Abstract
Paclitaxel and sirolimus are the two major drugs for the treatment of coronary arterial disease in current drug eluting stents. The two drugs can effectively inhibit the in-stent restenosis through their independent pathways and show synergistic effect in preventing tumor tissue growth. We hypothesize that the combination of the two drugs in a drug eluting stent (DES) can also effectively suppress the neointima growth in the stented artery. The present work was focused on the investigation of paclitaxel/sirolimus combination release profiles from a novel biodegradable polymer (poly (D, L-lactide -co-glycolide) / amorphous calcium phosphate, PLGA/ACP) coated stent both in vitro and in vivo. For the in vitro, the drug releasing profiles were characterized by measuring the drug concentration in a drug release medium (Dulbecco’s phosphate buffered saline, DPBS, pH 7.4) at predetermined time points. For the in vivo, a rat aorta stenting model was employed. The results showed that both paclitaxel and sirolimus had a two-phase release profile both in vitro and in vivo, which is similar to the drug release profile of their individual coated DESs, and there is no evident of interference between two drugs. The data suggest that paclitaxel and sirolimus can be combined pharmacokinetically in a DES for the treatment of coronary arterial diseases.
Keywords: Amorphous Calcium Phosphate, Drug-Eluting Stent, Paclitaxel, Poly (D, L-lactide -co-glycolide), Sirolimus
1. Introduction
Coronary arterial stenting with drug-eluting stent (DES) is a major therapy for the treatment of coronary arterial diseases in present interventional cardiology practice. Currently, four DESs have been approved by the FDA for the U.S. market including: Cypher® stent (Cordis, Miami, FL), Taxus® stent (Boston Scientific, Inc., Natick, MA), Endeavour® stent(Medtronic Minneapolis, MN) and Xience® stent(Abbott Laboratories, Abbott Park, IL). Among four approved DESs, besides Taxus® stent which is coated with anti-microtubule drug—paclitaxel, all other three DESs are coated with either sirolimus (Cypher® stent) or its analogs zotarolimus (Endeavour® stent) and everolimus (Xience® stent) [1]. Though paclitaxel or sirolimus used alone in DESs can effectively inhibit the restenosis formation [2, 3], restenosis in high risk patients such as small vessels, diabetes, and long segments of diffusely diseased arteries still remains unacceptably high (30%–60% in bare metal stents and 6%–18% in drug coated stents) [2–4]. Therefore, there still exists an unmet medical need for a more powerful anti-restenosis agent to curb the problem.
The in-stent restenosis (ISR) formation or the neointima growth in the stented arteries is a multiple factored sequential process involving smooth muscle cell (SMC) migration, extracellular matrix formation, macrophages recruitment, etc. over a period of several weeks [5–7]. This benign tissue growth process is similar to the tumor tissue growth [8], which had lead to the discovery of anti-tumor drugs such as paclitaxel and sirolimus as effective agents for the treatment of ISR [6, 8]. Drug combination therapy is an effective, well-known regimen used in the daily treatment of tumors clinically. The similar approaches have been investigated previously in the treatment of ISR with anti-proliferative drugs such as sirolimus combined with anti-thrombotic agents (Glycoprotein IIB/IIIA inhibitor or heparin) [9] or paclitaxel combined with nitric oxide [10]. However, the anti-restenosis effects of these combinations are limited primarily due to the physiochemical incompatibility among combined drugs. Local drugs that are retained within the blood vessel are more effective than those that are not [11]. Both heparin and nitric oxide compounds are so soluble and diffusible that they cannot stay simply in the artery for more than a few minutes after released [12]. In contrast, both sirolimus and paclitaxel are hydrophobic, and retained well in the blood vessel wall for up to three days through specifically binding to their individual binding proteins after they are released from stents [13]. The synergistic effect of sirolimus/paclitaxel combination in anti-tumor growth has been confirmed by a well designed study reported by Mondesire et al. [14]. In the study, investigators found that sirolimus is synergistic with paclitaxel, carboplatin and vinorelbine, and additive with doxorubicin and gemcitabine. The combination of sirolimus and paclitaxel leads to a significant reduction in tumor growth in vivo in a sirolimus-sensitive xenograft model. Hence, we hypothesize that the combination of the two drugs should be more effective in preventing ISR than their individual drug does when released from a coated DES due to their synergistic effect. The release kinetics of paclitaxel or sirolimus alone coated DES has been investigated extensively [15, 16], however, no studies were reported regarding their combination release profile from a biodegradable polymer coated stent. The purpose of this pilot study is to investigate the release kinetics of the drug combination loaded on a biodegradable stent coating (poly (D, L-lactide -co-glycolide) / amorphous calcium phosphate, PLGA/ACP) both in vitro and in vivo.
2. Experimental
2.1 DES preparation
Poly (D, L-lactide -co-glycolide) (PLGA, D, L lactide:glycolide=65:35), amorphous calcium phosphate nanoparticles (ACP, <150nm), methanol and ethanol were all purchased from Sigma-Aldrich (St. Louis, MO). Tetrahydrofuran (THF) was obtained from Fisher Scientific (Fair Lawn, NJ). The drug release medium was Dulbecco’s phosphate buffered saline (DPBS, pH 7.4, Solon, OH). Both paclitaxel and sirolimus were purchased from LC Labs (Woburn, MA).
Eighteen metal stents made from 316L stainless steel (13mm long) were provided by VasoTech, Inc (Lowell, MA). PLGA/ACP copolymer was made by blending PLGA and ACP nanoparticles at a ratio of 65:35 (w/w) in THF. The final polymer /drug coating matrix was made by dissolving equal amount of sirolimus and paclitaxel with the polymer/drug ratio of 65/35(w/w) in THF. Prior to coating, all stents were pre-weighed with a Cahn C-35 Ultra Microbalance (Thermo Fisher Scientific Inc., Waltham, MA), and then ultrasonically cleaned for 30 minutes (Branson 5210R-MT Ultrasonic Cleaner, Branson Ultrasonics Corporation, Danbury, CT). The stents were first spray-coated with the polymer/drug coating matrix by using a MediCoat DES 1000 Benchtop Stent Coating System (Sono-Tek Corp., Milton, NY) under a clean hood environment with total drug loading of 90±10μg on each stent, and then coated with a thin layer (approximately 10μm) of PLGA only over the drug layer. The final coating thickness was approximately 30±10μm.
2.2 Standard preparation
A standard curve was made by measuring the concentration of paclitaxel or sirolimus after the paclitaxel/sirolimus (50/50, w/w) combination was dissolved in a mixture of methanol/ethanol (50/50, v/v) in the range of 0 to 50μg/ml. The drug concentration was analyzed with an UV/VIS spectrometer (Lambda 25, PerkinElmer Inc., Waltham, MA) at the wavelength of 229nm (paclitaxel) and 277nm (sirolimus).
To minimize experimental errors from the drug loading, stent coating and drug releasing, three stents were incubated individually in 5ml drug extracting medium (50/50 methanol/ethanol, v/v) at room temperature for overnight after one hour’s sonication, and the real total amount of drug loading on each stent was measured with the same method as used in the standard preparation. The mean value and the drug difference between real measurement and the theoretical loading amount in each stent were calculated as the final reference.
2.3 In vitro drug release
Three drug-eluting stents were placed individually in three capped polypropylene tubes with 10ml DPBS in each and incubated at room temperature with constant shaking at 75rpm in a water bath (Precision Scientific Inc., Chicago, IL). At every three days post drug-releasing, 500μl supernatant was extracted carefully from the tube after the stent suspension was centrifuged at 6500rpm for 15min. The drug concentration in each tube was measured with the same UV/VIS spectrometer as used in the standard preparation. After each analysis, the supernatant solution was put back to the releasing tube in order to maintain the releasing medium at the same volume.
2.4 In vivo drug release
2.4.1 Animal preparation and stent implantation
All procedures involving animal use conformed with the “Guide for the Care and Use of Laboratory Animals“ published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996), and the animal study protocol was approved by Institutional Animal Care Committee at the Beth Israel Deaconess Medical Center, Harvard Medical School.
Twelve Sprague-Dawley male rats weighing from 400–500g were purchased from Charles River Laboratory (Wilmington, MA). All animals were fed aspirin-incorporated food (5mg/kg/day, Bio-Serv, Frenchtown, NJ) commencing three days before the surgery and maintained through the entire study period. All polymer coated stents were pre-mounted on VasoTech® miniature balloon catheters (1.5mm ×15mm, VasoTech, Inc.) and sterilized with Ethylene Oxide (ETO) for 60 minutes before implantation.
All animals were fasted 12 hours before the surgery. After the animal was fully anesthetized with constantly inhalation of a mixture of oxygen/isoflurane (1.5:2 pressure/pressure), the left iliac artery was exposed. The stent was inserted into the abdominal aorta 10mm above the bifurcation through left iliac arterial incision, and was deployed by inflating the balloon catheter to 10 ATM pressure for 30 seconds. The balloon catheter was deflated to maintain negative pressure for 30 seconds. The process was repeated three times to fully deploy the stent. The deflated catheter was then withdrawn slowly while leaving the stent in place. The artery was then sutured with 7–0 bio-absorbable suture. All animals were allowed to recover and returned to the animal care facility where they continued receiving antiplatelet therapy for the entire study period. Antibiotics (Gentamycin, 50mg/kg, IM, Abbott Laboratories, Abbott Park, Chicago, IL) were given to all animals for three days following the surgery. Postoperative analgesia was administrated, as needed, at the discretion of the attending veterinarian
2.4.2 Drug analysis
At predetermined days of 3,7,14 and 21 days post stent implantation, three animals in each time point were sacrificed. In the study, the rats were perfused with heparinized saline under deep anesthesia at 80mmHg pressure through the left ventricle until the perfusate from right atria was clear of blood. The stented arteries were harvested, and the tissue surrounding the stent was carefully removed under a stereomicroscope (American Scope Inc., CA) with a fine tweezers. The stents were then put into 5ml methanol/ethanol (50/50, v/v) releasing medium at room temperature for overnight after 1h sonication. After the stent suspension was centrifuged at 6500rpm for 15min, 500μl supernatant was extracted and the drug concentration was measured with the UV/VIS spectrometer.
2.5 Surface morphology
The surface morphology of coated stent was analyzed by a Field Emission Scanning Electron Microscope (FE-SEM, JEOL JSM-7401F, JEOL USA Inc., Peabody, MA) before and at 21 days after both in vitro and in vivo drug release studies. For the in vitro, the stent samples were dried in a vacuum oven at room temperature overnight before SEM observation. For the in vivo, the harvested stents were fixed by 4% buffered formaldehyde first after attached tissues were removed and then sequentially dehydrated by gradient ethanol (70%: 1h; 80%: 1h; 96%:1h, twice; 100%: 1h, twice) and finally dried in a vacuum oven at room temperature for the SEM observation.
3. Results and discussion
3.1 Standard curves
Using regression analysis, the following two equations for standard curves of paclitaxel and sirolimus were obtained respectively:
where Y is the absolute value of the absorbency of paclitaxel/sirolimus at 229nm/277nm, X is the concentration of paclitaxel/sirolimus (μg/ml) and R2 is the regression coefficient.
3.2 In vitro drug release kinetics
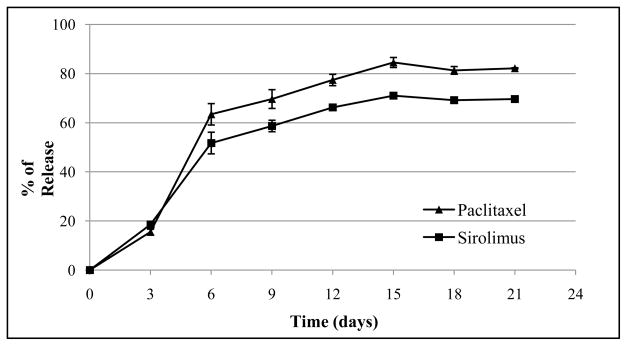
The in vitro release kinetics of paclitaxel and sirolimus was determined by an UV/VIS spectrometer for 21 days at regular intervals. Fig. 1 shows paclitaxel and sirolimus released from PLGA/ACP coated stents as the fraction of the total drug loading vs. the immersion time in the DPBS. As shown in the chart, two drugs have similar releasing behavior in the coated stent: burst release at first week (approximately 63.4% of paclitaxel and 51.7% of sirolimus were released) followed by a slow release phase to the end. At 21 days, approximately 82.1% of paclitaxel and 69.6% of sirolimus were released.
Fig. 1.
In vitro release profile of paclitaxel and sirolimus from PLGA/ACP coated stents in DPBS.
3.3 In vivo drug release kinetics
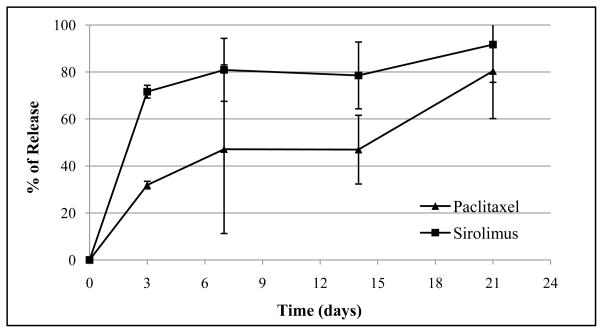
Fig. 2 is the release profile of paclitaxel and sirolimus in vivo. As shown in Fig. 2, both paclitaxel and sirolimus had similar releasing profiles as they were in vitro. However, the burst release effect was occurred significantly earlier in vivo (within the first 3 days) than that in vitro (one week). At 21 days post implantation, over 80% of both drugs were released (paclitaxel: 80.4%, sirolimus: 91.7%).
Fig. 2.
In vivo release profile of paclitaxel and sirolimus from PLGA/ACP coated stents in rats.
3.4 Morphological changes
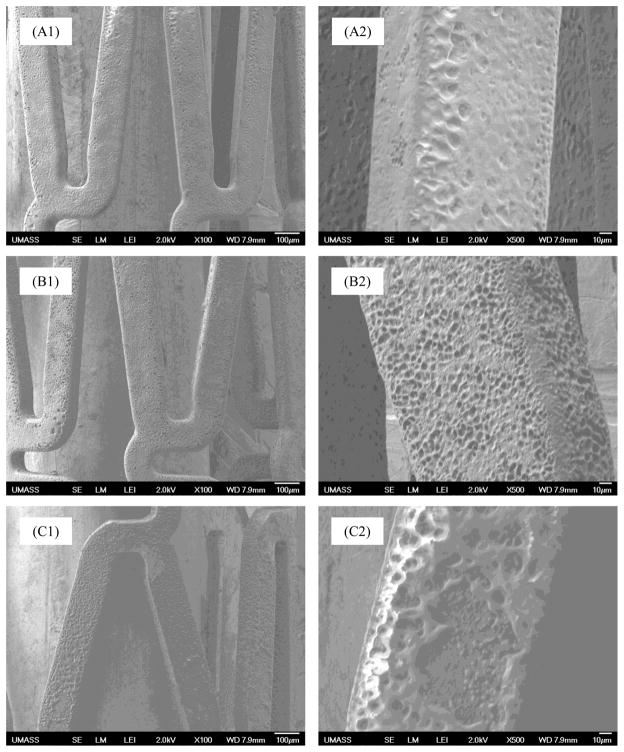
Fig. 3 is the morphological change of drug-eluting stent surface at prior and post in vitro and in vivo drug release studies at 21 days under the SEM. As shown in these SEM images, the stent surface changed from smooth before the study (Fig. 3A1 & A2), to porous structures at 21 days after both in vitro (Fig. 3B1 & B2) and in vivo (Fig. 3C1 & C2) studies. Microscopically, polymer degradation in vivo was more evident than in vitro.
Fig. 3.
SEM images of drug-eluting stent surface before (A1, A2) and after being incubated in DPBS (B1, B2) and implanted in rats (C1, C2) for 21 days, respectively (A1,B1,C1: lower power, A2,B2,C2: high power of A1,B1, and C1).
3.5 Drug release mechanism
In the present study, we investigated the drug release profiles of a paclitaxel/sirolimus combination coated stent both in vitro and in vivo. The two drugs had similar phase release profiles both in vitro and in vivo: a rapid burst release phase followed by a slow release phase. The results correlated well with the both drug release profiles when they were loaded individually [17], indicating that the drug releasing kinetics of both drugs were not affected when they were combined in a stent.
In vitro drug releasing from a biodegradable polymer coated stent is mainly through two mechanisms: 1) drug diffusion and 2) polymer’s degradation. At the early stage, it was generally thought that the burst release occurred via the dissolution of drugs from the surface of coated stents and rapid bulk erosion of the polymer [16]. After the initial burst release, both paclitaxel and sirolimus releasing profiles entered a slow release stage until to the end. Our morphological data (Fig. 3) showed that the polymer incorporated with drugs showed a smooth appearance, but changed gradually to a microporous structure at around 21 days post degradation. The data indicate that the drugs release mechanism in this period was controlled mainly through polymer’s degradation or peeling off, and the drugs from the core/inner of polymer matrix were released further via diffusion from the porous regions caused by the polymer degradation.
The in vivo drug release mechanism was more complex than in vitro since many factors such as blood flow, pH value, body temperature, cellular environment, etc. [18], were involved. Those factors will affect not only the drug release/uptake profile, but also the PLGA/ACP polymer’s degradation kinetics as shown in our data that both drugs had expedited burst releasing time in vivo as compared with that of in vitro. Other factors, such as polymer loss during the stent sterilization, expanding, implantation, may also potentially affect the total loss of the polymer and drugs.
It is interesting to note that though both drugs had similar phase release profile both in vitro and in vivo, the release rate of paclitaxel was a little higher than that of sirolimus in vitro while the paclitaxel release rate was much lower than sirolimus release rate and even a “late-onset burst release’ phenomenon was found in the terminal phase of paclitaxel release in vivo, which probably resulted from their different solubilities in different dissolutions, different absorption coefficient by cells and experimental/system errors. For better mimicking the in vivo conditions, a model with continuous flow media would be better than our current model. However, in vivo conditions cannot be fully represented by any in vitro models.
3.6 The potential benefit of paclitaxel/sirolimus combination
As stated hereinabove, paclitaxel, sirolimus and analogs of sirolimus have been proven to be clinically effective in preventing ISR and are in use worldwide. The drug dose in the first-generation DESs is probably excessive: Cypher® (sirolimus)--135μg/13mm stent with 68.4% release at 28 days; Taxus® (paclitaxel)--85μg/12mm stent with 10% release at 28 days; while it is relatively less in the second-generation DESs: Xience V® (everolimus) 56μg/12mm stent with 79.5% release at 28 days; Endeavor® (zotarolimus) 120μg/12mm stent with 95% release at 14 days [19–21]. Though higher dosage of anti-restenotic agents would suppress the neointima growth more effectively [17], too high dosage would also delay/inhibit the process of endothelialization, and furthermore, the residual drug in non-degradable polymer may incur the late-stage thrombosis (LST) --- the issue concerned most recently by the interventional cardiologists [22, 23]. Therefore, the trend for the drug-eluting stent is to use minimum drug, but remain maximum effect on restenosis inhibition. However, the efficacy and safety of our paclitaxel/sirolimus combination coated stent needs to be further proven in more extensive animal models or human studies.
3.7 Study limitations
There were two major limitations for the study: 1) the in vitro drug releasing model cannot fully represent the in vivo conditions; 2) there were only three samples at each time point in the in vivo study, which are statistically low. Therefore, the data generated from this in vitro and in vivo analysis should be further confirmed with studies in a clinical mimics’ situation at sufficient samples.
4. Conclusion
In the present study, we reported the release profiles of the paclitaxel and sirolimus combination coated stent both in vitro and in vivo. The results showed that the paclitaxel and sirolimus combination can be eluted almost completely within 21 days both in vitro and in vivo. Though paclitaxel and sirolimus had similar drug release kinetics both in vitro and in vivo, the burst release periods of both drugs in vivo are significantly shorter than that in vitro. The data indicate that the combination of two drugs in a drug eluting stent will not affect their individual drug release kinetic, and therefore can be combined pharmacokinetically in a drug eluting stent for the treatment of coronary arterial diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sheiban I, Villata G, Bollati M, Sillano D, Lotrionte M, Biondi-Zoccai G. Next-generation drug-eluting stents in coronary artery disease: focus on everolimus-eluting stent (Xience V®) Vasc Health Risk Manag. 2008;4:31–38. doi: 10.2147/vhrm.2008.04.01.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iijima R, Ikari Y, Miyazawa A, Nakajima H, Hara K. Predictors of restenosis after implantation of 2.5 mm stents in small coronary arteries. Circ J. 2004;68:236–240. doi: 10.1253/circj.68.236. [DOI] [PubMed] [Google Scholar]
- 3.Schofer J, Schlüter M, Gershlick AH, Wijns W, García E, Schampaert E, Breithardt G. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS) Lancet. 2003;362:1093–1099. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- 4.Muramatsu T, Tsukahara R, Ho M, Ito Y, Ishimori H, Hirano K, Nakano M, Matsushita M, Leung W. Clinical outcome of stent implantation in small coronary arteries using different type of coronary stents. J Invasive Cardiol. 2001;13:634–639. [PubMed] [Google Scholar]
- 5.Welt FGP, Rogers C. Inflammation and restenosis in the stent era, Arterioscler. Thromb Vasc Biol. 2002;22:1769–1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 6.Bennett MR. In-stent stenosis: pathology and implications for the development of drug eluting stents. Heart. 2003;89:218–224. doi: 10.1136/heart.89.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah PK. Inflammation, neointimal hyperplasia, and restenosis: as the leukocytes roll, the arteries thicken. Circulation. 2003;107:2175–2177. doi: 10.1161/01.CIR.0000069943.41206.BD. [DOI] [PubMed] [Google Scholar]
- 8.Cotran R, Kumar V, Collins T. WB Saunders. 6. Philadelphia: 1999. pp. 260–328. [Google Scholar]
- 9.Leon MB, Bakhai A. Drug-eluting stents and glycoprotein IIb/IIIa inhibitors: Combination therapy for the future. Am Heart J. 2003;146:S13–S17. doi: 10.1016/j.ahj.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Lin CE, Garvey DS, Janero DR, Letts LG, Marek P, Richardson SK, Serebryanik D, Shumway MJ, Tam SW, Trocha AM. Combination of paclitaxel and nitric oxide as a novel treatment for the reduction of restenosis. J Med Chem. 2004;47:2276–2282. doi: 10.1021/jm0304111. [DOI] [PubMed] [Google Scholar]
- 11.Nugent MA, Karnovsky MJ, Edelman ER. Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993;73:1051–1060. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- 12.Lovich MA, Edelman ER. Tissue average binding and equilibrium distribution: an example with heparin in arterial tissues. Biophys J. 1996;70:1553–1559. doi: 10.1016/S0006-3495(96)79719-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levin AD, Vukmirovic N, Hwang CW, Edelman ER. Specific binding to intracellular proteins determines arterial transport properties for rapamycin and paclitaxel. Proc Natl Acad Sci USA. 2004;101:9463–9467. doi: 10.1073/pnas.0400918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mondesire WH, Jian W, Zhang H, Ensor J, Hung MC, Mills GB, Meric-Bernstam F. Targeting Mammalian Target of Rapamycin Synergistically Enhances Chemotherapy-Induced Cytotoxicity in Breast Cancer Cells. Clin Cancer Res. 2004;10:7031–7042. doi: 10.1158/1078-0432.CCR-04-0361. [DOI] [PubMed] [Google Scholar]
- 15.Klugherz BD, Llanos G, Lieuallen W. Stent-based delivery of sirolimus for the prevention of restenosis. J Am Coll Cardiol. 2000;35:58A. [Google Scholar]
- 16.Kothwala D, Raval A, Choubey A, Engineer C, Kotadia H. Paclitaxel drug delivery from cardiovascular stent. Trends Biomater Artif Organs. 2006;19:88–92. [Google Scholar]
- 17.Pires NMM, van der Hoeven BL, de Vries MR, Havekes LM, van Vlijmen BJ, Hennink WE, Quax PHA, Jukema JW. Local perivascular delivery of anti-restenotic agents from a drug-eluting poly([epsilon]-caprolactone) stent cuff. Biomaterials. 2005;26:5386–5394. doi: 10.1016/j.biomaterials.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 18.Tracy MA, Ward KL, Firouzabadian L, Wang Y, Dong N, Qian R, Zhang Y. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials. 1999;20:1057–1062. doi: 10.1016/s0142-9612(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 19.Nakazawa G, Finn AV, Kolodgie FD, Virmani R. A review of current devices and a look at new technology: drug-eluting stents. Expert Rev Med Devices. 2009;6:33–42. doi: 10.1586/17434440.6.1.33. [DOI] [PubMed] [Google Scholar]
- 20.Serruys PW, Ruygrok P, Neuzner J, Piek JJ, Seth A, Schofer JJ, Richardt G, Wiemer M, Carrie D, Thuesen L. A randomised comparison of an everolimus-eluting coronary stent with a paclitaxel-eluting coronary stent: the SPIRIT II trial. Euro Intervention. 2006;2:286–294. [PubMed] [Google Scholar]
- 21.Kandzari DE, Leon MB. Overview of pharmacology and clinical trials program with the zotarolimus-eluting endeavor stent. J Interv Cardiol. 2006;19:405–413. doi: 10.1111/j.1540-8183.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 22.Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 23.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]