Conspectus
Enantioselective allylic substitution reactions comprise some of the most versatile methods for preparing enantiomerically enriched materials. These reactions form products that contain multiple functionalities by creating carbon–nitrogen, carbon–oxygen, carbon–carbon, and carbon–sulfur bonds. For many years, the development of catalysts for allylic substitution focused on palladium complexes. However, studies of complexes of other metals have revealed selectivities that often complement those of palladium systems. Most striking is the observation that reactions with unsymmetrical allylic electrophiles that typically occur with palladium catalysts at the less hindered site of an allylic electrophile occur at the more hindered site with catalysts based on other metals. In this Account, we describe an iridium precursor and a phosphoramidite ligand that catalyze reactions with a particularly broad scope of nucleophiles.
The active form of this iridium catalyst is not generated by the simple binding of the phosphoramidite ligand to the metal precursor. Instead, the initial phosphoramidite and iridium precursor react in the presence of base to form a metallacyclic species that is the active catalyst. This species is generated either in situ or separately in isolated form by reactions with added base. The identification of the structure of the active catalyst led to the development of simplified catalysts as well as the most active form of the catalyst now available, which is stabilized by a loosely bound ethylene. Most recently, this structure was used to prepare intermediates containing allyl ligands, the structures of which provide a model for the enantioselectivities discussed here.
Initial studies from our laboratory on the scope of iridium-catalyzed allylic substitution showed that reactions of primary and secondary amines, including alkylamines, benzylamines, and allylamines, and reactions of phenoxides and alkoxides occurred in high yields, with high branched-to-linear ratios and high enantioselectivities. Parallel mechanistic studies had revealed the metallacyclic structure of the active catalyst, and subsequent experiments with the purposefully formed metallacycle increased the reaction scope dramatically. Aromatic amines, azoles, ammonia, and amides and carbamates as ammonia equivalents all reacted with high selectivities and yields. Moreover, weakly basic enolates (such as silyl enol ethers) and enolate equivalents (such as enamines) also reacted, and other research groups have used this catalyst to conduct reactions of stabilized carbon nucleophiles in the absence of additional base.
One hallmark of the reactions catalyzed by this iridium system is the invariably high enantioselectivity, which reflects a high stereoselectivity for formation of the allyl intermediate. Enantioselectivity typically exceeds 95%, regioselectivity for formation of branched over linear products is usually near 20:1, and yields generally exceed 75% and are often greater than 90%. Thus, the development of iridium catalysts for enantioselective allylic substitution shows how studies of reaction mechanism can lead to a particularly active and a remarkably general system for an enantioselective process. In this case, a readily accessible catalyst effects allylic substitution, with high enantioselectivity and regioselectivity complementary to that of the venerable palladium systems.
1. Introduction
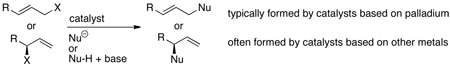
Catalytic allylic substitution (eq 1) was one of the first examples of organometallic catalysts applied to organic synthesis.1–3 For decades, the development of catalysts for these reactions focused on palladium complexes.2–4 The scope of nucleophiles that have been shown to undergo palladium-catalyzed allylic substitution is broad, and many ligands have been identified that create catalysts for enantioselective allylic substitution with high stereoselectivities. However, reactions catalyzed by complexes of other metals often occur with selectivities that differ from those of palladium. For this reason allylic substitution catalyzed by complexes of metals other than palladium has become a focus of recent research.
 |
(1) |
In particular, palladium catalysts usually form linear, achiral products from the reactions of allylic electrophiles that are substituted at only one terminus, whereas catalysts based on other metals typically form branched, chiral products from the reactions of such electrophiles (eq 1). Although complexes of many metals, including certain palladium systems,5 form products from substitution at the more hindered position of an allylic electrophile, few such complexes are readily accessible, give the branched isomer from a wide range of allylic electrophiles, and react with high enantioselectivities. The development of this chemistry with complexes of molybdenum and tungsten6–8 had progressed the furthest prior to the work described in this account, but reactions catalyzed by complexes of these metals encompassed only certain classes of carbon nucleophiles and did not encompass heteroatom nucleophiles.
Work from the laboratories of the authors, with contributions from Alexakis and Helmchen, led to complexes generated from an iridium precursor and a phosphoramidite ligand that catalyze allylic substitution of a broad range of carbon and heteroatom nucleophiles at the more substituted position of unsymmetrical allylic electrophiles with high enantioselectivity. Mechanistic studies of this process conducted in the authors’ laboratory revealed an unusual structure of the active catalyst that is far from that of the species formed by simple coordination of the phosphoramidite ligand to the iridium precursor. Identification of this catalyst structure led to dramatic improvements in rate and scope. This account describes asymmetric allylic substitution catalyzed by these new iridium complexes and how identification of the structure of the true catalyst was crucial to this development.
2. Prior studies on rhodium- and iridium-catalyzed allylic substitution
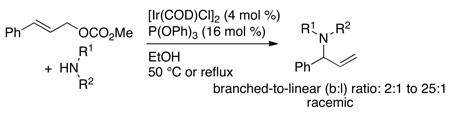
Prior to the work described in this account, the group of P. Andrew Evans had shown that optically enriched, branched allylic carbonates underwent nucleophilic substitution with a high degree of retention of configuration in the presence of rhodium catalysts (eq 2).9 In addition, Takeuchi and coworkers had shown that iridium complexes catalyze allylic alkylation and amination of linear allylic carbonates to generate branched substitution products (for an example, see eq 3).10–12 However, these rhodium and iridium catalysts were achiral. One early publication by Helmchen showed that iridium complexes of chiral phosphine-oxazoline ligands catalyzed asymmetric allylic substitution of soft carbon nucleophiles with excellent regioselectivity and high enantioselectivity (eq 4).13 Further studies showed that a catalyst generated from [Ir(COD)Cl]2 and a chiral phosphoramidite ligand containing a dimethyl amino group14 was more active than that containing the phosphine-oxazoline, but enantioselectivities of allylic alkylation reactions with this second system varied from 4% to 86% ee (eq 5), and those of allylic aminations were 11–13% ee (eq 6).14,15
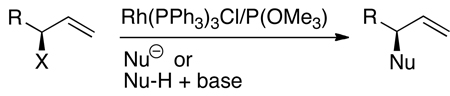 |
(2) |
 |
(3) |
 |
(4) |
 |
(5) |
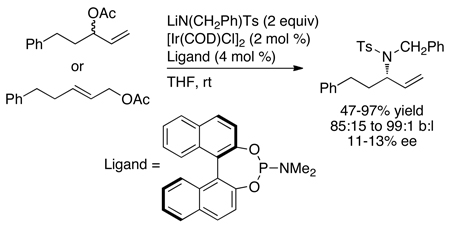 |
(6) |
3. Discovery of a general catalyst for iridium-catalyzed enantioselective allylic substitution
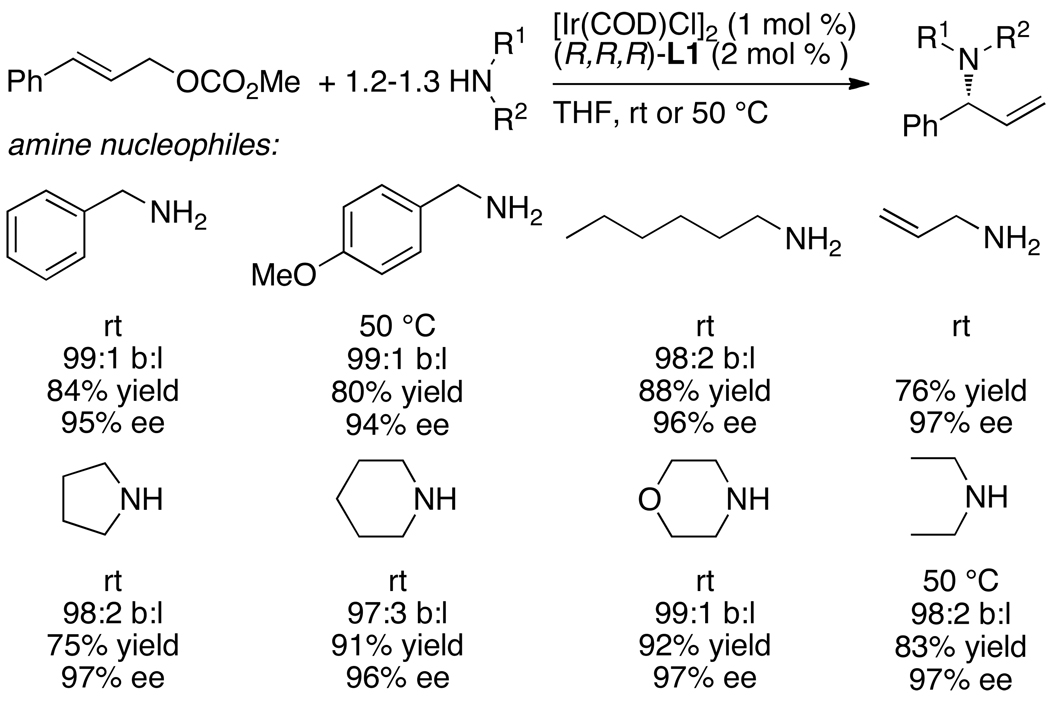
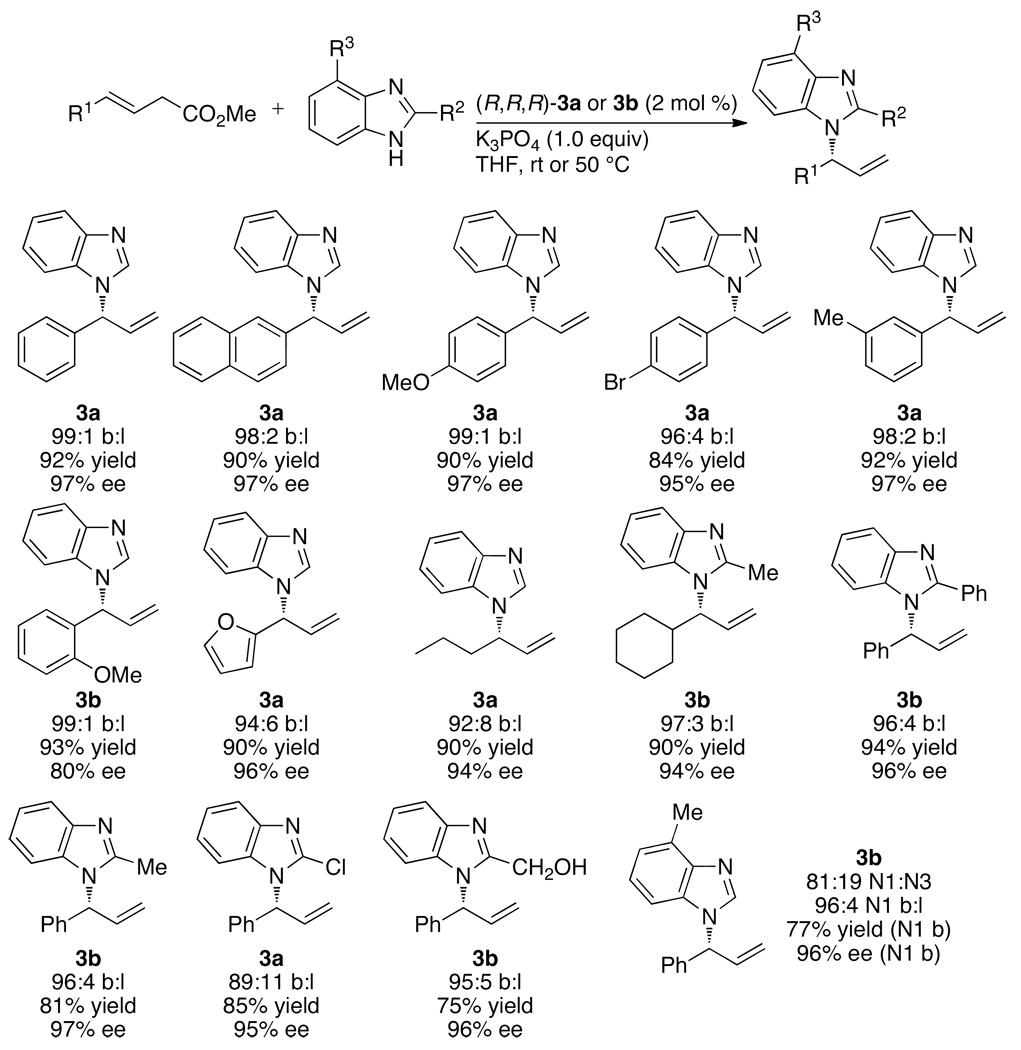
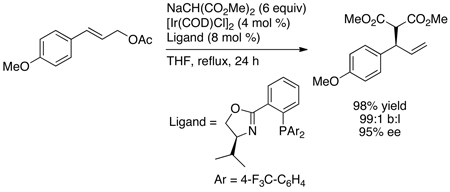
In contrast to this prior art, reactions between neutral amine nucleophiles and linear allylic carbonates conducted by Toshimichi Ohmura in the author’s laboratory with an iridium catalyst generated from the phosphoramidite L1 occurred in 58–95% yield, with high branch-to-linear ratios (11:1 to >99:1), and 94–97% enantioselectivities (eq 7 and Chart 1) in all but two cases.16 Moreover, these initial reactions occurred at room temperature with only 2 mol % iridium. Allylic alkylation catalyzed by [Ir(COD)Cl]2 and L1 was reported by Helmchen at a similar time,17 and these reactions occurred in 56–98% yield with regioselectivities between 1:1 and 10:1 and 82–94% ee (eq 8). The high selectivities and yields observed for the asymmetric allylic amination, in particular, marked a clear path for studies on the development of the scope of iridium-catalyzed allylic substitution, but the mechanistic complexity of this system and the importance of subsequent mechanistic studies were unanticipated. This complexity created several roadblocks and several resulting experimental detours.
Chart 1.
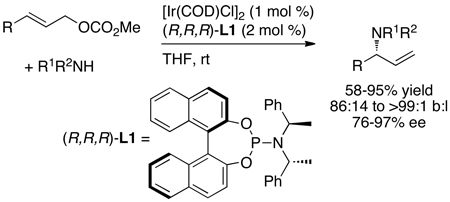 |
(7) |
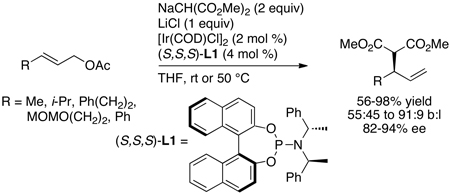 |
(8) |
The high enantioselectivity observed with a 1:1 ratio of iridium to phosphoramidite was initially perplexing. A highly enantioselective catalyst containing a single monodentate phosphorus ligand is rare; usually reactions of complexes of bidentate chiral ligands occur with higher enantioselectivity because the second point of binding dramatically decreases the number of degrees of freedom of the ligand.18 The origin of the observed enantioselectivity will be discussed in the section describing our studies on catalysts with edited stereochemistry.
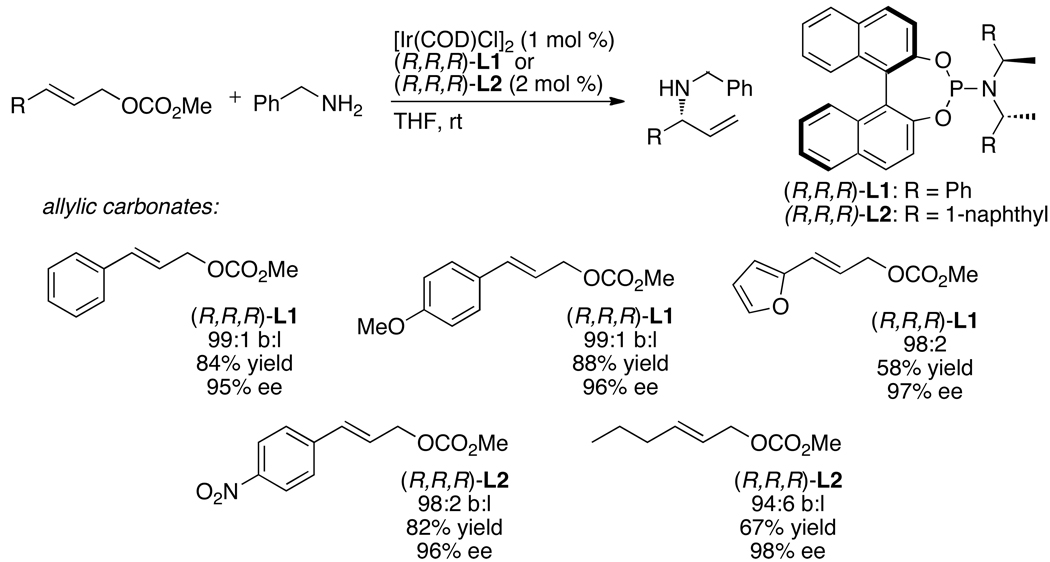
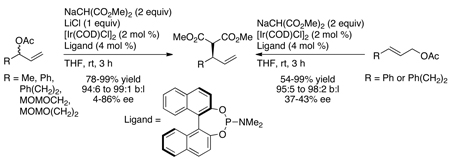
As shown in Chart 1, a broad range of primary benzylic, allylic and saturated aliphatic amines, as well as cyclic and acyclic secondary amines, underwent iridium-catalyzed allylic substitution in good yields with good branched-to-linear ratios and high enantioselectivities (typically 94–97% ee) at room temperature to 50 °C. Reactions of primary amines occurred with good selectivity for formation of the mono-allylation product (>98:2). As shown in Chart 2, the reaction also occurred with a series of allylic carbonates.16,19 These allylic substitutions even occurred with high enantioselectivities with purely straight-chain, aliphatic carbonates, as shown by the last example in Chart 2, which occurred in 98% ee.
Chart 2.
4. Initial scope of iridium-catalyzed allylic substitution
4.1. Reactions of oxygen nucleophiles
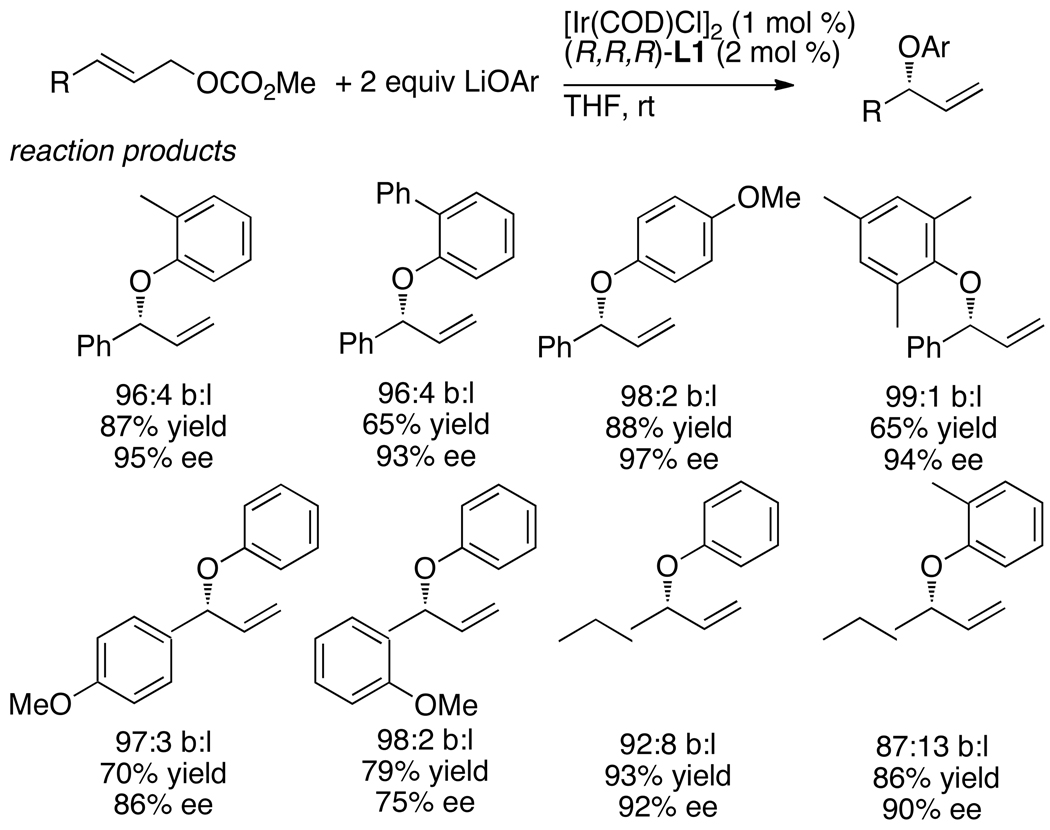
Allylic substitution to form the branched product also occurred with high yields and selectivities with oxygen nucleophiles.20,21 Allylic substitutions with phenoxide nucleophiles had been reported with rhodium22 and palladium catalysts,23,24 but enantioselective reactions that formed products from substitution at the more hindered position with high regioselectivity were rare. Toshimichi Ohmura and Fernando Lopez showed that reactions of lithium and sodium phenoxides with allylic carbonates occurred in good yield with moderate catalyst loadings and with good to excellent branch-to-linear ratios and enantioselectivities (Chart 3).20 With the system developed at that time (vide infra for improved catalysts), some of the reactions of the phenoxide nucleophiles occurred with lower enantioselectivities than had been observed with nitrogen nucleophiles. However, the enantioselectivities remained above 90% in most cases.
Chart 3.
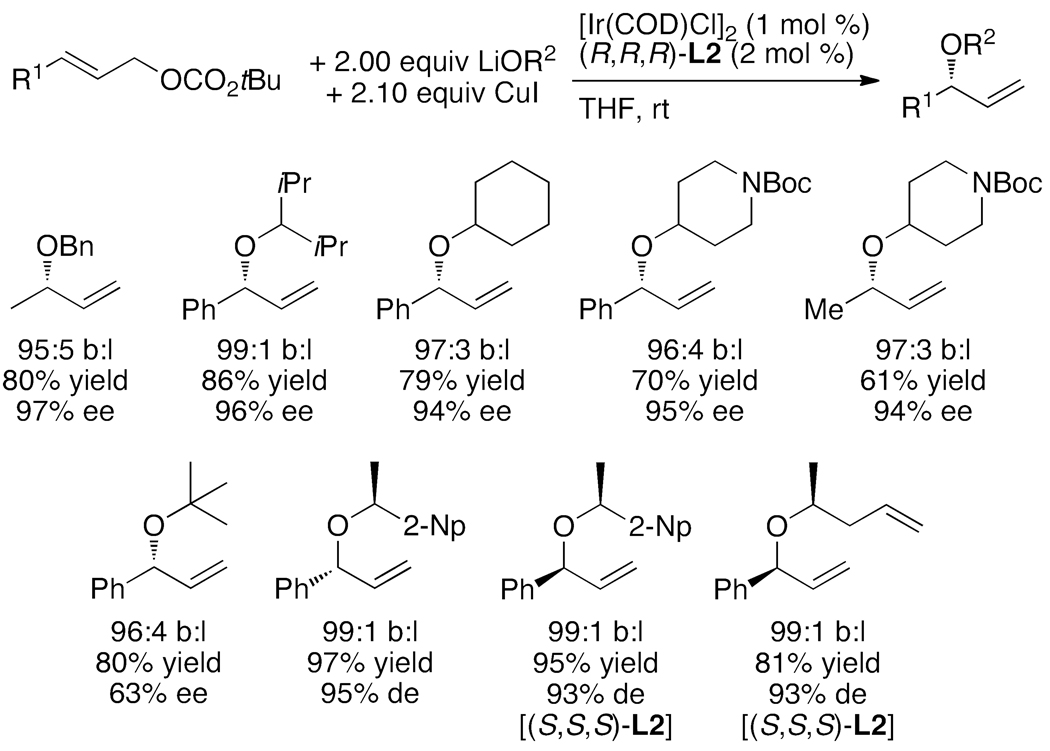
In contrast to reactions of phenoxide nucleophiles, there was little precedent for intermolecular enantioselective reactions of aliphatic alkoxides with allylic electrophiles. Evans had shown that achiral rhodium complexes catalyze stereoretentive allylic substitution with copper alkoxides,25,26 but no complex of any metal catalyzed intermolecular asymmetric allylic substitution of achiral allylic electrophiles with aliphatic alkoxide nucleophiles with both high yield and enantioselectivity. Using the catalyst generated from the phosphoramidite ligand L2 containing a bis(1-naphthylethyl)amino group on phosphorus, Chutian Shu developed reactions of aliphatic alkoxides that occurred in high yields and with enantioselectivities that were similar to those observed with nitrogen nucleophiles (Chart 4).21 Reactions of hindered and unhindered alkoxides occurred with high selectivities with aromatic and aliphatic allylic carbonates. In some cases, these reactions formed hindered ethers that are challenging to prepare by the Williamson ether synthesis due to competing elimination. In addition, these reactions occurred with allylic or homoallylic alcohols to form precursors to oxygen heterocycles with defined stereochemistry alpha to oxygen by olefin metathesis. The catalyst also controlled the diastereoselectivity of reactions of chiral alkoxides. The diastereoselectivities were nearly identical to the enantioselectivities of reactions of achiral alcohols. This first set of studies was conducted with copper alkoxides to temper the basicity of the nucleophile, but later studies by Satoshi Ueno showed that these reactions occurred in a similar fashion with the combination of alcohol and phosphate base.27 Related reactions of KOSiEt3 with aromatic, heteroaromatic, dienyl, and aliphatic allylic carbonates were shown by Carreira and co-workers to occur with this catalyst to form protected allylic alcohols in high ee (eq 9).28
Chart 4.
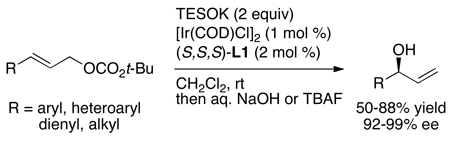 |
(9) |
4.2. Reactions of carbon nucleophiles
This combination of phosphoramidite and iridium precursor also catalyzed reactions of carbon nucleophiles with high selectivities. Work of other groups showed that this system catalyzes the reactions of stabilized, anionic carbon nucleophiles typically used for allylic substitution.29–32 Helmchen33–35 and You36 have subsequently shown that the this iridium-phosphoramidite complex catalyzes reactions of allylic carbonates with a series acidic carbon pronucleophiles under the salt-free conditions initially reported by Tsuji with palladium catalysts.37 Our studies aimed to exploit this iridium catalyst for reactions of nucleophiles that typically have been less amenable to allylic substitution processes.
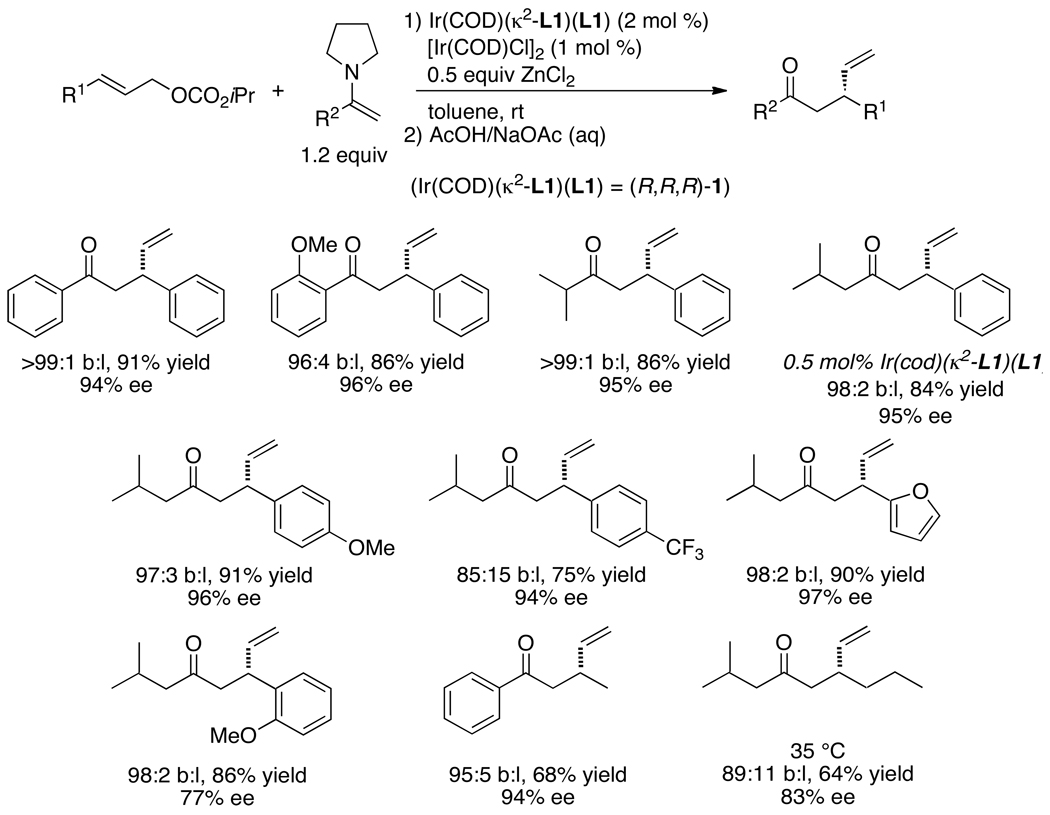
Tim Graening showed that an unusual combination of two different metal fluorides activated trimethylsilyl enol ethers toward iridium-catalyzed asymmetric allylic substitution (Chart 5).38 Reactions of allylic carbonates with silyl enol ethers and cesium fluoride alone as activator led to cleavage of the silyl enol ether to form the cesium enolate that reacted with unremarkable regio-and enantioselectivities. The same reaction, but with zinc fluoride alone as activator, occurred to low conversion. However, the reactions of allylic carbonates with silyl enol ethers and a combination of these two metal fluorides occurred to form homoallylic ketones containing a stereogenic center beta to the carbonyl group with high regioselectivity and enantioselectivity. Reactions of aromatic ketones occurred in high yield; reactions of aliphatic ketones occurred with high selectivities but in modest yields. Improved reactions with alternative equivalents of aliphatic ketone enolates are described later in this account.
Chart 5.
5. Mechanistic studies
5.1. Identification of a catalytically active cyclometallated species
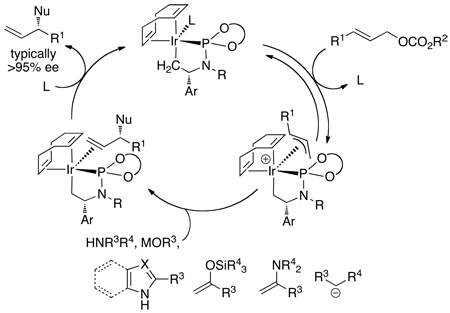
Although this iridium system catalyzed asymmetric allylation of many nitrogen, oxygen, and carbon nucleophiles, several classes of such nucleophiles did not react in the presence of the original catalyst. For example, weakly basic amines, such as aromatic amines, weakly basic carbon nucleophiles, such as enamines, and weakly basic heterocycles, such as imidazoles, failed to react in the presence of the original catalyst. Thus, we sought to identify intermediates in the allylic substitution process. These studies underscored how the active catalyst in a system can be much different than planned. Through a series of mechanistic studies, each leading to procedures that improved the reaction scope, we developed a detailed picture of the structure of the active catalyst, the relative rates of the individual steps of the catalytic cycle, and the origin of enantioselection.
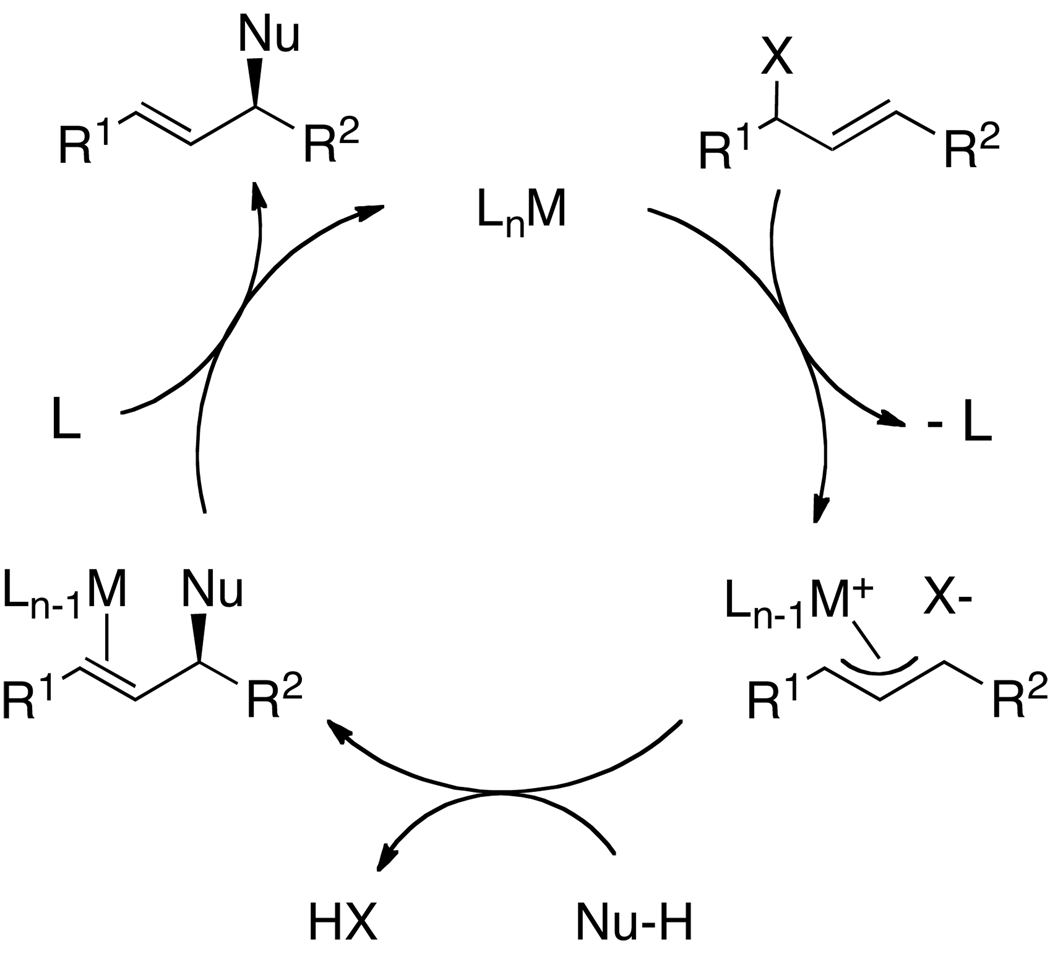
A typical catalytic cycle for allylic substitution1,3 occurs by oxidative addition of an allylic electrophile to a low-valent, transition-metal complex to generate an allyl-metal intermediate (Scheme 1). This intermediate then reacts with the nucleophile, most commonly by backside attack of the nucleophile on a cationic version of the allyl-metal intermediate. Dissociation of the product from the metal center regenerates the active catalyst. The identity of the low-valent metal complex and the allyl intermediate in our iridium-catalyzed process was unclear.
Scheme 1.
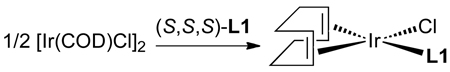 |
(10) |
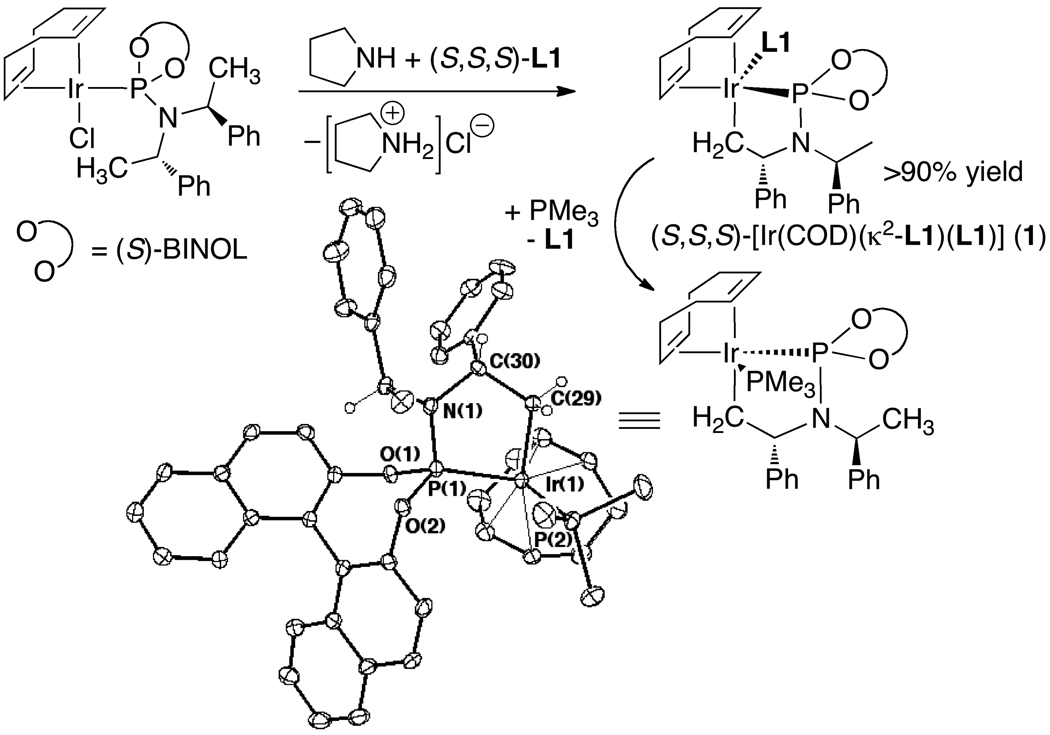
Initial studies by Christoph Kiener39 showed that [Ir(COD)Cl]2 and L1 formed the square planar complex in eq 10. However, this iridium(I) species did not react with the allylic carbonate, indicating that this iridium(I) species was not a catalytic intermediate. The true intermediate was elusive until Christoph identified the metallacyclic product 1 in Scheme 2 resulting from reaction of [Ir(COD)Cl(L1)] with an alkylamine. Intramolecular C–H activation of a ligand methyl group, followed by elimination of alkylamine•HCl, formed the five-coordinate metallacycle 1 containing one cyclometallated κ2-phosphoramidite and one κ1-phosphoramidite. Displacement of the κ1-phosphoramidite by trimethylphosphine yielded crystals suitable for X-ray diffraction and definitive identification of the structure of the metallacyclic core.
Scheme 2.
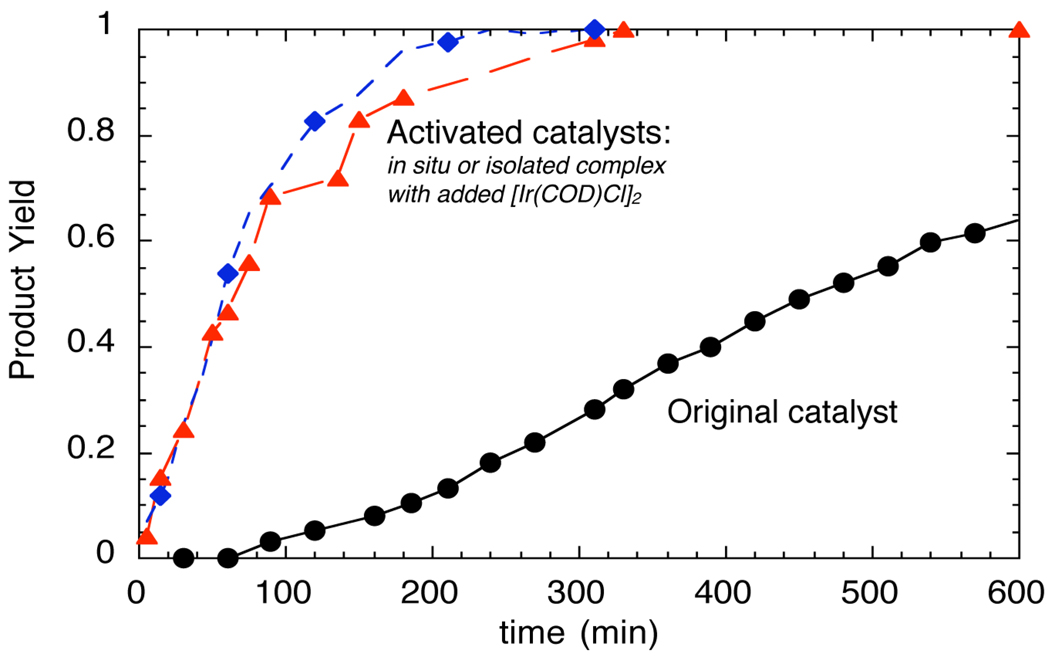
The formation of this cyclometallated species does not require it to be relevant to the catalytic cycle. Many palladacycles have been shown to be catalyst precursors, rather than the active catalyst.40,41 The profile of reactions catalyzed by the initial system generated in situ, the metallacyclic species generated prior to adding the reagents, and the isolated five-coordinate complex with an additional equivalent of [Ir(COD)Cl]2 in Figure 1 provides information on the competence of the metallacycle to be an intermediate in the catalytic process. Reactions in the presence of the pre-activated catalyst occurred faster than reactions with the original system, and the appearance of product during the reactions of the original catalyst occurred with a clear induction period. Thus, the cyclometallated core appeared to be present in the active catalyst, and cyclometallation is likely occurring during the induction period observed with the original system.
Figure 1.
Profile for the reaction of benzylamine with methyl cinnamyl carbonate catalyzed by the species generated in situ from [Ir(COD)Cl]2L1, and H2NPr (blue,  ), the combination of isolated metallacyclic complex 1 and [Ir(COD)Cl]2 (red,
), the combination of isolated metallacyclic complex 1 and [Ir(COD)Cl]2 (red,  ), or the original system generated by combining [Ir(COD)Cl]2 and L1 (black, ●).
), or the original system generated by combining [Ir(COD)Cl]2 and L1 (black, ●).
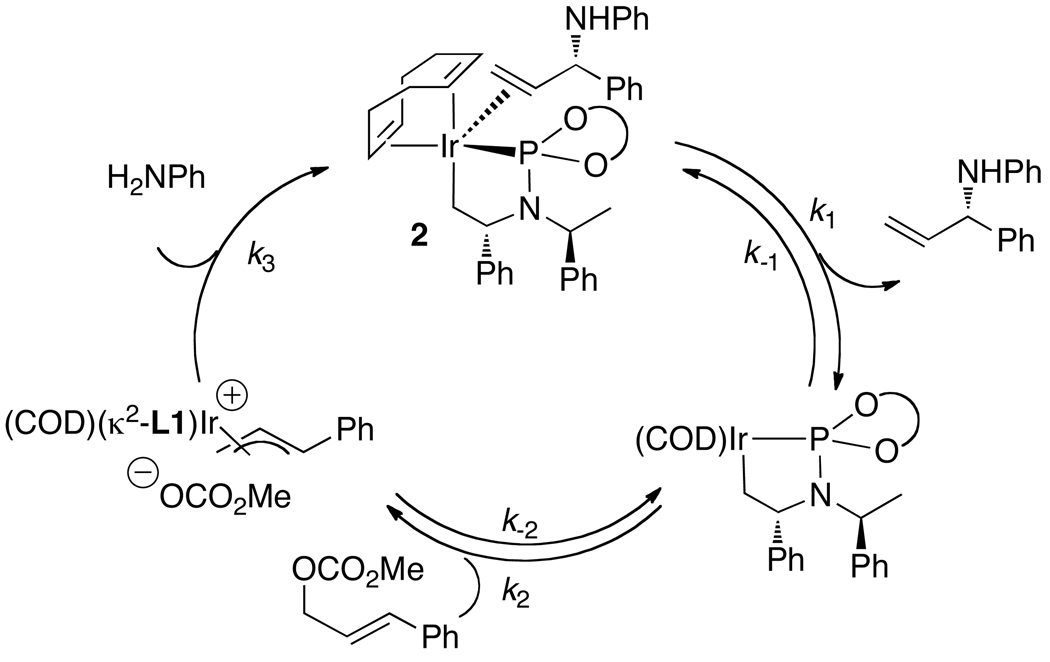
Based on this result, several procedures have been developed for the generation of the active catalyst in situ. The reaction of [Ir(COD)Cl]2 and ligand with propylamine, followed by evaporation of the volatile materials, generates a solid consisting of the five-coordinate species and remaining [Ir(COD)Cl]2. This solid was stored as a stable form of the metallacyclic catalyst. Now, this protocol or related protocols31,42,43 for the generation of the metallacyclic catalyst by addition of base to [Ir(COD)Cl]2 and L1 or a variant of L1 are common in reactions involving weakly basic nucleophiles.27,33–35,38,43–47
5.2. Identification of the catalyst resting state and analysis of the reaction kinetics
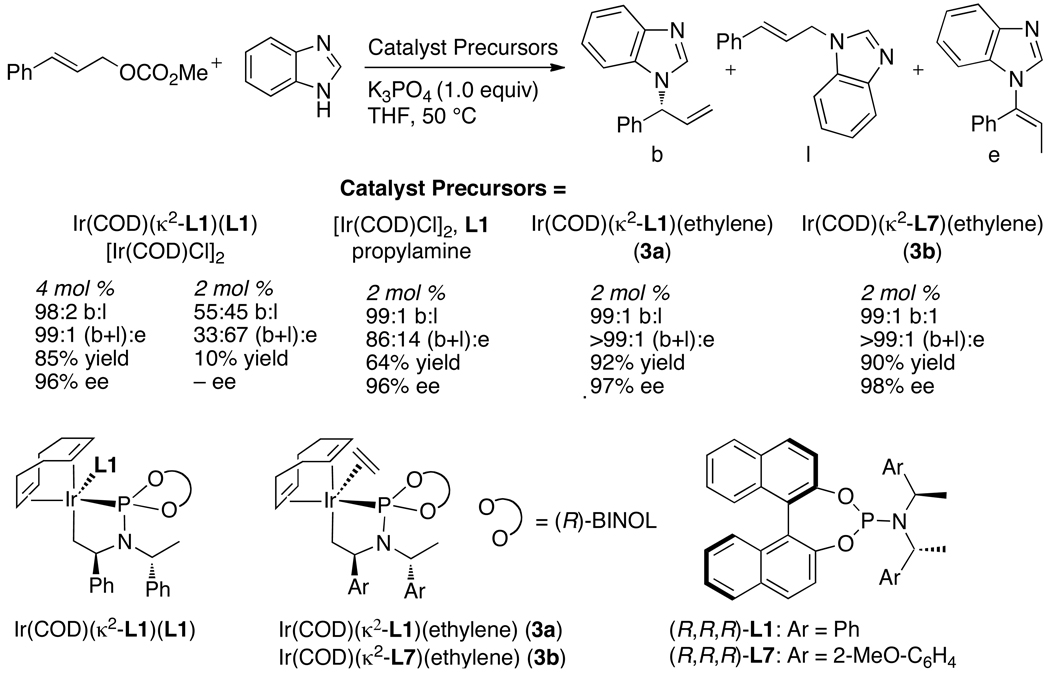
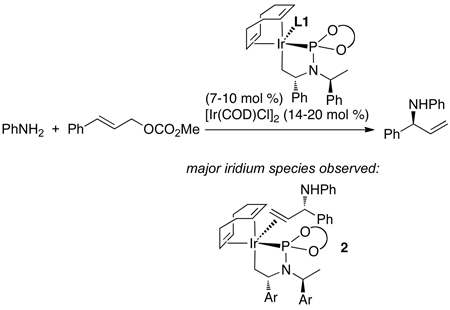
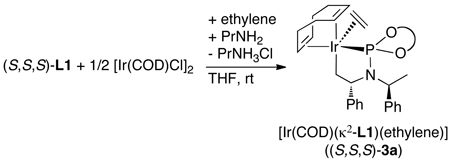
Dean Markovic then sought to identify the species contained within the catalytic cycle.48 By monitoring the reaction of aniline with methyl cinnamyl carbonate in the presence of the pre-activated iridium catalyst he showed that the major iridium complex in this system was 2 in eq 11 containing the coordinated allylic amine product. This complex was independently synthesized from [Ir(COD)Cl]2, the phosphoramidite ligand, the allylic amine and propylamine as base. A more stable analog (3a) containing ethylene in place of the allylic amine was prepared by a similar method (eq 12). This ethylene complex has been prepared on multi-gram scale in a single step from commercial precursors in yields above 90%.
 |
(11) |
 |
(12) |
 |
(13) |
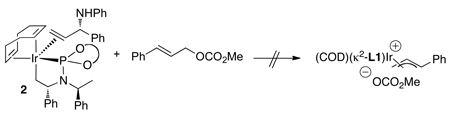
Studies on the generation of allyliridium complexes led to some unexpected information on the catalytic system. Treatment of the olefin adduct 2 with methyl cinnamyl carbonate led to no observable change in the starting olefin adduct (eq 13).48 This result could imply that the alkene adduct, and any allyl complex derived from it, are not relevant to the catalytic cycle. Alternatively, the allyliridium species could lie uphill thermodynamically from the olefin adduct and form reversibly. In the latter case, the reversible addition of the allylic carbonate would be followed by an irreversible reaction with the nucleophile (Scheme 3). This catalytic cycle would be first order in catalyst, inverse-first order in the bound olefin, and first order in both the electrophile and nucleophile. The experimental data matched this prediction.48
Scheme 3.
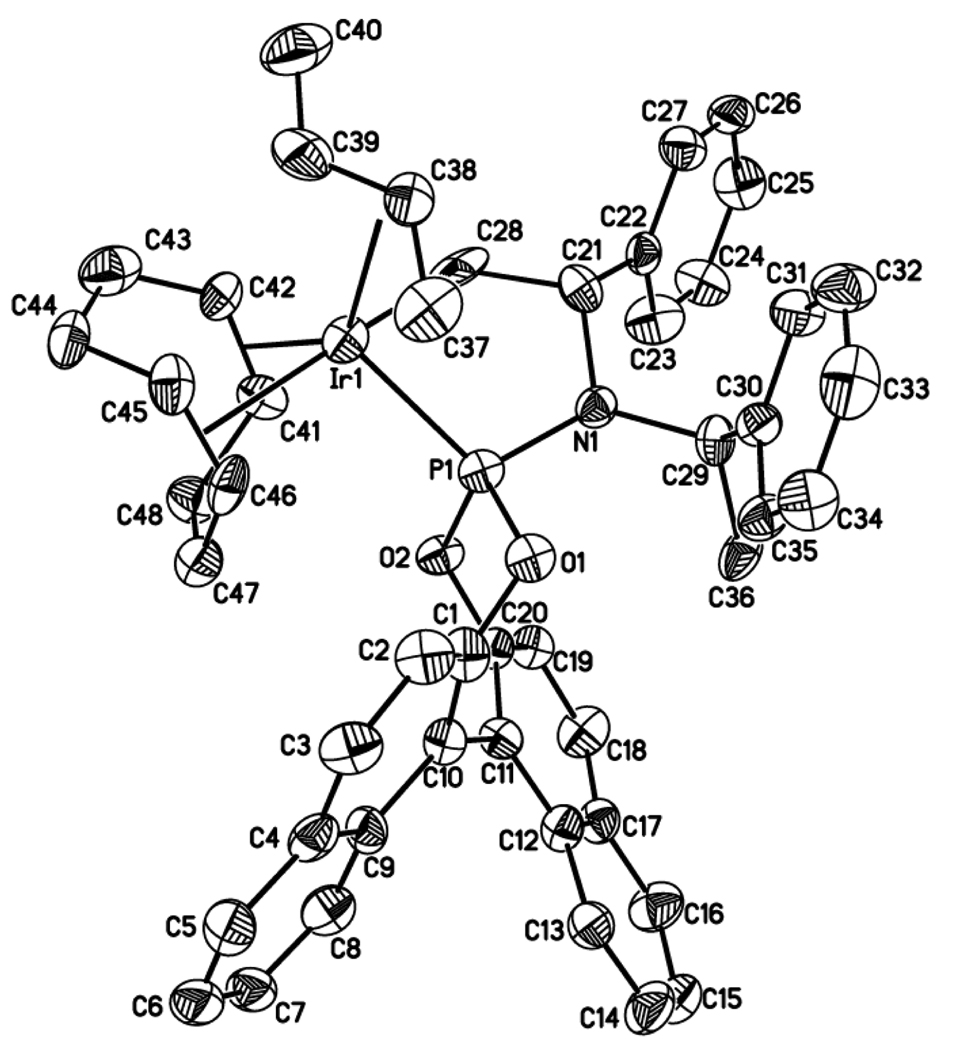
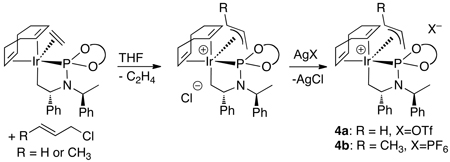
With further support of the relevance of the observed complexes to the catalytic chemistry, we resolved to isolate the allyliridium intermediates and succeeded by using more reactive allylic electrophiles.49 Reaction of the ethylene complex 3a with allyl chloride or crotyl chloride formed new iridium complexes that contain an allyl unit. Addition of a silver salt containing a poorly coordinating anion allowed us to isolate the stable allyliridium and crotyliridium species 4a and 4b (eq 14). Sherzod Madrahimov obtained single crystals of several allyliridium species that were suitable for X-ray diffraction; the structure of the crotyliridium intermediate 4b is shown in Figure 2.
Figure 2.
ORTEP diagram of crotyl complex 4b
 |
(14) |
The metallacyclic core of the proposed activated catalyst is maintained in the crotyliridium species 4b, and the cyclooctadiene ligand of the catalyst precursor remains bound. From this structure, the origin of enantioselectivity can be deduced. Stoichiometric reactions of this complex showed that the major diastereomer forms the major enantiomer of the product, and ongoing studies imply that this reaction occurs by backside attack of the nucleophile onto the allyl ligand. The nearly constant enantiomeric excess for a wide range of nucleophiles implies that the stereoselectivity for formation of the allyl intermediates or the thermodynamics of the resulting diastereomeric allyl complexes controls enantioselectivity.
The structure in Figure 2 shows why one face of the allyl ligand binds preferentially to the iridium fragment. The central allyl carbon C38 lies nearest C21 of the metallacycle, and this C21 contains a hydrogen atom on the side of the ring containing the allyl unit. The substituent on the allyl unit (C40 of the crotyl ligand) is located on the exterior of the complex, and a large group in this position would contact only solvent. Binding of the metal to the opposite face of the allyl group, while maintaining the orientation of the substituted and unsubstituted termini of the crotyl ligand, would lead to close contact of the C38 carbon with the cyclooctadiene ligand. Binding of the metal to the opposite face of the allyl group by exchanging the positions of the substituted and unsubstituted ends of the crotyl ligand would cause the C40 carbon on the allyl group to contact the BINOLate substituent on phosphorus. This analysis shows how the metallacyclic fragment, which lacks any degree of symmetry, selectively binds one face of the allyl unit over the other.
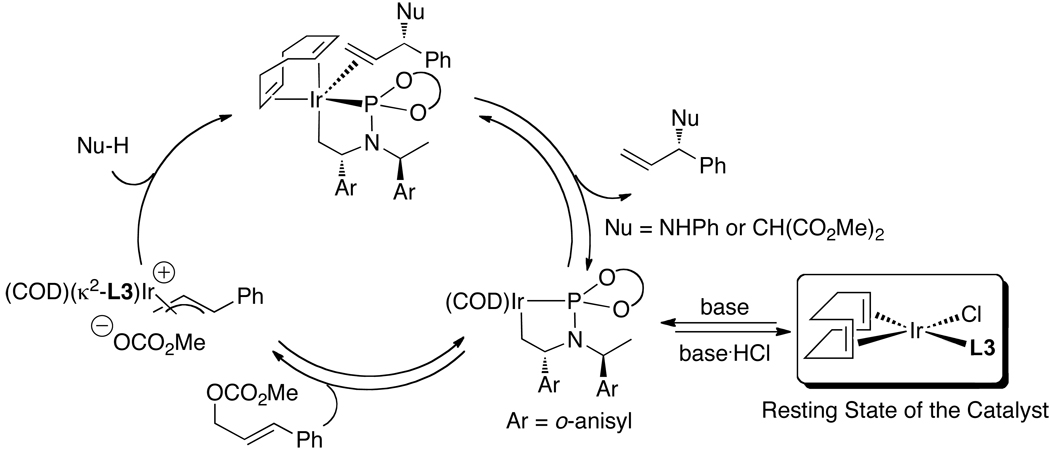
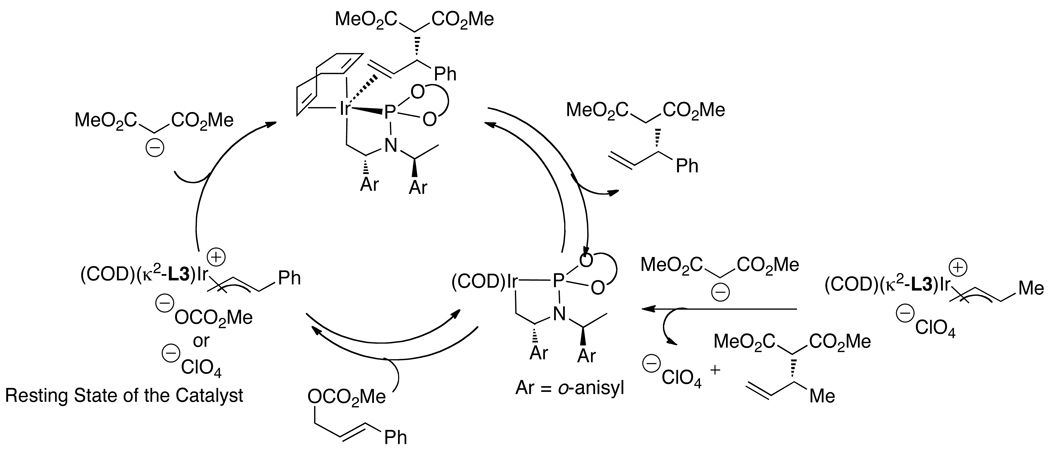
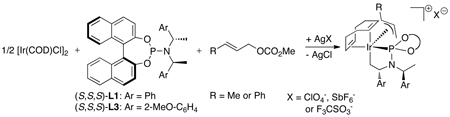
Helmchen and coworkers recently reported an alternative more direct method to synthesize allyliridium complexes.50,51 Crotyliridium and cinnamyliridium complexes were prepared in high yields from [Ir(COD)Cl]2, phosphoramidite ligands L1 or L3, a silver salt containing a weakly coordinating counterion, and either crotyl methyl carbonate or cinnamyl methyl carbonate (eq 15). In addition, they found that the resting state of the catalyst depends on the precursor and reaction conditions. When the reactions are conducted with the catalyst generated in situ and an ammonium salt is present, most of the active catalyst reacts with the acid to open the metallacycle core and form the active species [Ir(COD)(L3)Cl] as the major iridium complex in solution (Scheme 4). These results underscore the value of conducting the allylation reactions with the isolated metallacyclic species, such as ethylene complex 3a or allyliridium complexes themselves, to maximize the amount of active catalyst. When allylic alkylations with dimethyl mealonate were initiated with the allyliridium complex, the allyl complex was observed as the resting state (Scheme 5). The observation of these species contrasts with the observation of alkene complexes as the resting state when weakly coordinating anions are absent (eq 11). Thus, the factors that control the relative rates for the individual steps within the cycle have not yet been delineated.
Scheme 4.
Scheme 5.
 |
(15) |
6. Studies on catalysts having edited stereochemistry
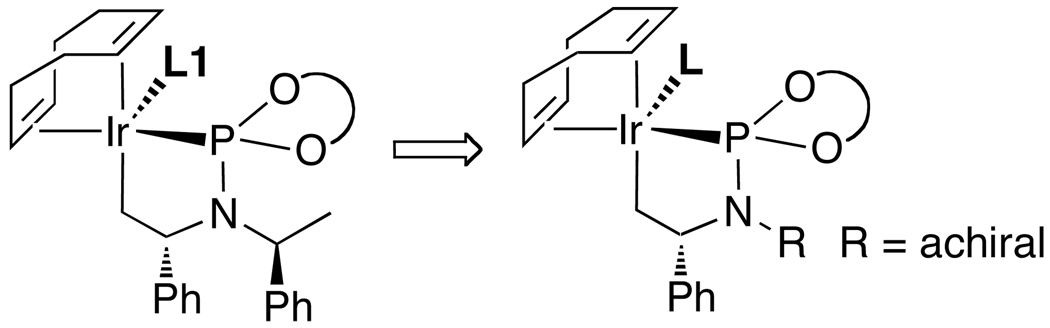
Based on the structure of the active catalyst, Andreas Leitner developed sterochemically edited complexes that are nearly equivalent in activity and selectivity as the original system.52,53 The phenethyl substituent attached the metallacyclic core of the structures in Scheme 2 and Figure 2 is distal from the metal center, and, we proposed that this substituent could be replaced by a group lacking any stereocenter (Figure 3). Thus, Andreas prepared a series of unsymmetrical phosphoramidite ligands containing a substituent on nitrogen derived from a symmetric ketone. The three ligands shown in Chart 6 show that the size of this substituent is important and that the second phenethyl group lacks an intimate role in controlling enantioselectivity.
Figure 3.
Strategy for elimination of one stereochemical element in the active catalyst
Chart 6.
The ligand containing a large cyclododecyl substituent (large cyclic ketones come from C4 precursors) generated a catalyst that reacted with essentially identical enantioselectivity to that of the original system containing the phosphoramidite bearing two phenethyl substituents on nitrogen. The enantioselectivities of reactions catalyzed by complexes of this ligand ranged from 95–98%, the branched-to-linear ratios ranged from roughly 20:1 to 100:1, and the yields remained high.
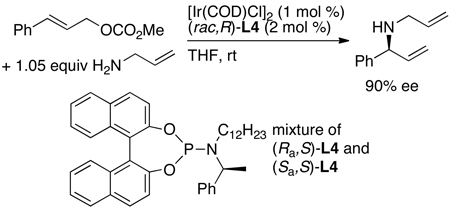
To determine whether the binaphtholate substituent on phosphorus or the phenethyl substituent remaining on the nitrogen was responsible for the absolute stereochemistry, we conducted two sets of experiments. First, we conducted the reaction with a mixture of diastereomeric catalysts derived from racemic binaphthol ((Sa,S)-L4 and (Ra,S)-L4).52 The allylic amine product formed with nearly the same enantiomeric excess as that of the original system (eq 16), implying that one diastereomer is much more reactive than the other. Further studies showed that faster cyclometallation of the (Sa,S)-diastereomer, relative to cyclometallation of the (Ra,S) diastereomer, accounts for the stereochemistry of the product from these experiments. Thus, these experiments do not reflect the relative rates of the two diastereomeric catalysts within the catalytic cycle.
 |
(16) |
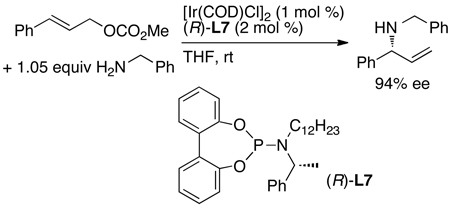
This conclusion led to studies of catalysts containing ligand L7, which bears a biphenolate group.52 The catalyst containing this ligand readily accesses both diastereomers of the active catalyst. Reactions catalyzed by the complex of (R)-L7 containing one phenethyl substituent as the only resolved stereochemical element occurred with enantioselectivities that were nearly as high as those of the original system, and the absolute stereochemistry of the product was the same as that derived from the original (R,R)-diastereomer of the phosphoramidite ligand (eq 17). This result implies that the R,R configurations of the ligands L4 and L7 are the most reactive within the catalytic cycle.
 |
(17) |
7. Further advances in ligand design
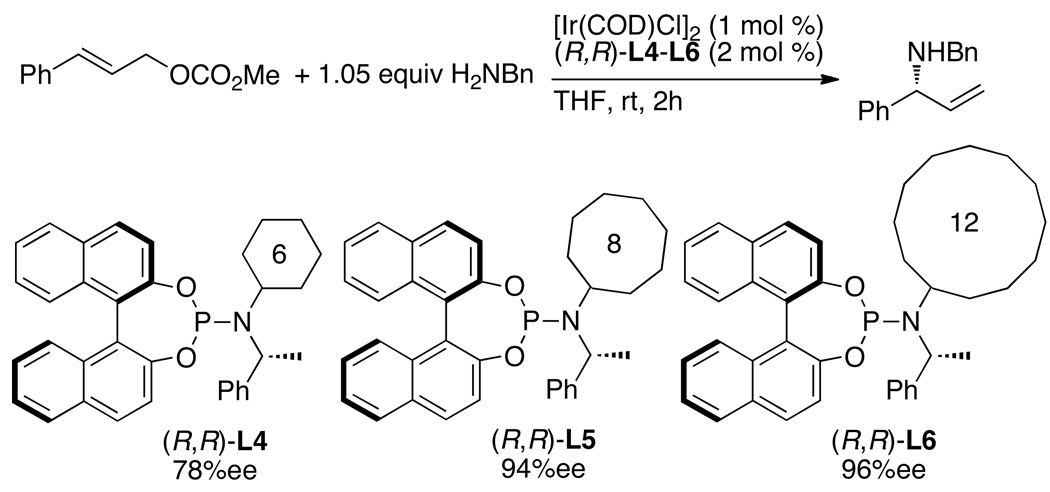
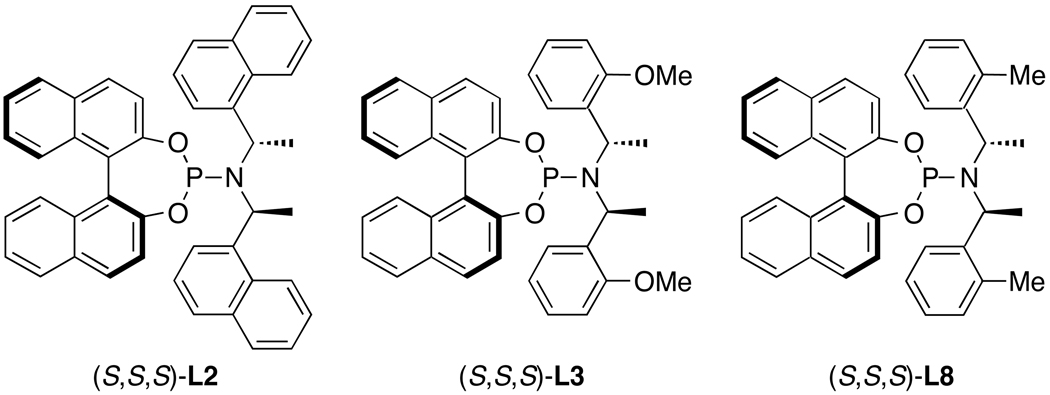
Although the combinations of [Ir(COD)Cl]2 with phosphoramidite ligands containing only one fixed stereochemical element, such as L7, form catalysts that generate highly enantioenriched allylic substitution products, catalysts prepared from the phosphoramidite ligand L1 and derivatives of this ligand containing ortho substitution on the aryl group of the amine moiety are used most commonly. Alexakis and co-workers showed that combinations of [Ir(COD)Cl]2 and phosphoramidite ligands L2, L3, and L8 containing 1-naphthyl, o-anisyl, and o-tolyl substitution on the amine moiety formed catalysts for allylic alkylation and amination reactions that are more active and enantioselective than those generated from [Ir(COD)Cl]2 and L1.29,32,54,55 A change in the steric environment near the allyl ligand is proposed to account for the improved enantioselectivity, but the origin of the increased activity of catalysts prepared from L2, L3, and L8 remains unclear. The phosphoramidite ligand L3 is now used as widely as the parent ligand L1 for iridium-catalyzed allylic substitution.
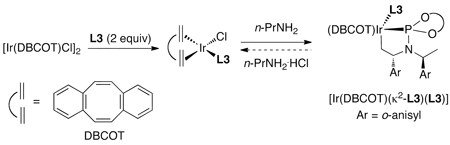
In addition, Helmchen and co-workers demonstrated that the identity of the diene ligand impacts the stability of metallacyclic core of the catalyst. The metallacycle [Ir(DBCOT)(κ2-L3)(L3)] formed from [Ir(DBCOT)Cl2 (DBCOT = dibenzo[a,e]cyclooctatetraene) and ligand L3 in the presence of an amine base (eq 18) is more stable to protonolysis by an amine hydrochloride, such as nPrNH2.HCl, than is the analogous complex prepared from [Ir(COD)Cl]2.56 Furthermore, reactions catalyzed by the metallacyclic iridium complex containing DBCOT can be conducted under air, provided that the metallacycle is formed initially under an inert atmosphere.
 |
(18) |
8. Increases in reaction scope with pre-activated cyclometallated catalysts
Synthesis of the catalyst in the absence of the nucleophile facilitated the development of enantioselective allylations of a series of reagents that are not sufficiently basic to generate the active catalyst. For example, reactions of enamines derived from ketones occurred in good yield with the metallacyclic catalyst generated independently to form, after hydrolysis, products from the α-allylation of a ketone.47 As shown in Chart 7, the scope of this process encompasses reactions of enamines from acetophenones and purely aliphatic ketones. This reaction occurs selectively at the methyl group of the enamine derived from a methyl alkyl ketone, due to selective reaction with one of two equilibrating enamines. Even reactions of enamines from aliphatic ketones with aliphatic allylic carbonates, although slow, formed the substitution product with substantial enantioselectivity.
Chart 7.
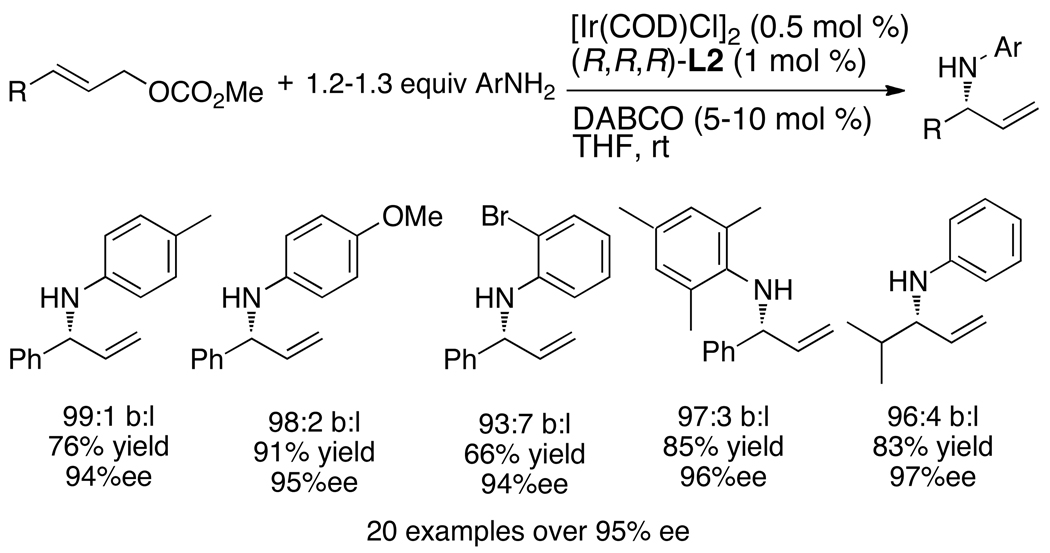
Independent generation of the metallacycle also enabled enantioselective allylation of aromatic amines.43 One can now appreciate that the allylation of aromatic amines did not occur with the original catalyst system because arylamines are not sufficiently basic to generate the active metallacycle. However, the reaction of a series of aromatic amines with a series of aromatic and aliphatic allylic carbonates occurred with high enantioselectivities, regioselectivities, and yields when catalyzed by a metallacycle generated independently by addition of an alkylamine (Chart 8). Aromatic amines are now among the most reactive nucleophiles for this iridium-catalyzed allylic substitution.
Chart 8.
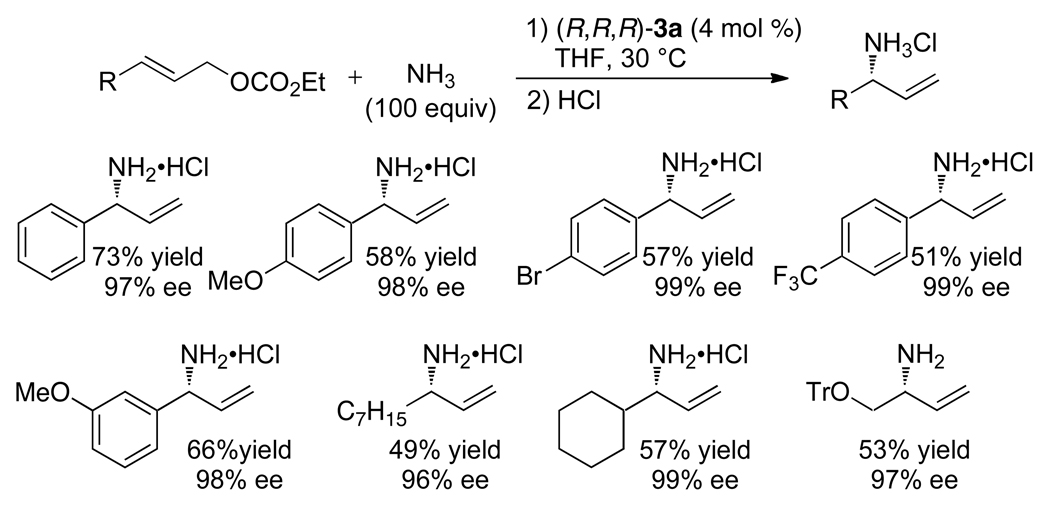
Use of the single-component, ethylene-ligated, metallacyclic catalyst 3a further improved the reaction scope. The reaction of ammonia to form primary allylamines57 requires a large excess of ammonia, and such reactions did not occur in high yield with the catalyst generated in situ because ammonia consumes the [Ir(COD)Cl]2 that is added to accept the κ1-phosphoramidite ligand. In contrast, reaction of ammonia with aliphatic and aromatic allylic carbonates in the presence of the isolated ethylene complex occurs in acceptable to good yields with exceptional enantioselectivities (Chart 9). Quenching of the initial primary allylic amine product with an acid chloride or anhydride generates the corresponding N-allylamide. Reactions of N,N-diacylamines, ortho-nosylamide, carbamates and trifluoroacetamide as ammonia equivalents have also been reported in the presence of the catalyst generated in situ or the an isolated ethylene complex.46,58–60
Chart 9.
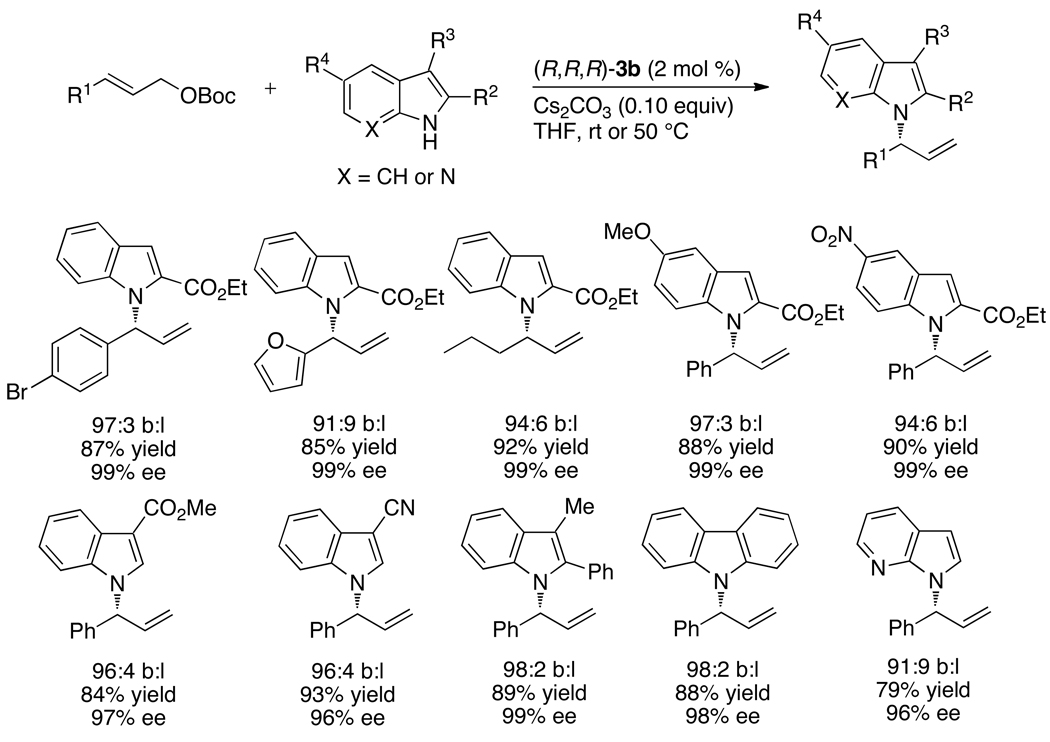
Finally, the experimental work of one of the authors showed that the ethylene adduct of the metallacyclic iridium complex catalyzed the unusual allylation of azole nucleophiles with high enantioselectivity and broad scope.61,62 Reactions of allylic carbonates with benzimidazole in the presence of the set of catalysts shown in Chart 10 occurred with the single-component ethylene adduct as catalyst in high yield, with high enantioselectivity and high branched-to-linear regioselectivity with low loading of catalyst.61 Reactions of a series of unsubstituted or 2-substituted benzimidazoles with aromatic, heteroaromatic, or aliphatic allylic carbonates and potassium phosphate as base occurred with the kinds of branched-to-linear selectivities and enantioselectivities that were observed with amine nucleophiles.61 Reactions of benzimidazoles containing a substituent at the 4-position were selective, although not exclusive, for allylation of the less hindered nitrogen.
Chart 10.
The substitution products were converted to benzimidazoles containing hydroxyalkyl and carboxyalkyl substituents with complete retention of the stereocenter by oxidative cleavage of the C=C bond, hydroboration of the C=C bond, oxidation of the resulting alkylborane, and oxidation of the resulting alcohols with TEMPO and iodosobenzene diacetate.
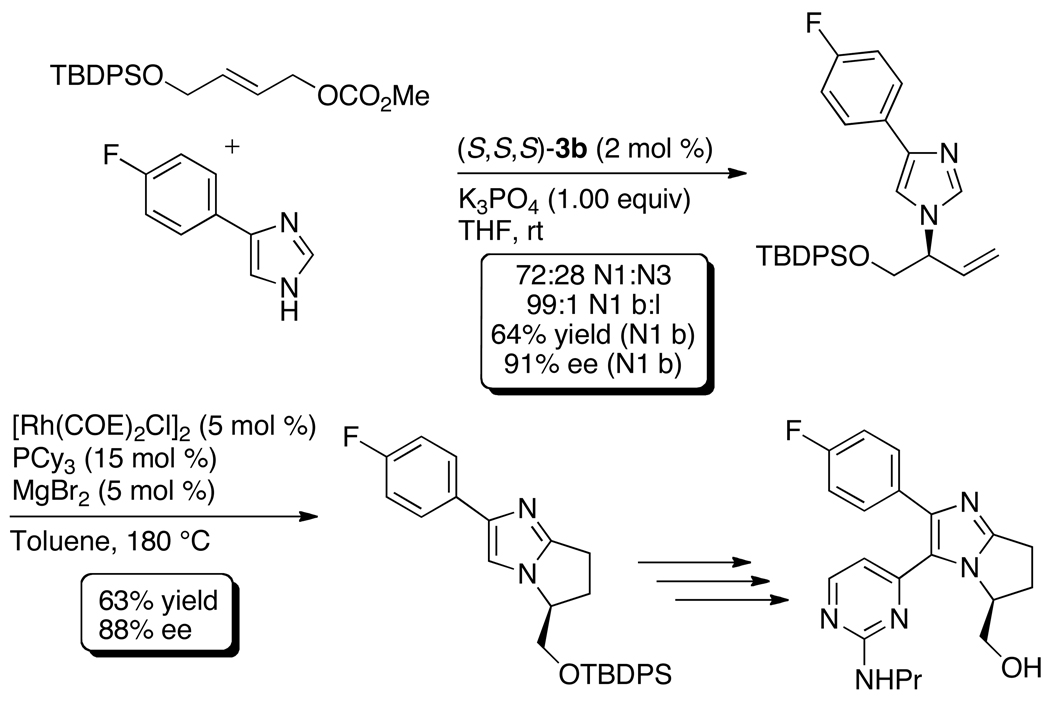
The N-allylation of azoles also occurred with imidazoles, including 4-phenylimidazole, 2,4- and 4,5-diphenylimidazoles. The allylation of 4-arylimidazoles created a short formal synthesis of the JNK-3 inhibitor in Scheme 6. The N-allylimidazole intermediate reported in a prior synthesis of this inhibitor63 was prepared by iridium-catalyzed reaction of 4-(4-fluorophenyl)-1H–imidazole with an allylic carbonate derived from trans-1,4-butenediol.
Scheme 6.
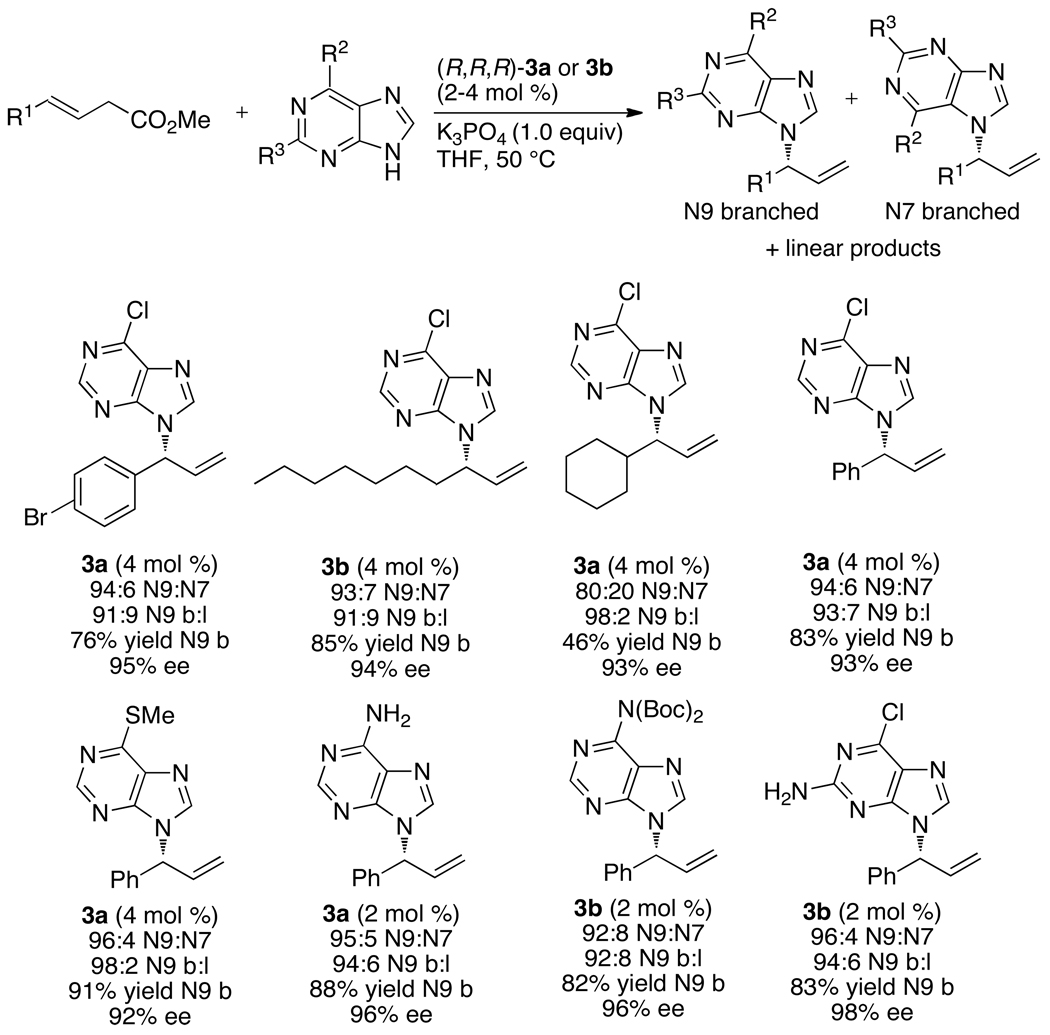
The allylation of azoles also encompassed the N-allylation of purines (Chart 12).61 Reactions of 6-chloropurine with a series of aromatic and aliphatic allylic carbonates occurred preferentially at N9 with >90% ee. Reactions with purines substituted in the 6-position with chloro-, thiomethyl-, amino-, or BOC-protected amino groups occurred with similar yields and enantioselectivities.
Chart 12.
The enantioselective allylation of azoles was also extended to the N-allylation of indoles (Chart 13).62 Although the asymmetric N-allylation of indole itself has not yet been achieved, the asymmetric allylation of slightly more electron-deficient indoles occurs with high yields and enantioselectivities. 2- or 3-Alkyoxycarbonyl-substituted indoles and 3-cyanoindole reacted with aromatic and aliphatic allylic carbonates in 99% ee with branched-to-linear ratios that typically ranged from 10:1 to 20:1. The less electron-poor 2-phenyl-3-methylindole, carbazole and 7-azaindole also underwent N-allylation with high yield and selectivities.
Chart 13.
9. Summary
The allylation of a series of carbon, nitrogen, and oxygen nucleophiles at the more substituted position of the allylic carbonate occurs in high yields, with high branched-to-linear regioselectivities, and high enantioselectivities with remarkably broad scope. Although the initial identification of the catalyst built upon analogies to key prior work, the factors that lead to high enantioselectivity were unanticipated. The identification of the structure of the active catalyst enabled a series of rational simplifications of the ligand, changes to reaction conditions and changes to catalyst precursors that created a system to effect allylic substitutions in a practical fashion with reagents that are less basic and nucleophilic than the aliphatic amines of our first experiments.
Such observations have opened the door for a large number of other research groups to investigate the capabilities of this iridium catalyst. For example, the author’s group has reported intermolecular reactions of sulfur nucleophiles,64 and You’s group has reported decarboxylative reaction of sulfur nucleophiles.65 Alexakis has reported iridium-catalyzed allylic arylation, although with modest regioselectivities.66,67 In addition, Han and You have shown that the iridium catalyst is suitable for decarboxylative reactions to generate carbamate68 and enolate69 nucleophiles. Moreover, recent studies of You have shown that indoles undergo allylation at carbon to form 3-allyl indole products in the presence of Cs2CO3 as base.44,45 Thus, an exploration of the scope of this reaction will certainly continue, and we hope that further generations of the catalyst will allow us to develop reactions of a series of nucleophiles and electrophiles that have not yet undergone the allylation process. Studies on these classes of iridium-catalyzed allylic substitutions are being pursued in the authors’ laboratory.
Figure 4.
Phosphoramidite ligands that generate active and selective iridium-phosphoramidite catalysts
Chart 11.
Acknowledgements
We thank the NSF for initial support and the NIH (GM-58108 and GM-55382) for current support of this work and Johnson-Matthey for gifts of iridium complexes.
Biographies
John Hartwig received his A.B. from Princeton University working with Maitland Jones and his Ph.D. with Bob Bergman and Richard Andersen at U.C. Berkeley. He conducted an ACS postdoctoral fellowship at MIT with Stephen Lippard. After 14 years at Yale University, he moved in 2006 to the University of Illinois, where is the Kenneth L. Rinehart Jr. Professor of Chemistry. His textbook on organometallic chemistry and catalysis “Organotransition Metal Chemistry: From Bonding to Catalysis” was recently published by University Science Books. In his spare time, he cooks and enjoys spending time with his daughters Amelia and Pauline.
Levi Stanley received his B.A. in chemistry from Augustana College (SD) in 2001, where he worked with Professors Gary Earl and Jetty Duffy-Matzner. In 2002 he began his graduate studies with Professor Mukund P. Sibi at North Dakota State University, where he developed enantioselective dipolar cycloadditions and studied applications of chiral relay ligands and auxiliaries in enantioselective catalysis. He received his Ph.D. in 2007 and is currently an NIH Postdoctoral Fellow with Professor John F. Hartwig at the University of Illinois studying iridium-catalyzed allylic substitution.
References
- 1.Hartwig JF. Organotransition Metal Chemistry. Sausalito, CA: University Science Books; 2010. Chapter 20: Allylic Substitution; pp. 967–1014. [Google Scholar]
- 2.Lu Z, Ma SM. Metal-Catalyzed Enantioselective Allylation in Asymmetric Synthesis. Angew. Chem. Int. Ed. 2008;47:258–297. doi: 10.1002/anie.200605113. [DOI] [PubMed] [Google Scholar]
- 3.Trost BM. Pd Asymmetric Allylic Alkylation (AAA). A Powerful Synthetic Tool. Chem. Pharm. Bull. 2002;50:1–14. doi: 10.1248/cpb.50.1. [DOI] [PubMed] [Google Scholar]
- 4.Trost BM. Asymmetric Allylic Alkylation, an Enabling Methodology. J. Org. Chem. 2004;69:5813–5837. doi: 10.1021/jo0491004. [DOI] [PubMed] [Google Scholar]
- 5.For a recent system and a discussion of prior work by Hayashi and Pfaltz, see You SL, Zhu XZ, Luo YM, Hou XL, Dai LX. Highly Regio-and Enantioselective Pd-Catalyzed Allylic Alkylation and Amination of Monosubstituted Allylic Acetates with Novel Ferrocene P,N-Ligands. J. Am. Chem. Soc. 2001;123:7471–7472. doi: 10.1021/ja016121w.
- 6.Trost BM, Hachiya I. Asymmetric Molybdenum-Catalyzed Alkylations. J. Am. Chem. Soc. 1998;120:1104–1105. [Google Scholar]
- 7.Glorius F, Pfaltz A. Enantioselective Molybdenum-Catalyzed Allylic Alkylation Using Chiral Bisoxazoline Ligands. Org. Lett. 1999;1:141–144. [Google Scholar]
- 8.Malkov AV, Baxendale IR, Dvorak D, Mansfield DJ, Kocovsky P. Molybdenum(II)- and Tungsten(II)-Catalyzed Allylic Substitution. J. Org. Chem. 1999;64:2737–2750. doi: 10.1021/jo9821776. [DOI] [PubMed] [Google Scholar]
- 9.Evans PA, Nelson JD. Conservation of Absolute Configuration in the Acyclic Alkylation Reaction: Evidence for an Enyl (σ+π) Organorhodium Intermediate. J. Am. Chem. Soc. 1998;120:5581–5582. [Google Scholar]
- 10.Takeuchi R, Kashio M. Highly Selective Allylic Alkylation with a Carbon Nucleophile at the More Substituted Allylic Terminus Catalyzed by an Iridium Complex: An Efficient Method for Constructing Quaternary Carbon Centers. Angew. Chem. Int. Ed. Engl. 1997;36:263–265. [Google Scholar]
- 11.Takeuchi R, Kashio M. Iridium Complex-Catalyzed Allylic Alkylation of Allylic Esters and Allylic Alcohols: Unique Regio- and Stereoselectivity. J. Am. Chem. Soc. 1998;120:8647–8655. [Google Scholar]
- 12.Takeuchi R, Ue N, Tanabe K, Yamashita K, Shiga N. Iridium Complex-Catalyzed Allylic Amination of Allylic Esters. J. Am. Chem. Soc. 2001;123:9525. doi: 10.1021/ja0112036. [DOI] [PubMed] [Google Scholar]
- 13.Janssen JP, Helmchen G. First Enantioselective Alkylations of Monosubstituted Allylic Acetates Catalyzed by Chiral Iridium Complexes. Tetrahedron Lett. 1997;38:8025–8026. [Google Scholar]
- 14.Bartels B, Helmchen G. Ir-Catalysed Allylic Substitution: Mechanistic Aspects and Asymmetric Synthesis with Phosphorus Amidites as Ligands. Chem. Commun. 1999:741–742. [Google Scholar]
- 15.Bartels B, Garcia-Yebra C, Rominger F, Helmchen G. Iridium-Catalysed Allylic Substitution: Stereochemical Aspects and Isolation of Ir-III Complexes Related to the Catalytic Cycle. Eur. J. Inorg. Chem. 2002:2569–2586. [Google Scholar]
- 16.Ohmura T, Hartwig JF. Regio- and Enantioselective Allylic Amination of Achiral Allylic Esters Catalyzed by an Iridium-Phosphoramidite Complex. J. Am. Chem. Soc. 2002;124:15164–15165. doi: 10.1021/ja028614m. [DOI] [PubMed] [Google Scholar]
- 17.Bartels B, Garcia-Yebra C, Helmchen G. Asymmetric IrI-Catalysed Allylic Alkylation of Monosubstituted Allylic Acetates with Phosphorus Amidites as Ligands. Eur. J. Org. Chem. 2003:1097–1103. [Google Scholar]
- 18.Dang TP, Kagan HB. The Asymmetric Synthesis of Hydratropic Acid and Amino-Acids by Homogeneous Catalytic Hydrogenation. J. Chem. Soc. D: Chem. Commun. 1971:481. [Google Scholar]
- 19.Leitner A, Shu CT, Hartwig JF. Effects of catalyst activation and ligand steric properties on the enantioselective allylation of amines and phenoxides. Org. Lett. 2005;7:1093–1096. doi: 10.1021/ol050029d. [DOI] [PubMed] [Google Scholar]
- 20.Lopez F, Ohmura T, Hartwig JF. Regio- and Enantioselective Iridium-Catalyzed Intermolecular Allylic Etherification of Achiral Allylic Carbonates with Phenoxides. J. Am. Chem. Soc. 2003;125:3426–3427. doi: 10.1021/ja029790y. [DOI] [PubMed] [Google Scholar]
- 21.Shu CT, Hartwig JF. Iridium-Catalyzed Intermolecular Allylic Etherification with Aliphatic Alkoxides: Asymmetric Synthesis of Dihydropyrans and Dihydrofurans. Angew. Chem. Int. Ed. 2004;43:4794–4797. doi: 10.1002/anie.200460214. [DOI] [PubMed] [Google Scholar]
- 22.Evans PA, Leahy DK. Regioselective and Enantiospecific Rhodium-Catalyzed Intermolecular Allylic Etherification with Ortho-Substituted Phenols. J. Am. Chem. Soc. 2000;122:5012–5013. [Google Scholar]
- 23.Dong Y, Teesdale-Spittle P, Hoberg JO. Regioselective Palladium-Catalyzed Allylic Alkylations. Tetrahedron Lett. 2005;46:353–355. [Google Scholar]
- 24.Trost BM, Toste FD. Catalytic Enantioselective Approach to Chromans and Chromanols. A Total Synthesis of (−)-Calanolides A and B and the Vitamin E Nucleus. J. Am. Chem. Soc. 1998;120:9074–9075. [Google Scholar]
- 25.Evans PA, Leahy DK. Regio- and Enantiospecific Rhodium-Catalyzed Allylic Etherification Reactions Using Copper(I) Alkoxides: Influence of the Copper Halide Salt on Selectivity. J. Am. Chem. Soc. 2002;124:7882–7883. doi: 10.1021/ja026337d. [DOI] [PubMed] [Google Scholar]
- 26.Evans PA, Leahy DK, Andrews WJ, Uraguchi D. Stereodivergent Construction of Cyclic Ethers by a Regioselective and Enantiospecific Rhodium-Catalyzed Allylic Etherification: Total Synthesis of Gaur Acid. Angew. Chem. Int. Ed. 2004;43:4788–4791. doi: 10.1002/anie.200460612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueno S, Hartwig JF. Direct, Iridium-Catalyzed Enantioselective and Regioselective Allylic Etherification with Aliphatic Alcohols. Angew. Chem. Int. Ed. 2008;47:1928–1931. doi: 10.1002/anie.200705267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyothier I, Defieber C, Carreira EM. Iridium-Catalyzed Enantioselective Synthesis of Allylic Alcohols: Silanolates as Hydroxide Equivalents. Angew. Chem. Int. Ed. 2006;45:6204–6207. doi: 10.1002/anie.200602408. [DOI] [PubMed] [Google Scholar]
- 29.Alexakis A, Polet D. Very Efficient Phosphoramidite Ligand for Asymmetric Iridium-Catalyzed Allylic Alkylation. Org. Lett. 2004;6:3529–3532. doi: 10.1021/ol048607y. [DOI] [PubMed] [Google Scholar]
- 30.Lipowsky G, Helmchen G. Regio- and Enantioselective Iridium-Catalysed Allylic Aminations and Alkylations of Dienyl Esters. Chem. Commun. 2004:116–117. doi: 10.1039/b311502j. [DOI] [PubMed] [Google Scholar]
- 31.Lipowsky G, Miller N, Helmchen G. Regio- and Enantioselective Iridium-Catalyzed Allylic Alkylation with in situ Activated P,C-Chelate Complexes. Angew. Chem. Int. Ed. 2004;43:4595–4597. doi: 10.1002/anie.200460016. [DOI] [PubMed] [Google Scholar]
- 32.Polet D, Alexakis A. Kinetic Study of Various Phosphoramidite Ligands in the Iridium-Catalyzed Allylic Substitution. Org. Lett. 2005;7:1621–1624. doi: 10.1021/ol050350w. [DOI] [PubMed] [Google Scholar]
- 33.Dahnz A, Helmchen G. Iridium-Catalyzed Enantioselective Allylic Substitutions with Aliphatic Nitro Compounds as Prenucleophiles. Synlett. 2006:697–700. [Google Scholar]
- 34.Forster S, Tverskoy O, Helmchen G. Malononitrile as Acylanion Equivalent. Synlett. 2008:2803–2806. [Google Scholar]
- 35.Gnamm C, Forster S, Miller N, Brodner K, Helmchen G. Enantioselective Iridium-Catalyzed Allylic Alkylations - Improvements and Applications Based on Salt-Free Reaction Conditions. Synlett. 2007:790–794. [Google Scholar]
- 36.Liu WB, Zheng SC, He H, Zhao XM, Dai LX, You SL. Iridium-Catalyzed Regio- and Enantioselective Allylic Alkylation of Fluorobis(phenylsulfonyl)methane. Chem. Commun. 2009:6604–6606. doi: 10.1039/b914315g. [DOI] [PubMed] [Google Scholar]
- 37.Tsuji J, Shimizu I, Minami I, Ohashi Y, Sugiura T, Takahashi K. Allylic Carbonates - Efficient Allylating Agents of Carbonucleophiles in Palladium Catalyzed-Reactions Under Neutral Conditions. J. Org. Chem. 1985;50:1523–1529. [Google Scholar]
- 38.Graening T, Hartwig JF. Iridium-Catalyzed Regio- and Enantioselective Allylation of Ketone Enolates. J. Am. Chem. Soc. 2005;127:17192–17193. doi: 10.1021/ja0566275. [DOI] [PubMed] [Google Scholar]
- 39.Kiener CA, Shu CT, Incarvito C, Hartwig JF. Identification of an Activated Catalyst in the Iridium-Catalyzed Allylic Amination and Etherification. Increased Rates, Scope, and Selectivity. J. Am. Chem. Soc. 2003;125:14272–14273. doi: 10.1021/ja038319h. [DOI] [PubMed] [Google Scholar]
- 40.Louie J, Hartwig JF. A Route To Pd(0) From Pd(II) Metallacycles in Amination and Cross-Coupling Chemistry. Angew. Chem. Int. Ed. Engl. 1996;35:2359–2361. [Google Scholar]
- 41.Weck M, Jones CW. Mizoroki-Heck Coupling Using Immobilized Molecular Precatalysts: Leaching Active Species from Pd Pincers, Entrapped Pd Salts, and PdNHC Complexes. Inorg. Chem. 2007;46:1865–1875. doi: 10.1021/ic061898h. [DOI] [PubMed] [Google Scholar]
- 42.Welter C, Koch O, Lipowsky G, Helmchen G. First Intramolecular Enantioselective Iridium-Catalysed Allylic Aminations. Chem. Commun. 2004:896–897. doi: 10.1039/b400023d. [DOI] [PubMed] [Google Scholar]
- 43.Shu CT, Leitner A, Hartwig JF. Enantioselective Allylation of Aromatic Amines After In Situ Generation of an Activated Cyclometalated Iridium Catalyst. Angew. Chem. Int. Ed. 2004;43:4797–4800. doi: 10.1002/anie.200460276. [DOI] [PubMed] [Google Scholar]
- 44.Liu WB, He H, Dai LX, You SL. Ir-Catalyzed Regio- and Enantioselective Friedel-Crafts-Type Allylic Alkylation of Indoles. Org. Lett. 2008;10:1815–1818. doi: 10.1021/ol800409d. [DOI] [PubMed] [Google Scholar]
- 45.Liu WB, He H, Dai LX, You SL. Synthesis of 2-Methylindoline- and 2-Methyl-1,2,3,4-tetrahydroquinoline-Derived Phosphoramidites and Their Applications in Iridium-Catalyzed Allylic Alkylation of Indoles. Synthesis. 2009:2076–2082. [Google Scholar]
- 46.Weihofen R, Tverskoy E, Helmchen G. Salt-Free Iridium-Catalyzed Asymmetric Allylic Aminations with N,N-Diacylamines and ortho-Nosylamide as Ammonia Equivalents. Angew. Chem. Int. Ed. 2006;45:5546–5549. doi: 10.1002/anie.200601472. [DOI] [PubMed] [Google Scholar]
- 47.Weix DJ, Hartwig JF. Regioselective and Enantioselective Iridium-Catalyzed Allylation of Enamines. J. Am. Chem. Soc. 2007;129:7720–7721. doi: 10.1021/ja071455s. [DOI] [PubMed] [Google Scholar]
- 48.Markovic D, Hartwig JF. Resting State and Kinetic Studies on the Asymmetric Allylic Substitutions Catalyzed by Iridium-Phosphoramidite Complexes. J. Am. Chem. Soc. 2007;129:11680–11681. doi: 10.1021/ja074584h. [DOI] [PubMed] [Google Scholar]
- 49.Madrahimov ST, Markovic D, Hartwig JF. The Allyl Intermediate in Regioselective and Enantioselective Iridium-Catalyzed Asymmetric Allylic Substitution Reactions. J. Am. Chem. Soc. 2009;131:7228–7229. doi: 10.1021/ja902609g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiess S, Raskatov JA, Gnamm C, Brodner K, Helmchen G. Ir-Catalyzed Asymmetric Allylic Substitutions with (Phosphoramidite)Ir Complexes-Resting States, Synthesis, and Characterization of Catalytically Active (π-Allyl)Ir Complexes. Chem. Eur J. 2009;15:11087–11090. doi: 10.1002/chem.200902065. [DOI] [PubMed] [Google Scholar]
- 51.Raskatov JA, Spiess S, Gnamm C, Brodner K, Rominger F, Helmchen G. Ir-Catalyzed Asymmetric Allylic Substitutions with Cyclometalated (Phosphoramidite)Ir Complexes-Resting States, Catalytically Active (π-Allyl)Ir Complexes and Computational Exploration. Chem. Eur J. 2010;16:6601–6615. doi: 10.1002/chem.200903465. [DOI] [PubMed] [Google Scholar]
- 52.Leitner A, Shekhar S, Pouy MJ, Hartwig JF. A Simple Iridium Catalyst with a Single Resolved Stereocenter for Enantioselective Allylic Amination. Catalyst Selection from Mechanistic Analysis. J. Am. Chem. Soc. 2005;127:15506–15514. doi: 10.1021/ja054331t. [DOI] [PubMed] [Google Scholar]
- 53.Leitner A, Shu CT, Hartwig JF. Editing the Stereochemical Elements in an Iridium Catalyst for Enantioselective Allylic Amination. Proc. Natl. Acad. Sci. USA. 2004;101:5830–5833. doi: 10.1073/pnas.0307967101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Polet D, Alexakis A, Tissot-Croset K, Corminboeuf C, Ditrich K. Phosphoramidite Ligands in Iridium-Catalyzed Allylic Substitution. Chem. Eur J. 2006;12:3596–3609. doi: 10.1002/chem.200501180. [DOI] [PubMed] [Google Scholar]
- 55.Tissot-Croset K, Polet D, Alexakis A. A Highly Effective Phosphoramidite Ligand for Asymmetric Allylic Substitution. Angew. Chem. Int. Ed. 2004;43:2426–2428. doi: 10.1002/anie.200353744. [DOI] [PubMed] [Google Scholar]
- 56.Spiess S, Welter C, Franck G, Taquet JP, Helmchen G. Iridium-Catalyzed Asymmetric Allylic Substitutions - Very High Regioselectivity and Air Stability with a Catalyst Derived from Dibenzo[a, e]cyclooctatetraene and a Phosphoramidite. Angew. Chem. Int. Ed. 2008;47:7652–7655. doi: 10.1002/anie.200802480. [DOI] [PubMed] [Google Scholar]
- 57.Pouy MJ, Stanley LM, Hartwig JF. Enantioselective, Iridium-Catalyzed Monoallylation of Ammonia. J. Am. Chem. Soc. 2009;131:11312–11313. doi: 10.1021/ja905059r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pouy MJ, Leitner A, Weix DJ, Ueno S, Hartwig JF. Enantioselective Iridium-Catalyzed Allylic Amination of Ammonia and Convenient Ammonia Surrogates. Org. Lett. 2007;9:3949–3952. doi: 10.1021/ol701562p. [DOI] [PubMed] [Google Scholar]
- 59.Weix DJ, Markovic D, Ueda M, Hartwig JF. Direct, Intermolecular, Enantioselective, Iridium-Catalyzed Allylation of Carbamates to Form Carbamate-Protected, Branched Allylic Amines. Org. Lett. 2009;11:2944–2947. doi: 10.1021/ol901151u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh OV, Han H. Iridium(I)-catalyzed regio- and enantioselective allylic amidation. Tetrahedron Lett. 2007;48:7094–7098. doi: 10.1016/j.tetlet.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanley LM, Hartwig JF. Regio- and Enantioselective N-Allylations of Imidazole, Benzimidazole, and Purine Heterocycles Catalyzed by Single-Component Metallacyclic Iridium Complexes. J. Am. Chem. Soc. 2009;131:8971–8983. doi: 10.1021/ja902243s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stanley LM, Hartwig JF. Iridium-Catalyzed Regio- and Enantioselective N-Allylation of Indoles. Angew. Chem. Int. Ed. 2009;48:7841–7844. doi: 10.1002/anie.200904338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rech JC, Yato M, Duckett D, Ember B, LoGrasso PV, Bergman RG, Ellman JA. Synthesis of Potent Bicyclic Bisarylimidazole c-Jun N-Terminal Kinase Inhibitors by Catalytic C–H Bond Activation. J. Am. Chem. Soc. 2006;129:490–491. doi: 10.1021/ja0676004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ueda M, Hartwig JF. Iridium-Catalyzed, Regio- and Enantioselective Allylic Substitution with Aromatic and Aliphatic Sulfinates. Org. Lett. 2010;12:92–94. doi: 10.1021/ol9023248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu QL, Liu WB, Dai LX, You SL. Iridium-Catalyzed Enantioselective Allylic Substitution of O-Allyl Carbamothioates. J. Org. Chem. 2010;75:4615–4618. doi: 10.1021/jo1006152. [DOI] [PubMed] [Google Scholar]
- 66.Alexakis A, El Hajjaji S, Polet D, Rathgeb X. Iridium-Catalyzed Asymmetric Allylic Substitution with Aryl Zinc Reagents. Org. Lett. 2007;9:3393–3395. doi: 10.1021/ol0713842. [DOI] [PubMed] [Google Scholar]
- 67.Polet D, Rathgeb X, Falciola CA, Langlois JB, El Hajjaji S, Alexakis A. Enantioselective Iridium-Catalyzed Allylic Arylation. Chem. Eur. J. 2009;15:1205–1216. doi: 10.1002/chem.200801879. [DOI] [PubMed] [Google Scholar]
- 68.Singh OV, Han H. Iridium(I)-Catalyzed Regio- and Enantioselective Decarboxylative Allylic Amidation of Substituted Allyl Benzyl Imidodicarbonates. J. Am. Chem. Soc. 2007;129:774–775. doi: 10.1021/ja067966g. [DOI] [PubMed] [Google Scholar]
- 69.He H, Zheng XJ, Yi U, Dai LX, You SL. Ir-Catalyzed Regio- and Enantioselective Decarboxylative Allylic Alkylations. Org. Lett. 2007;9:4339–4341. doi: 10.1021/ol7019394. [DOI] [PubMed] [Google Scholar]