food impaction and solid food dysphagia. Although tissue fibrosis and remodeling can be quantified using histological specimens to assess lamina propria fibrosis, this is typically not done in clinical practice. EoE disease activity is usually monitored qualitatively by describing the extent of endoscopically visible mucosal abnormalities (e.g.: rings, furrows, strictures, exudates) and quantitatively by the eosinophil count in the mucosal biopsies at the time patients present for clinical evaluation. Consequently, there is considerable variability in diagnostic criteria and interpretation, and clinical presentation.4, 17 Given that solid food dysphagia and food impaction are likely dependent on the opening aperture of the esophagus during bolus transit, an attractive alternative is to measure esophageal distensibility and compliance. Findings from this study support the contention that esophageal opening dimensions during distention are reduced in EoE and that this characteristic is not correlated with either the degree of mucosal eosinophil inflitration or specific endoscopic features of EoE. Thus, distensibility may be a useful objective measure to characterize disease severity in the context of dysphagia and food impaction.
The pressure-volume curves in Figure 3B and D, reflective of the wall characteristics of the entire 8-cm esophageal segment undergoing distension, showed significantly reduced compliance among the EoE patients at low (<20 mm Hg) but not higher distension pressures. Although this discrepancy was not specifically addressed in our protocol, there are several possible explanations. While low pressure distension results in cylindrical geometry and, hence, wall characteristics of the entire esophageal segment, higher pressure distension was more likely to accentuate focal areas of restriction as evident in Figure 2C. Note that the bag volumes and distension pressure in the control subject (Figure 2A) and the EoE patient (Figure 2C) are similar despite there being an obvious stricture in the EoE patient. Thus, it appears that the greater pressure/greater volume distention phase of the study protocol is insensitive to defining regional differences in compliance. Larger distension volumes probably also elicit contractile tone in the circular and longitudinal muscle that would confound the compliance measurement. This variability in geometry during distension, that appears to be common is EoE, also illustrates the limitation of using a barostat to study esophageal compliance as the volume increase is not uniform along the axial length of the esophagus. It also highlights the advantage of impedance planimetry in its ability to define the focus of obstruction that mediates the symptom of dysphagia.
The phenotypic presentation of EoE is heterogeneous. Although the diagnosis and management are currently guided by the eosinophil count within the esophageal biopsies,4 this is a patchy abnormality. The eosinophil counts between proximal and distal esophageal biopsies vary within an individual,12, 18 and there is often a disconnect between eosinophil count and endoscopic features of EoE.19, 20 Although the latter may be due to patchy eosinophil infiltration, biopsy sampling errors, or gastroesophageal reflux, it is also likely that the presentation of EoE may have distinct phenotypic expressions related to genetic predisposition to potential antigens.21 Our results also suggest that there is substantial heterogeneity in distensibility in EoE patients regardless of PPI treatment or endoscopic features (Table 2). In effect, the distensile properties were independent of the degree of tissue eosinophilia (Table 4) and this suggests that EoE may have subtypes akin to Crohn's disease: fibrostenotic and inflammatory.
Although our study cannot distinguish the etiology of the restriction and reduced compliance, the results suggest that it is a fixed defect not altered by contractile function in the esophageal body. The distension plateau represents a reliable indicator of the minimal opening diameter of the esophagus during distention and this measurement was not altered by increasing intra-bag pressure or contractile activity in the esophageal body. Distention of the bag within the esophageal body can cause intermittent secondary and/or non-propagating tertiary contractions and not controlling this pharmacologically to distinguish the active and passive components of compliance was a limitation of this study. However, the distention plateau was maintained between periods of active contraction or post-contractile quiescence suggesting that the defect is related to restrictions in the passive mechanical properties of the esophageal wall. This finding is in line with previous manometric data suggesting that contractile activity of the circular muscle is relatively normal in EoE. 22-29 Thus, it appears that the defect is fixed at the extreme of distention, but the esophageal wall is still collapsible and amenable to contraction.
Recent insights into the pathogenesis of EoE using intraluminal ultrasound support normal contractile function of the circular muscle and lumen collapse during peristalsis; however, the function of the longitudinal muscle was suggested to be altered in EoE patients compared to controls23. The longitudinal muscle response to edrophonium was markedly reduced in EoE patients and there was a clear dissociation between circular and longitudinal muscle contraction during peristalsis. These findings were not associated with abnormal thickening or enhancement of the esophageal musculature, and thus, we believe that the defect in restriction of radial diameters is still likely confined to the thickened mucosa and submucosa exhibited on high-resolution intraluminal ultrasound. Although we speculate that the reduction in longitudinal muscle function could be related to restriction of axial mobility from fibrosis in the mucosa and submucosa, it is also possible that the longitudinal muscle may be altered by the cytokine cascade associated with EoE.
Our study was limited to the assessment of the distal smooth muscle esophagus; however, EoE can affect the entire esophagus including the proximal striated muscle segment. Considering that the sensitivity and neuromuscular control of the proximal esophagus are different than the distal smooth muscle segment studied in this protocol, it may be possible that different distensibility plateaus may be found in the proximally. Thus, future studies should include measures of both proximal and distal esophageal segments.
One could argue that the current study is limited by inclusion of both EoE patients on and off PPI therapy, as the overlap of GERD and EoE is an important diagnostic issue. The interaction between GERD and EoE is complex and it may range from one causing the other to coexistence of the two,30 and it remains unclear to what extent GERD-EoE patients will respond to PPI therapy.31 Despite these important issues, our results were not confounded by PPI status as regression analysis revealed that PPI status had no bearing on esophageal distensibility in our EoE cohort (Table 4). Once again, the esophageal distensibility in EoE patients did not correlate with the eosinophil count noted on the day of the EndoFLIP® procedure (Table 4); hence, esophageal distensibility may serve as an independent marker of disease severity.
In conclusion, this experiment confirmed that the EndoFLIP®, a new commercially available technology utilizing high-resolution impedance planimetry, is capable of evaluating esophageal distensibility in EoE. These findings also represent the first quantification of reduced esophageal distensibility in EoE and provide insight into the mechanical dysfunction associated with dysphagia and impaction. EoE patients appear to have a restriction in radial distention that is associated with preserved luminal collapse during peristalsis. We hypothesize that measuring esophageal distensibility may be an important adjunct to the clinical management of EoE that potentially provides a more objective measure from which to guide medical and dilation therapy. However, future studies done before and after therapy are required to determine whether reduced distensibility is responsive to treatment focused on reducing inflammation and fibrosis, and whether these changes are associated with improvement in patient reported symptom severity reported outcomes.
Acknowledgments
Funding: This work was supported by R01 DK56033 (PJK) and R01 DK079902 (JEP) from the Public Health Service; EndoFLIP® equipment supported and sponsored by Crospon™ Ltd., Galway, Ireland
Abbreviations
- CSA
cross sectional area
- EGJ
esophagogastric junction
- EoE
eosinophilic esophagitis
Footnotes
Conflicts of Interest: No conflicts of interest exist – MAK, IH, PJK, JR, DL; Crospon Inc. - JEP, Advisory Board; the potential conflict of interest was disclosed to the study participants.
Contribution: Monika A. Kwiatek: conception and study design, study supervision, data collection, modeling, analysis and interpretation, statistical analysis, manuscript drafting, editing, critical revision and final approval; Ikuo Hirano: conception and study design, study supervision, data collection, analysis and interpretation, manuscript drafting, editing, critical revision and final approval; Peter J. Kahrilas: conception and study design, obtained funding, data collection and interpretation, manuscript drafting, editing, critical revision and final approval; Jami Rothe: data collection and interpretation; technical support; manuscript drafting and final approval; Daniel Luger: data collection and interpretation; technical support; manuscript drafting, and final approval; John E. Pandolfino: conception and study design, study supervision, obtained funding, data collection, analysis and interpretation, manuscript drafting, editing, critical revision and final approval.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Croese J, Fairley SK, Masson JW, et al. Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc. 2003;58:516–22. doi: 10.1067/s0016-5107(03)01870-4. [DOI] [PubMed] [Google Scholar]
- 2.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–1. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 3.Straumann A, Simon HU. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol. 2005;115:418–9. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Furuta GT, Liacouras CA, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–63. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Aceves SS, Ackerman SJ. Relationships between eosinophilic inflammation, tissue remodeling, and fibrosis in eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29:197–211. xiii–xiv. doi: 10.1016/j.iac.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feczko PJ, Halpert RD, Zonca M. Radiographic abnormalities in eosinophilic esophagitis. Gastrointest Radiol. 1985;10:321–4. doi: 10.1007/BF01893121. [DOI] [PubMed] [Google Scholar]
- 7.Vasilopoulos S, Murphy P, Auerbach A, et al. The small-caliber esophagus: an unappreciated cause of dysphagia for solids in patients with eosinophilic esophagitis. Gastrointest Endosc. 2002;55:99–106. doi: 10.1067/mge.2002.118645. [DOI] [PubMed] [Google Scholar]
- 8.Potter JW, Saeian K, Staff D, et al. Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc. 2004;59:355–61. doi: 10.1016/s0016-5107(03)02713-5. [DOI] [PubMed] [Google Scholar]
- 9.Attwood SE, Lamb CA. Eosinophilic oesophagitis and other non-reflux inflammatory conditions of the oesophagus: diagnostic imaging and management. Best Pract Res Clin Gastroenterol. 2008;22:639–60. doi: 10.1016/j.bpg.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Kwiatek MA, Kahrilas PJ, Soper NJ, et al. Esophagogastric Junction Distensibility After Fundoplication Assessed with a Novel Functional Luminal Imaging Probe. J Gastrointest Surg. 2009;14:268–276. doi: 10.1007/s11605-009-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwiatek MA, Pandolfino JE, Hirano I, et al. Esophagogastric junction distensibility assessed with an endoscopic functional luminal imaging probe (EndoFLIP) Gastrointest Endosc. 2010;72:272–8. doi: 10.1016/j.gie.2010.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonsalves N, Policarpio-Nicolas M, Zhang Q, et al. Histopathologic variability and endoscopic correlates in adults with eosinophilic esophagitis. Gastrointest Endosc. 2006;64:313–9. doi: 10.1016/j.gie.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 13.McMahon BP, Frokjaer JB, Liao D, et al. A new technique for evaluating sphincter function in visceral organs: application of the functional lumen imaging probe (FLIP) for the evaluation of the oesophago-gastric junction. Physiol Meas. 2005;26:823–36. doi: 10.1088/0967-3334/26/5/019. [DOI] [PubMed] [Google Scholar]
- 14.Protheroe C, Woodruff SA, de Petris G, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–755. e11. doi: 10.1016/j.cgh.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blanchard C, Mingler MK, Vicario M, et al. IL-13 involvement in eosinophilic esophagitis: transcriptome analysis and reversibility with glucocorticoids. J Allergy Clin Immunol. 2007;120:1292–300. doi: 10.1016/j.jaci.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Vicario M, Blanchard C, Stringer KF, et al. Local B cells and IgE production in the oesophageal mucosa in eosinophilic oesophagitis. Gut. 59:12–20. doi: 10.1136/gut.2009.178020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellon ES, Aderoju A, Woosley JT, et al. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol. 2007;102:2300–13. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 18.Shah A, Kagalwalla AF, Gonsalves N, et al. Histopathologic variability in children with eosinophilic esophagitis. Am J Gastroenterol. 2009;104:716–21. doi: 10.1038/ajg.2008.117. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Gonsalves N, Rao S, et al. Characteristic endoscopic features of eosinophilic esophagitis in patients without tissue eosinophilia: true or falso negative? Gastroenterology. 2007;132:A610. [Google Scholar]
- 20.Bonis PAL, Furuta GT. In: Clinical manifestations and diagnosis of eosinophilic esophagitis. Talley NJ, Ginsburg CH, editors. Vol. 2010. 2010. UpToDate. [Google Scholar]
- 21.Rodrigo S, Abboud G, Oh D, et al. High intraepithelial eosinophil counts in esophageal squamous epithelium are not specific for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103:435–42. doi: 10.1111/j.1572-0241.2007.01594.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Ghosh SK, Pandolfino JE, et al. Esophageal dysmotility in eosiniphilic esophigitis: analysis using high resolution esophageal manometry. Gastroenterology. 2007;132:A6. [Google Scholar]
- 23.Korsapati H, Babaei A, Bhargava V, et al. Dysfunction of the longitudinal muscles of the oesophagus in eosinophilic oesophagitis. Gut. 2009;58:1056–62. doi: 10.1136/gut.2008.168146. [DOI] [PubMed] [Google Scholar]
- 24.Attwood SE, Smyrk TC, Demeester TR, et al. Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci. 1993;38:109–16. doi: 10.1007/BF01296781. [DOI] [PubMed] [Google Scholar]
- 25.Nurko S, Rosen R. Esophageal dysmotility in patients who have eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2008;18:73–89. ix. doi: 10.1016/j.giec.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucendo AJ, Castillo P, Martin-Chavarri S, et al. Manometric findings in adult eosinophilic oesophagitis: a study of 12 cases. Eur J Gastroenterol Hepatol. 2007;19:417–24. doi: 10.1097/MEG.0b013e328010bd69. [DOI] [PubMed] [Google Scholar]
- 27.Lucendo AJ, Pascual-Turrion JM, Navarro M, et al. Endoscopic, bioptic, and manometric findings in eosinophilic esophagitis before and after steroid therapy: a case series. Endoscopy. 2007;39:765–71. doi: 10.1055/s-2007-966738. [DOI] [PubMed] [Google Scholar]
- 28.Hejazi RA, Reddymasu SC, Sostarich S, et al. Disturbances of Esophageal Motility in Eosinophilic Esophagitis: A Case Series. Dysphagia. 2009 doi: 10.1007/s00455-009-9248-6. [DOI] [PubMed] [Google Scholar]
- 29.Bassett J, Maydonovitch C, Perry J, et al. Prevalence of esophageal dysmotility in a cohort of patients with esophageal biopsies consistent with eosinophilic esophagitis. Dis Esophagus. 2009;22:543–8. doi: 10.1111/j.1442-2050.2009.00949.x. [DOI] [PubMed] [Google Scholar]
- 30.Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102:1301–6. doi: 10.1111/j.1572-0241.2007.01179.x. [DOI] [PubMed] [Google Scholar]
- 31.Shah A, Hirano I. Treatment of eosinophilic esophagitis: drugs, diet, or dilation? Curr Gastroenterol Rep. 2007;9:181–8. doi: 10.1007/s11894-007-0016-1. [DOI] [PubMed] [Google Scholar]