Abstract
Neurosteroids hold great promise for the treatment of diseases of the central nervous system (CNS). We compared the uptake by 11 brain regions and appearance in blood of tritium-labeled pregnenolone and progesterone after intranasal and intravenous (IV) injection. Both neurosteroids appeared in blood and brain after either method of administration, but with important differences in uptake. Bioavailability based on appearance in arterial serum showed that about 23% and 14% of the intranasal administered doses of pregnenolone and progesterone, respectively, entered the blood. Brain levels were about two fold lower after intranasal administration for the two neurosteroids. With intranasal administration, brain levels of the two steroids did not vary over time (2–120 min), whereas brain levels were higher early (10 min or less) after i.v. administration. With i.v. administration, uptake by brain regions did not vary, whereas the olfactory bulb, hippocampus, and hypothalamus had high uptake rates after intranasal administration. Intranasal administration of prenenolone improved memory, whereas progesterone decreased anxiety, thus demonstrating that therapeutic levels of neurosteroids can be delivered to the brain by intranasal administration. The neurosteroids were rapidly degraded after i.v. or intranasal delivery, but pregnenolone was more resistant to degradation in brain after intranasal administration and in serum after i.v. administration. These results show that either the i.v. or intranasal routes of administration can deliver neurosteroids to blood and brain, but that the two routes have significant differences with intranasal administration favoring some brain regions.
Keywords: intranasal, neurosteroid, pregnenolone, progesterone, memory, anxiety
1. INTRODUCTION
Neurosteroids accumulate in the brain after local synthesis or after metabolism of adrenal steroids or gonadal steroids (Baulieu & Robel, 1990). Neurosteroids are synthesized in the central and the peripheral nervous system, in glial cells, and also in neurons from cholesterol or steroidal precursors imported from peripheral sources. Neurosteroids are potent endogenous neuromodulators that have long been known to have diverse actions in the central nervous system (Gyermek et al., 1967). Neuroactive steroids have been claimed to have specific roles in normal or pathological brain function including effects on cognition, anxiety, depression, feeding, emotion, motivation, traumatic brain injury, and motor skills (Eser et al., 2008; Morley et al., 1984; Farr et al., 58 A.D.; Flood et al., 1992; Zheng, 2009; Stein, 2001). Neurosteroids exert their effects on the central nervous system either by regulating the expression of specific hormone-sensitive genes or by modulating the activity of neurotransmitter receptors, such as the type A γ-aminobutyric acid (GABA), the N-methyl-D-aspartate (NMDA), the nicotinic, the dopamine D1, and the sigma 1 (σ1) receptors and the α1 adrenoreceptor (Farr et al., 58 A.D.) (Shiraishi et al., 2002; Collier et al., 2007; Zheng, 2009).
Two powerful neurosteroids are pregnenolone and progesterone. Pregnenolone is the main precursor of various steroid hormones, including progesterone. Pregnenolone occurs as unconjugated steroid, and its fatty acid or sulfate esters. The CNS effects of pregnenolone and pregnenolone sulfate include modulation of GABA receptors and effects on seizure susceptibility, anxiety, depression, and memory processes with some of these processes mediated through the hippocampus (Flood et al., 1992; Meyer et al., 2002). Interestingly, dose-response curves for memory-enhancing steroids are different from those usually obtained with excitatory substances. In a study by Morley et. al (Flood et al., 1992), pregnenolone sulfate was effective over a wide dose range (10,000 fold). Alone or in conjunction with other treatments, pregnenolone might be more effective in treatment of memory deficits than substances having narrower therapeutic windows. Progesterone also affects a wide range of activities including cognition and anxiety with some of the effects being mediated through the hippocampus (Orr et al., 2009; Bitran et al., 1995).
Neuroactive steroids administered by several different routes can produce CNS effects. Neurosteroids rapidly cross the blood-brain barriers (BBB) after parenteral administration (Marynick et al., 1976), which can produce significant concentrations in the CNS. Systemic administration of steroids is useful in clinical practice; however, systemic delivery has the potential to cause unwanted side effects. As examples, oral medications because of first pass effects require high doses and injections are not popular with many patients. Recent evidence suggests that the delivery of intranasal steroids with absorption through the cribiform plate may be a more optimal way to deliver steroids to either the blood or brain and might be used for the treatment of CNS disease states, with less peripheral side effects (Banks et al., 2009).
The first aim of our study was to determine the relative efficiencies of intranasal versus intravenous (IV) administration for delivery of pregnenolone and progesterone to blood. The second aim was to determine whether intranasal administration targeted the brain more so than i.v. administration. The third aim was to determine whether intranasal delivery could deliver sufficient amounts of neurosteroids to the brain to induce effects on the central nervous system. The fourth aim was to compare the relative enzymatic stability in blood and brain after i.v. and intranasal administration.
2. MATERIALS AND METHODS
2.1 Pharmacokinetics of I.V. and Intranasal Administration
All animal studies were conducted under protocols approved by the local IACUC. Food and water were available ad lib until time of study. Male CD-1 mice aged 6–8 weeks and weighing 25–30 g from our in-house colony (VA-St. Louis) received an injection of 0.25 μCi of tritium-labeled pregnenolone (H-Preg, specific activity 12.6 Ci/mmol; Perkin Elmer, Boston, MA), pregnenolone sulfate (H-Preg-S, specific activity 10–20 Ci/mmol; American Radiolabeled Chemical Inc, St. Louis, MO) or 0.25 μCi tritium-labeled progesterone (H-Prog, specific activity 96.6 Ci/mmol; Perkin Elmer) by intranasal (dissolved in a proprietary oleogel, M & P Pharma AG, Stans, Switzerland) or i.v. (saline) administration. At 2, 5, 10, 15, 20, 30, 60, or 120 minutes after administration (n=5/time point), blood was collected from a cut in the carotid artery and the brains immediately harvested. The brains were dissected into 11 different brain regions (olfactory bulb, frontal cortex, parietal cortex, occipital cortex, striatum, hippocampus, hypothalamus, pon-medulla, cerebellum, thalamus, midbrain) and weighed. Whole blood was centrifuged at 4500 × G for 10 minutes and serum obtained. One ml of BTS-450 solubilizer (Beckman, Fullerton, CA) was added to each brain region and arterial serum (50 microl). Ten ml of Ready Organic Liquid Scintillation cocktail (Beckman, Fullerton, CA) was added to each vial and the samples were dark adapted for 24 hours. The levels of radioactivity in the brain regions and serum were determined in a beta counter (Wallac 1409 OSA). Results were expressed as brain/serum ratios (units of microl/g), percent of the administered or injected dose taken up per g of tissue (%inj/g), or percent of the dose present in one ml of serum (%inj/ml) which were calculated with the following equations:
As a quality check, brain/serum ratios were found to be approximately 2000 microl/g for progesterone. As the vascular space is only about 10 microl/g, the calculation of %Injection/g was considered accurate without correcting for vascular space.
2.2 Behavioral Effects After Intranasal Administration
CD-1 male mice aged 6–8 weeks weighing 25–30 g from our in house colony were trained in an active avoidance T-maze to test the effects of intranasal pregnenolone on memory. The T-maze consisted of a black plastic alley with a start box at one end and two goal boxes at the other. The start box was separated from the alley by a plastic guillotine door, which prevented movement down the alley until the training began. An electrifiable stainless steel rod floor ran throughout the maze to deliver scrambled footshock. In a training trial, a door was raised and a buzzer sounded. After 5 seconds, the footshock was administered until the mouse found the correct goal box located in one of the arms at the top of the maze. Mice were trained to effect. Immediately after training or 24 hours after training, the mice were lightly anesthetized with isoflurane and pregnenolone or vehicle (oleogel without pregnenolone) was administered intranasally. One week later, memory was tested in mice by determining the number of trials it took for them to reach the criterion of 5 avoidances in 6 consecutive trials.
CD-1 male mice were used to test the effects of intranasal progesterone on anxiety in the elevated plus maze. The procedure used was similar to that described by Lister (Lister, 1987) with progesterone administered 30 min prior to testing. The experimental apparatus is shaped like a “plus” sign and consists of a central platform, two open arms and two equal-sized closed arms opposite to each other. The maze is made of black Plexiglas, elevated to a height of 50 cm above the floor and illuminated by a dim light. The test consisted of placing a mouse in the central platform facing an enclosed arm and allowing it to freely explore the maze for 5 min. The test arena was wiped with a damp cloth after each trial. The number of entries into the open and closed arms and the time spent in open arms was measure by an observer blind to the drug treatment. Decreased anxiety was indicated by an increase in time spent in the open arms and an increase in the number of open arm entries.
2.3 Stability After I.V. or Intranasal Administration
Male 8 week old CD-1 mice were anesthetized with urethane and administered 1 μCi of H-Preg or H-Prog by the i.v. (jugular vein) or intranasal route. Carotid artery blood and whole brains (minus olfactory bulbs) were harvested 10 or 60 min later. To assess the amount of degradation during subsequent processing, other brains were harvested from mice not administered radioactivity and 104 dpm of H-Preg or H-Prog added to the blood or applied to the brain in silico (processing controls). The arterial blood was centrifuged at 5400 × g for 10 min and the serum obtained was frozen at −70 °C until processing. Each brain was homogenized in 2 ml of ice cold lactated Ringer’s solution containing 1% bovine serum albumin adjusted to pH 3.4 with phosphoric acid. The homogenate was centrifuged at 5400 × g for 20 min and 1 ml of supernatant collected and stored at −70 °C until analysis. Prior to analysis, the serum and brain samples were thawed and centrifuged at 22,000 × g 20 min. The resulting supernatant was injected onto a C-18 column (Phenomenex Onyx monolithic #CHO-7642) and 1ml fractions collected every 30 sec. For the H-Preg, the samples were eluted in solvent A (water) against an increasing gradient of solvent B (methanol), increasing solvent B from 10% at 0.01min to 90% by 20 min with H-Preg eluting at fraction 18. For the H-Prog, the samples were eluted in solvent A (water with 0.1% H2PO4) against a gradient of solvent B (acetonitrile), maintaining solvent B at 40% for 5 min, increasing solvent B to 90% by 15 min, and reducing solvent B back to 40% by 20 min; H-Prog eluted at fraction 9. The results were corrected for degradation during processing by dividing the percent of radioactivity representing intact material in a biological sample by the percent of radioactivity representing intact material in the processing control.
2.4 Statistics
Means are expressed with their standard error of the mean (S.E.M.). One way analysis of variance (ANOVA) was performed followed by Newman-Keuls multiple comparison test using the statistical software package Prism 5.0 (GraphPad Inc, San Diego, CA). Statistical significance is reported at P<0.05. The area under the curve (AUC) was calculated with the statistical software in Prism 5.0.
3. Results
3.1 Uptake into Blood After Intranasal or I.V. Administration
We first determined whether H-Preg (Fig. 1, upper panel) and H-Prog (Fig. 1, lower panel) entered the blood after intranasal administration. We found that radioactivity appeared in blood after the intranasal administration of either neurosteroid. Calculation of the AUC from 0 to 120 min gave a value of 235 %Inj-min/ml after i.v. injection and a value of 54 %Inj-min/ml after the intranasal injection of H-Preg. This shows that the bioavailability of intranasal H-Preg was about 23% and that blood levels averaged about 4.35 higher after i.v. administration when compared to intranasal administration. For H-Prog, the AUC was 125 %Inj-min/ml after i.v. administration and 17 %Inj-min/ml after intranasal administration. This gave a bioavailability of 14% for intranasal H-Prog and indicated that blood levels averaged about 7.35 times higher after i.v. than after intranasal administration.
Fig. 1.
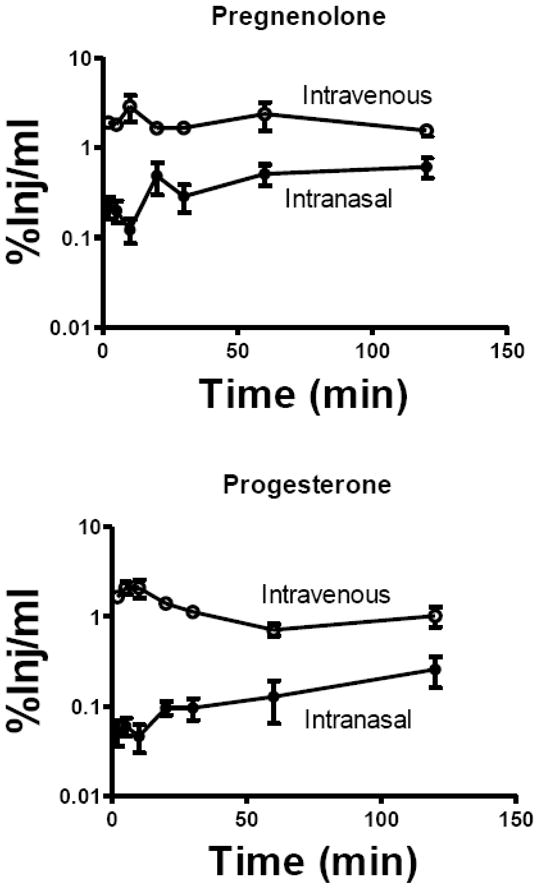
Levels of Radioactivity in Serum After the Intranasal or Intravenous Administration of 3H-Pregnenolone or 3H-Progesterone. Results are expressed as the percent of the injected dose present in one ml of arterial serum (%Inj/ml). Upper panel shows results for pregnenolone which had an area under the curve for the first 120 min of 235 %Inj-min/ml after intravenous injection and 54 %Inj-min/ml after intranasal administration. Lower panel shows results for progesterone which had an area under the curve of 125 %Inj-min/ml after intravenous injection and 17 %Inj-min/ml after intranasal administration. N = 3/time point. Means are shown with their S.E.M.
3.2 Uptake of Pregnenolone by Brain After Intranasal or I.V. Administration
After intranasal administration, levels of H-Preg did not show statistically significant variation with time for any brain region. Fig. 2 upper left panel shows the results for the hippocampus as a representative brain region. Because %Inj/g did not vary over time, we combined values across time to compute the average %Inj/g for the 120 min experiment ( Fig. 2, upper right panel). These values of uptake by brain regions did vary significantly: F(10, 372) = 16.2, P<0.001.
Fig. 2.
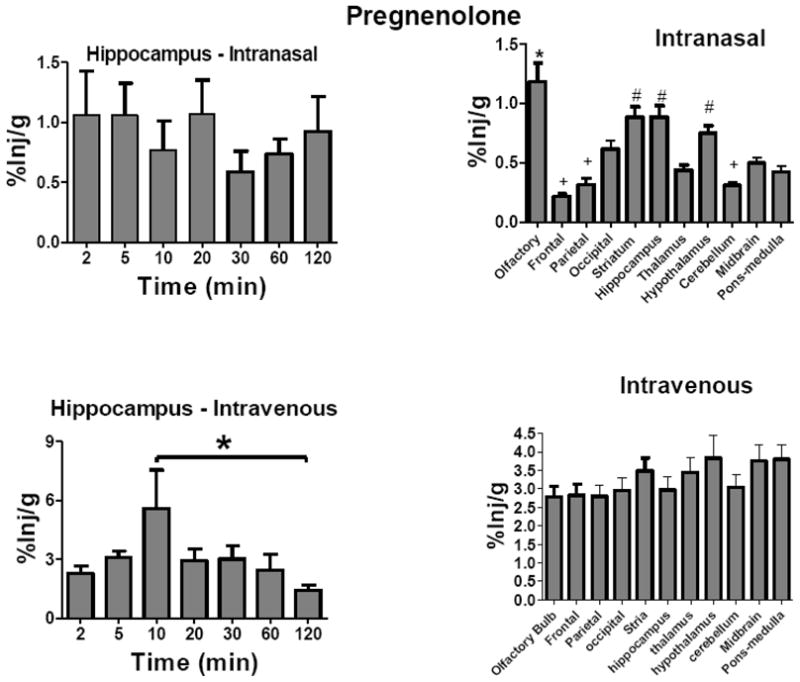
Uptake Over Time and Brain Distribution of 3H-Pregnenolone After Intravenous or Intranasal Administration. Results are expressed as the percent of the administered dose present per g of brain region (%Inj/g). Letters indicate groups that are not statistically different from one another. Upper left panel shows hippocampus as a representative brain region for uptake of H-Preg after its intranasal administration. Neither the hippocampus nor any other brain region showed a time-dependent change in %Inj/g after intranasal administration (n = 5/time point). Brain regions, however, showed statistically significant differences after intranasal administration (upper right panel; n =35/regionm * = different from all others, # = different from frontal cortex, thamalus, occipital cortex, cerebellum, and pons medulla). Lower left panel shows hippocampus as a representative brain region after the intravenous administration of H-Preg, with the 10 min time point being statistically different from the 120 min time point (n = 5 time point). Brain regions, however, showed no statistical differences after intravenous administration (lower right panel, n = 35/region). Means are shown with their S.E.M.
After i.v. administration, levels of H-Preg varied with time in the frontal cortex, parietal cortex, occipital cortex, thalamus, midbrain and pons-medulla, with the 10 min value being the highest in each of these regions. There was not a statistically significant difference with time after the i.v. administration of H-Preg for the olfactory bulb, striatum, or hypothalamus. The hippocampus showed a trend (P = 0.089) and Newman-Keuls multiple comparison test showed the 10 min and 120 min values to differ (P<0.05). There were no differences in uptake among brain regions after the i.v. administration of H-Preg (Fig. 2, lower right panel).
Five mice were given injections of H-Preg-S by i.v. or intranasal administration and serum and brains harvested 10 min later. The values of 0.87 ± 0.10 %Inj/ml and 0.17 ± %Inj/ml after i.v. or intranasal administration, respectively, were similar to the results found after H-Preg administration (Fig 1). Values for the hippocampus were 0.40 ± 0.6 %Inj/g and 0.36± 0.02 %Inj/g after i.v. or intranasal administration, respectively, and were lower than the values for H-Preg (Fig 2, lower left panel).
3.3 Uptake of Progesterone by Brain After Intranasal or I.V. Administration
H-Prog was studied after its intranasal and i.v. administration. After intranasal administration, H-Prog did not show statistically significant variation with time for any brain region. Fig. 3 upper left panel shows the results for the hippocampus. However, uptake by brain regions did vary significantly ( Fig. 3, upper right panel: F(10, 374) = 5.56, P<0.001.
Fig. 3.
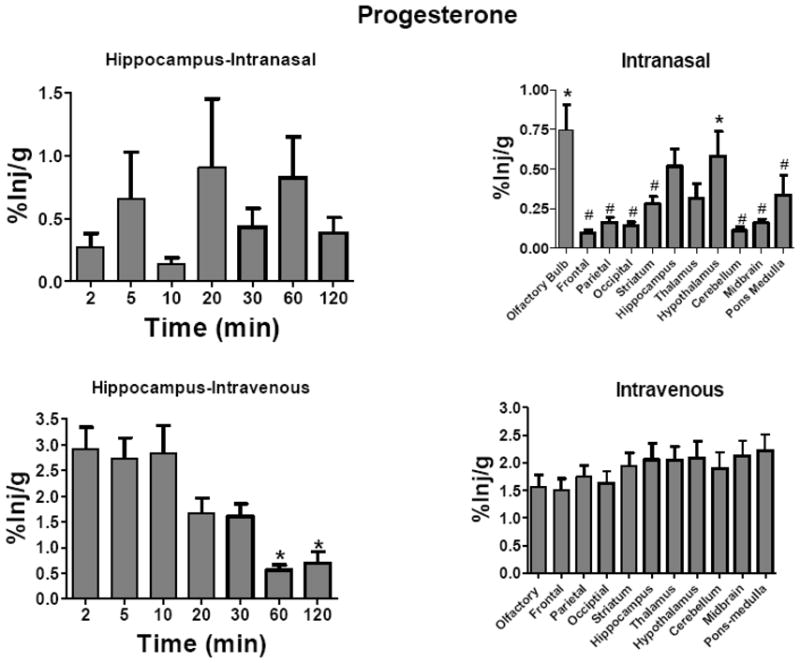
Uptake Over Time and Brain Distribution of 3H-Progesterone After Intravenous or Intranasal Administration. Results are expressed as the percent of the administered dose present per g of brain region (%Inj/g). Letters indicate groups that are not statistically different from one another. Upper left panel shows hippocampus as a representative brain region for uptake of H-Prog after its intranasal administration. Neither the hippocampus nor any other brain region showed a time-dependent change in %Inj/g after intranasal administration (n = 5/time point). Brain regions, however, showed statistically significant differences after intranasal administration (upper right panel; n =35/region, * = different from frontal cortex, parietal cortex, occipital cortex, cerebellum, and midbrain, # = different from olfactory bulb and hypothalamus). Lower left panel shows hippocampus as a representative brain region after the intravenous administration of H-Prog. Earlier time points (2–10 min) were higher than later time points (n = 5 time point). Brain regions, however, showed no statistical differences after intravenous administration (lower right panel, n = 35/region). Means are shown with their S.E.M.
After i.v. administration, levels of H-Prog varied with time in all brain regions, with the highest time points always being the 2–10 min values. Fig. 3 lower left panel shows representative results for the hippocampus: F(6, 27) = 8.97, P<0.001. Newman-Keuls multiple comparison test showed that the 2, 5, and 10 min values were statistically different from the 60 and 120 min values. There were no differences in uptake among brain regions after the i.v. administration of H-Prog (Fig. 3, lower right panel).
3.4 Behavioral Effects of Prenenolone and Progesterone After Intranasal Administration
The effect of intranasal pregnenolone on memory was tested in the active avoidance T-maze (Fig. 4). One way ANOVA showed a significant effect [F(4,44) = 7.58, P<0.001] and Newman-Keuls multiple comparison test showed that the 200 pg/mouse dose given immediately after training differed from vehicle (p<0.01). Giving 200 pg/mouse 24 h after the training episode, a time after which memory consolidation has been completed, did not improve memory.
Fig. 4.
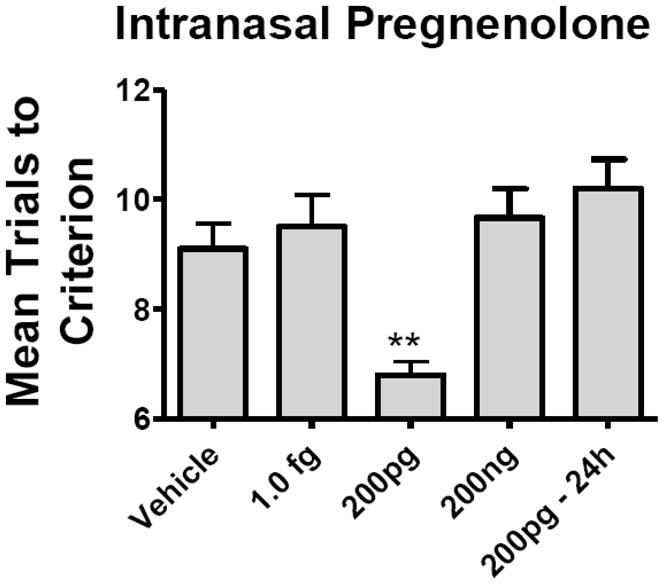
Effects of Intranasal Pregnenolone on Memory. The 200 pg/mouse given immediately after training produced a decrease in mean trials to criterion, demonstrating an improvement in memory as assessed in the active avoidance T-maze (n = 10/time point). The 1.0 fg and 200 ng doses were without effect. Giving pregnenolone 24h after the training episode was without effect (200pg – 24 h). Means are shown with the S.E.M.
Intranasal progesterone at a dose of 7.5 μg/mouse but not at 0.75 μg/mouse significantly improved performance in the elevated plus maze, a measure of anxiety. One way ANOVA showed a statistically significant effect for number of entries (Fig. 5, upper panel): F(2,48) = 10.4, P<0.001; Newman-Keuls multiple comparison test showed the 7.5 μg dose to be different from control (P<0.001). Time spent in open arms was also increased as determined by one way ANOVA ( Fig. 5, lower panel): F(2,48) = 5.34, P<0.01. Newman-Keuls multiple comparison test showed the 7.5 μg dose to be different from control (P<0.01).
Fig. 5.
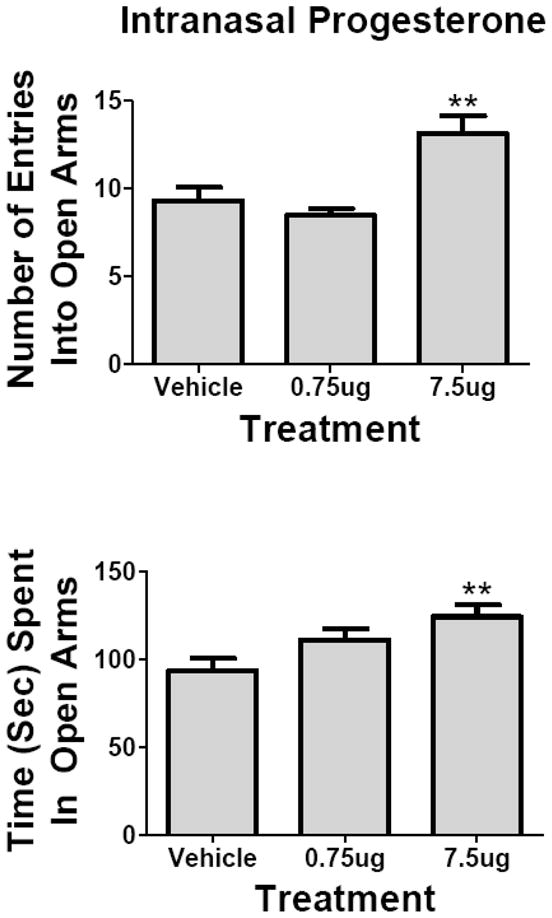
Effects of Intranasal Progesterone on Anxiety. The elevated plus maze was used as a test of anxiety ( n = 17/group). The 7.5 μg/mouse dose but not the 0.75 μg/mouse dose increased the number of entries into the open arms (upper panel) and increased the time spent in the open arms (lower panel), indicative of a decrease in anxiety. Means are shown with their S.E.M.
3.4 Stability of Progesterone and Pregnenolone: I.V. and Intranasal Administration
Stability of H-Preg and H-Prog was measured in serum and brain at 10 min and 60 min after their i.v. or intranasal administration (Table 1). A representative HPLC result plotting the mean of the two HPLC determinations for both brain and serum 10 min after the i.v. administration of H-Preg is shown in Fig. 6. About 40% of the radioactivity in brain represented intact H-Preg after intranasal administration, whereas 10–26% represented intact H-Preg after i.v. administration. Brain and serum levels of intact H-Preg were similar after i.v. administration, whereas much less radioactivity represented intact H-Preg in serum after intranasal administration. In comparison to H-Preg, less radioactivity eluted as intact H-Prog in brain and serum after i.v. administration and in brain after intranasal administration. Intact radioactivity was similar after intranasal or i.v. administration in brain and serum for H-Prog.
Table I.
HPLC of H-Preg and H-Prog in Brain and Serum after Intranasal or Intravenous Administration. Means are given with their S.E.M. and units are % of total recovered dpm eluting as intact neurosteroid.
| H-Pregnenolone | ||
|---|---|---|
| 10 min | 60 min | |
| Intranasal - Brain | 35 ± 2.6 | 45 ± 18 |
| Intranasal - Serum | 3.7 ± 0.60 | 7.0 ± 0.54 |
| Intravenous - Brain | 26.0 ± 1.9 | 10 ± 1.2 |
| Intravenous - Serum | 26 ± 6.6 | 10 ± 3.7 |
| H-Progesterone | ||
| 10 min | 60 min | |
| Intranasal - Brain | 9.5 ± 0.9 | 20 ± 2.6 |
| Intranasal - Serum | 13 ± 2.5 | 18.2 ± 0 |
| Intravenous - Brain | 7.9 ± 1.1 | 9.8 ± 2.0 |
| Intravenous - Serum | 13 ± 0.33 | 7.3 ± 0.75 |
10 min and 60 min values are means ± S.E.M., n = 2
Fig. 6.
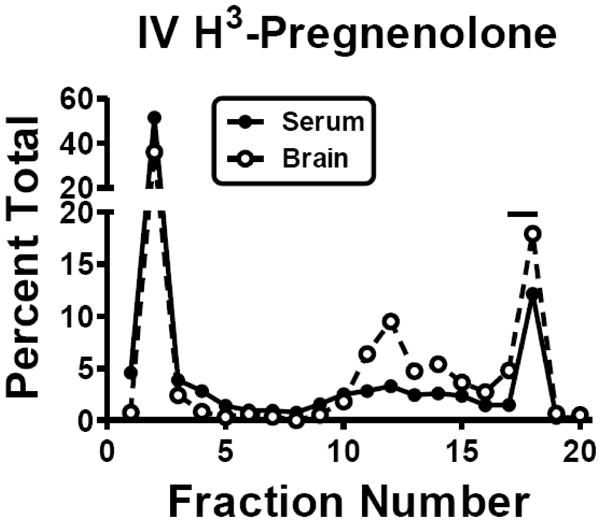
Representative HPLC of Neurosteroid Stability in Serum and Brain. The results are the mean of two HPLC determinations for serum and for brain after the i.v. injection of H-Peg. Units are the percent of total radioactivity recovered that elutes per fraction. The bar over fraction 17–18 indicates where H-Pregnenolone elutes.
4. Discussion
Two neurosteroids, pregnenolone and progesterone, have profound effects on the CNS and have potential as treatments for CNS diseases (Flood et al., 1992; Sadri-Bakili et al., 2008; Stein, 2001; Eser et al., 2008). Pregnenolone and pregnenolone sulfate are reported to improve memory (Flood et al., 1992; Meyer et al., 2002; Valée et al., 2001; Park, 2006). Pregnenolone sulfate has been shown at a narrow dose range to increase neuronal activity by inhibiting GABAergic and by stimulating glutamatergic neurotransmission (Sadri-Bakili et al., 2008). It has been proposed that these actions of pregnenolone underlie its neuropharmacological effects, and in particular its influence on memory processes. Our study shows that pregnenolone enters brain tissue after either i.v. or intranasal administration, consistent with their known permeation across the BBB (Kumar et al., 1982). However, there were differences between these two routes in targeting central and peripheral tissues as discussed below.
Progesterone has been shown to possess anti-anxiolytic properties after peripheral or central administration (Bitran et al., 1995) (Wang, 1997). Progesterone is also useful in post-injury recoveries, including traumatic brain injury, stroke and spinal cord injuries (Stein, 2001). Here, we used the elevated plus maze to measure the effects of intranasal progesterone on anxiety. In this test, mice have a choice to spend more or less time in open vs closed arms. Mice with lower levels of anxiety enter the open arms more often and spend more time in the open arms. Here, we found that mice given 7.5 microg/mouse of progesterone by nasal administration entered the open arms more and spent more time there, consistent with an anti-anxiolytic effect of progesterone. Higher and lower doses of progesterone were without effect, consistent with the inverted-U dose-response relation between dose of progesterone and the anti-anxiolytic effect (Sadri-Bakili et al., 2008) (Schumacher et al., 2007; Andréen et al., 2009; de Souza Silva et al., 2008).
These results as discussed above show that either intranasal or i.v. administration results in the appearance of the neurosteroids in both brain and blood. The results also show that the classic central actions of memory enhancement with pregnenolone and reduction in anxiety with progesterone can be achieved after the intranasal administration of these steroids. However, there were several differences in results between the i.v. and the intranasal route. First, based on the AUCs of Fig. 1, only about 23% and 14% of the intranasal dose of pregnenolone and progesterone, respectively, entered the blood. Similarly, brain levels were lower after intranasal than after i.v. administration. Temporal and brain region differences also occurred between the two routes. Whereas the levels in brain for both pregnenolone and progesterone varied little over time after intranasal administration, intravenous administration tended to produce early peaks in brain levels with declines after 10 min; in contrast, concentrations of pregnenolone or progesterone varied little among brain regions after intravenous injections, there was a great deal of statistically significant variation after intranasal administration. Although the olfactory bulb is expected to have high concentrations of any material administered by the intranasal route, the hippocampus, hypothalamus, and striatum took up large amounts of pregnenolone and the uptake of progesterone by the hippocampus and hypothalamus was not statistically different from the uptake by the olfactory bulb. These results show that intranasal and i.v. administration target tissues, especially brain regions, differently. This is likely to have important consequences for the subsequent CNS actions of the neurosteroids.
These results also offer interesting comparisons with testosterone given by intranasal administration to adult female mice (Banks et al., 2009). Rapid degradation and a favoring of uptake by the olfactory bulb, striatum, and hippocampus were also found for testosterone. However, testosterone differed in that blood levels after i.v. and intranasal administration were essentially identical after abut 30 min and intranasal administration produced higher levels of brain uptake than did i.v. administration. Steroid uptake by brain by either route is likely influenced by age, species, and gender (Brinton et al., 2008; Korneyev, 1993; Guerra-Araiza, 2002; Guerra-Araiza, 2001). These patterns differ even more so from those for peptides given by intranasal administration. Peptides show even more variation among region and temporal patterns with intranasal administration favoring brain uptake over entry into blood (During et al., 2003; Banks et al., 2004; Nonaka et al., 2008). It also appears that regional uptake of some peptides can be controlled or directed by including cyclodextrins in the intranasal administration (Nonaka et al., 2008).
Finally, we examined the degradation of the neurosteroids in brain and blood after intranasal or intravenous administration. Steroid dehydroxylases are common in peripheral and central tissues, including nasal mucosa (Brittebo & Rafter, 1984). Degradation or other modifications have made it difficult to detect pregnenolone sulfate in the brain (Schumacher et al., 2008). For pregnenolone, intranasal administration was associated with higher stability in brain and intravenous administration with higher stability in blood. However, progesterone showed little variation as a function of route of administration. These results clearly show that the neurosteroids are not stable in brain or blood by either route of administration. However, what effect this rapid degradation would have on the calculations shown in Fig. 2 are not straightforward, especially in the interpretation of brain values. This is because it is unclear whether degradation occurred before or after entering the brain. If the former, then values in Fig. 2 should be corrected for degradation, but if the latter, then the values as shown in Fig. 2 reflect at least initially levels in brain. It should be noted that neurosteroids injected directly into brain are also likely rapidly degraded and so values shown in Fig. 2 likely represent a good comparison for doses given i.v., intranasally, or directly into the brain.
In conclusion, our results show that intranasal administration can deliver pregnenolone sulfate and progesterone to both the brain and the blood. Intranasal administration produced lower levels in blood and brain than i.v. administration, but with brain levels being relatively higher than blood after i.v. administration. We also found that intranasal administration targeted specific brain regions, especially the hippocampus, hypothalamus, and striatum, an effect not seen after i.v. administration. Pregnenolone improved memory and progesterone reduced anxiety after intranasal administration, demonstrating that therapeutic levels of neurosteroids can be reached in brain after intranasal administration. Pregnenolone, but not progesterone, was less degraded in brain after intranasal than i.v. administration. Overall, these results show that intranasal administration is a viable route for both the central and peripheral delivery of the neurosteroids pregnenolone and progesterone.
Acknowledgments
Supported by Mattern Pharmaceutical and NSR01 051334.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andréen L, Nyberg S, Turkmen S, van Wingen G, Ferández G, Bäckström T. Sex steroid induced negative mood may be explained by the paradoxical effect mediated by GABAA modulators. Psychoneuroendocrinol. 2009;34:1121–1132. doi: 10.1016/j.psyneuen.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Banks WA, During MJ, Niehoff ML. Brain uptake of glucagon-like peptide-1 antagonist exendin(9–39) after intranasal administration. J Pharmacol Exp Ther. 2004;309:469–475. doi: 10.1124/jpet.103.063222. [DOI] [PubMed] [Google Scholar]
- Banks WA, Morley JE, Niehoff ML, Mattern C. Delivery of testosterone to the brain by intranasal administration: Comparison to intravenous testosterone. J of Drug Targeting. 2009;17:759–765. doi: 10.1080/10611860802382777. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Neurosteroids: A new brain function? J of Steroid Chem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, Mcleod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABA (A) receptors. J Neuroendocrinol. 1995;7:171–177. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk RZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittebo EB, Rafter JJ. Steroid metabolism by rat nasal mucosa: studies on progesterone and testosterone. J Steroid Biochem. 1984;20:1147–1151. doi: 10.1016/0022-4731(84)90358-3. [DOI] [PubMed] [Google Scholar]
- Collier TL, Waterhouse RN, Kassiou M. Imaging sigma receptors: applications in drug development. Current Pharm Design. 2007;13:51–72. doi: 10.2174/138161207779313740. [DOI] [PubMed] [Google Scholar]
- de Souza Silva MA, Topic B, Huston JP, Mattern C. Intranasal administration of progesterone increases dopaminergic activity in amygdala and neostriatum of male rats. Neurosci. 2008;157:196–203. doi: 10.1016/j.neuroscience.2008.09.003. [DOI] [PubMed] [Google Scholar]
- During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nature Med. 2003;9:1173–1179. doi: 10.1038/nm919. [DOI] [PubMed] [Google Scholar]
- Eser D, Baghai TC, Schuele C, Nothdurfter C, Ruppercht R. Neuroactive steroids as endogenous modulators of anxiety. Current Pharm Design. 2008;14:3225–3253. doi: 10.2174/138161208786848838. [DOI] [PubMed] [Google Scholar]
- Farr SA, Flood JF, Scherrer JF, Kaiser FE, Taylor GT, Morley JE. 58 A.D. Effect of ovarian steroids on footshock avoidance learning and retention in female mice. Physiol Behav. 58:715–723. doi: 10.1016/0031-9384(95)00124-2. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci USA. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Araiza C. Progesterone receptor isoforms expression in the prepuberal and adult male rat brain. Brain Res Bull. 2001;54:13–17. doi: 10.1016/s0361-9230(00)00410-x. [DOI] [PubMed] [Google Scholar]
- Guerra-Araiza C. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull. 2002;59:105–109. doi: 10.1016/s0361-9230(02)00845-6. [DOI] [PubMed] [Google Scholar]
- Gyermek L, Genther G, Fleming N. Some effects of progesterone and related steroids on the central nervous system. Int J Neuropharmacol. 1967;6:191–198. doi: 10.1016/0028-3908(67)90005-6. [DOI] [PubMed] [Google Scholar]
- Korneyev A. Regional and interspecies differences in brain progesterone metabolism. J Neurochem. 1993;61:2041–2047. doi: 10.1111/j.1471-4159.1993.tb07440.x. [DOI] [PubMed] [Google Scholar]
- Kumar TCA, David GFX, Sankaranarayanan A, Puri V, Sundram KR. Pharmacokinetics of progesterone after its administration to ovariectomized rhesus monkeys by injection, infusion, or nasal spraying. Proc Nat Acad Sci USA. 1982;79:4185–4189. doi: 10.1073/pnas.79.13.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacol (Berlin) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Marynick SP, Haven WW, Ebert MH, Loriaux DL. Studies on the transfer of steroid hormones across the blood-cerebrospinal fluid barrier in Rhesus monkey. Endocrinol. 1976;99:400–405. doi: 10.1210/endo-99-2-400. [DOI] [PubMed] [Google Scholar]
- Meyer DA, Carta M, Partridge LD, Covey DF, Valenzuela CF. Neurosteroids enhance spontaneous release in hippocampal neurons. Possible role of metabotropic sigma1-like receptors. J Biol Chem. 2002;277:28725–28732. doi: 10.1074/jbc.M202592200. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS, Grace M, Kneip J, Gosnell BA. The effect of ovariectomy, estradiol and progesterone on opioid modulation of feeding. Physiol Behav. 1984;33:237–241. doi: 10.1016/0031-9384(84)90105-7. [DOI] [PubMed] [Google Scholar]
- Nonaka N, Farr SA, Kageyama H, Shioda S, Banks WA. Delivery of galanin-like peptide to the brain: Targeting with intranasal delivery and cyclodextrins. J Pharmacol Exp Ther. 2008;325:513–519. doi: 10.1124/jpet.107.132381. [DOI] [PubMed] [Google Scholar]
- Orr PT, Lewis MC, Frick KM. Dorsal hippocampal progesterone infusions enhance object recognition in young female mice. Pharm Biochem Behav. 2009;93:177–182. doi: 10.1016/j.pbb.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR. Permissive influence of stress in the expression of a U-shaped relationship between serum corticosterone levels and spatial memory errors in rats. Dose Response. 2006;4:55–75. doi: 10.2203/dose-response.004.01.005.Park. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadri-Bakili G, Janis GC, Pierce RC, Gibbs TT, Farb DH. Nanomolar concentrations of pregnenolone sulfate enhance striatal dopamine overflow in vivo. J Pharmacol Exp Ther. 2008;327:840–845. doi: 10.1124/jpet.108.143958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu E. Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev. 2007;28:387–439. doi: 10.1210/er.2006-0050. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Liere P, Akwa Y, Rajkowski K, Griffiths W, Bodin K, Sjovall J, Baulieu EE. Pregnenolone sulfate in the brain: A controversial neurosteroid. Neurochem Int. 2008;52:522–540. doi: 10.1016/j.neuint.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Shiraishi M, Shibuya I, Minami K, Uezono Y, Okamoto T, Yanagihara N, Ueno S, Ueta Y, Shigematsu A. A neurosteroid anesthetic, alphaxalone, inhibits nicotinic acetylcholine receptors in cultured bovine adrenal chromaffin cells. Anesth Analg. 2002;95:900–906. doi: 10.1097/00000539-200210000-00020. [DOI] [PubMed] [Google Scholar]
- Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- Valée M, Mayo W, Le Moal M. Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging. Brain Res Rev. 2001;37:301–312. doi: 10.1016/s0165-0173(01)00135-7. [DOI] [PubMed] [Google Scholar]
- Wang MD. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intavenous infusion. J Steroid Biochem Mol Biol. 1997;62:299–306. doi: 10.1016/s0960-0760(97)00041-1. [DOI] [PubMed] [Google Scholar]
- Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Prog Neurobiol. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]


