Abstract
Background & Aims
Premature neonates are predisposed to necrotizing enterocolitis (NEC), an idiopathic, inflammatory bowel necrosis. We investigated the hypothesis that NEC occurs in the preterm intestine due to incomplete ‘non-inflammatory’ differentiation of intestinal macrophages, which increases the risk of a severe mucosal inflammatory response to bacterial products.
Methods
We compared inflammatory properties of human/murine fetal, neonatal, and adult intestinal macrophages. To investigate gut-specific macrophage differentiation, we next treated monocyte-derived macrophages with conditioned media from ex planted human fetal and adult intestinal tissues. Transforming growth factor-beta (TGF-β) expression and bioactivity were measured in fetal/adult intestine and in NEC. Finally, we used wild-type and transgenic mice to investigate the effects of deficient TGF-β signaling on NEC-like inflammatory mucosal injury.
Results
Intestinal macrophages in the human preterm intestine (fetus/premature neonate), but not in full-term neonates and adults, expressed inflammatory cytokines. Macrophage cytokine production was suppressed in the developing intestine by TGF-β, particularly the TGF-β2 isoform. NEC was associated with decreased tissue expression of TGF-β2 and decreased TGF-β bioactivity. In mice, disruption of TGF-β signaling worsened NEC-like inflammatory mucosal injury, whereas enteral supplementation with recombinant TGF-β2 was protective.
Conclusions
Intestinal macrophages progressively acquire a non-inflammatory profile during gestational development. TGF-β, particularly the TGF-β2 isoform, suppresses macrophage inflammatory responses in the developing intestine and protects against inflammatory mucosal injury. Enterally-administered TGF-β2 protected mice from experimental NEC-like injury.
Keywords: necrotizing enterocolitis, macrophage, newborn, inflammation, TGF-β
Introduction
Premature neonates born before 32 weeks of estation or with a birth weight <1500 grams are predisposed to necrotizing enterocolitis (NEC), an idiopathic, inflammatory bowel necrosis characterized by pneumatosis intestinalis (accumulation of gaseous products of bacterial fermentation within the bowel wall), inflammation, and tissue necrosis.1–2 Existing data indicate that bacterial flora normally present in the gut lumen, not specific bacterial pathogens, play a major role in the pathogenesis of NEC.1–2 The pathophysiological importance of bacteria in NEC is underscored by the frequent detection of bacteria and pneumatosis in intestinal tissue, occurrence of NEC only after postnatal bacterial colonization and never in the sterile intrauterine microenvironment prior to birth, inability to induce NEC-like lesions in germ-free experimental animals, and by observations that enteral antibiotics may reduce the incidence of NEC in preterm infants.2–3 Based on current evidence, NEC is believed to occur when mucosal injury or altered permeability in the preterm intestine permits the translocation of luminal bacteria across the epithelial barrier, which, in turn, triggers a severe inflammatory response.2, 4
Gut mucosal injury and bacterial translocation are frequent events in critically-ill patients of all ages, but unlike in premature infants, these invading bacteria do not evoke a NEC-like inflammatory response in the mature intestinal mucosa. Bacteria that breach the gut epithelial barrier are normally eliminated by resident macrophages in the lamina propria, the first phagocytic cells of the innate immune system to encounter these microorganisms.5–6 Unlike macrophages in other organ systems that release cytokines/chemokines upon phagocytosis of bacteria to stimulate a local inflammatory response, intestinal macrophages are profoundly suppressed for cytokine production.6 This unique dichotomy of phagocytic versus inflammatory properties in intestinal macrophages plays an important role in maintaining the normal absence of inflammation in the gut mucosa (despite close proximity to immunostimulatory bacteria), as illustrated by spontaneous onset of enterocolitis in genetically-modified mice with defects in differentiation of intestinal macrophages.6–9 In this context, we hypothesized that the risk of NEC in the premature intestine is related to the state of differentiation of intestinal macrophages. We postulated that the non-inflammatory differentiation of intestinal macrophages is a function of gestational maturation and is therefore, incomplete in the preterm intestine, increasing the risk of a severe mucosal inflammatory response to bacterial products. In this study, we investigate maturational changes in gut macrophage differentiation in the context of NEC using a variety of in-vitro and in-vivo models.
Methods
Human intestinal tissue samples
Human intestinal tissues were collected after approval by local Institutional Review Boards. Fetal intestinal tissue (11–24 wks, n=25) was obtained at elective terminations of pregnancy. Tissue samples of advanced NEC (n=8) were compared with healthy tissue margins obtained during resection for indications other than NEC (premature neonates: repair of ostomy, n=5; gestational age 27, 27.5, 28.5, 30.5, and 32 wks; full-term neonates: atresia/obstruction; n=3). Adult tissues were obtained during bariatric surgery (n=5).
Immunohistochemistry
Tissue sections (and cells) were stained for macrophage markers (HAM56 or F4/80), tumor necrosis factor (TNF)-α, interleukin (IL)-8, TGF-β2, and TGF-β receptors using our previously-described fluorescence protocol6, 10–12 (included in online supplement).
Murine intestinal macrophages
Murine studies were approved by the local Institutional Animal Care and Use Committee. Murine intestinal macrophages were isolated by standard methods including density centrifugation and adherence to polystyrene,8, 13 described in the online supplement.
Real-time polymerase chain reaction (PCR)
Inflammatory cytokines and TGF-β isoforms were measured by our previously-described quantitative PCR method using SYBR green.14–15
Tissue-conditioned media (T-CMs)
We prepared T-CMs from fresh human and murine intestinal tissue by using a previously-reported protocol.16 Briefly, fresh intestinal tissue was cleaned, IECs were removed by dispase/ethylenediaminetetraacetic acid to expose the lamina propria, and the remaining tissue was incubated overnight in serum-free RPMI. The exfoliated epithelial cells were incubated overnight in a separate plate to prepare epithelial-conditioned media (E-CMs). In some experiments, we used intact tissue (with epithelium) to prepare the T-CMs. A detailed protocol is included in the online supplement.
Treatment of monocyte-derived macrophages with T-CMs
Blood monocytes from healthy adult volunteers were isolated by Ficoll-Hypaque centrifugation and immunoselection with CD14 microbeads (Miltenyi).10 Monocyte-derived macrophages (20,000/well) were incubated in 96-well plates with T-CM (250, 500, and 1000 µg total protein/mL) × 2–24 hrs and then stimulated with 500 ng/mL E. coli LPS (pre-determined optimum; Sigma) for up to 18 hrs.
Measurement of inflammatory cytokines
TNF-α (human and murine), IL-6, IL-1β, and IL-8 were measured by commercially-available ELISA kits (R&D, Minneapolis, MN).
Neutrophil chemotaxis
Culture supernatants from monocyte-derived macrophages were tested for neutrophil chemotactic activity using our fluorescence-based protocol17 described in the online supplement.
NF-κB activation
Macrophages were treated with T-CMs and LPS as above. NF-κB p65 phosphorylation was measured by ELISA (SuperArray Biosciences).
TGF-bioactivity
TGF-β bioactivity was measured by a quantitative luciferase assay; T-CMs were added for 16 hrs to mink lung epithelial cells transfected with a luciferase plasmid containing the TGF-β–responsive promoter of the platelet activator inhibitor-1 (PAI-1) gene;18 Smad2/3 phosphorylation (ser423, ser425) was measured by western blots using polyclonal antibodies against total and phosphorylated smad 2 (Santa Cruz) and appropriate secondary reagents.19 TGF-β activity was neutralized in vitro by addition of excess (15 or 50 µg/mL) neutralizing anti-human TGF-β antibody.
Assays for TGF-β isoforms
Total and active TGF-β1, TGF-β2, and TGF-β3 were measured by ELISA (R&D). T-CMs containing only one unique TGF-β isoform were derived from 20–24 wk T-CMs (n=3) by removing two of the three TGF-β isoforms by immunoprecipitation;19 the presence/absence of TGF-β isoforms was confirmed by ELISA. Macrophages were treated with parent or derivative T-CM prior to LPS stimulation. In some experiments, we treated macrophages with 15.6–2000 pg/mL recombinant human TGF-β1, TGF-β2, and TGF-β3 (R&D) instead of T-CMs before stimulation with LPS.
Mice
DNIIR mice20 were treated with zinc sulfate (50 µg/gm/day subcutaneous; pre-determined optimum dose) for up to 7 days. Identification of DNIIR mice and measurement of DNIIR mRNA20 is described in the online supplement. To confirm the loss of TGF-β-mediated signaling upon zinc supplementation, we administered recombinant TGF-β2 (100 ng, intra-peritoneal) in DNIIR mice (after 0, 3, and 7 days of zinc supplementation) and measured smad phosphorylation in intestinal tissue after 1 hour.
Gut mucosal injury was induced in 10–12 day old mouse pups by intraperitoneal administration of PAF (50 µg/kg) and LPS (1 mg/kg).21–22 Mice were sacrificed 2 hrs after PAF and LPS administration and mucosal injury was graded on a 5-point scale: grade 0: no injury; grade 1: mild separation of lamina propria; grade 2: moderate separation of submucosa; grade 3: severe separation and/or edema in submucosa; grade 4: transmural injury.23 To investigate whether enterally-administered TGF-β2 protected against mucosal injury, we treated some mice with 100 ng recombinant TGF-β2 2 hours prior to PAF and LPS administration. The protective effect of TGF-β2 were further confirmed in a second experimental model of NEC24 where 10-day-old mouse pups were separated from the dam, provided formula feedings every 3 hours and exposed to hypoxia (5% oxygen for 2 min) twice daily prior to feedings. The formula was prepared by mixing 15gm of Similac 60/40 (Abbott Laboratories, Abbott Park, IL) in 75 ml of Esbilac canine milk replacer (PetAg, Hampshire, IL); 200 µL/5 gm body weight by gavage over 2–3 min. Pups were sacrificed after 4 days and intestinal injury was measured as above.
Statistical analysis
Statistical analyses were performed using the software package SigmaStat version 5.1 (Systat, San Jose, CA) as described in figure legends.
Results
Macrophages in the preterm human intestine express inflammatory cytokines
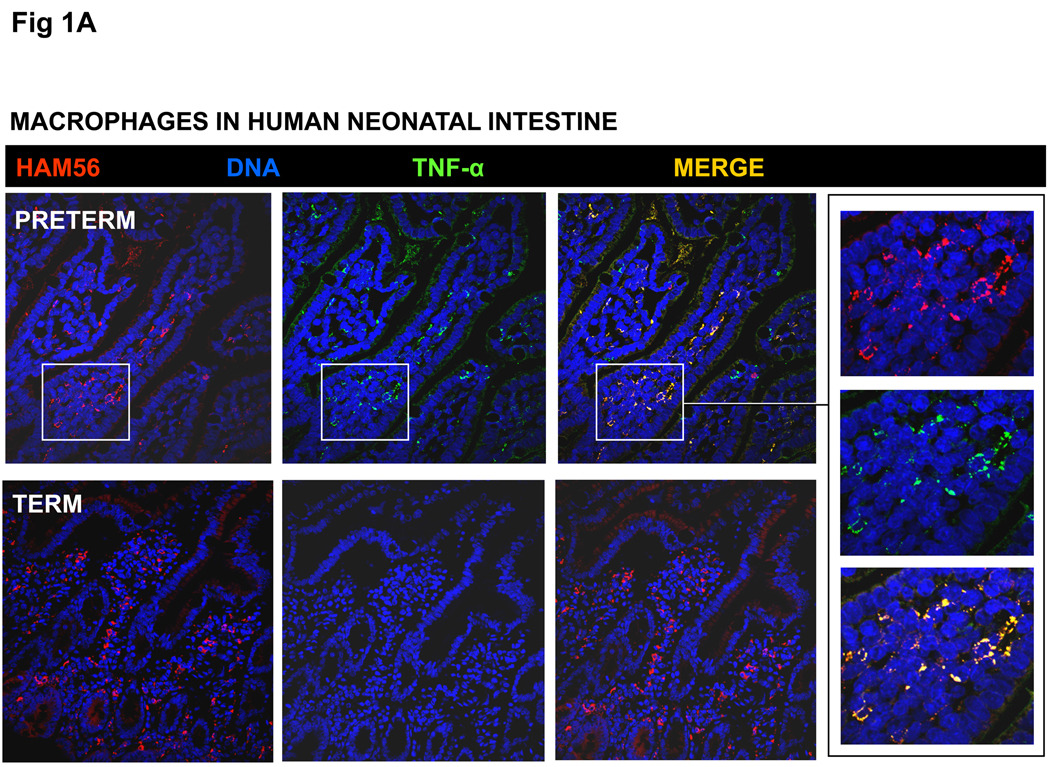
To investigate our hypothesis that the inflammatory downregulation of intestinal macrophages is a function of gestational maturation, we first compared macrophages in intestinal tissue samples from human fetuses, premature and full-term neonates, and adults by Immunohistochemistry for inflammatory cytokines. Consistent with existing information,6, 25 macrophages in the full-term neonatal and adult intestine did not express TNF-α or IL-8/CXCL8. However, macrophages in preterm intestine (from fetuses and premature neonates) showed strong immunoreactivity for TNF-α (Fig. 1A) and IL-8 (not depicted).
Fig. 1.
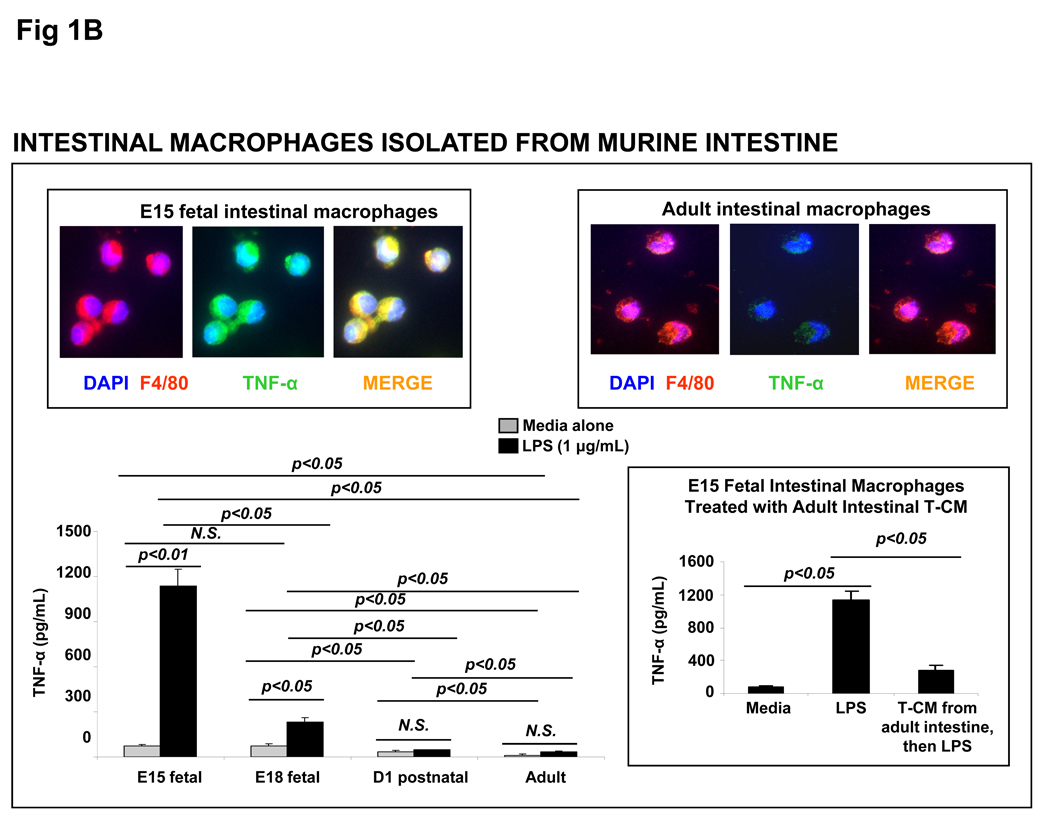
(A) Macrophages in the preterm human intestine express TNF-α. Photomicrographs of jejunum (magnification 100×) show the distribution of immunoreactivity for the macrophage marker HAM56 (red) and TNF-α (green). Co-localization is shown in a computer-merged image (yellow). Macrophages in preterm (26-wk premature infant) but not in the full-term intestine show strong immunoreactivity for TNF-α. Inset: high-magnification images (400×) of selected area highlight TNF-α expression in HAM56+ macrophages in the preterm intestine. (B) Macrophages isolated from the murine fetal intestine express TNF-α upon LPS stimulation in vitro: Bar diagram shows TNF-α concentration (means ± SEM) in culture supernatants from primary murine intestinal macrophages. Macrophages from the E15 and E18 murine fetuses, but not those from the P1 pups or adult mice, show LPS-induced TNF-α expression. Photomicrographs above the bar diagram show co-localization of F4/80 (a murine macrophage marker) and TNF-α in the fetal, but not in adult murine intestinal macrophages. Inset: bar diagram shows that tissue-conditioned media prepared from the adult mouse intestine suppressed LPS-induced TNF-α production in E15 murine fetal intestinal macrophages. Experiments represent 3 T-CMs per group; replicates were averaged. * indicates p<0.05 in Kruskall-Wallis H test.
Macrophages isolated from the murine fetal intestine express TNF-α upon LPS stimulation in vitro but become tolerant to LPS upon exposure to media conditioned with adult intestinal tissue
We next investigated developmental changes in the inflammatory properties of intestinal macrophages in vitro. Because primary human fetal intestinal macrophages were not available (limited availability of fresh human fetal tissue), we compared murine CD11b+ F4/80+ CD11cint intestinal macrophages isolated from fetal, neonatal, and adult mouse intestine as a model system.8 In support of our immunohistochemical data from the human intestine, murine intestinal macrophages from embryonic day 15 (E15) and E18 fetuses, but not from newborn pups or adults, produced TNF-α following LPS stimulation in vitro (Fig. 1B).
We have shown previously that intestinal macrophages are derived from ‘proinflammatory’ blood monocytes, which differentiate under the influence of the extracellular matrix (ECM) in the lamina propria to acquire a ‘non-inflammatory’ profile.5, 10 To explain the intact inflammatory responses of fetal intestinal macrophages, we hypothesized that tissue factor(s) that suppress the inflammatory responses of macrophage precursors in the intestine are expressed as a function of gestational maturation, and are therefore, deficient in the fetus. To test this possibility, we treated E15 intestinal macrophages (exposed in vivo to an immature matrix) and ‘supplied’ the differentiating factor(s) missing from the fetal intestine by adding media conditioned with ex planted adult mouse jejunum. In support of our hypothesis, adult T-CMs suppressed fetal intestinal macrophages for LPS-induced TNF-α production (inset).
Human peripheral blood monocyte-derived macrophages develop LPS-tolerance upon exposure to media conditioned with human adult, but not fetal, intestinal tissue
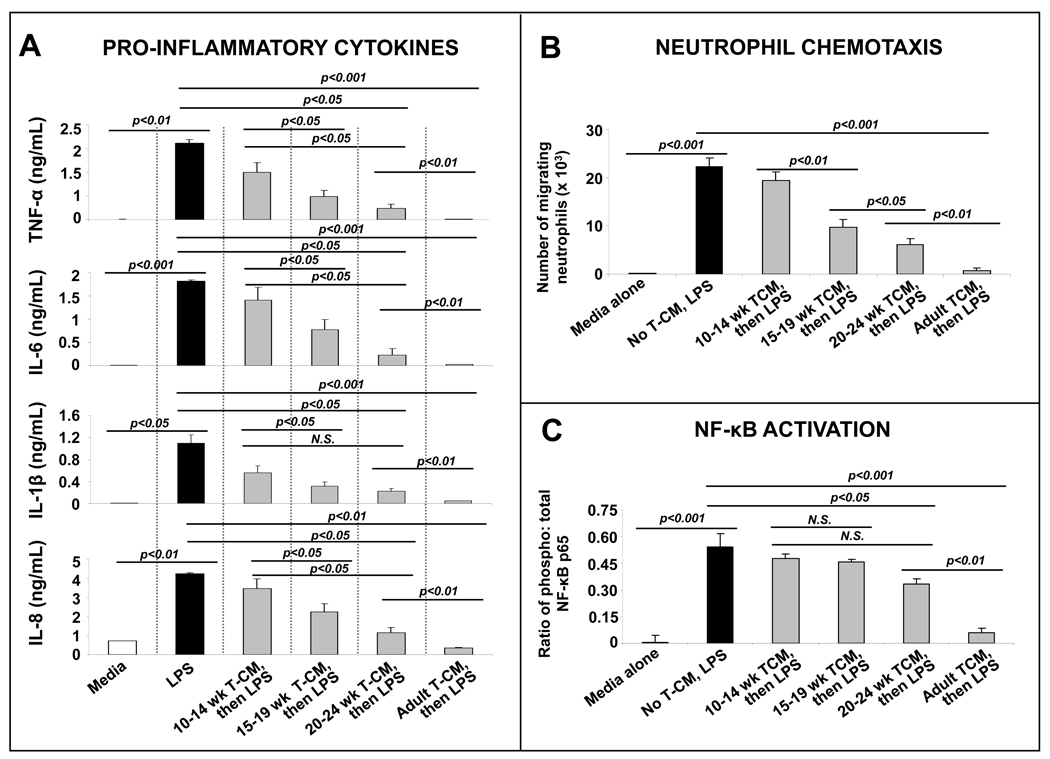
The inflammatory downregulation of intestinal macrophages can be recapitulated in vitro by exposing monocyte-derived macrophages to media conditioned with ex planted adult intestinal tissue.10 To investigate whether the non-inflammatory differentiation of intestinal macrophages undergoes a similar ‘maturation’ in human and murine fetal intestine, we treated monocyte-derived macrophages with T-CMs prepared from fetal tissue of various gestational ages. Consistent with our hypothesis, we found that the suppressive effect of fetal T-CMs on LPS-induced macrophages cytokine production increased with gestational age but remained significantly less than that of adult T-CM (Fig. 2A; supporting data from a real-time PCR array shown in Supplementary fig. 1). T-CM suppression of macrophage cytokine production followed a similar pattern when macrophages were stimulated with heat-killed Listeria monocytogenes (to stimulate toll-like receptor-2) instead of LPS (not depicted).
Fig. 2.
Human peripheral blood monocyte-derived macrophages develop LPS-tolerance upon exposure to media conditioned with human adult, but not fetal, intestinal tissue (A) Bar diagrams show TNF-α, IL-6, IL-1β, and IL-8 concentrations (means ± SEM) in culture supernatants from monocyte-derived macrophages treated with T-CMs. Fetal T-CMs became more effective in suppressing macrophage cytokine production with gestational maturation but remained inferior to adult T-CMs. Data representative of 3 independent experiments, each performed with tissues from 3–5 fetuses per fetal group and 3 adults. (B) T-CM suppression of macrophage cytokine production shown in panel A correlated with a corresponding reduction in the neutrophil chemotactic activity of these culture supernatants. Bar diagram (means ± SEM) show the number of neutrophils migrating across a polycarbonate filter in a microchemotaxis chamber towards culture supernatants from experiments in panel A. Data are representative of 3 independent experiments, each performed with 3–5 supernatants from each group; (C) Unlike T-CMs prepared from adult intestinal tissue, fetal T-CMs did not block LPS-induced NF-κB activation in macrophages. Bar diagram (means ± SEM) shows ratio of phosphorylated: total NF-κB p65. Data represent 3–4 T-CMs per group. All experimental groups in the 3 panels were compared by Kruskall-Wallis H-test.
The maturational increase in T-CM suppression of macrophage cytokine production was not affected by variations in the protocol for T-CM preparation, such as the presence or absence of IECs on ex planted intestinal tissue. A similar (albeit weaker) maturational pattern was detected when culture supernatants from exfoliated IECs were added to macrophage cultures instead of T-CMs (Supplementary fig. 2). Finally, as T-CMs from premature and full-term neonatal tissue were not available, we used tissue lysates of frozen intestinal tissue samples from 20–24 wk fetuses, term neonates, and adults in some experiments. These lysates were less efficacious than T-CMs but showed a similar maturational increase in the suppression of LPS-induced TNF-α production (not depicted).
Media conditioned with human intestinal tissue induce LPS-tolerance in human peripheral blood monocyte-derived macrophages by providing TGF-β
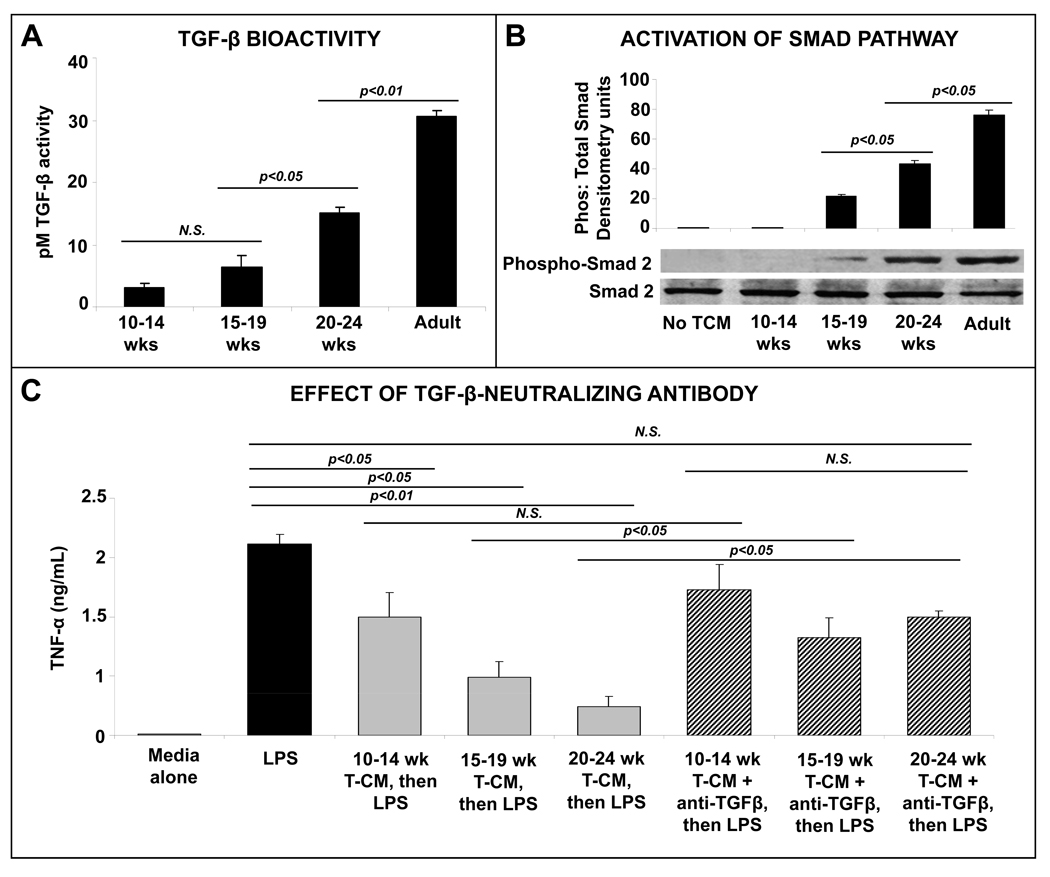
To understand the inflammatory downregulation of intestinal macrophages, we evaluated TGF-β-, IL-10-, and programmed death-1-ligand 1 (PD1L1)-mediated effects as possible mechanisms.9 TGF-β was the most plausible mediator of these effects because the inflammatory downregulation of intestinal macrophages can be reproduced by exposing macrophages to ECM products present in T-CMs, and preformed TGF-β, but not IL-10 and PD1L1, is stored in intestinal ECM.5 Consistent with the maturational increase in T-CM suppression of macrophage cytokine production, we detected increasing TGF-β bioactivity in these T-CMs (Fig. 3A). Similarly, T-CM treatment of macrophages induced the phosphorylation of smad2 (a signaling event specifically associated with TGF-β), which became progressively more robust with increasing gestational age of the intestinal tissue sample (Fig. 3B). The role of TGF-β in T-CM suppression of macrophage cytokine production was confirmed by the reversal of T-CM effects upon neutralization of TGF-β (Fig. 3C).
Fig. 3.
Media conditioned with human intestinal tissue induce LPS-tolerance in human peripheral blood monocyte-derived macrophages by providing TGF-β: (A) TGF-β bioactivity, measured as activation of the platelet activator inhibitor-1 promoter in a luciferase assay, increases in intestinal tissue-conditioned media with maturation. Data represent n = 3–5 samples per group; (B) T-CM activation of smad signaling in human monocyte-derived macrophages increases with maturation. Bar diagram shows densitometric analysis of blots (means ± SEM). Data are representative of 3 independent experiments, each performed with a distinct set of T-CMs and utilized 2 different monocyte donors; (C) T-CM suppression of LPS-induced cytokine production in human monocyte-derived macrophages was reversed by neutralization of TGF-β in the conditioned media. Bar diagram shows LPS-induced TNF-α (means ± SEM) production in macrophages. Data represent n = 4–6 T-CMs per group. All experimental groups in the 3 panels were compared by Kruskall-Wallis H-test.
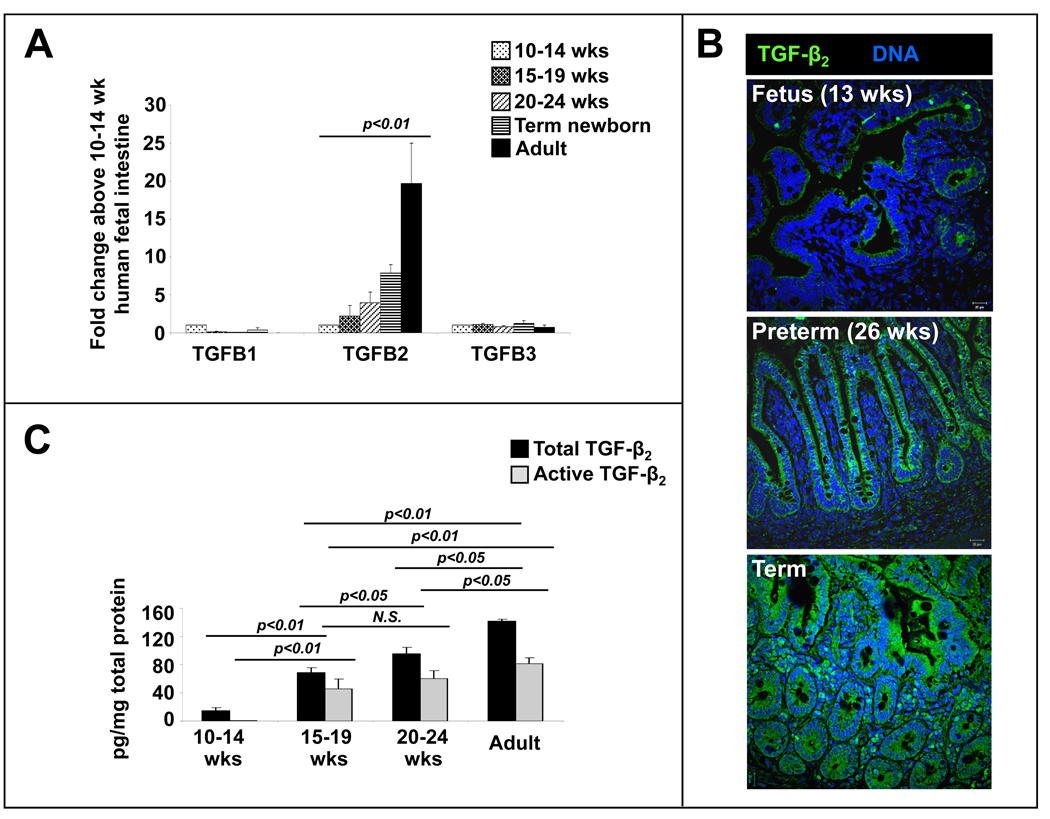
Media conditioned with human intestinal tissue suppress cytokine production in human monocyte-derived macrophages primarily via the TGF-β2 isoform
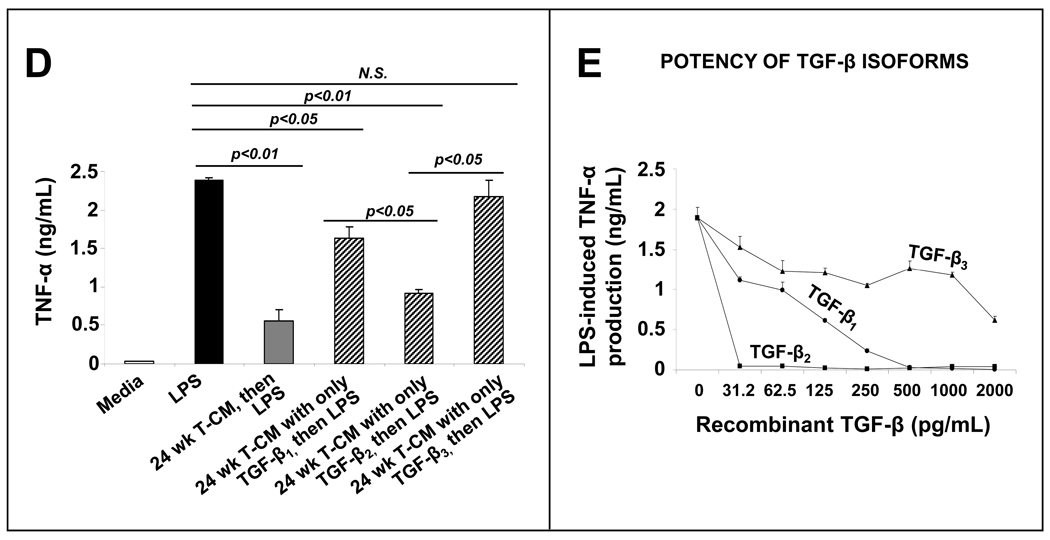
The expression of TGF- β2, but not TGF-β1 or TGF-β3, increased in intestinal tissue with maturation (Fig. 4A–C). To ascertain the relative contribution of the three TGF-β isoforms to the anti-inflammatory effects of T-CMs, we removed two of three isoforms in different aliquots of T-CMs by immunoprecipitation. T-CM derivatives containing TGF-β2 were most effective in suppressing LPS-induced cytokine production (Fig. 4D). TGF-β1 had a smaller effect, whereas TGF-β3 did not affect macrophage cytokine production. These effects were blocked by neutralizing isoform-specific antibodies (R&D, not depicted). In support of these data, we found that recombinant human TGF-β2 was more potent than recombinant TGF-β1 or recombinant TGF-β3 in suppressing macrophage cytokine production (inset).
Fig. 4.
Media conditioned with human intestinal tissue suppress cytokine production in human monocyte-derived macrophages primarily via the TGF-β2 isoform: (A) mRNA expression of TGF-β2, but not of TGF-β1 or TGF-β3, increases with intestinal maturation. Data depicted as fold-change above 10–14 wk fetal intestine (means ± SEM) and represent n = 3–4 per group; (B) TGF-β2 immunoreactivity (green) increases in the intestine with gestational maturation. TGF-β2 was detected in epithelium and in cells in the lamina propria. Nuclear staining (blue) was obtained with DAPI. (C) Concentrations of active and total TGF-β2 increased in T-CMs with maturation. Bar diagrams show means ± SEM. Data represent n=3–5 per group; (D) TGF-β2 is the most important TGF-β isoform in T-CM downregulation of macrophage cytokine production. We removed two of the three TGF-β isoforms by immunoprecipitation in separate T-CM aliquots to obtain T-CM derivatives containing only one of the three TGF-β isoforms. Bar diagram (means ± SEM) shows that T-CMs containing TGF-β2 were most effective in suppressing LPS-induced TNF-α production in macrophages. Data are representative of 3 independent experiments, each performed with T-CMs derived from 3–4 subjects in each group; Inset: recombinant TGF-β2 is the most potent of the three isoforms in suppressing LPS-induced TNF-α production in macrophages. Data are representative of 3 independent experiments. All experimental groups in the 3 panels were compared by Kruskall-Wallis H-test.
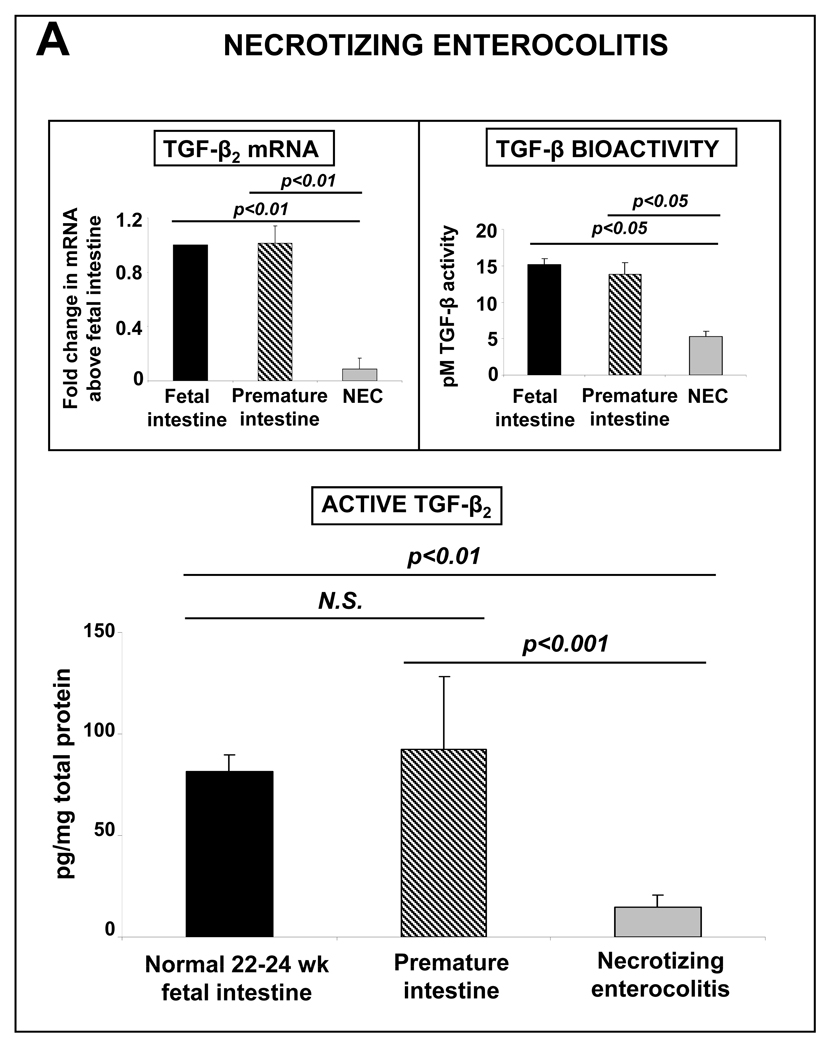
TGF-β2 expression is decreased in intestinal tissues samples resected from patients with NEC
Based on our findings, we argued that macrophages in preterm intestine, which are yet to undergo inflammatory downregulation, should predispose all premature infants to inflammatory mucosal injury. In this context, the actual 5–15% incidence of NEC in premature infants appeared to be surprisingly low. To investigate whether clinically-evident NEC occurs in infants with the lowest levels of TGF-β2 expression, we compared tissue expression of TGF-β2 in NEC (n=8; mean gestational age ± standard error = 28±1.5 wks) versus intestinal conditions other than NEC (n=5; 29.1±2 wks) and normal midgestation fetal intestine (n=6; 22±0.4 wks). TGF-β2 expression and TGF-β bioactivity were lower in NEC than in the controls (Fig. 4). The concentrations of TGF-β1 were also lower in NEC (not depicted), while TGF-β3 was expressed near the lower limit of detection.
TGF-β protects mouse pups against NEC-like intestinal injury
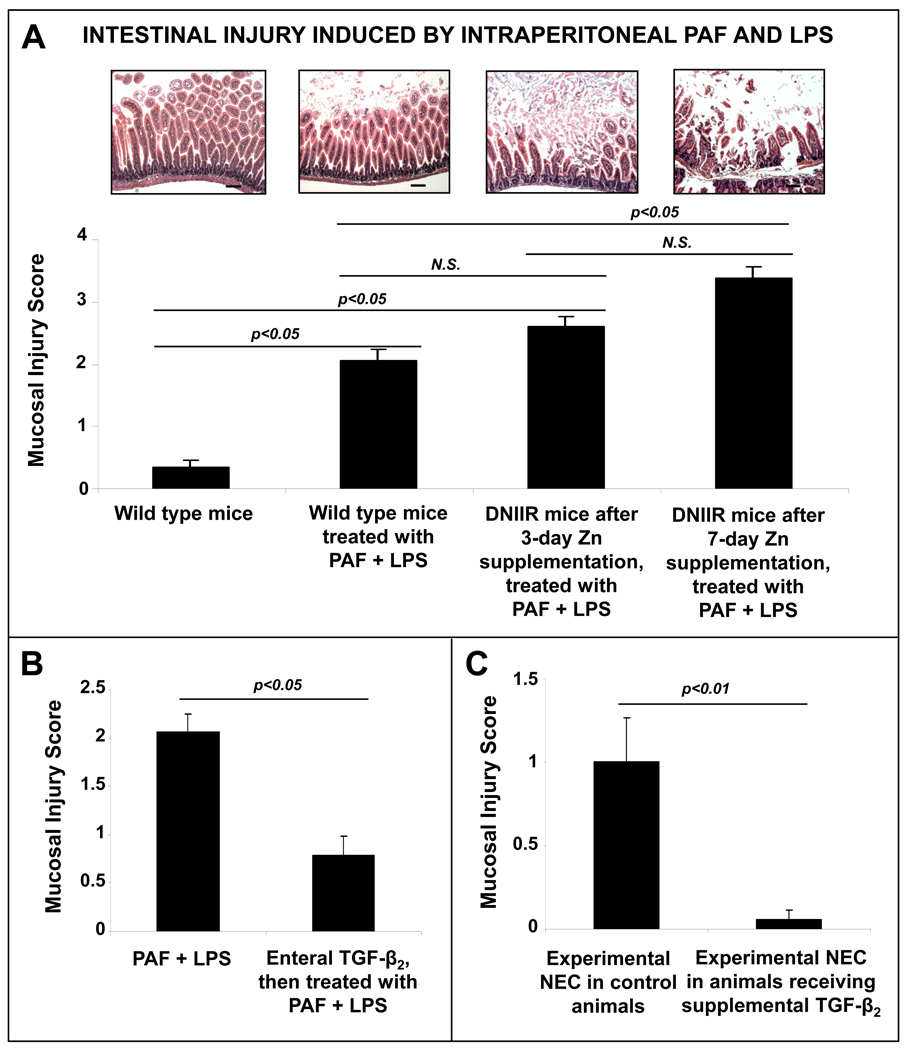
We next used a murine model to investigate whether decreased TGF-β-mediated signaling could worsen inflammatory mucosal injury. Although TGF-β2 was the most potent of the three TGF-β isoforms in suppressing macrophage cytokine production, TGF-β1 and TGF-β3 showed similar effects at high concentrations (Fig. 4D). In the intestine, TGF-β is normally stored in a bound-form in the lamina propria where these peptides are anchored to ECM proteins.5 Depending on the physical proximity to a differentiating macrophage, these matrix stores of TGF-β could theoretically provide biologically-relevant local concentrations of TGF-β1 and/or TGF-β3 in the immediate microenvironment of the macrophage. Based on these considerations, we could not exclude the possibility of some redundancy in the anti-inflammatory effects of TGF-β isoforms and therefore, opted against the use of isoform-specific deletion/neutralization models. We used a transgenic mouse that can be induced to lose TGF-β receptor II (TGF-β RII) expression and consequently, all TGF-β-mediated signaling. . In these DNIIR mice,20 which express a kinase-defective TGF-β RII transgene upon zinc supplementation, we were able to induce a partial deficiency of TGF-β effects after 3 days of zinc supplementation and a complete abrogation of all TGF-β signaling after 7 days (supplementary Fig. 3). To determine whether TGF-β protects the neonatal intestine against inflammatory injury, we induced gut mucosal injury by intraperitoneal administered PAF and LPS in wild-type, 3-day-zinc DNIIR, and 7-day-zinc DNIIR mice (n=18 per group). The severity of intestinal injury worsened with increasing loss of TGF-β effects in the intestine (Fig. 6A).
Fig. 6.
TGF-β protects mouse pups against NEC-like intestinal injury: (A) Loss of TGF-β signaling in mice worsened NEC-like intestinal injury induced by intraperitoneal administration PAF and LPS. Bar diagram (means ± SEM) shows the severity of mucosal injury on a 5-point scale in wild type mice, transgenic DNIIR mice with a partial deficiency of TGF-β signaling (3 days of zinc supplementation) and DNIIR mice with complete disruption of TGF-β signaling (7 days of zinc supplementation); n =18 mice per group. Injury scores in DNIIR mice treated with zinc for 3 or 7 days but not treated with PAF and LPS were similar to WT controls (0.24 ±0.11 and 0.26±0.20, respectively; not depicted in the bar diagram). Groups were compared by Kruskall-Wallis H-test. (B) Enteral administration of TGF-β2 prior to PAF and LPS administration in mouse pups reduced the severity of NEC-like intestinal injury; n =18 mice per group, comparison by Mann-Whitney U-test. (C) Enteral administration of TGF-β2 once a day in formula-fed mouse pups reduced the severity of intestinal injury induced by hypoxic stress; n=18 mice per group, comparison by Mann-Whitney U-test.
We next investigated whether enterally-administered recombinant TGF-β2 could protect against inflammatory mucosal injury in mouse pups. The enteral route was chosen because (1) TGF-β2 is normally expressed in human milk in high concentrations26–27 and (2) TGF-β receptors (TGF-β RI, TGF-β RII, and TGF-β RIII) are widely-expressed in human fetal as well as murine intestine (Supplementary fig. 4). Administration of recombinant TGF-β2 (100 ng; dose calculated to provide 10 times the amount of TGF-β2 received by a rodent pup in one day)28 by gavage 2 hours prior to PAF and LPS administration protected these pups against mucosal injury (Fig. 6B). We then confirmed the protective effects of TGF-β2 in another experimental model of NEC, where formula-fed 10-day-old mouse pups exposed to hypoxia and hypothermia develop intestinal injury. Similar to the results in our PAF-LPS model, daily administration of TGF-β2 (100 ng; one single dose in morning for 4 days) was protective against mucosal injury in this model (Fig. 6C). In addition to the reduced severity of intestinal injury, we also noted a reduction in the frequency of injury (9 control pups with intestinal injury versus 1 pup with injury in TGF-β2–treated group, p<0.05)
Discussion
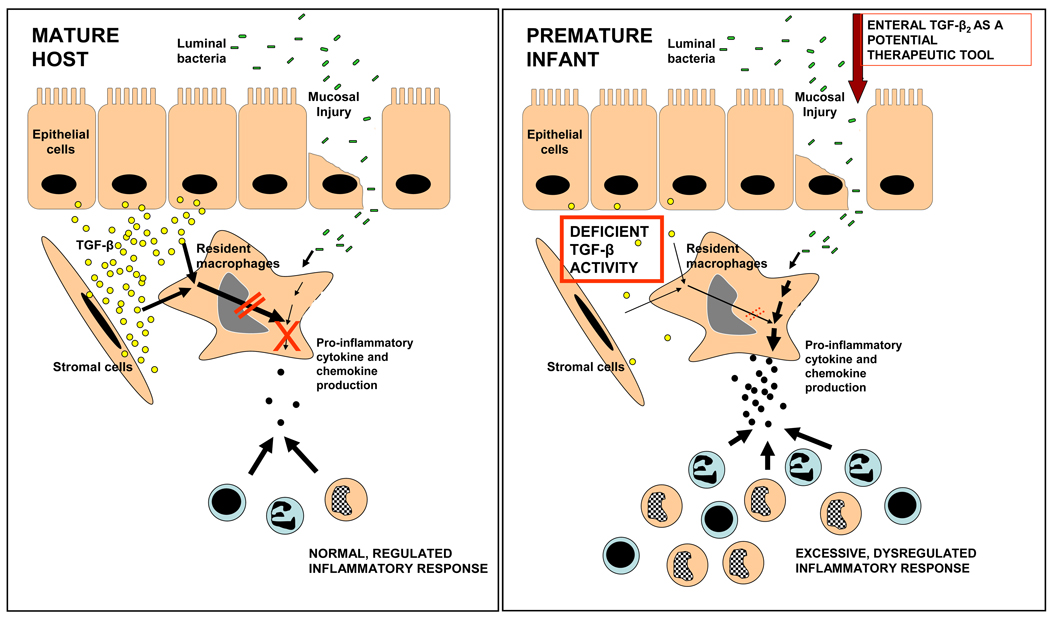
We present a detailed investigation into the normal development of LPS-tolerance in intestinal macrophages and a novel pathophysiological model for NEC with possible therapeutic implications. In contrast to the ‘non-inflammatory’ functional profile of intestinal macrophages seen in the mature host, macrophages in the preterm intestine can respond to bacterial products to produce a robust inflammatory response. In the normal fetus, intestinal macrophages undergo progressive inflammatory downregulation and resemble macrophages in the adult intestine by term gestation. However, in the event of a midgestation delivery, when the inflammatory downregulation of the resident macrophages is still incomplete, ‘premature’ bacterial colonization of the intestinal mucosa may predispose these infants to inflammatory mucosal injury as seen during NEC (Fig. 7).
Fig. 7.
Incomplete development of macrophage tolerance to bacterial products predisposes the preterm intestine to NEC. In the mature intestine (schematic representation on the left), epithelial and stromal cell-derived TGF-β attenuates the inflammatory responses of intestinal macrophages to luminal bacteria or their products. In contrast, in the premature infant (right), the inflammatory responses of intestinal macrophages remain intact because TGF-β expression, and therefore, mucosal tolerance to bacterial products, are deficient. Bacterial products trigger an intense inflammatory reaction, causing widespread tissue damage. Enteral supplementation of recombinant TGF-β2 is a potential therapeutic strategy to prevent NEC in neonates.
The progressive suppression of inflammatory responses of intestinal macrophages we observed during gestational development mirrors a similar maturational reduction in the expression of inflammatory cytokines in the gut epithelium, indicating that these changes may be a part of a larger, more generalized change in the inflammatory milieu in the intestine.25, 29–30 Because IECs are a major source of TGF-β2, these cells may conceivably play a regulatory role in the differentiation of macrophage precursors in the intestine. Interestingly, intestinal macrophages become tolerant to bacterial products in utero as part of an intrinsic, developmentally-regulated program, which contrasts with the development of LPS-tolerance in alveolar macrophages in the postnatal period following exposure to bacterial flora.31 Although TGF-β2 is widely expressed in the lung, these developmental differences between intestinal vs. alveolar macrophages could be related to the absence of TGF-β2 expression in the distal airspaces and alveoli where most macrophages are normally located.32–33.
Immunomodulatory effects of TGF-β2 have been previously reported in diverse conditions such as in systemic inflammatory response syndrome, traumatic brain injury, and atopy.34–36 While TGF-β2 shares the Smad signaling pathways with the other isoforms of TGF-β, the absence of the arg-gly-asp (RGD) integrin-binding motif in TGF-β2 indicates that activation/stabilization mechanisms other than those related to integrin αvβ6 may assume greater importance in TGF-β2–mediated signaling.37 TGF-β2 usually requires betaglycan (TGF-β receptor III) as a co-receptor for efficient binding to TGF-β receptors I and II,38 although emerging evidence indicates that certain splice variants of TGF-β R II may bind TGF-β2 in the absence of betaglycan. Phenotypic differences between mice lacking individual TGF-β isoforms emphasize the existence of hitherto unknown differences in the intracellular signals activated by these isoforms.39
We report for the first time that TGF-β2 expression and TGF-β bioactivity is decreased in NEC. TGF-β2 expression was decreased in NEC to levels even lower than the premature/fetal intestine, suggesting that genetic/epigenetic variability in TGF-β2 expression may increase the risk of NEC in a premature neonate. The low levels of TGF-β we observed in NEC are unique to NEC and are not due to consumption during mucosal inflammation; TGF-β expression is increased in mucosal inflammatory states such as ulcerative colitis and Crohn’s disease.40 Whereas TGF-β effects have not been investigated in NEC, the protective effect of TGF-β in our model is consistent with previous reports where TGF-β1 protected against experimental colitis induced in mice by rectal administration of trinitrobenzene sulfonic acid or by adoptive transfer of Th1 cells.41–42
Several studies have now shown that cytokines and growth factors present in amniotic fluid and human milk can survive digestion in the stomach and the proximal small intestine by binding to cognate receptors on the epithelium, due to deficiency of digestive enzymes during early neonatal period, or because of an intrinsic resistance to gastric and enteral proteases in many cytokines due to the presence of specific structural motifs that render these peptides resistant to digestion.27 The neonatal intestinal epithelium can absorb intact proteins and other macromolecules, indicating that enterally-administered cytokines such as TGF-β2 may be bioavailable in the mucosa.27 TGF-β2 is expressed in high concentrations in amniotic fluid and human milk and is therefore, normally ingested by the fetus and newborn infant in large amounts.26–27 In contrast, the absence of TGF-β2 in infant formula calls for speculation that the protective effect of breast milk against NEC (as compared to formula) may be, at least partially, due to TGF-β2 (Fig. 7). Since recombinant TGF-β2 can be easily synthesized in large amounts, our findings justify aggressive evaluation of the safety and efficacy of enterally-administered TGF-β2 as a prophylactic strategy against NEC.
Supplementary Material
Fig. 5.
TGF-β2 expression is decreased in intestinal tissues samples resected from patients with NEC: TGF-β2 expression in tissue samples of NEC (n=8) was lower than in the mid-gestation fetal (n=6) and preterm neonatal intestine (n=5). Data show measurements by ELISA (means ± SEM). Insets show TGF-β2 mRNA expression (top left) and TGF-β bioactivity (top right). Groups were compared by Kruskall-Wallis H-test.
Acknowledgments
Sources of Funding: Supported by the NIH awards HD059142, HD043397, American Gastroenterological Association 2006 Research Scholar Award, and a research grant from the CACA Jones Family Foundation (A.M.), NIH grants HD046513, HL092906, ATS PH-06-006 (N.A.), and ES015323 (M.A.). Blood monocytes and adult jejunal tissue were received from Core 2 of the UAB Mucosal Immunology and HIV Center (DK64400). The work was made possible in part by the Research Facilities Improvement Grant C06RR15490 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A.M. designed the study and wrote the manuscript; D.R.K, N.A., J.M.U., M.A., V.B., and R.K.O. contributed to study design; R.S., C.A., S.K.J., and R.A.D. provided critical biological samples, reagents, or mice and contributed to study design; T.N., and M.S. performed critical laboratory assays. All authors contributed to the manuscript and approved the final version
Conflicts of interest: The authors have no conflicts to disclose
REFERENCES
- 1.Frost BL, Jilling T, Caplan MS. The importance of pro-inflammatory signaling in neonatal necrotizing enterocolitis. Semin Perinatol. 2008;32:100–106. doi: 10.1053/j.semperi.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsueh W, Caplan MS, Tan X, MacKendrick W, Gonzalez-Crussi F. Necrotizing enterocolitis of the newborn: pathogenetic concepts in perspective. Pediatr Dev Pathol. 1998;1:2–16. doi: 10.1007/s100249900002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bury RG, Tudehope D. Enteral antibiotics for preventing necrotizing enterocolitis in low birthweight or preterm infants. Cochrane Database Syst Rev. 2001 doi: 10.1002/14651858.CD000405. CD000405. [DOI] [PubMed] [Google Scholar]
- 4.Neu J. Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am. 1996;43:409–432. doi: 10.1016/S0031-3955(05)70413-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smythies LE, Maheshwari A, Clements R, Eckhoff D, Novak L, Vu HL, Mosteller-Barnum LM, Sellers M, Smith PD. Mucosal IL-8 and TGF-beta recruit blood monocytes: evidence for cross-talk between the lamina propria stroma and myeloid cells. J Leukoc Biol. 2006;80:492–496. doi: 10.1189/jlb.1005566. [DOI] [PubMed] [Google Scholar]
- 6.Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 8.Kamada N, Hisamatsu T, Okamoto S, Sato T, Matsuoka K, Arai K, Nakai T, Hasegawa A, Inoue N, Watanabe N, Akagawa KS, Hibi T. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 9.Denning TL, Wang YC, Patel SR, Williams IR, Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 10.Maheshwari A, Smythies LE, Wu X, Novak L, Clements R, Eckhoff D, Lazenby AJ, Britt WJ, Smith PD. Cytomegalovirus blocks intestinal stroma-induced down-regulation of macrophage HIV-1 infection. J Leukoc Biol. 2006;80:1111–1117. doi: 10.1189/jlb.0306230. [DOI] [PubMed] [Google Scholar]
- 11.Maheshwari A, Kurundkar AR, Shaik SS, Kelly DR, Hartman Y, Zhang W, Dimmitt R, Saeed S, Randolph DA, Aprahamian C, Datta G, Ohls RK. Epithelial Cells in Fetal Intestine Produce Chemerin to Recruit Macrophages. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1–G10. doi: 10.1152/ajpgi.90730.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maheshwari A, Christensen RD, Calhoun DA. ELR+ CXC chemokines in human milk. Cytokine. 2003;24:91–102. doi: 10.1016/j.cyto.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Kanai T, Watanabe M, Okazawa A, Sato T, Yamazaki M, Okamoto S, Ishii H, Totsuka T, Iiyama R, Okamoto R, Ikeda M, Kurimoto M, Takeda K, Akira S, Hibi T. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn's disease. Gastroenterology. 2001;121:875–888. doi: 10.1053/gast.2001.28021. [DOI] [PubMed] [Google Scholar]
- 14.Wittwer CT, Herrmann MG, Moss AA, Rasmussen RP. Continuous fluorescence monitoring of rapid cycle DNA amplification. Biotechniques. 1997;22:130–131. 134–138. doi: 10.2144/97221bi01. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin JT, Smith RJ, Halloran BA, Day TJ, Kelly DR, Prince LS. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L550–L558. doi: 10.1152/ajplung.00329.2006. [DOI] [PubMed] [Google Scholar]
- 16.Smythies LE, Wahl LM, Smith PD. Isolation and purification of human intestinal macrophages. In: Coligan JE, Kruisbeek AM, Marguilies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. 6B. Volume 7. New York, NY: Current Protocols; 2006. pp. 1–9. [DOI] [PubMed] [Google Scholar]
- 17.Fox SE, Lu W, Maheshwari A, Christensen RD, Calhoun DA. The effects and comparative differences of neutrophil specific chemokines on neutrophil chemotaxis of the neonate. Cytokine. 2005;29:135–140. doi: 10.1016/j.cyto.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor-beta: structural features and mechanisms of activation. Kidney Int. 1997;51:1376–1382. doi: 10.1038/ki.1997.188. [DOI] [PubMed] [Google Scholar]
- 19.Maheshwari A, Voitenok NN, Akalovich S, Shaik SS, Randolph DA, Sims B, Patel RP, Killingsworth CR, Fallon MB, Ohls RK. Developmental changes in circulating IL-8/CXCL8 isoforms in neonates. Cytokine. 2009;46:12–16. doi: 10.1016/j.cyto.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsueh W, Gonzalez-Crussi F, Arroyave JL. Platelet-activating factor: an endogenous mediator for bowel necrosis in endotoxemia. FASEB J. 1987;1:403–405. doi: 10.1096/fasebj.1.5.3678700. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Rozenfeld RA, Qu X, Huang W, Gonzalez-Crussi F, Hsueh W. P-selectin-deficient mice are protected from PAF-induced shock, intestinal injury, and lethality. Am J Physiol. 1997;273:G56–G61. doi: 10.1152/ajpgi.1997.273.1.G56. [DOI] [PubMed] [Google Scholar]
- 23.Musemeche C, Caplan M, Hsueh W, Sun X, Kelly A. Experimental necrotizing enterocolitis: the role of polymorphonuclear neutrophils. J Pediatr Surg. 1991;26:1047–1049. doi: 10.1016/0022-3468(91)90671-f. discussion 1049-50. [DOI] [PubMed] [Google Scholar]
- 24.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A Critical Role for TLR4 in the Pathogenesis of Necrotizing Enterocolitis by Modulating Intestinal Injury and Repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 25.Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. J Exp Med. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penttila IA, van Spriel AB, Zhang MF, Xian CJ, Steeb CB, Cummins AG, Zola H, Read LC. Transforming growth factor-beta levels in maternal milk and expression in postnatal rat duodenum and ileum. Pediatr Res. 1998;44:524–531. doi: 10.1203/00006450-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Maheshwari A. Role of cytokines in human intestinal villous development. Clin Perinatol. 2004;31:143–155. doi: 10.1016/j.clp.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Penttila I. Effects of transforming growth factor-beta and formula feeding on systemic immune responses to dietary beta-lactoglobulin in allergy-prone rats. Pediatr Res. 2006;59:650–655. doi: 10.1203/01.pdr.0000203149.75465.74. [DOI] [PubMed] [Google Scholar]
- 29.Nanthakumar NN, Fusunyan RD, Sanderson I, Walker WA. Inflammation in the developing human intestine: A possible pathophysiologic contribution to necrotizing enterocolitis. Proc Natl Acad Sci U S A. 2000;97:6043–6048. doi: 10.1073/pnas.97.11.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claud EC, Lu L, Anton PM, Savidge T, Walker WA, Cherayil BJ. Developmentally regulated IkappaB expression in intestinal epithelium and susceptibility to flagellininduced inflammation. Proc Natl Acad Sci U S A. 2004;101:7404–7408. doi: 10.1073/pnas.0401710101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin SM, Frevert CW, Kajikawa O, Wurfel MM, Ballman K, Mongovin S, Wong VA, Selk A, Martin TR. Differential regulation of membrane CD14 expression and endotoxin-tolerance in alveolar macrophages. Am J Respir Cell Mol Biol. 2004;31:162–170. doi: 10.1165/rcmb.2003-0307OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatherer D, Ten Dijke P, Baird DT, Akhurst RJ. Expression of TGF-beta isoforms during first trimester human embryogenesis. Development. 1990;110:445–460. doi: 10.1242/dev.110.2.445. [DOI] [PubMed] [Google Scholar]
- 33.de Bortoli C, Chailley-Heu B, Bourbon JR. Production of transforming growth factor (TGF) beta by fetal lung cells. Biol Cell. 1995;84:215–218. doi: 10.1016/0248-4900(96)89431-7. [DOI] [PubMed] [Google Scholar]
- 34.Hatsushika K, Hirota T, Harada M, Sakashita M, Kanzaki M, Takano S, Doi S, Fujita K, Enomoto T, Ebisawa M, Yoshihara S, Sagara H, Fukuda T, Masuyama K, Katoh R, Matsumoto K, Saito H, Ogawa H, Tamari M, Nakao A. Transforming growth factor-beta(2) polymorphisms are associated with childhood atopic asthma. Clin Exp Allergy. 2007;37:1165–1174. doi: 10.1111/j.1365-2222.2007.02768.x. [DOI] [PubMed] [Google Scholar]
- 35.Rimaniol AC, Lekieffre D, Serrano A, Masson A, Benavides J, Zavala F. Biphasic transforming growth factor-beta production flanking the pro-inflammatory cytokine response in cerebral trauma. Neuroreport. 1995;7:133–136. [PubMed] [Google Scholar]
- 36.Stoiser B, Knapp S, Thalhammer F, Locker GJ, Kofler J, Hollenstein U, Staudinger T, Wilfing A, Frass M, Burgmann H. Time course of immunological markers in patients with the systemic inflammatory response syndrome: evaluation of sCD14, sVCAM-1, sELAM-1, MIP-1 alpha and TGF-beta 2. Eur J Clin Invest. 1998;28:672–678. doi: 10.1046/j.1365-2362.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 37.Roberts AB, Sporn MB. Differential expression of the TGF-beta isoforms in embryogenesis suggests specific roles in developing and adult tissues. Mol Reprod Dev. 1992;32:91–98. doi: 10.1002/mrd.1080320203. [DOI] [PubMed] [Google Scholar]
- 38.Brown CB, Boyer AS, Runyan RB, Barnett JV. Requirement of type III TGF-beta receptor for endocardial cell transformation in the heart. Science. 1999;283:2080–2082. doi: 10.1126/science.283.5410.2080. [DOI] [PubMed] [Google Scholar]
- 39.Kitisin K, Saha T, Blake T, Golestaneh N, Deng M, Kim C, Tang Y, Shetty K, Mishra B, Mishra L. Tgf-Beta signaling in development. Sci STKE. 2007;2007 doi: 10.1126/stke.3992007cm1. cm1. [DOI] [PubMed] [Google Scholar]
- 40.Del Zotto B, Mumolo G, Pronio AM, Montesani C, Tersigni R, Boirivant M. TGF-beta1 production in inflammatory bowel disease: differing production patterns in Crohn's disease and ulcerative colitis. Clin Exp Immunol. 2003;134:120–126. doi: 10.1046/j.1365-2249.2003.02250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168:900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 42.Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGF-beta-bearing regulatory cells. J Immunol. 2005;174:3237–3246. doi: 10.4049/jimmunol.174.6.3237. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.