Abstract
Although arterial limb tourniquet is one of the first-line treatments to prevent exsanguinating hemorrhage in both civilian pre-hospital and battlefield casualty care, prolonged application of a limb tourniquet can lead to serious ischemia-reperfusion injury. However, the underlying pathomechanisms of tourniquet-induced ischemia-reperfusion injury are still poorly understood. Using a murine model of acute limb ischemia-reperfusion, we investigated if acute limb ischemia-reperfusion injury is mediated by superoxide overproduction and mitochondrial dysfunction. Hind limbs of C57/BL6 mice were subjected to 3 h ischemia and 4 h reperfusion via placement and release of a rubber tourniquet at the greater trochanter. Approximately 40% gastrocnemius muscle suffered infarction in this model. Activities of mitochondrial electron transport chain complexes including complex I, II, III, and IV in gastrocnemius muscle were decreased in the ischemia-reperfusion group compared to sham. Superoxide production was increased while activity of manganese superoxide dismutase (MnSOD, the mitochondria-targeted SOD isoform) was decreased in the ischemia-reperfusion group compared to sham group. Pretreatment with tempol (a SOD mimetic, 50 mg/kg) or co-enzyme Q10 (50 mg/kg) not only decreased the superoxide production, but also reduced the infarct size and normalized mitochondrial dysfunction in the gastrocnemius muscle. Our results suggest that tourniquet-induced skeletal muscle ischemia-reperfusion injuries including infarct size and mitochondrial dysfunction may be mediated via the superoxide over-production and reduced antioxidant activity. In the future, this murine ischemia-reperfusion model can be adapted to mechanistically evaluate anti-ischemic molecules in tourniquet-induced skeletal muscle injury.
Keywords: Infarct size, Ischemia-reperfusion injury, Mitochondria, Superoxide, Tourniquet
1. Introduction
Much evidence has indicated that hemorrhage from extremity injuries is as the most common cause of preventable death in both civilian and battlefield medicine (Holcomb et al., 2007; Keel and Trentz, 2005). The use of arterial tourniquet as a means to achieve hemostasis is clinically common in both trauma and other clinical settings (Beekley et al., 2008; Doyle and Taillac, 2008; Lee et al., 2007). Over 20,000 tourniquet applications are performed daily worldwide to produce bloodless operating fields in orthopedic, musculoskeletal reconstructive procedures, and vascular surgeries (Hammers et al., 2008). However, it has been found that tourniquets also induced many complications including ischemia-reperfusion injury, which greatly limits the clinical utilization of the tourniquet. Evidence from animal experiments showed that two hours of tourniquet-induced ischemia led to high levels of both lactic acid and creatine phosphokinase, suggesting muscle damage was occurring (Heppenstall et al., 1979). Most of clinical studies support that no more than 60–90 minutes of operative tourniquet are acceptable to safely use this technique (Doyle and Taillac, 2008). Recent military experience also support the safety of these short tourniquet times for prehospital patients (Bellamy, 1984; Lakstein et al., 2003; Sebesta, 2006). We clearly understand that longer tourniquet time is needed in many emergency cases for effective life saving. Therefore, it is very important to explore the mechanism(s) responsible to the tourniquet-induced ischemia-reperfusion injury and to discover the useful strategies for preventing the skeletal muscle from the ischemic reperfusion injury.
Ischemia-reperfusion injury has been characterized by necrosis and mitochondrial dysfunction. Although the mechanism(s) underlying the ischemia-reperfusion injury are complex and not thoroughly understood, some studies have pointed to mitochondria as the central players in cell survival, not only because of their roles in energy production for the cell but also because of their involvement in the generation of reactive oxygen species (including superoxide) and the regulation of programmed cell death in cardiac myocytes (Honda et al., 2005; Murphy and Steenbergen, 2008). Using a validated model of tourniquet-induced-acute murine hindlimb ischemia-reperfusion (Crawford et al., 2007), this study investigated the prophylactic effects of co-enzyme Q10 and superoxide dismutase mimetic (tempol) on the ischemia-reperfusion-induced skeletal muscle infarct size and mitochondrial dysfunction to clarify if superoxide overproduction leads to skeletal muscle ischemia-reperfusion injury, including mitochondrial dysfunction and cellular injury. Preliminary results from this study have been published in an abstract form (Tu et al., 2009).
2. Material and methods
2.1. Animals
Male C57BL6 mice (10–12 weeks of age, 22–35 g, n=108, Charles River Laboratory) were housed under controlled temperature and humidity and a 12:12-h dark-light cycle, and were provided water and mouse chow ad libitum. Experiments were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and were carried out in accordance with the National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.2. Drug treatments
Mice were assigned randomly to one of four groups: sham, ischemia-reperfusion, tempol plus ischemia-reperfusion, and co-enzyme Q10 plus ischemia-reperfusion. Thirty minutes before tourniquet or sham procedure, mouse was intraperitoneally administered vehicle (sham and ischemia-reperfusion groups) or tempol (Alexis Biochemicals Co., CA, USA; 4-hydroxy-2,2,6,6-tetramethyl-piperidinyloxy, a superoxide dismutase mimetic, 50 mg/kg). In the group of co-enzyme Q10 plus ischemia-reperfusion, mouse was intraperitoneally treated with co-enzyme Q10 (MP Biomedicals, OH, USA; 50 mg/kg) at 24 h and 2 h before tourniquet, which based on the uptake and distribution of co-enzyme Q10 (Miles, 2007).
2.3. Acute hindlimb ischemia-reperfusion model
Mice were anesthetized with an anesthetic cocktail consisting of 0.1 mg/g ketamine and 0.01 mg/g xylazine, given as an intraperitoneal injection (0.01 ml/g body weight). The level of anesthesia was continuously monitored by observing the respiratory patterns and toe pinch reflex. Anesthesia was maintained throughout the duration of experiments with additional anesthetic cocktail (0.1 ml) as needed. After the induction of anesthesia, fur was completely removed from both hind limbs with an electric shaver to facilitate the measurement of limb perfusion. The animals were restrained on a heating pad to maintain body temperature at 37°C.
Unilateral hind limb ischemia was induced by placing an orthodontic rubber band at the hip joint using a McGivney hemorrhoidal ligator (Crawford et al., 2007). After 3 h ischemia, the orthodontic rubber band tourniquet was released and the hindlimb underwent 4 h reperfusion. Sham-operated animals were subjected to the same procedure except for the application of the orthodontic rubber band (i.e., no ischemia). During the entire procedure, mice were kept hydrated with intraperitoneal injection of 0.2 ml normal saline every 2 h.
2.4. Skeletal muscle blood flow measurement
Skeletal muscle blood flow was measured by planting a transonic flow probe in the gastrocnemius muscles. The probe was connected to a Transonic Laser Doppler blood flowmeter (BLF21, Transonic System Inc) and PowerLab 8/30 (ADInstruments). Blood flow was recorded by Labchart 6 at baseline and after the application and release of tourniquet.
2.5. Measurement of infarct size
Triphenyl tetrazolium chloride assay was used to measure infarct size of skeletal muscle induced by ischemia-reperfusion (Lie et al., 1971). At the end of the ischemia-reperfusion protocol (3 h ischemia and 4 h reperfusion), gastrocnemius muscles from sham and ischemia-reperfusion animals were immediately harvested and washed with 0.9% normal saline. After the adherent fascias, fat, and tendons were removed, the muscles were cut into 1.5-mm transverse slices using a sharp knife. These thin slices were again washed free of blood with cold normal saline, blotted dry with paper towels, and incubated in 1% triphenyl tetrazolium chloride (Sigma-Aldrich, St. Louis, MO) solution at room temperature for 1 h. The slice images were digitalized and the muscles were separated into viable skeletal muscles with a dark purple-red color stain and the infarcted muscles with pale brown color stain. Infarct size in each slice was quantified using Adobe Photoshop CS3 (Photoshop Extended, Adobe Systems Incorporated, CA, USA) and reported as the ratio (%) of the infarcted muscle to total gastrocnemius muscles (viable plus infarcted muscles).
2.6. Spectrophotometric electron transport chain measurements
Assays were performed in gastrocnemius muscle homogenates using a spectrophotometer (DU Series 640; Beckman Instruments, Fullerton, CA). Complex I (NADH dehydrogenase) enzyme activity was measured as a function of the decrease in absorbance from NADH oxidation by decylubiquinone before and after rotenone addition (Birch-Machin et al., 1994). The specific activity of complex I reported in this study is rotenone-sensitive NADH dehydrogenase (ubiquinone) activity. Complex II (succinate dehydrogenase) activity was measured as a function of the decrease in absorbance from 2, 6-dichloroindophenol reduction (Birch-Machin et al., 1994). Complex III (ubiquinol cytochrome c oxidoreductase) activity was determined as a function of the increase in absorbance from cytochrome c reduction (Krahenbuhl et al., 1994). The nonenzymatic reduction in cytochrome c was measured under the same conditions after the addition of antimycin A and was subtracted from the total activity of complex III to calculate the activity specifically due to complex III. Complex IV (cytochrome c oxidoreductase) activity was determined as a function of the decrease in absorbance from cytochrome c oxidation (Birch-Machin et al., 1994). Specificity of the respiratory complexes activities was determined by monitoring changes in absorbance in the presence of the specific inhibitors: complex I (rotenone, 5 µM), complex II (malonate, 10 mM), complex III (antimycin A, 5 µM), and complex IV (KCN, 2 mM). The nmol/min/unit of citrate synthase was used to express the activities of complex I, II, and III; whereas k/min/unit of citrate synthase was used to present the activity of complex IV, where k is the first-order velocity constant {k=[2.3 log A (timeo)/A (time0 + 1 min)]/min}. Citrate synthase activity was measured spectrophotometrically at 412 nm from whole muscle homogenates as previously described (Srere, 1969).
2.7. In situ detection of superoxide anion production
In situ detection of superoxide anion production was performed as described previously (Li et al., 2007). Fresh gastrocnemius muscles were frozen and cut into 20 µM-thick sections and arranged in separate wells in a 24-well plate bathed in PBS solution with 5 µM dihydroethidium (Molecular Probes, Carlsbad, CA). Plates were incubated in the dark at 37°C for 30 min. After being placed on glass slides, the muscle slices were observed under a Leica fluorescent microscope with appropriate excitation/emission filters. Pictures were captured using a digital camera system.
2.8. Measurement of superoxide anion production
Superoxide anion production was measured in gastrocnemius muscle homogenates using the lucigenin chemiluminescence method described previously (Li et al., 2007). The homogenate (0.3 ml) was placed in 0.5 ml microfuge containing dark–adapted lucigenin (5 µM), and then read in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA). Light emission was recorded for 5 min and expressed as mean light units (MLU)/min/100 µg protein. Total protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce; Rockford, IL).
2.9. Western blot analysis for manganese superoxide dismutase (MnSOD)
After an acute ischemia-reperfusion experiment, gastrocnemius muscles were quickly harvested and stored in liquid nitrogen (−80°C) until analysis. At the time of analysis, stored gastrocnemius muscles were thawed and homogenized at 4°C. The protein was extracted with a lysing buffer (10 mM Tris, 1 mM EDTA, 1 % SDS, pH 7.4) plus a protease inhibitor cocktail (100 µl/ml). Following centrifugation at 12,000 g for 20 min at 4 °C, the protein concentration in the supernatant was determined using a BCA protein assay kit (Pierce Chemical, Rockford, IL). The protein sample was mixed with loading buffer containing β-mercaptoethanol and heated at 100°C for 5 min. Equal amounts of the protein were loaded. Protein was fractionated in a 10% polyacrylamide gel along with molecular weight standards and transferred to PVDF membrane. The membrane was probed with rabbit anti MnSOD antibody (Abcam, Cambridge, MA) and a peroxidase-conjugated rabbit anti-goat IgG (Pierce Chemical, Rockford, IL). The signal was detected using enhanced chemiluminescence substrate (Pierce Chemical, Rockford, IL) and the bands analyzed using UVP BioImaging Systems. The target protein was controlled by probing western blot with mouse anti-muscle actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and normalizing target protein intensity to that of actin.
2.10. MnSOD Activity measurement
MnSOD activity was measured by a modification of the nitrite method (Beauchamp and Fridovich, 1971; Elstner and Heupel, 1976; Oyanagui, 1984). Briefly, a SOD activity reference set (0, 6.25, 12.5, 25, 50, 100, 200, and 400 ng MnSOD/20 µl) was prepared with standard MnSOD (Sigma-Aldrich, St. Louis, MO). The unknowns were prepared in duplicates by adding muscle homogenates at dilution of 1/20 to the activity reference sets. The signal reduction of the reference and sample sets was plotted as a function of the concentration of MnSOD in the activity reference set. The shift of the X-intercept at the 50% end point of the sample curve relative to the 50% end point of the reference curve was a linear function (R2 = 0.997) of the concentration of MnSOD added to the reference set. Standard SOD activity units in each sample were computed as nanogram equivalents of standard MnSOD and normalized to citrate synthase.
2.11. Data analysis
All data are presented as mean ± S.E.M. SigmaStat 3.5 (Systat Software, Chicago, IL) was used for data analysis. A one-way ANOVA, with a Bonferroni procedure for post hoc was used in the comparison of all parameters. Normal distribution of data was confirmed with Kolmogorov-Smirnov test and equal variance with Levene’s test. Statistical significance was accepted when P<0.05.
3. Results
3.1. Identification of tourniquet-induced ischemia-reperfusion model
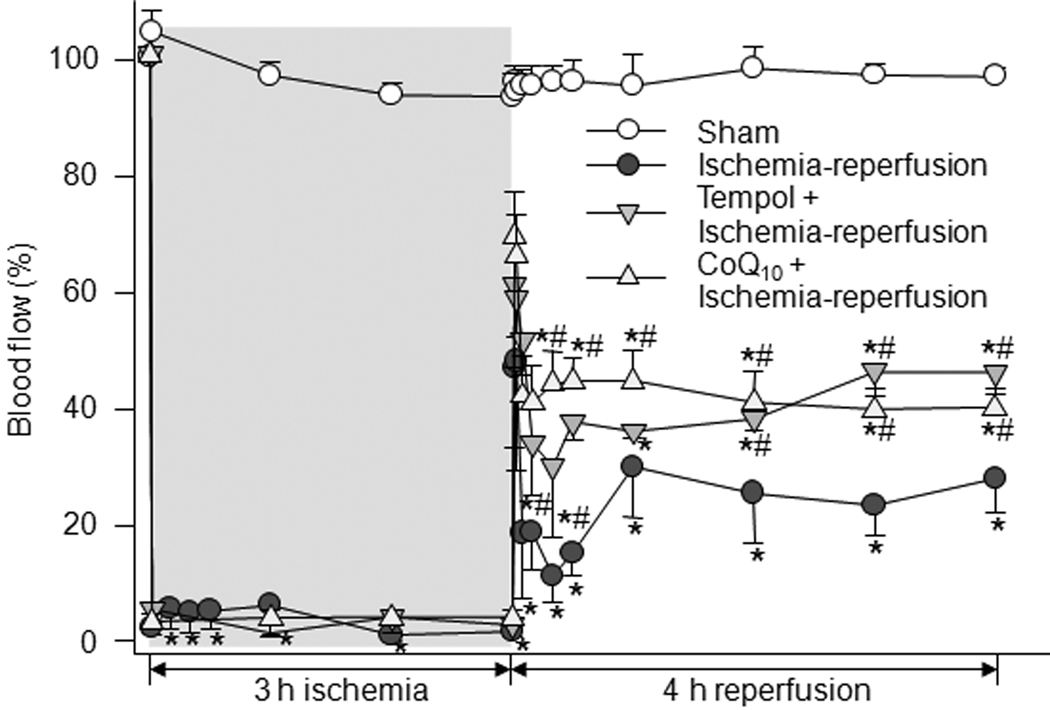
Although the use of tourniquets to induce limb ischemia in a rodent model is well published, we wished to verify muscle ischemia and reperfusion in this model by measuring blood flow to gastrocnemius muscle of the ischemic limb. Blood flow to gastrocnemius muscle during the ischemia-reperfusion protocol was measured by a Transonic Doppler flowmeter planted in the muscle belly. Blood flow data were summarized in Figure 1. Blood flow dropped to about 2% of baseline after placement of tourniquet and remained steady during 3 h ischemia. Upon tourniquet release which marks the beginning of the reperfusion phase, a rapid and transient increase in the blood flow to approximately 50% of baseline was observed, which was followed by a decline to a steady state of about 30% of baseline. In sham animals, there was no significant change of blood flow during 7 h ischemia-reperfusion protocol (Fig.1). Pretreatment with tempol (50 mg/kg) and co-enzyme Q10 (50 mg/kg) prior to ischemia significantly improved the blood flow during reperfusion (Fig. 1).
Figure 1.
Time courses of change in blood flow of gastrocnemius muscle in sham (n=8), tourniquet-induced ischemia-reperfusion (n=8), tempol (50 mg/kg) + ischemia-reperfusion (n=5), and CoQ10 (50 mg/kg) + ischemia-reperfusion (n=6) groups. CoQ10, co-enzyme Q10. Data are mean ± S.E.M. *P<0.05 vs. sham; #P<0.05 vs. ischemia-reperfusion group.
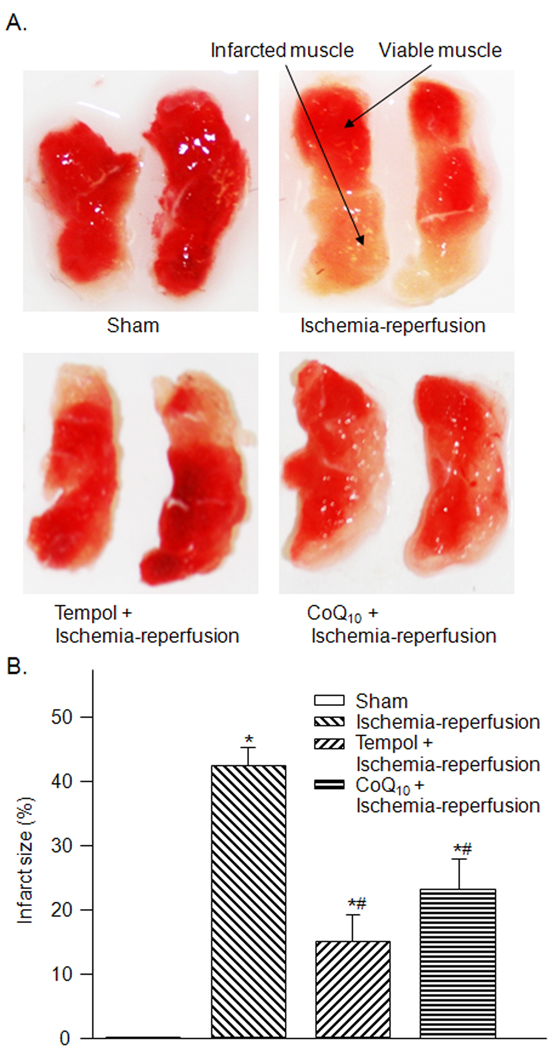
3.2. Infarct size in sham and tourniquet group
The mice in sham group suffered no skeletal muscle infarction as measured by triphenyl tetrazolium chloride histochemistry. Ischemia-reperfusion significantly increased the infarct size of gastrocnemius muscle (42.4 ± 2.7 %) compared to that in the sham group (P< 0.05, Fig. 2-A and -B). Tempol and co-enzyme Q10 markedly reduced, but did not abolish, the infarct size induced by ischemia-reperfusion (Fig. 2).
Figure 2.
The representative (A) and summary (B) data for infarct size in gastrocnemius muscles from sham (n=8), tourniquet-induced ischemia-reperfusion (n=10), tempol + ischemia-reperfusion (n=7), and CoQ10 + ischemia-reperfusion (n=6) groups. Infarct size is presented as the ratio (%) of infracted area to total gastrocnemius muscle. Data are mean ± S.E.M. *P<0.05 vs. sham; #P<0.05 vs. ischemia-reperfusion group.
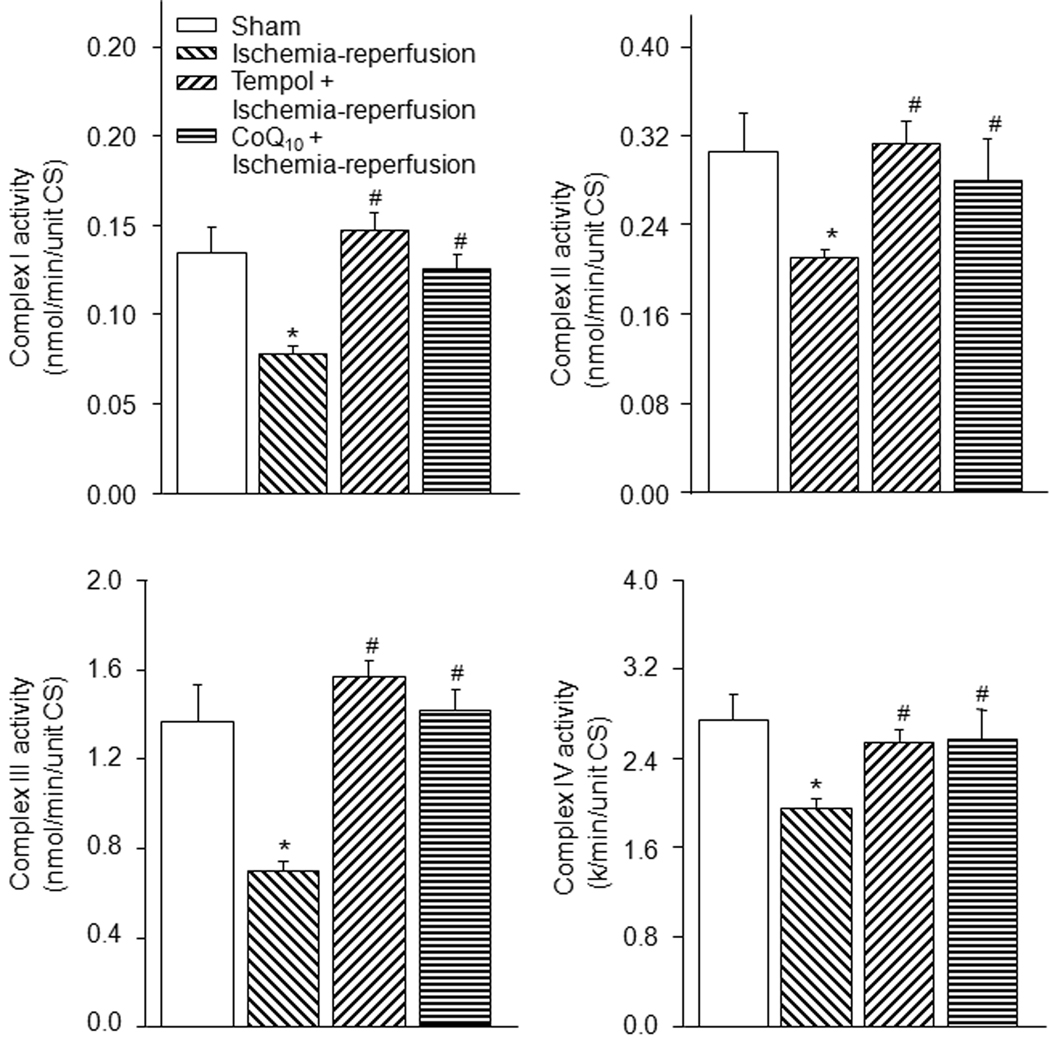
3.3. Mitochondrial electron transport chain complex activity in gastrocnemius muscles
Spectrophotometric measurement of mitochondrial complex I through IV activities showed activities of complex I, II, III, and IV were markedly decreased in the ischemia-reperfusion group as compared to the sham group (Fig. 3, P<0.05). Tempol and co-enzyme Q10 normalized the activities of the complex enzymes in the ischemia-reperfusion group towards the levels seen in sham group (Fig. 3).
Figure 3.
Mitochondrial complex enzyme activities in gastrocnemius muscles from sham (n=10), tourniquet-induced ischemia-reperfusion (n=13), tempol + ischemia-reperfusion (n=7), and CoQ10 + ischemia-reperfusion (n=8) groups. Data are mean ± S.E.M. *P<0.05 vs. sham; #P<0.05 vs. ischemia-reperfusion group.
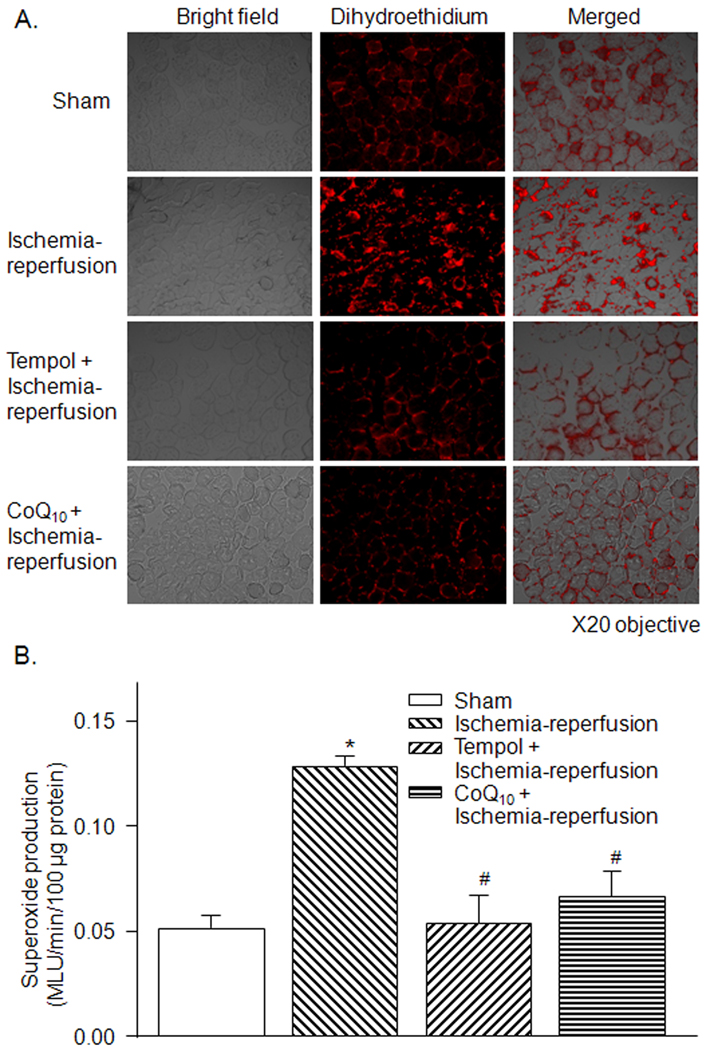
3.4. Superoxide production in gastrocnemius muscles
On dihydroethidium staining, superoxide production in the gastrocnemius muscles was qualitatively higher in the ischemia-reperfusion group compared to the sham group (Fig. 4-A). Quantitatively, lucigenin chemiluminescent data showed that superoxide production was significantly elevated in the ischemia-reperfusion group compared to the sham group (Fig. 4-B). Tempol and co-enzyme Q10 significantly inhibited superoxide production in the ischemia-reperfusion group (Fig. 4-A and -B).
Figure 4.
A, superoxide production in gastrocnemius muscles from sham, tourniquet-induced ischemia-reperfusion, tempol + ischemia-reperfusion, and CoQ10 + ischemia-reperfusion groups, as assessed by dihydroethidium fluorescence. B, superoxide production in homogenates of gastrocnemius muscles from sham, tourniquet-induced ischemia-reperfusion, tempol + ischemia-reperfusion, and CoQ10 + ischemia-reperfusion groups, as measured by lucigenin chemiluminescence. MLU: mean light units. Data are mean ± S.E.M., n=7 mice in each group. *P<0.05 vs. sham; #P<0.05 vs. ischemia-reperfusion group.
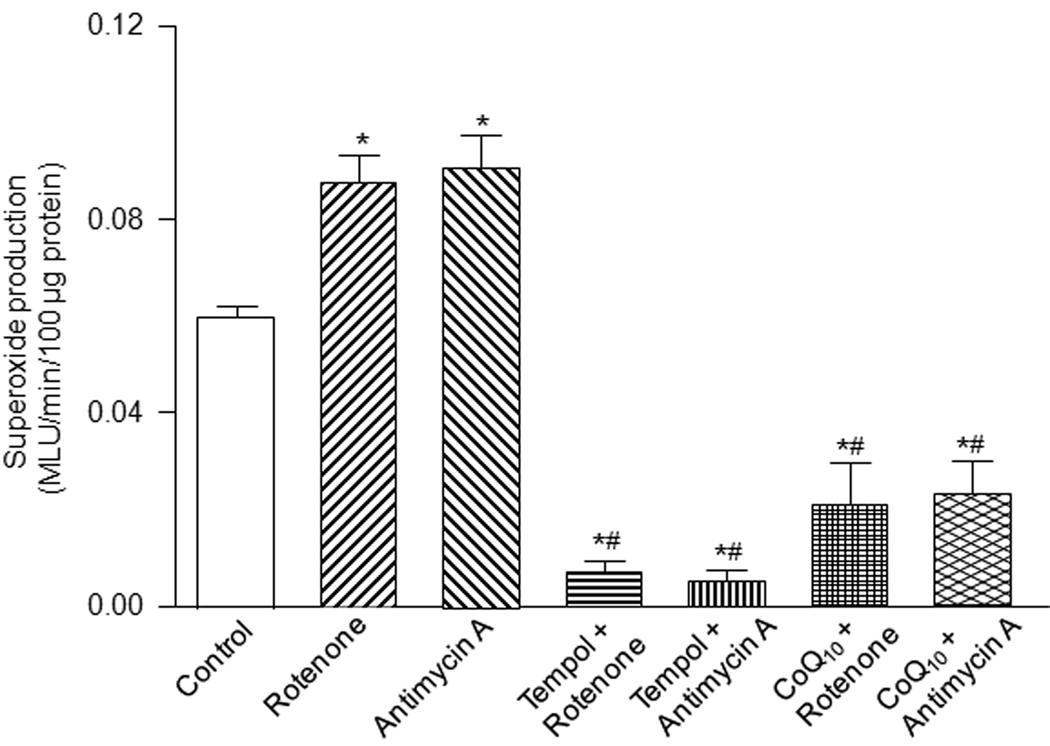
In addition, in order to assess the effects of tempol and co-enzyme Q10 on the mitochondria-derived superoxide production, we also used rotenone (mitochondrial complex I inhibitor, 5 µM) and antimycin A (mitochondrial complex III inhibitor, 5 µM) to produce superoxide (Plecita-Hlavata et al., 2009) in gastrocnemius muscle homogenates from sham mice. As shown in Fig. 5, rotenone and antimycin A induced a remarkable increase of the superoxide production in sham gastrocnemius muscle homogenates after 20 min of rotenone or antimycin A treatment. Tempol (1 mM) and co-enzyme Q10 (100 µM) decreased not only the mitochondrial complex inhibitor-induced superoxide production but also basal superoxide production in sham gastrocnemius muscles (Fig. 5).
Figure 5.
Effects of tempol (1 mM) and CoQ10 (100 µM) on rotenone (5 µM)- and antimycin A (5 µM)-enhanced superoxide production in gastrocnemius muscle homogenates from sham mice (n=5), as measured by lucigenin chemiluminescence. Data are mean ± S.E.M. *P<0.05 vs. control; #P<0.05 vs. rotenone or antimycin A.
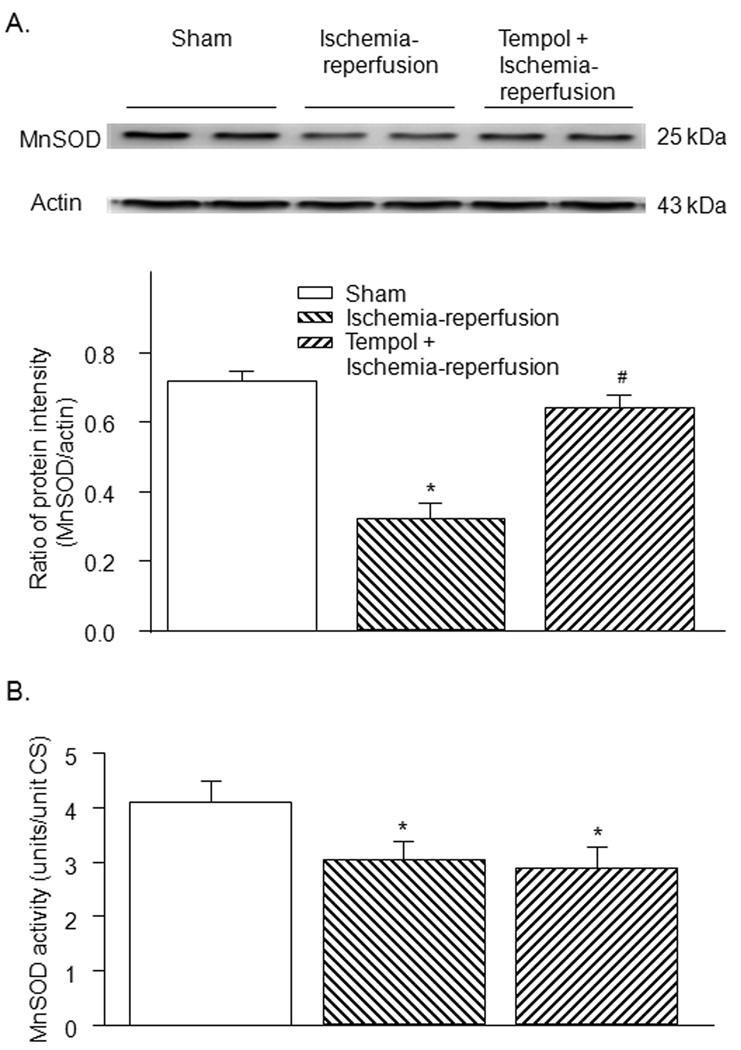
3.5. Protein expression and activity of MnSOD in gastrocnemius muscles
Protein expression and activity of MnSOD, the principal mitochondrial superoxide scavenger, were lower in the ischemia-reperfusion group compared to those in the sham group (Fig. 6). Tempol increased the MnSOD protein expression (Fig. 6-A) but did not affect the MnSOD activity in the ischemia-reperfusion group (Fig. 6-B). Similarly, MnSOD protein expression but not MnSOD activity was normalized by co-enzyme Q10 in the ischemia-reperfusion group (see Fig. 1s in supplementary data for detail).
Figure 6.
Protein expression (A) and activity (B) of MnSOD in gastrocnemius muscle from sham, tourniquet-induced ischemia-reperfusion, tempol + ischemia-reperfusion, and CoQ10 + ischemia-reperfusion groups. Data are mean ± S.E.M., n=6 mice in each group. *P<0.05 vs. sham; #P<0.05 vs. ischemia-reperfusion group.
4. Discussion
In the present study, we reproduced the critical limb syndrome in a murine model subjected to 3 h ischemia followed by 4 h reperfusion. A clinically significant infarct size of about 40 % in gastrocnemius muscles (Fig. 2) was achieved with this model. Mitochondrial respiratory function, measured by complex I through IV activities, was markedly reduced in the ischemia-reperfusion group compared to the sham group (Fig 3). Concurrently, superoxide production in gastrocnemius muscles was significantly elevated (Fig. 4) and protein expression and activity of MnSOD, the main mitochondrial superoxide scavenger, were decreased in the ischemia-reperfusion group (Fig. 6). Pretreatment with tempol (SOD mimetic) or co-enzyme Q10, significantly inhibited superoxide production, improved the mitochondrial function, and decreased the infarct size in tourniquet-induced ischemia-reperfusion gastrocnemius muscles. These results strongly suggest that superoxide overproduction mediates the ischemia-reperfusion injury including cellular injury and mitochondrial dysfunction in skeletal muscles.
Since exsanguination is the most common cause of preventable trauma deaths, hemorrhage control usually takes precedence over fluid resuscitation (Bickell et al., 1994). A controversial topic for decades (Klenerman, 1962), arterial tourniquet has become one of the first-line treatments of exsanguinating hemorrhage in civilian and military casualties who incur severe penetrating and blunt extremity injuries, either as an isolated injury or a part of multisystem trauma. While beneficial, prolonged applications of tourniquet can lead to ischemia-reperfusion injuries of the underlying skeletal muscles. These injuries range from minor skeletal muscle dysfunction to muscle necrosis to even limb loss and systemic life-threatening shock (Mabry, 2006). Evidence from animal experiments showed muscle injury begins after 2 h of tourniquet-induced ischemia, as evidenced by the elevated levels of lactic acid and creatine phosphokinase (Heppenstall et al., 1979). Most clinical studies support the traditional 60–90 minutes as the upper limits of safe tourniquet time in the operative theaters (Doyle and Taillac, 2008). The safety of these shorter tourniquet times is also re-affirmed for pre-hospital patients in recent military experience (Beekley et al., 2008; Lakstein et al., 2003; Sebesta, 2006). Under certain tactical conditions in the civilian and military pre-hospital setting, however, longer tourniquet times may sometimes be necessary or unavoidable.
The timing and choice of revascularization method for acute limb ischemia in critical limb syndrome depends on patient’s level of limb ischemia. Using the clinical tools of motor, sensory assessment and Doppler study, a popular staging method is to categorize acute limb ischemia into Category I (Viable – No immediate threat), Category IIA (marginally threatened – Salvageable if promptly treated), Category IIB (Immediately threatened – Salvageable if immediately revascularized), and Category III (Irreversible – Major tissue loss, permanent nerve damage) (Ouriel et al., 1994). This animal model utilized in this study was chosen to simulate acute limb ischemia IIB to III - severe enough to be clinically relevant, but that is not too severe to go past the point of non-viability and death for the entire limb. A rubber band tourniquet was applied to the hind limbs of mice for 3 h and released to begin the reperfusion. Hind limb muscles were harvested and examined at the end of 4 h of reperfusion. Our data for 1 and 2 h of ischemia (unpublished) showed that 3 h of ischemia gave the optimal and clinically relevant muscle infarction of 40%. The reperfusion time of 4 h was selected based on clinical and animal models showing stable pathology after 4 h of reperfusion (Blaisdell, 2002; Hua et al., 2005). Our preliminary data also found there is no significant difference between 4 h reperfusion injury and 24 h reperfusion injury (data not shown). During the application of the rubber band tourniquet, ischemia was confirmed by the near complete occlusion of blood flow to gastrocnemius muscle (Fig. 1), consistent with data in a previous study (Crawford et al., 2007). The reperfusion was marked by post-ischemic hyperemia and partially recovered reflow (Fig.1), consistent with previous clinical observations (Blaisdell, 2002).
Consistent with previous studies using cardiac and other types of tissue (Solaini and Harris, 2005), results in this study suggest that superoxide anion in skeletal muscles significantly contribute to ischemia-reperfusion-induced mitochondrial and cellular injury in skeletal muscles. First, the elevated superoxide production in the ischemia-reperfusion group in this study parallels the low reflow (Fig. 1), with previous studies having shown that these microcirculatory changes are caused by ROS-mediated endothelial damage (Menger et al., 1992). Second, the elevated superoxide production in the ischemia-reperfusion group in this study is associated with 40% skeletal muscle necrosis and significant skeletal muscle mitochondrial dysfunction (Figs 2 and 3), an association that heretofore was not causally established in skeletal muscle. Third, tempol (a SOD mimetic) or co-enzyme Q10 (an endogenous antioxidant) scavenges the superoxide and prevents the gastrocnemius against the ischemia-reperfusion injuries (low blood flow, mitochondrial dysfunction and necrosis).
A number of papers have identified mitochondria as the principal source of superoxide in striated muscle (Sadek et al., 2003). While < 2% of reactive oxygen speicies in the form of superoxide anion is produced by mitochondria under physiologic condition, superoxide production by mitochondria is significantly elevated in ischemia-reperfusion (Becker et al., 1999). Low levels of reactive oxygen species during ischemia cause the electron transport chain damage, leading to inefficient transfer of electrons, and in turn, increased production of reactive oxygen species (Lesnefsky et al., 2004; Sack, 2006). At the onset of reperfusion, the additional burst of reactive oxygen species causes further damage to the electron transport chain and membrane damage via lipid peroxidation, leading to further oxidative damage to the cell and loss of cell viability (Kim et al., 2006). In our present study, the activities of mitochondrial complex I, II, III, and IV were decreased in ischemia-reperfusion (Fig.3); and complex I and III inhibitors significantly elevated the superoxide production in sham gastrocnemium muscles (Fig. 5). Tempol and co-enzyme Q10 conversely improved the mitochondrial dysfunction (Fig. 3) accompanied by inhibiting ischemia-reperfusion-induced superoxide production (Fig. 4) and mitochondrial complex inhibitor (rotenone or antimycin A)-enhanced superoxide production (Fig. 5). Based on these results, it is reasonable to assume that mitochondria also serve as producers of superoxide besides serving as targets for reactive oxygen species-induced injury in the pathophysiological conditions.
Superoxide anion in mitochondria is normally scavenged under physiologic conditions by MnSOD, which converts superoxide into hydrogen peroxide (H2O2), and H2O2 is subsequently reduced to water by mitochondrial glutathione peroxidase. MnSOD protein expression and activities were reduced in the ischemia-reperfusion group as compared to shams (Fig. 6). Similar to the study by Kim (Kim et al., 2002), in which a deficiency in MnSOD was found to exacerbate cerebral infarction after focal cerebral ischemia-reperfusion in mice, the harmful effects of superoxide production in this study were probably magnified by the inability of the endogenous antioxidant MnSOD (Fig. 6). These results suggest the endogenous cellular compensatory antioxidant mechanism failed to offset the increase in oxidative stress, either because the cellular antioxidant defenses were being overwhelmed by reactive oxygen species or there existed a post-translational modification of MnSOD induced by the ischemia-reperfusion injury.
Even though pretreatment of tempol or co-enzyme Q10 significantly reduced superoxide overproduction and normalized the activities of mitochondrial complex enzymes, tempol or co-enzyme Q10 did not increase MnSOD activity. Although the present study cannot explain this phenomenon, one possible explanation is that tempol and co-enzyme Q10 did not stop the ischemia-reperfusion-induced MnSOD nitration because some studies have shown that MnSOD nitration decreases the MnSOD activity (Filipovic et al., 2007; Redondo-Horcajo et al., 2010; Tangpong et al., 2008), which needs further study. Nevertheless, this lack of effect by tempol or co-enzyme Q10 on MnSOD activity may explain the partial improvement in the size muscle infarction by tempol or co-enzyme Q10 in the ischemia-reperfusion, and it is possible that other factors (such as calcium overload) also contribute to the ischemia-reperfusion injuries besides superoxide.
In conclusion, using a murine model that simulates clinical acute limb ischemia caused by prolonged tourniquet application, we have shown that superoxide overproduction may be associated with mitochondrial dysfunction, cellular injury and cellular death in the skeletal muscle, and antioxidants can partially prevent the skeletal muscle from the ischemia-reperfusion injuries.
Supplementary Material
Acknowlegements
The authors wish to thank Michael T. Watkins, MD, Department of Surgery, Division of Vascular Surgery, Massachusetts General Hospital, Harvard Medical for his generous help with model development; Zhen Zhu, MD, Ilir Frokkaj, MD, Stanley Swanson, BS, Tanuja Lakshmi, MD, and George P. Casale, PhD, Department of Surgery, University of Nebraska Medical Center for their skilled technical support. This work was supported in part by National Heart, Lung, and Blood Institute Grant HL-098503 to Y.L. Li.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
No conflicts of interest are declared by the authors.
References
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Becker LB, vanden Hoek TL, Shao ZH, Li CQ, Schumacker PT. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am. J. Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- Beekley AC, Sebesta JA, Blackbourne LH, Herbert GS, Kauvar DS, Baer DG, Walters TJ, Mullenix PS, Holcomb JB. Prehospital tourniquet use in Operation Iraqi Freedom: effect on hemorrhage control and outcomes. J. Trauma. 2008;64:S28–S37. doi: 10.1097/TA.0b013e318160937e. [DOI] [PubMed] [Google Scholar]
- Bellamy RF. The causes of death in conventional land warfare: implications for combat casualty care research. Mil. Med. 1984;149:55–62. [PubMed] [Google Scholar]
- Bickell WH, Wall MJ, Jr, Pepe PE, Martin RR, Ginger VF, Allen MK, Mattox KL. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N. Engl. J. Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- Birch-Machin MA, Briggs HL, Saborido AA, Bindoff LA, Turnbull DM. An evaluation of the measurement of the activities of complexes I-IV in the respiratory chain of human skeletal muscle mitochondria. Biochem. Med. Metab. Biol. 1994;51:35–42. doi: 10.1006/bmmb.1994.1004. [DOI] [PubMed] [Google Scholar]
- Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc.Surg. 2002;10:620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- Crawford RS, Hashmi FF, Jones JE, Albadawi H, McCormack M, Eberlin K, Entabi F, Atkins MD, Conrad MF, Austen WG, Jr, Watkins MT. A novel model of acute murine hindlimb ischemia. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H830–H837. doi: 10.1152/ajpheart.00581.2006. [DOI] [PubMed] [Google Scholar]
- Doyle GS, Taillac PP. Tourniquets: a review of current use with proposals for expanded prehospital use. Prehosp. Emerg. Care. 2008;12:241–256. doi: 10.1080/10903120801907570. [DOI] [PubMed] [Google Scholar]
- Elstner EF, Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal. Biochem. 1976;70:616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- Filipovic MR, Stanic D, Raicevic S, Spasic M, Niketic V. Consequences of MnSOD interactions with nitric oxide: nitric oxide dismutation and the generation of peroxynitrite and hydrogen peroxide. Free Radic. Res. 2007;41:62–72. doi: 10.1080/10715760600944296. [DOI] [PubMed] [Google Scholar]
- Hammers DW, Merritt EK, Matheny W, Adamo ML, Walters TJ, Estep JS, Farrar RP. Functional deficits and insulin-like growth factor-I gene expression following tourniquet-induced injury of skeletal muscle in young and old rats. J. Appl. Physiol. 2008;105:1274–1281. doi: 10.1152/japplphysiol.90418.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppenstall RB, Balderston R, Goodwin C. Pathophysiologic effects distal to a tourniquet in the dog. J. Trauma. 1979;19:234–238. doi: 10.1097/00005373-197904000-00003. [DOI] [PubMed] [Google Scholar]
- Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, Butler FK. Causes of death in U.S. Special Operations Forces in the global war on terrorism: 2001–2004. Ann. Surg. 2007;245:986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann. N.Y. Acad. Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- Hua HT, Albadawi H, Entabi F, Conrad M, Stoner MC, Meriam BT, Sroufe R, Houser S, LaMuraglia GM, Watkins MT. Polyadenosine diphosphate-ribose polymerase inhibition modulates skeletal muscle injury following ischemia reperfusion. Arch. Surg. 2005;140:344–351. doi: 10.1001/archsurg.140.4.344. [DOI] [PubMed] [Google Scholar]
- Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Kim GW, Kondo T, Noshita N, Chan PH. Manganese superoxide dismutase deficiency exacerbates cerebral infarction after focal cerebral ischemia/reperfusion in mice: implications for the production and role of superoxide radicals. Stroke. 2002;33:809–815. doi: 10.1161/hs0302.103745. [DOI] [PubMed] [Google Scholar]
- Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2024–H2034. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- Klenerman L. The tourniquet in surgery. J. Bone Joint Surg..Br. 1962;44-B:937–943. doi: 10.1302/0301-620X.44B4.937. [DOI] [PubMed] [Google Scholar]
- Krahenbuhl S, Talos C, Wiesmann U, Hoppel CL. Development and evaluation of a spectrophotometric assay for complex III in isolated mitochondria, tissues and fibroblasts from rats and humans. Clin. Chim. Acta. 1994;230:177–187. doi: 10.1016/0009-8981(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Lakstein D, Blumenfeld A, Sokolov T, Lin G, Bssorai R, Lynn M, Ben-Abraham R. Tourniquets for hemorrhage control on the battlefield: a 4-year accumulated experience. J. Trauma. 2003;54:S221–S225. doi: 10.1097/01.TA.0000047227.33395.49. [DOI] [PubMed] [Google Scholar]
- Lee C, Porter KM, Hodgetts TJ. Tourniquet use in the civilian prehospital setting. Emerg. Med. J. 2007;24:584–587. doi: 10.1136/emj.2007.046359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J. Biol. Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- Li YL, Gao L, Zucker IH, Schultz HD. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc. Res. 2007;75:546–554. doi: 10.1016/j.cardiores.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie JT, Holley KE, Kampa WR, Titus JL. New histochemical method for morphologic diagnosis of early stages of myocardial ischemia. Mayo Clin. Proc. 1971;46:319–327. [PubMed] [Google Scholar]
- Mabry RL. Tourniquet use on the battlefield. Mil. Med. 2006;171:352–356. doi: 10.7205/milmed.171.5.352. [DOI] [PubMed] [Google Scholar]
- Menger MD, Pelikan S, Steiner D, Messmer K. Microvascular ischemia-reperfusion injury in striated muscle: significance of "reflow paradox". Am. J. Physiol. Heart Circ. Physiol. 1992;263:H1901–H1906. doi: 10.1152/ajpheart.1992.263.6.H1901. [DOI] [PubMed] [Google Scholar]
- Miles MV. The uptake and distribution of coenzyme Q10. Mitochondrion. 2007;7 Suppl:S72–S77. doi: 10.1016/j.mito.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol. Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouriel K, Shortell CK, DeWeese JA, Green RM, Francis CW, Azodo MV, Gutierrez OH, Manzione JV, Cox C, Marder VJ. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J. Vasc. Surg. 1994;19:1021–1030. doi: 10.1016/s0741-5214(94)70214-4. [DOI] [PubMed] [Google Scholar]
- Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984;142:290–296. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- Plecita-Hlavata L, Jezek J, Jezek P. Pro-oxidant mitochondrial matrix-targeted ubiquinone MitoQ10 acts as anti-oxidant at retarded electron transport or proton pumping within Complex I. Int. J. Biochem. Cell Biol. 2009;41:1697–1707. doi: 10.1016/j.biocel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Redondo-Horcajo M, Romero N, Martinez-Acedo P, Martinez-Ruiz A, Quijano C, Lourenco CF, Movilla N, Enriquez JA, Rodriguez-Pascual F, Rial E, Radi R, Vazquez J, Lamas S. Cyclosporine A-induced nitration of tyrosine 34 MnSOD in endothelial cells: role of mitochondrial superoxide. Cardiovasc. Res. 2010;87:356–365. doi: 10.1093/cvr/cvq028. [DOI] [PubMed] [Google Scholar]
- Sack MN. Mitochondrial depolarization and the role of uncoupling proteins in ischemia tolerance. Cardiovasc. Res. 2006;72:210–219. doi: 10.1016/j.cardiores.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Sadek HA, Nulton-Persson AC, Szweda PA, Szweda LI. Cardiac ischemia/reperfusion, aging, and redox-dependent alterations in mitochondrial function. Arch. Biochem. Biophys. 2003;420:201–208. doi: 10.1016/j.abb.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Sebesta J. Special lessons learned from Iraq. Surg. Clin. North Am. 2006;86:711–726. doi: 10.1016/j.suc.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Solaini G, Harris DA. Biochemical dysfunction in heart mitochondria exposed to ischaemia and reperfusion. Biochem. J. 2005;390:377–394. doi: 10.1042/BJ20042006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srere PA. Citrate synthase. Methods Enzymol. 1969;13:3–11. [Google Scholar]
- Tangpong J, Sompol P, Vore M, St CW, Butterfield DA, St Clair DK. Tumor necrosis factor alpha-mediated nitric oxide production enhances manganese superoxide dismutase nitration and mitochondrial dysfunction in primary neurons: an insight into the role of glial cells. Neuroscience. 2008;151:622–629. doi: 10.1016/j.neuroscience.2007.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu HY, Li YL, Tran TP. A murine model of acute hindlimb ischemia/reperfusion injury. FASEB J. 2009;23:763.1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.