Abstract
Several studies have reported favipiravir (T-705) to be effective in treating a number of viral diseases modeled in rodent systems. Notably, the related pyrazine derivative, T-1106, was found to be more effective than T-705 in treating yellow fever virus infection in hamsters. Based on these findings, we hypothesized that T-1106 may be more effective in treating hepatotropic Punta Toro virus (PTV, Phlebovirus) infection in rodents. In cell culture, the inhibitory concentrations of the compounds against various phleboviruses ranged from 3–55 µM for T-705 and 76–743 µM for T-1106. In PTV-challenged hamsters, a model that generally presents with high liver viral loads, T-1106 was more effective at reducing mortality. However, in mice infected with PTV, a model wherein systemic infection is more prominent, the greater efficacy exhibited by T-1106 in the hamster system was not apparent. In contrast, T-705 was superior in preventing mortality in hamsters challenged with Pichinde virus (PICV, Arenavirus), an infection characterized as diffuse and pantropic. Remarkably, T-1106 has proven more active in vivo than would have been expected from our cell culture results, and our in vivo findings suggest that it is more effective in infections characterized predominantly by high levels of hepatic viral burden.
Keywords: Punt Toro virus, Rift Valley fever, Arenavirus, Phlebovirus, T-705, T-1106
1. Introduction
Rift Valley fever (RVF) is a viral zoonosis that primarily affects ungulates, and to a lesser extent, humans (Morrill and McClain, 1996). The etiological agent, Rift Valley fever virus (RVFV), is a mosquito-borne RNA virus member of the Bunyaviridae family, genus Phlebovirus. It is classified as “Category A Pathogen” by the NIAID and meets selection criteria for dual DHHS and USDA “Select Agent” status, underscoring the importance of RVFV in the context of national security and global public health. Punta Toro virus (PTV) is a phlebovirus related to the highly pathogenic RVFV, which causes a severe hepatotropic disease in mice and hamsters (Anderson et al., 1990; Fisher et al., 2003; Pifat and Smith, 1987) similar to that reported for RVF (Peters and Meegan, 1981). PTV infection in humans is generally asymptomatic or limited to a mild febrile illness (Peters and LeDuc, 1984). Small animal and cell culture model systems based on infection with the less biohazardous PTV and RVFV vaccine strain, MP-12, respectively, are often employed to evaluate experimental therapies early in the preclinical development process (Gowen et al., 2009; Gowen et al., 2006a; Gowen et al., 2006b; Gowen et al., 2008b; Gowen et al., 2007a; Gowen et al., 2007b; Sidwell et al., 1988; Smee et al., 1991). Ultimately, advanced studies employing authentic RVFV strains in rodent or nonhuman primate models are required for continued preclinical development (Gowen and Holbrook, 2008; Peters et al., 1986).
There are currently no FDA-approved antivirals for the treatment of RVF. Ribavirin has shown some efficacy in animal model systems of RVF (Huggins, 1989; Peters et al., 1986), but due to concerns regarding its toxicity and limited data in humans, it is only indicated under compassionate use guidelines in the event of emergency (Borio et al., 2002). Recently, we reported on the activity of favipiravir (T-705, 6-fluoro-3-hydroxy-2-pyrazinecarboxamide) against PTV infection in mice and hamsters, and against several other bunyaviruses including the RVFV vaccine strain in cell culture (Gowen et al., 2007a). Moreover, T-705 has also been shown to be effective in treating orthomyxovirus (influenza viruses), arenavirus (Pichinde virus), and flavivirus (West Nile and yellow fever viruses) infections in animal models (Furuta et al., 2002; Gowen et al., 2008a; Julander et al., 2009; Morrey et al., 2008; Sidwell et al., 2007), and is currently in clinical trials for the treatment of influenza virus infections.
T-1106 (3,4-dihydro-3-oxo-4-β-d-ribofuranosyl-2-pyrazinecarboxamide), a related pyrazine derivative, has been shown to be more efficacious than T-705 for treating yellow fever virus (YFV) infection in hamsters (Julander et al., 2009). YFV infection causes a severe hepatic disease in hamsters, suggesting that T-1106 may be a better option for treating viral syndromes that principally target the liver. Because PTV infection causes a severe liver disease in rodents, we hypothesized that T-1106 may be a better option for treating PTV-infected animals. Thus, in the present study, we compared the inhibitory effects of T-1106 and T-705 in the mouse and hamster models of acute PTV disease. In addition, the antiviral activities of the pyrazine derivatives were compared in the hamster Pichinde arenavirus (PICV) infection model, as well as several phlebovirus and arenavirus cell culture model systems.
2. Materials and methods
2.1. Animals
Female 6 week-old Syrian hamsters were obtained from Charles River Laboratories (Wilmington, MA). Female 6 week-old C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Hamsters and mice were acclimated to the Laboratory Animal Research Facility at Utah State University for 6 days prior to use. Animals were 7–8 weeks of age at time of challenge. All procedures complied with USDA guidelines and were approved by the Utah State University Institutional Animal Care and Use Committee.
2.2. Viruses
PTV, Adames strain, was received from Dr. Dominique Pifat of the U. S. Army Medical Research Institute for Infectious Diseases, Ft. Detrick (Frederick, MD). The virus used was from a stock prepared following 4 passages of the original virus through LLC-MK2 monkey kidney cells (ATCC; American Type Culture Collection, Manassas, VA) and one passage in hamsters. Clarified viral stocks made from pooled hamster liver homogenates containing high titers of infectious PTV were diluted in minimal essential medium (MEM, Hyclone, Logan, UT) just prior to infectious challenge by the subcutaneous (s.c.) route. Sandfly fever virus, Naples strain (SFNV, Phlebovirus) and Tacaribe virus (TCRV, Arenavirus), strain TRVL 11573, were from ATCC. The RVFV and Junin virus (JUNV, Arenavirus) vaccine strains, MP-12 and Candid 1, respectively, were provided by Dr. Robert Tesh (World Reference Center for Emerging Viruses and Arboviruses, University of Texas Medical Branch, Galveston, TX). PICV (Arenavirus), strain An 4763, was provided by Dr. David Gangemi (Clemson University, Clemson, SC). The virus was passed once through hamsters and a stock made from pooled hamster liver homogenates. PICV was inoculated (0.2 mL) via the intraperitoneal (i.p.) route.
2.3. Test articles
T-705 (MW 157.1) and T-1106 (MW 269.2) were provided by the Toyama Chemical Company, Ltd. (Tokyo, Japan). The structures and basic properties of these pyrazine derivatives are described elsewhere (Furuta et al., 2009). Both T-705 and T-1106 were suspended in 0.4% carboxymethylcellulose (CMC) for oral administration (p.o.). For cell culture studies, the compounds were dissolved in minimal essential medium (MEM).
2.4. Cell culture antiviral assays
The monkey kidney cell lines, Vero and Vero 76 were obtained from ATCC and maintained in MEM supplemented with 0.18 % NaHCO3 and 10 % fetal bovine serum (FBS, Hyclone). Viruses were diluted in culture medium containing 2% FBS to a cell culture 50% infectious dose (CCID50) that produced maximal cytopathic effect (CPE) by visual examination in preliminary virus titration experiments. Varying concentrations of the pyrazine derivatives were added to test wells at the time of infection. Vero and Vero 76 cells were ~70 and 90 % confluent at time of infection and treatment. For toxicity determinations, drugs were added in the absence of viral challenge. Plates were incubated at 37°C, 5 % CO2, until virus-infected control wells were observed to have maximal viral CPE (4–5 days for phleboviruses, 7–8 days for the arenaviruses), at which time the plates were processed to assess cell viability by neutral red (NR) vital dye uptake as previously described (Gowen et al., 2007a). The mean effective drug concentration (EC50) and the concentration that reduced cell viability by 50% (CC50) were determined by regression analysis. Virus yield reduction (VYR) data were determined as the concentration of drug reducing virus yield by 1 log10 (EC90) based on regression analysis. Selectivity index (SI) values were calculated as the CC50/EC50 for the CPE reduction (CPER) NR-based assays, and as CC50/EC90 for the VYR assays.
2.5. In vivo challenge studies
For the PTV studies, hamsters or mice were weighed on the morning of infection and grouped so that the average weight per group across the entire experiment varied by less than 5% between groups. Animals in each group (n=15) were treated orally with pyrazine derivatives (25–100 mg/kg/day) or 0.4% CMC placebo (n=25) for 5–6 days beginning 24–48 h after challenge. Hamsters were inoculated with 50 plaque-forming units (PFU) of PTV and mice were inoculated with 5 × 103 PFU. Five animals from each treatment group were sacrificed on day 3 (mice) or 4 (hamsters) of infection to measure viremia, liver virus burden, and serum alanine aminotransferase (ALT) activity. Serum was collected for assaying systemic viral burden and ALT activity and livers were harvested, homogenized, and clarified for viral titer determination as described below. The remaining 10–20 animals were observed 21 days for mortality. Three sham-infected controls were included for comparison to establish baselines for all test parameters. The PICV hamster challenge (~2 PFU by intraperitoneal route) efficacy study was conducted similarly, except that treatment was initiated on day 4 and lasted 7 days. Improvement in survival outcome, and reduction in viral burden and ALT levels were used to measure therapeutic efficacy.
2.6. Determination of liver and serum virus titers
Virus titers were assayed by infectious cell culture assay as previously described (Gowen et al., 2007a). Liver homogenates or sera were serially diluted and added in triplicate to Vero cell monolayers in 96-well microplates. Viral CPE was determined 7–8 days after exposure to the samples and the 50% endpoints were calculated as described (Reed and Muench, 1938). The assay detection range was 2.75 – 9.5 log10 CCID50/g of liver or 1.75 – 8.5 CCID50/ml of serum. In samples presenting with no detectable liver or serum virus, a value of < 2.8 log10 or < 1.8 log10, respectively, was assigned. In cases wherein virus exceeded the detection range, a value of > 9.5 log10 or > 8.5 log10 was assigned. For the purpose of statistical analysis, values of 1.8, 2.8, 8.5, or 9.5 log10 were assigned as needed for samples with undetectable or saturated virus levels.
2.7. Measurement of serum ALT activity
Serum ALT activity, a robust marker indicative of liver disease (Amacher, 1998), was measured in serum samples using the ALT (SGPT) Reagent Set (Pointe Scientific, Lincoln Park, MI). The reagent volumes were adjusted for analysis on 96-well microplates for large sample numbers.
2.8. Statistical analysis
Kaplan-Meier survival plots and all statistical evaluations were done using Prism (GraphPad Software, CA). The log-rank test was employed for survival analysis. For analyzing differences in viral titers and ALT levels, a one-way analysis of variance (ANOVA) with Newman-Keuls post test or the Kruskal-Wallis test with the Dunn’s post-test was performed based on Gaussian distribution of the data.
2.9. Molar comparison
For pyrazine derivative comparisons on a molar basis, percent survival, virus titer, and ALT reduction data were considered following conversion of mg/kg doses of T-705 or T-1106 to moles/kg. Regression analysis was used to determine the 50% effective dose (ED50), defined as the moles of drug required to protect 50% of challenged animals from mortality, or reduce viral load or ALT by 50% compared to placebo. The determined ED50s facilitated comparison at the molar level since the disparity in MW between the two pyrazine derivatives resulted in different molar quantities favoring T-705 when comparing equivalent mg/kg doses.
3. Results
3.1. Pyrazine derivative inhibition of phleboviral CPE and yield in cell culture
We have previously reported on the anti-phleboviral activity of T-705 in cell culture (Gowen et al., 2007a). In those studies, the activity of T-705 was compared to ribavirin, and T-705 was dissolved in dimethyl sulfoxide (DMSO) prior to dilution in culture media for analysis. Here, we compared the activity of T-705 with T-1106, having dissolved both compounds in MEM. A series of experiments evaluating the inhibitory capacity of T-705 and T-1106 against RVFV, PTV, and SFNV are summarized in Table 1. The potency of T-705 was considerably greater, as reflected by EC50 values ranging from 27–55 µM by CPE reduction assay and 3–19 µM EC90 by VYR, compared to T-1106 EC50 and EC90 values in the range of 111–743 µM and 76–576 µM, respectively. Activity of both pyrazine analogs was weakest against PTV.
Table 1.
In vitro inhibitory effects of T-705 and T-1106 against phlebovirusesa.
| Virus | Assay b | T-705 c | T-1106 c | ||||
|---|---|---|---|---|---|---|---|
| CC50 ± SD | EC50/90 ± SD | SI d | CC50 ± SD | EC50/90 ± SD | SI d | ||
| RVFV | CPER | >6365 ± 0 | 27 ± 5 | >236 | >3715 ± 0 | 111 ± 58 | >33 |
| VYR | 12 ± 4 | >530 | 146 ± 129 | >25 | |||
| PTV | CPER | 3734 ± 1037 | 55 ± 35 | >68 | >3715 ± 0 | 743 ± 297 | >5 |
| VYR | 19 ± 11 | >197 | 576 ± 450 | >6 | |||
| SFNV | CPER | 5856 ± 168 | 30 ± 15 | 195 | >3715 ± 0 | 152 ± 13 | >24 |
| VYR | 3 ± 1 | 1952 | 76 ± 15 | >49 | |||
Data are the mean and standard deviations from 3–6 separate experiments in Vero 76 cells.
Cytopathic effect reduction (CPER) based on neutral red dye uptake by viable cells; virus yield reduction (VYR).
CC50 and EC50/90 values are in µM.
Selectivity index (SI) = CC50/EC50/90.
3.2. Efficacy of pyrazine derivatives in PTV-challenged hamsters
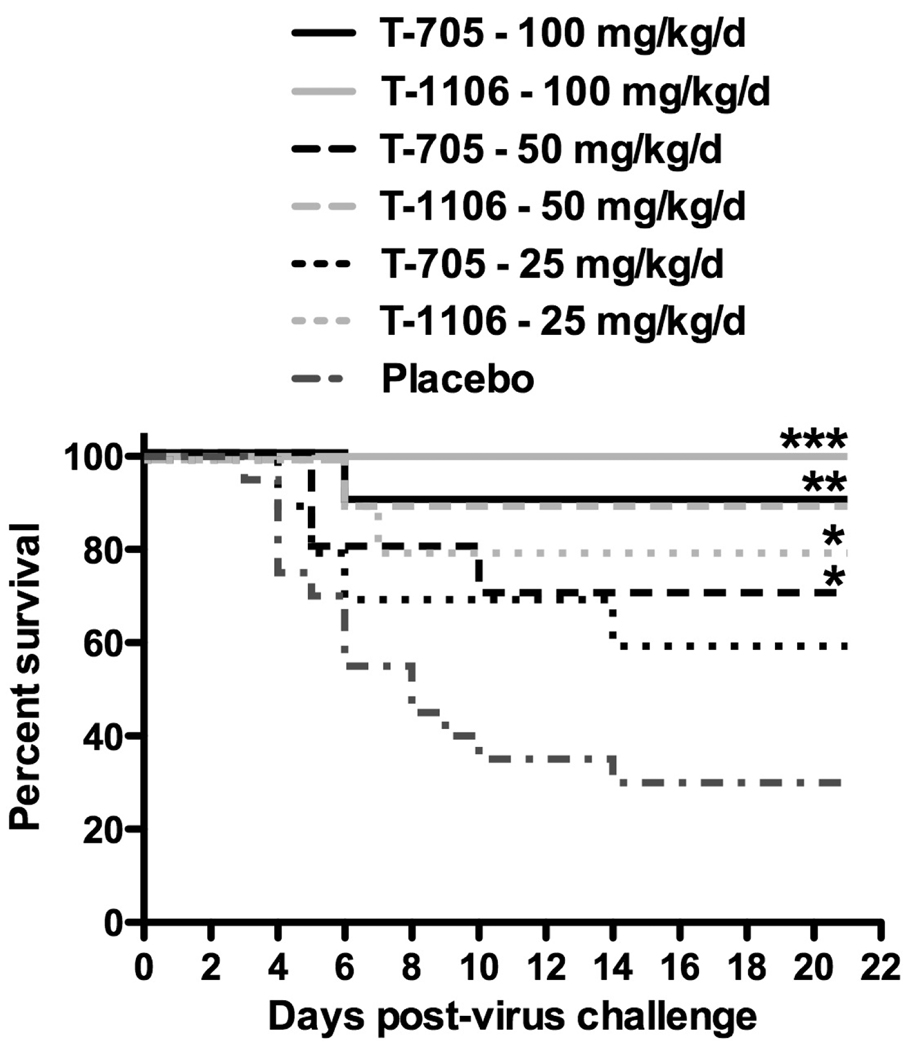
To test our hypothesis that T-1106 may be more effective than T-705 for treating viral infections wherein prominent liver disease is a salient feature, we conducted an experiment directly comparing the activities of the pyrazine derivatives on a mg/kg basis against hepatotropic PTV infection in hamsters. As seen in Figure 1, the T-1106 survival curves for all tested doses of 100, 50, and 25 mg/kg/day trended towards greater efficacy compared to the equivalent mg/kg T-705-treated animals when initiating treatment 24 h post virus challenge. When compared on a molar basis, T-1106 was 9.4 times more potent than T-705 based on ED50 determination. Significant protection was observed between all T-1106 groups and the placebo. The two highest doses of T-705 also provided significant protection. When compared at molar equivalence, there were no appreciable differences in the effect of the pyrazine derivative treatments on viral burden or ALT (Table 2).
Figure 1. Effect of T-705 and T-1106 treatment on survival outcome of advanced PTV infections in hamsters.
Daily doses of 100, 50, or 25 mg/kg of T-1106, T-705, or 0.4% CMC placebo were given p.o., twice per day for 6 days, starting 24 h after virus challenge. Treatments groups, n=10; placebo, n=20. *P< 0.05, **P<0.01, ***P < 0.001 compared to placebo-treated animals. Based on molar conversion of the data, regression analysis, and extrapolation, the ED50 for T-705 is 112 µmoles/kg/day, and the ED50 for T-1106 is 12 µmoles/kg/day.
Table 2.
Effect of oral T-705 and T-1106 on PTV infection in hamsters.
| Disease parametera | ||||
|---|---|---|---|---|
| Dosage | Mean virus titerb ± SD | |||
| Treatment | mg/kg/day / µmoles/kg |
Liver | Serum | ALTc ± SD |
| T-705 | 100 / 637 | < 3.9 ± 1.5 (40)* | < 3.1 ± 1.9 (40) | 38 ± 19 |
| 50 / 318 | < 3.6 ± 1.7 (40)* | < 4.9 ± 2.5 (20) | 52 ± 79 | |
| 25 / 159 | < 5.9 ± 1.8 (80) | < 5.4 ± 3.4 (60) | 820 ± 1148 | |
| T-1106 | 100 / 371 | < 4.3 ± 2.1 (40) | < 3.6 ± 2.6 (40) | 446 ± 959 |
| 50 / 186 | < 4.9 ± 1.5 (80) | < 3.9 ± 2.2 (40) | 802 ± 1149 | |
| 25 / 93 | < 4.6 ± 1.7 (80)* | < 4.8 ± 2.9 (60) | 251 ± 438 | |
| 0.4 % CMC | - | 7.2 ± 1.4 (100) | < 4.8 ± 3.4 (100) | 779 ± 1044 |
| Sham-infected | - | < 2.8 (0) | < 1.8 (0) | 63 ± 55 |
Determined on day 4 of infection; 5 hamsters per treatment group.
Log10 cell culture 50% infectious dose (CCID50)/g of liver or ml of serum. Percentage of animals presenting with detectable virus levels are indicated in parentheses.
Alanine aminotransferase; measured in international units per liter.
P< 0.05 compared to 0.4% CMC placebo-treated hamsters.
3.3. Efficacy of pyrazine derivatives in PTV-challenged mice
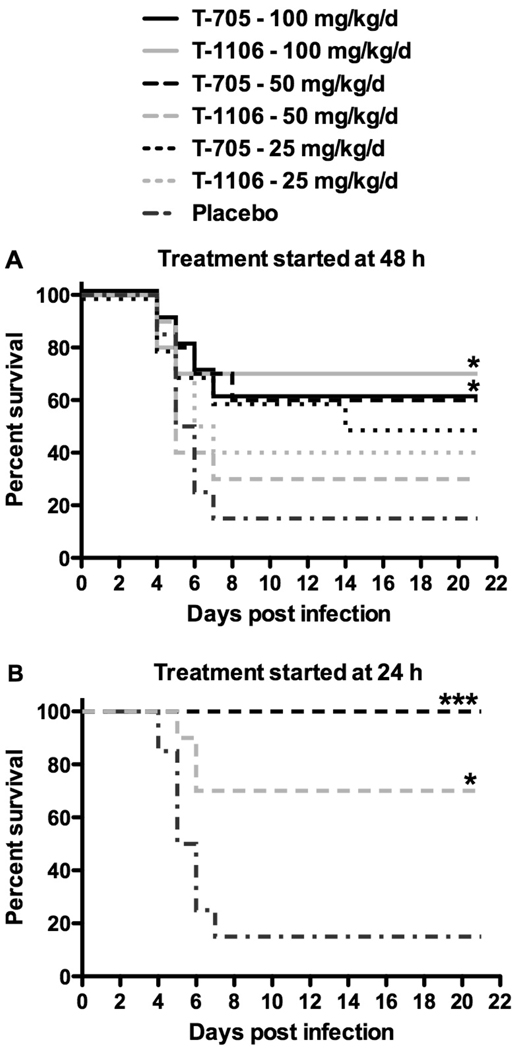
We next examined the efficacy of T-705 and T-1106 treatments in mice challenged with PTV, as there are distinct differences in disease pathogenesis between the mouse and hamster PTV infection models. In mice, pathology is more pronounced in the white pulp of the spleen, whereas severe inflammation and lesions are observed in the red pulp in hamsters (Anderson et al., 1990; Pifat and Smith, 1987). Moreover, significant enteritis is observed in PTV-infected hamsters, but not mice. As seen in Figure 2A, with the exception of the highest dose (100 mg/kg/day) treatment, T-705 appeared to be slightly more effective than T-1106 by mg/kg comparison at improving survival outcome when initiating therapy 2 days after challenge. When we compared effectiveness based on molar equivalence, the ED50 was 159 µmoles/kg/day for T-705 and 201 µmoles/kg/day for T-1106. Included as a positive control, the intermediate dose of 50 mg/kg/day of T-705 started on day 1 of infection was also more efficacious than the same mg/kg T-1106 treatment (Figure 2B). Nevertheless, the molar equivalents for the 50-mg/kg/day doses are 318 moles/kg/day of T-705 and 186 moles/kg/day for T-1106, and therefore, comparison at this level cannot be made.
Figure 2. Effect of oral T-705 and T-1106 treatment on survival outcome of PTV-infected mice.
A) T-705 and T-1106 (100, 50 and 25 mg/kg/day) or placebo was given p.o. 48 h after virus challenge twice per day for 5 days. B) As positive controls, T-705 and T-1106 (50 mg/kg/day) were also given p.o. 24 h after virus challenge twice per day for 5 days. Treatments groups, n=10; placebo, n=20. *P < 0.05; **P < 0.01 ***P < 0.001 compared to placebo-treated mice. Based on molar conversion of the data, regression analysis, and interpolation, the ED50 for T-705 is 159 µmoles/kg/day, and the ED50 for T-1106 is 201 µmoles/kg/day.
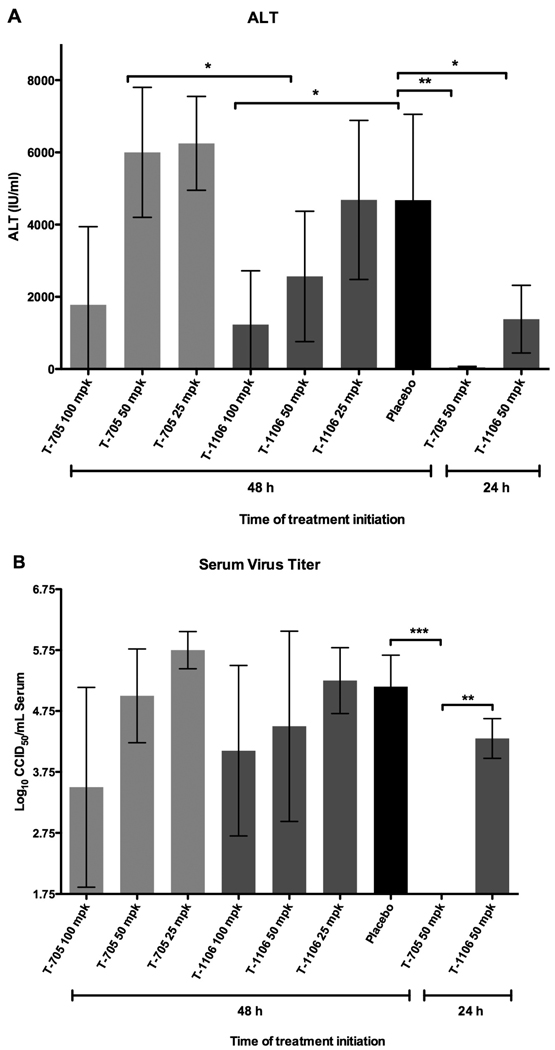
As previously seen in the mouse PTV infection model (Gowen et al., 2007a), only low-level infectious liver virus was detectable in a small percentage of the placebo-treated mice sacrificed on day 3 of infection (data not shown) despite clear evidence of pronounced liver dysfunction (high ALT levels) seen in the placebo-treated animals (Figure 3A). Consequently, there was little to no liver virus in the drug-treated animals (data not shown). Notably, there did appear to be an improvement in ALT in mice treated with T-1106, as compared to T-705, when treatment was delayed until 48 h (Figure 3A). If considered in the context of molar equivalence, the impact of T-1106 is even more pronounced. It is difficult to draw any conclusions from the 24 h ALT comparison since 42% less moles of T-1106 were used. T-705 and T-1106 serum viral load reductions for the 48 h treatment groups were comparable at the mg/kg level (Figure 3B), suggesting that on a per mole basis, T-1106 was superior. When the treatment was initiated at 24 h, T-705 was significantly better than the equivalent mg/kg treatment regimen of T-1106 at reducing systemic viral load (Figure 3B). Nevertheless, as alluded to above, unequal comparison at the molar level complicates the interpretation of this result.
Figure 3. Disease parameter assessment following treatment of PTV-infected mice with oral T-705 and T-1106.
Mice were treated as described in Figure 2. Serum ALT and virus titer are shown for 5 animals per group sacrificed on day 3 of infection. *P< 0.05, **P<0.01, ***P < 0.001. mpk, mg/kg/day. Molar conversions: 100, 50, and 25 mg are equal to 637, 318, and 159 µmoles of T-705, and 371, 186, and 93 µmoles of T-1106, respectively.
3.4. Efficacy of pyrazine derivatives in arenavirus challenged hamsters and cell culture
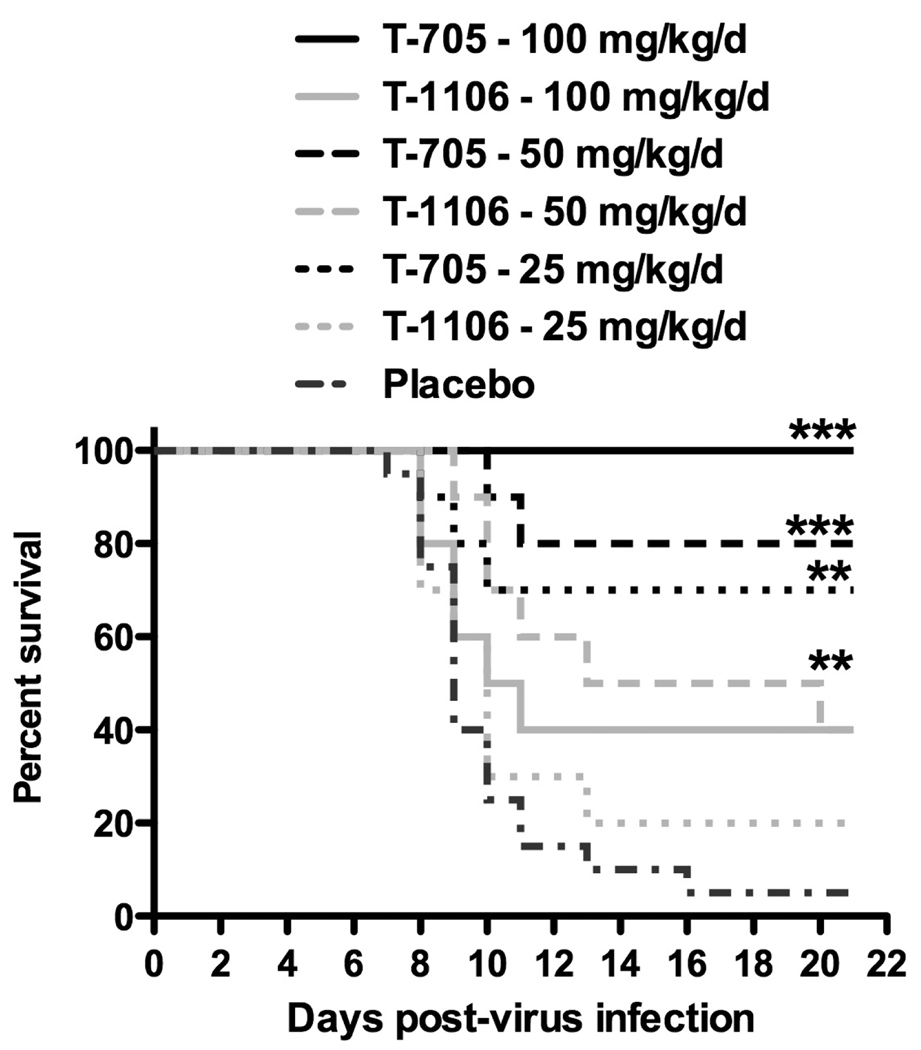
To compare efficacy in a hamster infection model wherein disease is based on a diffuse systemic viral infection, T-705 and T-1106 were evaluated for their ability to treat PICV infection in hamsters when initiating treatment on day 4 of the infection. We have previously reported T-705 to be effective against PICV (Gowen et al., 2008a; Gowen et al., 2007a). Treatment with 100 mg/kg/day, starting 4 or 5 days post virus challenge protected 100% of infected animals (Gowen et al., 2008a). As expected, the 100-mg/kg/day dose of T-705 offered complete protection against an LD95 challenge dose of PICV (Figure 4). In contrast, the equivalent mg/kg T-1106 dose failed to significantly protect infected animals, as determined by log-rank analysis (P = 0.06), yet it clearly had a beneficial effect, as 4 of the 10 challenged hamsters survived the infection. At the intermediate dose of 50 mg/kg/day, T-705 protected 80% of the animals, whereas T-1106 once again protected 40% (Figure 4). At the lowest dose of 25 mg/kg/day, T-705 protected 70% of the infected hamsters, while only 20% survived in the T-1106-treated group. At all doses, T-705 significantly protected the animals, with reduced efficacy evident as the treatment dose was decreased. On the other hand, T-1106 was inferior in its ability to offer complete protection, although the 50-mg/kg/day dose, and to a lesser degree the 100 mg/kg/day dose, were found to reduce mortality and improve survival outcome (Figure 4). Even taking in to account the molar disparity associated with the direct mg/kg comparison between the two compounds, T-705 (ED50 = 72 µmoles/kg/day) was still 6.1 times more potent than T-1106 (ED50 = 441 µmoles/kg/day) in the PICV hamster model system.
Figure 4. Survival outcome following treatment of PICV-infected hamsters with T-705, T-1106 or placebo.
Drugs were given orally twice per day for 7 days. All treatments were initiated on day 4. T-705 and T-1106 were administered at daily doses of 100, 50 or 25 mg/kg. Treatments groups, n=10; placebo, n=20. **P<0.01, ***P < 0.001 compared to placebo-treated animals. Based on molar conversion of the data, regression analysis, and extrapolation, the ED50 for T-705 is 72 µmoles/kg/day, and the ED50 for T-1106 is 441 µmoles/kg/day.
Both the 50- and 100-mg/kg/day doses of T-705 significantly limited viral burden in the serum and liver, as determined on day 7 of the infection (Table 3). These treatment regimens also reduced ALT below that observed for the placebo group. Despite lower viral titers and values for liver disease markers, the low-dose T-705 group and all of the T-1106 treatment groups were not found to be significantly different compared to the placebo-treated group (Table 3). Notably, the 100-mg/kg/day dose of T-1106 (molar equivalent dose of 371 µmoles/kg/day) was clearly less effective than the 50-mg/kg/day dose of T-705 (molar equivalent dose of 318 µmoles/kg/day). It was only possible to perform accurate regression analysis on ALT based on molar dose response. T-705 was 2.6-fold more potent that T-1106 in this regard. In cell culture studies, the pattern of inhibition against a panel of arenavirueses (TCRV, PICV, JUNV) was similar to that seen with the phleboviruses, wherein T-705 had greater potency (Table 4).
Table 3.
Effect of oral T-705 and T-1106 on PICV infection in hamsters.
| Disease parametera | ||||
|---|---|---|---|---|
| Dosage | Mean virus titerb ± SD | |||
| Treatment | mg/kg/day / µmoles/kg |
Liver | Serum | ALTc ± SD |
| T-705 | 100 / 637 | < 3.3 ± 0.5 (60)** | < 1.8 (0)** | 25 ± 10** |
| 50 / 318 | 5.8 ± 1.1 (100)** | < 3.8 ± 1.6 (80)** | 199 ± 180** | |
| 25 / 159 | 7.6 ± 0.8 (100) | 6.6 ± 1.0 (100) | 859 ± 782 | |
| T-1106 | 100 / 371 | 7.4 ± 0.4 (100) | 6.5 ± 1.0 (100) | 961 ± 1071 |
| 50 / 186 | 7.1 ± 1.5 (100) | < 5.4 ± 2.1 (80) | 1026 ± 1289 | |
| 25 / 93 | 7.7 ± 0.6 (100) | 6.5 ± 1.1 (100) | 1354 ± 961 | |
| 0.4 % CMC | - | 8.2 ± 0.6 (100) | > 7.3 ± 1.1 (100) | 1742 ± 953 |
| Sham-infected | - | < 2.8 (0) | < 1.8 (0) | 18 ± 4 |
Determined on day 7 of infection; 5 hamsters per treatment group.
Log10 cell culture 50% infectious dose (CCID50)/g of liver or ml of serum. Percentage of animals presenting with detectable virus levels are indicated in parentheses.
Alanine aminotransferase; measured in international units per liter.
P< 0.01 compared to 0.4% CMC placebo-treated hamsters.
Table 4.
In vitro inhibitory effects of T-705 and T-1106 against arenavirusesa.
| Virus | Assay b | T-705 c | T-1106 c | ||||
|---|---|---|---|---|---|---|---|
| CC50 ± SD | EC50/90 ± SD | SI d | CC50 ± SD | EC50/90 ± SD | SI d | ||
| TCRV | CPER | >4859 ± 2033 | 26 ± 13 | >187 | >3715 ± 0 | 619 ± 205 | >6 |
| VYR | 12 ± 4 | >405 | 296 ± 273 | >13 | |||
| JUNV | CPER | >5411 ± 832 | 19 ± 5 | >285 | >3715 ± 0 | 128 ± 44 | >29 |
| VYR | 14 ± 3 | >387 | 114 ± 28 | >33 | |||
| PICV | CPER | 4859 ± 409 | 25 ± 6 | 194 | >3715 ± 0 | 154 ± 39 | >24 |
| VYR | 24 ± 5 | 202 | 173 ± 93 | >21 | |||
Data are the mean and standard deviations from 3-6 separate experiments in Vero (JUNV and PICV) or Vero 76 (TCRV) cells.
Cytopathic effect reduction (CPER) based on neutral red dye uptake by viable cells; virus yield reduction (VYR).
CC50 and EC50/90 values are in µM.
Selectivity index (SI) = CC50/EC50/90.
4. Discussion
The objective of the present study was to compare the efficacy of T-705 against hepatotropic phleboviral disease to that of the related pyrazine derivative, T-1106. The latter has been previously reported to have superior activity to that of T-705 in the YFV hamster infection model (Julander et al., 2009). The current hypothesis is that T-1106 may biodistribute preferentially to the liver, and thereby is more effective against viral diseases that principally target the liver. Studies in rats support this premise, as liver tissue:plasma ratios for T-1106 are ~50-fold greater than those observed for T-705 (Y. Furuta, unpublished data). Comprehensive analyses of T-1106 and T-705 tissue distribution, concentration, and conversion to the active triphosphate forms in infected and uninfected hamsters and mice are needed to definitively address the question in the context of the PTV and PICV infection models.
It would be of interest to evaluate the inhibitory activity of the pyrazine derivatives in hepatocytes and cells of the myelomonocytic lineage, as the latter are likely targets for initial RVFV and PTV infection and dissemination to the liver (Anderson et al., 1990; Geisbert and Jahrling, 2004). In addition, analysis of the conversion of T-705 and T-1106 to their active triphosphate forms in different cell types may provide insights into the differences in efficacy observed against viral disease that differ in their capacity to produce severe liver disease. In cell culture experiments based on infection of Vero cells, a similar inhibition pattern was seen when comparing the activity profiles of both compounds against selected phleboviruses and arenaviruses, likely due to similar mechanisms of action. It will be important to perform the aforementioned studies to investigate whether the enhanced potency of T-705 in Vero cell types will be borne out in studies employing more relevant primary cell types.
On a mg/kg basis, the improved T-1106 efficacy observed in the PTV hamster phlebovirus infection model was not as dramatic as the protection offered by T-705 in the PICV hamster arenavirus infection model. However, when compared on a molar basis, this comparison shifted towards T-1106 having the more profound difference in efficacy in the PTV-infected hamsters. Although T-705 appeared to be more effective at reducing day-4 hepatic viral burden and limiting liver disease as measured by ALT, at the molar level, and due to dose response variability, the differences were not appreciable. It is conceivable that at this stage in the infection and disease process that the differences that ultimately dictate survival are not resolved. This was not the case in the PICV-infected hamsters, as the disease parameters assessed on day 7 corroborated the improved efficacy seen in the context of survival.
Collectively, the data support the idea that T-705 may be a better treatment option for more diffuse viral infections such as the characteristically pantropic arenaviral infections, whereas viral disease where liver involvement is more prominent may respond better to T-1106. Recent studies employing the YFV hamster model also suggest that T-1106 is more effective than T-705 for treating what is arguably the most severe and dramatic virally-induced liver disease model (Julander et al., 2009; Sbrana et al., 2004; Tesh et al., 2001; Xiao et al., 2001). Further studies investigating the efficacy of these pyrazine derivatives in small animal models based on RVFV infection are needed. This need is underscored by the fact that in cell culture studies we found RVFV to be ~5 times more sensitive to the inhibitory activity of T-1106.
Notably, hamsters are much more sensitive to PTV infection compared to mice, as considerably lower (~650 CCID50 less) infectious virus is required to produce lethal disease. In hamsters challenged with PTV, animals present with significant liver infections as reflected by 8–9 log10 CCDID50/g of liver tissue viral burdens (Gowen et al., 2008b). In contrast, mice present with 4–5 log10 less infectious viral loads (Gowen et al., 2006a; Gowen et al., 2006b; Gowen et al., 2007a). Based on these findings, we suspect that liver virus is controlled by day 3, but cytokine storm-driven immunopathological events leads to the pronounced hepatocellular necrosis associated with PTV infection of mice. The fact that infectious liver viral loads do not appear to be a major feature of PTV infection in mice may explain why, unlike in hamsters infected with PTV, the effectiveness T-1106 was less dramatic. The more predominant systemic infection of mice with PTV could explain why T-705 was as effective or better than T-1106 for the treatment of disease in the mouse model. Further characterization of PTV infection in mice is necessary to better understand the nature of the severe liver disease, which is characterized by excessively high ALT concentrations and massive hepatocellular necrosis (Pifat and Smith, 1987), despite only limited infectious viral burden.
Acknowledgements
We thank Heather Greenstone and Peter Silvera for critical review of the manuscript. We also thank Kevin Bailey and Deanna Larsen for technical support.
This work was supported by contract grant NO1-AI-15435, NO1-AI-30048, and NO1-AI-30063 (awarded to Southern Research Institute) from the Virology Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
References
- Amacher DE. Serum transaminase elevations as indicators of hepatic injury following the administration of drugs. Regul. Toxicol. Pharmacol. 1998;27:119–130. doi: 10.1006/rtph.1998.1201. [DOI] [PubMed] [Google Scholar]
- Anderson GW, Jr, Slayter MV, Hall W, Peters CJ. Pathogenesis of a phleboviral infection (Punta Toro virus) in golden Syrian hamsters. Arch. Virol. 1990;114:203–212. doi: 10.1007/BF01310749. [DOI] [PubMed] [Google Scholar]
- Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB, Ksiazek T, Johnson KM, Meyerhoff A, O'Toole T, Ascher MS, Bartlett J, Breman JG, Eitzen EM, Jr, Hamburg M, Hauer J, Henderson DA, Johnson RT, Kwik G, Layton M, Lillibridge S, Nabel GJ, Osterholm MT, Perl TM, Russell P, Tonat K. Hemorrhagic fever viruses as biological weapons: medical and public health management. JAMA. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- Fisher AF, Tesh RB, Tonry J, Guzman H, Liu D, Xiao SY. Induction of severe disease in hamsters by two sandfly fever group viruses, Punta toro and Gabek Forest (Phlebovirus, Bunyaviridae), similar to that caused by Rift Valley fever virus. Am. J. Trop. Med. Hyg. 2003;69:269–276. [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, Nomura N, Egawa H, Minami S, Watanabe Y, Narita H, Shiraki K. In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob. Agents Chemother. 2002;46:977–981. doi: 10.1128/AAC.46.4.977-981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, Gowen BB, Julander JG, Morrey JD. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat. Med. 2004;10:S110–S121. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- Gowen BB, Fairman J, Dow S, Troyer R, Wong MH, Jung KH, Melby PC, Morrey JD. Prophylaxis with cationic liposome-DNA complexes protects hamsters from phleboviral disease: importance of liposomal delivery and CpG motifs. Antiviral Res. 2009;81:37–46. doi: 10.1016/j.antiviral.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Fairman J, Smee DF, Wong MH, Jung KH, Pace AM, Heiner ML, Bailey KW, Dow SW, Sidwell RW. Protective immunity against acute phleboviral infection elicited through immunostimulatory cationic liposome-DNA complexes. Antiviral Res. 2006a;69:165–172. doi: 10.1016/j.antiviral.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Gowen BB, Holbrook MR. Animal models of highly pathogenic RNA viral infections: Hemorrhagic fever viruses. Antiviral Res. 2008;78:79–90. doi: 10.1016/j.antiviral.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Gowen BB, Smee DF, Wong MH, Hall JO, Jung KH, Bailey KW, Stevens JR, Furuta Y, Morrey JD. Treatment of late stage disease in a model of arenaviral hemorrhagic Fever: T-705 efficacy and reduced toxicity suggests an alternative to ribavirin. PLoS ONE. 2008a;3:e3725. doi: 10.1371/journal.pone.0003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Smee DF, Wong MH, Judge JW, Jung KH, Bailey KW, Pace AM, Rosenberg B, Sidwell RW. Recombinant Eimeria protozoan protein elicits resistance to acute phlebovirus infection in mice but not hamsters. Antimicrob. Agents Chemother. 2006b;50:2023–2029. doi: 10.1128/AAC.01473-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Blatt LM, Sidwell RW. Prophylactic and therapeutic intervention of Punta Toro virus (Phlebovirus, Bunyaviridae) infection in hamsters with interferon alfacon-1. Antiviral Res. 2008b;77:215–224. doi: 10.1016/j.antiviral.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, Furuta Y, Sidwellz RW. In Vitro and In Vivo Activities of T-705 against Arenavirus and Bunyavirus Infections. Antimicrob. Agents Chemother. 2007a;51:3168–3176. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen BB, Wong MH, Jung KH, Sanders AB, Mitchell WM, Alexopoulou L, Flavell RA, Sidwell RW. TLR3 is essential for the induction of protective immunity against Punta Toro Virus infection by the double-stranded RNA (dsRNA), poly(I:C12U), but not Poly(I:C): differential recognition of synthetic dsRNA molecules. J. Immunol. 2007b;178:5200–5208. doi: 10.4049/jimmunol.178.8.5200. [DOI] [PubMed] [Google Scholar]
- Huggins JW. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad-spectrum antiviral drug. Rev. Infect. Dis. 1989;11 Suppl 4:S750–S761. doi: 10.1093/clinids/11.supplement_4.s750. [DOI] [PubMed] [Google Scholar]
- Julander JG, Shafer K, Smee DF, Morrey JD, Furuta Y. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob. Agents Chemother. 2009;53:202–209. doi: 10.1128/AAC.01074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrey JD, Taro B, Siddharthan V, Wang H, Smee DF, Christensen AJ, Furuta Y. Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antiviral Res. 2008 doi: 10.1016/j.antiviral.2008.07.009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrill JC, McClain DJ. Epidemiology and pathogenesis of the Rift Valley fever and other phleboviruses edn. New York and London: Plenum Press; 1996. [Google Scholar]
- Peters CJ, LeDuc JW. Bunyaviruses, phleboviruses, and related viruses. In: Belshe RB, editor. Textbook of human virology, edn. Littleton: PSG Publishing Co.; 1984. pp. 547–598. [Google Scholar]
- Peters CJ, Meegan JM. Rift Valley Fever. In: Beran GW, editor. Handbook series of zooneses, edn. Boca Raton: CRC Press; 1981. pp. 403–420. [Google Scholar]
- Peters CJ, Reynolds JA, Slone TW, Jones DE, Stephen EL. Prophylaxis of Rift Valley fever with antiviral drugs, immune serum, an interferon inducer, and a macrophage activator. Antiviral Res. 1986;6:285–297. doi: 10.1016/0166-3542(86)90024-0. [DOI] [PubMed] [Google Scholar]
- Pifat DY, Smith JF. Punta Toro virus infection of C57BL/6J mice: a model for phlebovirus-induced disease. Microb. Pathog. 1987;3:409–422. doi: 10.1016/0882-4010(87)90011-8. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Sbrana E, Xiao SY, Guzman H, Ye M, Travassos da Rosa AP, Tesh RB. Efficacy of post-exposure treatment of yellow fever with ribavirin in a hamster model of the disease. Am. J. Trop. Med. Hyg. 2004;71:306–312. [PubMed] [Google Scholar]
- Sidwell RW, Barnard DL, Day CW, Smee DF, Bailey KW, Wong MH, Morrey JD, Furuta Y. Efficacy of Orally Administered T-705 on Lethal Avian Influenza A (H5N1) Virus Infections in Mice. Antimicrob. Agents Chemother. 2007;51:845–851. doi: 10.1128/AAC.01051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidwell RW, Huffman JH, Barnett BB, Pifat DY. In vitro and in vivo Phlebovirus inhibition by ribavirin. Antimicrob. Agents Chemother. 1988;32:331–336. doi: 10.1128/aac.32.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee DF, Huffman JH, Gessaman AC, Huggins JW, Sidwell RW. Prophylactic and therapeutic activities of 7-thia-8-oxoguanosine against Punta Toro virus infections in mice. Antiviral Res. 1991;15:229–239. doi: 10.1016/0166-3542(91)90069-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh RB, Guzman H, da Rosa AP, Vasconcelos PF, Dias LB, Bunnell JE, Zhang H, Xiao SY. Experimental yellow fever virus infection in the Golden Hamster (Mesocricetus auratus). I. Virologic, biochemical, and immunologic studies. J. Infect. Dis. 2001;183:1431–1436. doi: 10.1086/320199. [DOI] [PubMed] [Google Scholar]
- Xiao SY, Zhang H, Guzman H, Tesh RB. Experimental yellow fever virus infection in the Golden hamster (Mesocricetus auratus). II. Pathology. J. Infect. Dis. 2001;183:1437–1444. doi: 10.1086/320200. [DOI] [PubMed] [Google Scholar]