Abstract
Chronic obstructive pulmonary disease (COPD) is a major public health problem, associated with considerable morbidity and health care costs. The global burden of COPD morbidity is predicted to rise substantially in the coming decade, but could be moderated by better use of existing management strategies. Smoking cessation, medication therapy, and pulmonary rehabilitation have all been shown to diminish morbidity and improve patient outcomes. But each of these strategies requires adherence. Adherence is crucial for optimizing clinical outcomes in COPD, with nonadherence resulting in a significant health and economic burden. Suboptimal medication adherence is common among COPD patients, due to a number of factors that involve the medication, the delivery device, the patient, and the health professionals caring for the patient. Lack of medication adherence needs to be identified and addressed by using simplified treatment regimens, increasing patient knowledge about self-management, and enhancing provider skills in patient education, communication, and adherence counseling. This article reports some of the challenges of medication nonadherence faced by the clinician in the management of COPD, and suggests ways to evaluate and improve adherence effectively in primary care.
Keywords: chronic obstructive pulmonary disease, adherence, clinician
Introduction
Nonadherence in patients with chronic diseases is estimated to cost $300 billion in the US annually, with COPD among the conditions with the lowest levels of adherence.1 Acute and maintenance treatment of COPD relies on inhaled agents to manage and control symptoms and/or complications of the disease, and prevent exacerbations.2 Therefore, adherence to inhaled agents is required to manage and control symptoms and to prevent or delay complications of COPD, including exacerbations.2,11 The rate of >50% poor adherence in the COPD population3–8 is not surprising, because people with COPD usually have multiple morbidities and take an average of six medications.9 In 2005, the global mortality rate for COPD was over three million. This figure is set to rise by over 30% in the coming decade, unless adequate preventive strategies are implemented.10
While everyone agrees that nonadherence is a major problem, there is no universal agreement on how to define adherence. Older definitions of adherence often used the term compliance, suggesting a more paternalistic relationship where patients play a passive role in their care, ie, the patient is “compliant” if they follow “orders”. An alternative definition that is gaining wide acceptance is that adherence is the degree of concordance (or agreement) between the health professional’s recommended therapy and the patient’s actual behavior. Concordance indicates a partnership between the patient and health care provider, and adherence implies that patients and clinicians work together in this partnership. Adherence therefore implies that the patient has an active role in consenting to, and following, prescribed treatments. Using the concept of concordance provides many additional avenues to work towards improved adherence.
This article reports on the proceedings of an advisory board discussing the “Implications of inhalation delivery systems for COPD therapies” (held in New York City, NY, March 25–26, 2009), and discusses the challenges surrounding nonadherence in COPD patients, assesses nonadherence and the determinants for nonadherence, and addresses how adherence could be improved by better clinician-patient communication.
Challenge of nonadherence in COPD
There are a number of reasons for suboptimal adherence to medications in patients with COPD, as well as other chronic diseases.11 Adherence tends to decline over time with chronic medication use, and is inversely related to the number of medications taken by the patient.11,12 These factors alone make adherence an even larger problem with COPD patients who require lifelong therapy after symptomatic COPD is diagnosed. In the Lung Health Study, a subset of 5887 COPD patients were studied for compliance with inhaler use by both self-report and canister weight. Inhaler compliance demonstrated a consistent decline over five years from >60% at year 1 to <50% compliance by year 5.13 These findings are consistent with another five-year study of medication use in obstructive lung disease patients, where 51% acquired their prescribed drugs less than once per year.4 In a Swedish study of patients aged ≥60 years with COPD or asthma, only 28% of patients had “satisfactory” refill patterns (ie, dispensed refills covering >80% of the prescribed treatment time) of their inhaled corticosteroid (ICS).14 In a study of 11,376 COPD patients in the last year of life, only 52% used any COPD medications.14
Data on adherence issues among patients with asthma are useful in projecting potential issues for COPD patients. Like COPD, asthma is a chronic condition that relies heavily on inhaled medications and less on the ingestion of tablets. Patients with COPD, as well as those with asthma, may be on as many as 3–4 different types of inhalers that require mastery. The challenges of inhaler mastery for the COPD patient are more numerous than for asthma, because COPD patients are usually older with multiple comorbidities, as well as impaired physical and cognitive function that may interfere with inhaler adherence and proper use. Some studies suggest that adherence may be worse for those using medications for COPD than for other chronic diseases such as diabetes, where adherence was 68% versus only 42% for asthma/COPD medications.6
COPD is a chronic disease and in many patients, includes the need to treat one or more exacerbations each year. Drug therapy with long-acting bronchodilators, ICS, or a combination of both, has been shown to reduce exacerbations in patients with moderate to severe COPD.15 However, patients often report they do not see an obvious link between taking their medication and an immediate effect on their symptoms.
Furthermore, prior to progressing to severe disease, many patients do not consider their condition sufficiently serious to require long-term therapy.6,16 Adding to this disconnect between their COPD and its treatment, many patients discontinue their medication in response to a decline in symptoms17 or the complexity of the treatment regimen.5 Patients report they worry that they will develop tolerance to the inhaled medications if they take them regularly over the long term, believing that the medication will be less effective when they really need it, such as during an exacerbation.18
Determinants of adherence
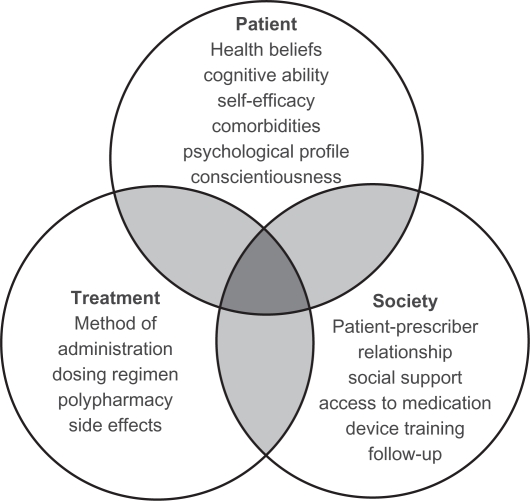
Adherence in patients with COPD is affected by multiple factors associated with the patient, their clinician, and society11 (see Figure 119). Patient-related factors include health beliefs, cognitive ability, self-efficacy, comorbidities, psychologic profile, and conscientiousness. For example, depression is a major cause of nonadherence in COPD patients, but is seldom recognized or acknowledged.16,20,21 Treatment-related factors include the need to use multiple inhalers, each requiring a different technique for administration, posing challenges for the older COPD patient. Societal factors include the patient-prescriber relationship, social support, access to medication, device training, and follow-up. Training the COPD patient on the various inhaler devices is fraught with the potential for incorrect delivery and administration of the medication, even among patients who want to take their inhaler correctly.
Figure 1.
Patient adherence in chronic obstructive pulmonary disease is multifactorial and is influenced by the patient, the clinician, and society.
Copyright© 2010. Reproduced with permission from BMH Publishing Group Ltd. Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63:831–838.19
There are a number of other factors that, perhaps unexpectedly, are not reliably associated with adherence, including age, gender, race, income, education, personality, and adherence to other medications.6,16 It is important to understand that human behavior is a key consideration when evaluating what influences adherence.22 For example, it seems reasonable to assume that patients who seek help from their clinician will want to take the medication they are prescribed, and that it would be illogical not to comply with the advice and instructions they receive. Nonadherence may appear to be an irrational act from the clinician’s perspective, but completely rational from the patient’s perspective.
Assessing adherence
Assessing adherence is a complex issue, with many aspects of adherence remaining poorly defined and understood. At present, there is no general agreement about how to define “optimal” or “adequate” adherence, or which of these should be measured in trials and clinical practice. Questions remain about what is an “acceptable” level of adherence to achieve disease control. For example, if the patient’s symptoms are under control and they are satisfied with symptom control, is that level of adherence acceptable? Different medications, regimens, and behaviors all have a varying impact on adherence. The main methods for assessing adherence are clinician estimates, patient self-reporting, pill counts/weighing, pharmacy records, biologic markers, and electronic monitoring. The advantages and disadvantages of these adherence measures are summarized in Table 1.
Table 1.
Advantages and disadvantages of different adherence measures
| Advantages | Disadvantages | |
|---|---|---|
| Clinician estimates | Easy to obtain | Unreliable |
| Patient self-reports | Easy to obtain | Unreliable |
| Pill counts/weighing | Easy to obtain | Overestimates use |
| Pharmacy records | Confirms prescription filling | Incomplete, biased estimates |
| Biologic measures | Confirms ingestion | Expensive, invasive, insensitive to inhaled drugs, affected by pharmacokinetics and polypharmacy |
| Electronic monitoring | Patterns of use, ingestion | Expensive, limited availability and use, malfunctions |
Clinician estimates, such as patient self-reports, have been shown to be unreliable.19,23 Studies in asthma patients have shown that patient self-reporting and pill counts can yield very misleading results, primarily because patients want their clinicians to be happy with them. Patients will tend to exaggerate their medication use in self-reports.24–26 Pill (or dose) counting on the other hand can lead to overestimates. In the latter case, patients have been found to empty pills from their packaging or empty their inhalers deliberately when they realize a primary care visit is imminent.27 Pharmacy data sets have been used, but only confirm that a prescription has been filled, not if the drug has been inhaled or ingested. Biologic measures would appear to be precise; however, they are not currently able to detect the presence of inhaled drugs and are only available for a limited number of oral medications. Electronic monitoring can provide more accurate data on patterns of medication use24 but these monitors are expensive, of limited availability, do not necessarily indicate the patient has taken the dose, and are susceptible to malfunction. Improved electronic monitoring devices are needed to evaluate each of available types/designs of inhaled devices.
Patient-clinician communication
Effective communication between the clinician and patient is crucial for optimizing adherence.11,22 Patients with poor adherence have been shown to have a low level of satisfaction and faith in their clinician.22 In a study of ICS use in asthma patients, the most powerful predictor of nonadherence was the belief that they did not need to take as much ICS as the doctor prescribed.18 Assessing and discussing the patients’ illness perceptions and attempting to bring perception in closer alignment with evidence has been shown to improve patients’ quality of life and clinical outcomes.28
Proper education ensures the patient is fully informed about the important aspects of their treatment regimen, from the practicalities of administration to the rationale for taking the medication.29–32 In practice, most patients receive inadequate education about proper use of their drug therapy.33 An observational study of 44 physicians demonstrated that full medication directions (specific medication name, purpose of medication, dosing quantity and frequency, adverse effects, and duration of treatment regimen) were conveyed to less than 60% of 185 patients.33
Many primary care clinicians are not fully aware of COPD management guidelines, eg, the Global Initiative for Chronic Obstructive Lung Disease2 and, therefore, may not be sufficiently equipped to educate patients about all aspects of managing their disease.34,35 Unfortunately, education is given a low priority, and budgetary constraints often lead to insufficient numbers of educators devoted to the needs of the pulmonary patient.
Tailoring treatment to fit the individual’s needs, abilities, and preferences is a key element of COPD management. This can be best accomplished by having patients as active participants in treatment decisions. COPD patients with self-management plans tend to have better awareness and adherence.20 Communication at the time of a new prescription plays an important role in future adherence, including whether the first prescription is filled. Therefore, when initiating new therapies, the clinician may increase their success in adherence of their patient by: explaining the roles of the different types of therapy in disease management, then asking the patient about their understanding of each prescribed therapy, and any gaps in the patient’s knowledge or misunderstandings can then be addressed, describing the medical criteria for assessing disease control and asking the patient about their expectations and what criteria they use for decision-making (eg, long-term safety concerns); assessing each patient’s preferences and possible barriers to adherence, including fears, beliefs, financial, social, cultural, and practical issues, such as transportation;36 providing written medication plans for the patient to take home, especially for those with cognitive issues or anxiety, who may not be able to retain information given verbally; encouraging telephone calls/email contact between visits with the clinician, a medical assistant, or nurse, to clarify questions about their medications (clinicians’ concern that they will be inundated with patient queries has not been borne out in practice); and discussing adherence in a nonthreatening way is pivotal to gaining the patient’s trust to discuss their difficulties with adherence. Acknowledge to the patient that adherence to a regime, especially a regimen requiring multiple medications, is difficult. Effective communication needs to be maintained throughout ongoing management so that adherence can be explored in a nonthreatening and nonjudgmental manner. The physician/clinician should discuss issues related to adherence, such as satisfaction with treatment, criteria for disease control and efficacy (from both the medical and patient’s perspective), long-term safety concerns, news about relevant clinical studies in the media, and new therapies.
Clinicians need to be vigilant for “red flags” indicating poor adherence (Table 2). Areas that are associated with poor adherence include denial, disruption (eg, personal crises), depression, and dementia. The clinician should not anticipate good adherence when any of these four factors are present.
Table 2.
Red flags for poor adherence
| Red flag | Details |
|---|---|
| Denial | Patient beliefs about illness and therapy |
| Disruption | Personal and family crises |
| Depression | Apathy and withdrawal |
| Dementia | Psychiatric or related to substance abuse |
Addressing adherence problems
Nonadherence can be either unintentional or intentional.11,30 Three forms of nonadherence have been identified, ie, erratic, unwitting, and intelligent.26 Erratic nonadherence occurs when the patient understands and agrees with therapy but has difficulty consistently maintaining the regimen. This difficulty may be attributable to a busy schedule, to lack of attention to detail, or to inadvertently forgetting to take their medication. Patients prone to this type of nonadherence may be forgetful, disorganized, or may simply run out of medication. Patients with complex medication regimens or a frequent change of schedule may also have episodes of erratic nonadherence. Using a once-daily medication may increase adherence in this group.37
Unwitting nonadherence describes when both the patient and provider mistakenly believe that the patient is adherent. For example, unwitting nonadherence may occur in the patient who misunderstands their medication regimen or forgets instructions, perhaps due to language barriers or cultural differences. Unwitting nonadherence is a particular danger in COPD patients due to the complexity of inhaler use. Were they properly trained? Were they observed in their use of each of their inhalers? Were they observed on subsequent visits? Patient retention of the various steps in inhaler use can easily confuse the patients. Patients with cognitive impairment, depression, or anxiety disorders have difficulty recalling instructions and should be provided with written instructions.
Intelligent nonadherence occurs when the patient deliberately alters or discontinues therapy based on reasoned decision-making about their perceptions of the drug’s efficacy, likelihood of long-term harm, and their social circumstances.38 Signs of this type of nonadherence include comments about feeling better and no longer needing the medication, concern about toxicity, perceived ineffectiveness of therapy, fear of tolerance, and a general opinion that taking as little medicine as possible is best. Once the type of non-adherence has been identified, adherence interventions can be matched to the source of nonadherence. Table 3 describes interventions for these three types of nonadherence.
Table 3.
Matching adherence interventions to the type of nonadherence
| Type of nonadherence | Adherence interventions |
|---|---|
| Erratic | Simplify and tailor regimen |
| Implement behavioral strategies such as cueing (eg, storing medication next to toothbrush), reminders and reinforcement | |
| Self-monitoring and support, with monitoring from others | |
| Unwitting | Review of adherence behavior |
| Written or visual medication plans | |
| Patient education in disease management | |
| Intelligent | Patient education and counseling |
| Negotiate therapy | |
| Link therapy with personal goals |
Conclusion
Adherence to medication in COPD is crucial for optimizing clinical outcomes, and nonadherence results in a significant health and economic burden. Suboptimal adherence is common among COPD patients and results from a complex interplay of medication, patient, and provider factors. Adherence could be improved by using simplified treatment regimens, increasing patient knowledge about self-management, and enhancing provider skills in patient education, communication, and adherence counseling. The challenge of adherence is determining acceptable levels of adherence and simple, but reliable devices to measure adherence. Given the numerous inhalers available to treat COPD, electronic monitors to evaluate all of these medications have not as yet been developed. Clinical trials using electronic monitoring, therefore, need to be conducted to investigate the feasibility of adherence interventions, their effects on clinical outcomes, and the durability of results.
Acknowledgments
This article was developed on the basis of presentations and discussions at the “Implications of Inhalation Delivery Systems for COPD Therapies” Advisory Board, New York City, NY, USA, March 25–26, 2009. This meeting, author’s participation, and manuscript preparation were supported by Boehringer Ingelheim Pharmaceuticals Inc. and Pfizer Inc. The authors wish to thank Dr Susan Bartlett for her presentation and input on this subject, on which this manuscript was based. Medical writing assistance was provided by Gill Sperrin, CBIOL MSB, of Envision Scientific Solutions. The article reflects the concepts of the authors and is their sole responsibility. It was not reviewed by Boehringer Ingelheim Pharmaceuticals Inc., and Pfizer Inc., except to ensure medical and safety accuracy.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.DiMatteo MR. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 2.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Bethesda, MD: National Heart, Lung, and Blood Institute; Available at: http://www.goldcopd.org. Accessed 2010 May. [Google Scholar]
- 3.Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118:899–904. doi: 10.1016/j.jaci.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Haupt D, Krigsman K, Nilsson JL. Medication persistence among patients with asthma/COPD drugs. Pharm World Sci. 2008;30:509–514. doi: 10.1007/s11096-008-9197-4. [DOI] [PubMed] [Google Scholar]
- 5.Krigsman K, Moen J, Nilsson JL, Ring L. Refill adherence by the elderly for asthma/chronic obstructive pulmonary disease drugs dispensed over a 10-year period. J Clin Pharm Ther. 2007;32:603–611. doi: 10.1111/j.1365-2710.2007.00866.x. [DOI] [PubMed] [Google Scholar]
- 6.Krigsman K, Nilsson JL, Ring L. Adherence to multiple drug therapies: Refill adherence to concomitant use of diabetes and asthma/COPD medication. Pharmacoepidemiol Drug Saf. 2007;16:1120–1128. doi: 10.1002/pds.1433. [DOI] [PubMed] [Google Scholar]
- 7.Krigsman K, Nilsson JL, Ring L. Refill adherence for patients with asthma and COPD: Comparison of a pharmacy record database with manually collected repeat prescriptions. Pharmacoepidemiol Drug Saf. 2007;16:441–448. doi: 10.1002/pds.1321. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization . Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization; Available at: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. Accessed 2010 May 4. [Google Scholar]
- 9.Dolce JJ, Crisp C, Manzella B, Richards JM, Hardin JM, Bailey WC. Medication adherence patterns in chronic obstructive pulmonary disease. Chest. 1991;99:837–841. doi: 10.1378/chest.99.4.837. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . Chronic obstructive pulmonary disease (COPD). Fact sheet No. 315. Geneva, Switzerland: World Health Organization; Available at: http://www.who.int/mediacentre/factsheets/fs315/en/print.html. Accessed 2010 May 4. [Google Scholar]
- 11.Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:371–384. doi: 10.2147/copd.s3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23:1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 13.Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on 1994; the rate of decline of FEV1. The Lung Health Study. JAMA. 272:1497–1505. [PubMed] [Google Scholar]
- 14.Jung E, Pickard AS, Salmon JW, Bartle B, Lee TA. Medication adherence and persistence in the last year of life in COPD patients. Respir Med. 2009;103:525–534. doi: 10.1016/j.rmed.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: Scientific review. JAMA. 2003;290:2301–2312. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- 16.DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: A meta-analysis. Med Care. 2007;45:521–528. doi: 10.1097/MLR.0b013e318032937e. [DOI] [PubMed] [Google Scholar]
- 17.Menckeberg TT, Bouvy ML, Bracke M, Hugtenburg JG, Lammers JW, Raaijmakers JA. Patients’ understanding of the reasons for starting and discontinuing inhaled corticosteroids. Br J Clin Pharmacol. 2008;66:255–260. doi: 10.1111/j.1365-2125.2008.03168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le TT, Bilderback A, Bender B, et al. Do asthma medication beliefs mediate the relationship between minority status and adherence to therapy? J Asthma. 2008;45:33–37. doi: 10.1080/02770900701815552. [DOI] [PubMed] [Google Scholar]
- 19.Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63:831–838. doi: 10.1136/thx.2007.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dowson CA, Town GI, Frampton C, Mulder RT. Psychopathology and illness beliefs influence COPD self-management. J Psychosom Res. 2004;56:333–340. doi: 10.1016/S0022-3999(03)00040-0. [DOI] [PubMed] [Google Scholar]
- 21.Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5:105–116. doi: 10.1080/15412550801941190. [DOI] [PubMed] [Google Scholar]
- 22.George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128:3198–3204. doi: 10.1378/chest.128.5.3198. [DOI] [PubMed] [Google Scholar]
- 23.Jerant A, DiMatteo R, Arnsten J, Moore-Hill M, Franks P. Self-report adherence measures in chronic illness: Retest reliability and predictive validity. Med Care. 2008;46:1134–1139. doi: 10.1097/MLR.0b013e31817924e4. [DOI] [PubMed] [Google Scholar]
- 24.Milgrom H, Bender B, Ackerson L, Bowry P, Smith B, Rand C. Noncompliance and treatment failure in children with asthma. J Allergy Clin Immunol. 1996;98:1051–1057. doi: 10.1016/s0091-6749(96)80190-4. [DOI] [PubMed] [Google Scholar]
- 25.Coutts JA, Gibson NA, Paton JY. Measuring compliance with inhaled medication in asthma. Arch Dis Child. 1992;67:332–333. doi: 10.1136/adc.67.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rand C. I took the medicine like you told me, doctor: Self-report of adherence with medical regimens. In: Stone AA, Turkkan JS, Bachrach CA, editors. The Science of Self-Report. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. [Google Scholar]
- 27.Rand CS, Wise RA, Nides M, et al. Metered-dose inhaler adherence in a clinical trial. Am Rev Respir Dis. 1992;146:1559–1564. doi: 10.1164/ajrccm/146.6.1559. [DOI] [PubMed] [Google Scholar]
- 28.Kaptein AA, Scharloo M, Fischer MJ, et al. Illness perceptions and COPD: An emerging field for COPD patient management. J Asthma. 2008;45:625–629. doi: 10.1080/02770900802127048. [DOI] [PubMed] [Google Scholar]
- 29.Falvo D, Woehlke P, Deichmann J. Relationship of physician behavior to patient compliance. Patient Couns Health Educ. 1980;2:185–188. doi: 10.1016/s0738-3991(80)80101-7. [DOI] [PubMed] [Google Scholar]
- 30.George J, Kong DC, Stewart K. Adherence to disease management programs in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:253–262. [PMC free article] [PubMed] [Google Scholar]
- 31.Hulka BS, Cassel JC, Kupper LL, Burdette JA. Communication, compliance, and concordance between physicians and patients with prescribed medications. Am J Public Health. 1976;66:847–853. doi: 10.2105/ajph.66.9.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med. 2004;19:1096–1103. doi: 10.1111/j.1525-1497.2004.30418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tarn DM, Heritage J, Paterniti DA, Hays RD, Kravitz RL, Wenger NS. Physician communication when prescribing new medications. Arch Intern Med. 2006;166:1855–1862. doi: 10.1001/archinte.166.17.1855. [DOI] [PubMed] [Google Scholar]
- 34.Foster JA, Yawn BP, Maziar A, Jenkins T, Rennard SI, Casebeer L. Enhancing COPD management in primary care settings. Med Gen Med. 2007;9:24. [PMC free article] [PubMed] [Google Scholar]
- 35.Yawn BP, Wollan PC. Knowledge and attitudes of family physicians coming to COPD continuing medical education. Int J Chron Obstruct Pulmon Dis. 2008;3:311–317. doi: 10.2147/copd.s2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roter DL, Hall JA. Strategies for enhancing patient adherence to medical recommendations. JAMA. 1994;271:80. doi: 10.1001/jama.271.1.80. [DOI] [PubMed] [Google Scholar]
- 37.Breekveldt-Postma NS, Koerselman J, Erkens JA, Lammers JW, Herings RM. Enhanced persistence with tiotropium compared with other respiratory drugs in COPD. Respir Med. 2007;101:1398–1405. doi: 10.1016/j.rmed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of Medicare beneficiaries: Prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14:553–560. doi: 10.18553/jmcp.2008.14.6.553. [DOI] [PMC free article] [PubMed] [Google Scholar]