Abstract
Two naturally occurring dietary sources of vitamin E [i.e. RRR-α-tocopherol (αT), and RRR-γ-tocopherol (γT)], the manufactured synthetic form of vitamin E, all-racemic-α-tocopherol (all-rac-αT), as well as, a potent antitumor analog of vitamin E, RRR-α-tocopherol ether-linked acetic acid analog (α-TEA) were assessed for anticancer actions. Data showed that γT, all-rac-αT and α-TEA but not αT or αT + γT significantly inhibited tumor burden of human MDA-MB-231 cells in nude mice. Immunohistochemical analyses of tumor tissue showed that all-rac-αT and α-TEA increased apoptosis and decreased proliferation in tumor cells while γT was associated with increased tumor cell apoptosis only. In vitro data showed α-TEA and γT but not all-rac-αT or αT to inhibit colony formation and induce apoptosis. Anticancer actions of α-TEA and γT involved DR5 protein upregulation, Survivin protein downregulation and PARP cleavage all of which were blocked by co-treatment with αT. In summary, both γT and α-TEA exhibited promising anticancer properties in vivo and in vitro, whereas all-rac-αT exhibited promising anticancer properties in vivo only. Importantly, αT not only failed to exhibit anticancer properties but it reduced anticancer actions of γT in vivo and γT and α-TEA in vitro.
Keywords: Anticancer actions, Apoptosis, cell proliferation, Human breast cancer, Vitamin E
1 Introduction
Vitamin E is a general term used to refer to a group of eight chemically different compounds produced by plants known as tocopherols (α, β, γ and δ) and tocotrienols (α, β, γ and δ), as well as synthetic vitamin E, all-racemic-α-tocopherol (all-rac-αT; also referred to in the literature and on vitamin supplements as dl-α-tocopherol) which is prepared commercially and consists of a mixture of eight stereoisomeric forms of α-tocopherol, only one of which is equivalent to a “natural” plant-produced form; namely, RRR-α-tocopherol (αT) [1]. Both αT and all-rac-αT can be purchased as acetate or succinate derivatives which are manufactured in order to protect their antioxidant capacity from destruction by air or sunlight. Synthetic vitamin E and its acetate derivative (all-rac-αTAc) are common components of dietary supplements. Significant sources of αT and γT are polyunsaturated plant oils and products made from them such as margarines and salad dressings.
Despite many research efforts, the biological functions of natural and synthetic vitamin E compounds remain to be clearly defined [2–5]. Pre-clinical cell culture and animal model studies suggest promise of certain vitamin E forms as anticancer agents [6–12]. Two extensive reviews concluded that vitamin E from dietary sources may provide women with modest protection from breast cancer; however, there was no evidence that vitamin E supplements (αT or all–rac-αTAc) conferred any protection against breast cancer [13, 14]. Furthermore, double-blind, placebo-controlled clinical trials failed to provide science-based support for either αT or all-rac-αTAc as anticancer agents, with the possible exception of all-rac-αTAc as a chemopreventive agent for prostate cancer in heavy smokers [reviewed in 8]. Recent findings from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) concluded that neither selenium nor vitamin E (all–rac-αTAc), alone or in combination at doses and formulations used, prevented prostate cancer in a population of relatively healthy men [15]. In contrast, there is increasing evidence suggesting that γT, the most abundant form of vitamin E in the American diet, may possess anticancer properties [13, 16–21].
Breast cancer is the most commonly diagnosed cancer in U.S. women, exceeding 211,000 cases annually, with approximately 88% surviving 5 years [22]. Survivors are seeking answers on how to prevent recurrent cancer, especially women with estrogen receptor negative cancers where preventive options are limited. A recent study reports that the use of vitamin and mineral supplements is widespread among cancer survivors, with breast cancer survivors reporting the highest use (75–87%), despite inconclusive evidence that it is beneficial [23]. Thus, there is a need to better understand how vitamin E compounds, found naturally in the diet or consumed as food additives or in dietary supplements, may influence breast cancer development and recurrence.
The goal of this study was to evaluate the anticancer actions of two natural forms of vitamin E (αT and γT), the manufactured synthetic form of vitamin E (all-rac-αT), and a combination of αT + γT in nude mice xenografted with MDA-MB-231 human breast cancer cells, as well as in cell culture. Additionally, this study sought to test the possibility that supplemental αT might block the anticancer actions of γT.
2 Materials and methods
2.1 Vitamin E compounds
all-rac-αT was purchased from Sigma Chemical Co (St. Louis, MO, USA). αT (RRR-α-tocopherol) was purchased from TAMA Biochemical Company, LTD (Tokyo, Japan) and γT (RRR-γ-tocopherol) was a gift from TAMA Biochemical Company, LTD (Tokyo, Japan). α-TEA (RRR-α-tocopherol ether-linked acetic acid analog) was used as a positive control and it was prepared as previously described [24].
2.2 Xenograft study
All animal experiments were conducted according to `Guidelines for the Humane Treatment of Animals' as designated by the University of Texas Institutional Animal Care and Use Committee (Animal permit No 06033002). Immune incompetent Nu/Nu female BALB/c mice at 6 weeks of age were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were injected subcutaneously with a total of 2 × 106 MDA-MB-231-GFP human breast cancer cells (gift from Dr. LuZhe Sun, Department of Structural Biology, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA) in 100 μl media containing 50% matrigel (BD Biosciences, Franklin Lakes, NJ) in the inguinal area at a point equal distant from the fourth and fifth nipples on the right side. One week after tumor cell injection, the mice were randomly assigned to 6 groups (10 mice/group): control, αT, γT, all-rac-αT, α-TEA and αT + γT when tumors reached an average volume of 25 mm3 (control = 25 mm3, all-rac-αT = 24 mm3, αT = 24 mm3, γT = 26 mm3, α-TEA = 26 mm3, αT + γT = 26 mm3) and fed test diets purchased from Harlan Teklad (Madison, WI, USA). Control diet contained tocopherol-stripped semi-purified AIN-76A diet with 33 IU all-rac-α-tocopherol acetate per kg diet to meet the estimated vitamin E nutrient requirement for mice [25]. To this basal (control) diet the different forms of vitamin E were added at 500 mg/kg each (thus, the combination diet contained 500 mg/kg αT +500 mg/kg γT). HPLC analyses of the actual concentrations of supplemented vitamin E forms in the diets (mg/kg diet) following radiation to permit food entry into the barrier facility housing the nude mice were as follows: αT = 378, all-rac-αT = 400, γT = 358, α-TEA = 243, αT + γT = 456 and 506, respectively, for each of the diets. The average food intake was 3.7 ± 0.1 g/d/mouse in control and treatment groups. To put this into perspective for physiological relevance, the 500 mg RRR-α-tocopherol /kg diet provides approximately 400 IU vitamin E to an individual mouse on a day-by-day basis, an amount typically found in one soft gel vitamin E capsule based on body surface area equivalency [26].
Tumors were measured every other day, and tumor volumes were calculated using the formula: volume (mm3) = (width × width × length/2). Body weights were determined weekly. Animals were euthanized after 24 days of dietary treatment.
2.3 Tumor immunohistochemistry
Immediately upon collection, tumors were fixed in formalin for immunohistochemical analyses. Deparaffinized 5 μm sections were examined for apoptosis and cell proliferation using reagents supplied in the ApopTag in situ Apoptosis Detection kit (Intergen, Purchase, NY, USA), and antibodies specific for nuclear antigen Ki-67 (DAKO Corp, Carpinteria, CA, USA). Brown TUNEL stained nuclei were scored as positive for apoptosis in 15 microscopic fields (400 ×)/tumor. Ki-67 positive stained cells were counted in 5 microscopic fields (400 ×)/tumor as previously described [27]. Tumor tissues from 5 individual mice in each group were analyzed in the both TUNEL and Ki67 assays.
2.4 HPLC detection of vitamin E compounds in serum and diets
Lipids were extracted from sera and diets and αT and γT levels were measured in sera and αT, γT, and α-TEA levels were measured in diets using reverse-phase high performance liquid chromatography (HPLC) with fluorometric detection as described by Tirmenstein et al. [28]. Each sample (40 μL) was injected into a Waters 717 HPLC equipped with an autosampler. The mobile phase consisted of 96% methanol (HPLC grade; Fisher Chemicals, Gibbstown, NJ), 4% water, and 0.001% glacial acetic acid. Samples were separated on a Waters spherisorb ODS-2 5u (250 × 4.6-mm) column (Alltech, Deerfield, IL, USA) and analyzed for αT and γT with excitation and emission wavelengths of 290 and 330 nm nm, respectively. Quantification of the separated compounds was performed based on the internal standard method using γ-tocotrienol as the internal standard and Millennium-32 chromatography manager software for data analyses (Waters Corp., Milford, MA, USA).
2.5 Cell culture
To further evaluate the anticancer effects of vitamin E compounds tested in vivo, we used the same cell line, MDA-MB-231-GFP human breast cancer cells to conduct in vitro study. Cells were maintained in MEM medium supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, UT), 2 mM glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin, 1 × MEM non-essential amino acid solution (Sigma) and 2 × MEM vitamins solution (Sigma). For experiments, FBS was reduced to 2% to better mimic the in vivo low serum exposure of these type cells.
2.6 Colony formation assay
Effects of vitamin E compounds on colony formation of MDA-MB-231-GFP cells were determined as described previously with the following modifications [29]. Vitamin E compounds were dissolved in DMSO at 200 mM, then further diluted in ethanol to achieve 40 mM stock solutions. Equivalent levels of DMSO/ethanol (1:4) were used as vehicle controls (VEH). Cells were seeded at 1000 cells/100 mm plate and incubated for 3 days to allow small colony formation. Cells were treated with vitamin E compounds or VEH at indicated concentrations for 10 days. Cells were washed with PBS, fixed with methanol and stained with 0.1% methylene blue in PBS. Colonies were analyzed and measured by ImageJ 1.41(National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/download.html) (30). Colony forming cells are expressed as cell survival (%), which was calculated as number of colonies in treatment/number of colonies in control × 100%. IC50 values for inhibition of colony formation were calculated using CalcuSyn software (Biosoft, Ambridge, UK).
2.7 Evaluation of apoptosis
Apoptosis was quantified using the Annexin V-PE assay following the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). This assay measures amount of phosphatidylserine on the outer surface of the plasma membrane (a biochemical alteration unique to membranes of apoptotic cells). Fluorescence was measured using FACSCalibur flow cytometry and data were analyzed using CellQuest software (BD Biosciences, San Jose, CA, USA). Cells displaying phosphatidylserine on their surface (positive for annexin-V fluorescence) were considered to be apoptotic [31].
2.8 Western blot analyses
Western blot analyses to assess protein levels in whole cell extracts were performed as described previously [31]. Antibodies to poly (ADP-ribose) polymerase (PARP) and Survivin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies to DR5 were purchased from Cell Signaling Technology (Beverly, MA, USA). Antibodies to GAPDH were produced in house. Following transfer, blots were reacted with primary antibody in 0.1% BSA/TBST overnight at 4°C, washed three times with TBST and reacted with horseradish peroxidaseconjugated goat anti-rabbit or rabbit anti-mouse (Jackson Immunoresearch, Rockford, IL, USA) secondary antibodies.
2.9 Statistical analyses
Tumor growth was evaluated by transforming volumes using a logarithmic transform (base 10) and analyzed using a nested two-factor analysis of variance (ANOVA) with SPSS software (SPSS Inc, Chicago, IL, USA). Differences in number of TUNEL and Ki-67 positive cells, and serum levels of αT and γT were determined using Mann-Whitney rank test with Prism software version 4.0 (Graphpad, San Diago, CA, USA). A level of p < 0.05 was regarded as statistically significant. Student t-test was used for in vitro studies.
3 Results
3.1 The ability of different vitamin E compounds to reduce tumor growth
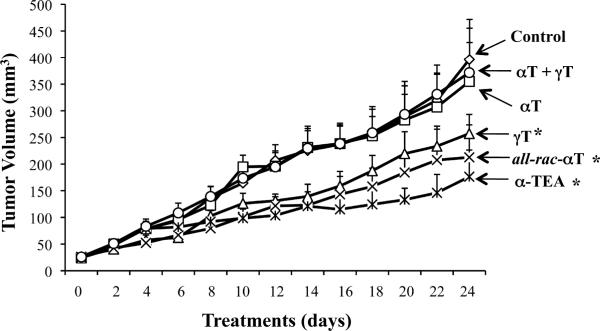
Tumor volumes (mean + S.E) through time for treatment and control groups are presented in Fig. 1. Data show a significant reduction of tumor volume in γT, all-rac-αT and α-TEA supplemented groups in comparison to basal control diet group (p < 0.05, < 0.01 and < 0.01, respectively). There were no significant differences in tumor volumes in αT and αT + γT groups in comparison with the control group and there were no significant differences in tumor volumes among γT, all-rac-αT and α-TEA groups. These data suggest that γT, all-rac-αT and α-TEA, but not αT, possess antitumor activity, and importantly, αT has the ability to block γT's antitumor efficacy as measured by reduction in tumor burden. The average body weight of mice at the beginning of the experiment was 18.5 ± 0.4 and at the end of the experiment was 22.1 ± 0.9. There were no significant differences in body weights among mice in control and treatment groups.
Figure 1.
Evaluation of tumor growth. Supplemented diets were initiated at day 0 which was 7 days after tumor cell injections when all tumors were approximately the same size (i.e. 25 mm3). Tumor volumes (mm3) were determined at two-day intervals and are depicted as mean ± S.E. *designates significant difference from control group (p < 0.05).
Visible metastases in lungs were counted at sacrifice. The incidences of visible lung metastases were 70, 60, 30, 30, 30, 40% in control, αT, γT, all-rac-αT, α-TEA and αT + γT groups, respectively. Attempts to determine GFP-labeled tumor cells in lung for analyses of micrometastases failed due to inadequate GFP expression by the tumor cells. Apparently, the transgene was lost when cells were grown in vivo.
3.2 γT, all-rac-αT and α-TEA, but not αT or αT + γT, induce tumor cells to undergo apoptosis
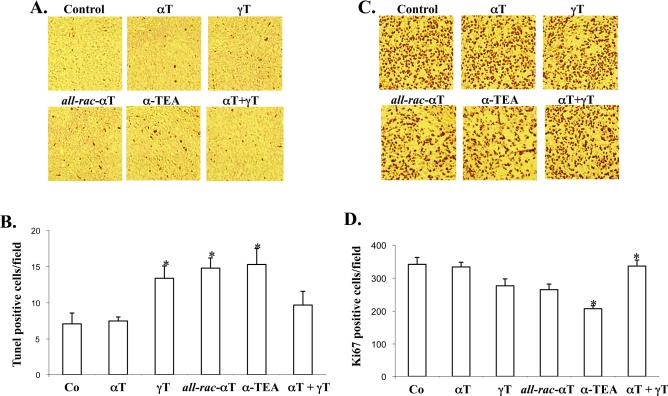
The levels of TUNEL positive cells in γT, all-rac-αT and α-TEA groups, but not in the αT or αT + γT groups, were significantly increased by 1.9, 2.1 and 2.1 fold, respectively, in comparison to control group (p < 0.03, p < 0.016 and p < 0.036, respectively (Fig. 2A).
Figure 2.
Assessment of biomarkers of antitumor action. Measures of apoptosis (TUNEL) and proliferation (Ki67) were detected by immunohistochemical analyses of tumor tissue. A and C are photo-picture of representative of TUNEL (A) and Ki67 (B), respectively. B and D are presented as mean ± S.E. of 5 samples in each group. * designates significant difference from control group (p < 0.05).
3.3 all-rac-αT and α-TEA but not γT, αT or αT + γT, inhibit tumor cell proliferation
Tumor cell proliferation was determined by immunohistochemical analyses of Ki-67, a nuclear antigen expressed in actively proliferating cells and presented as average number of Ki-67 positive stained cells counted in 5 microscopic fields at 400 × magnification/tumor ± S.E from 5 individual mice. The levels of Ki-67 positive cells in all-rac-αT and α-TEA groups, (but not αT or αT + γT groups), were significantly decreased by 23 and 39%, respectively, in comparison to control group (p < 0.032, and p < 0.008, respectively; (Fig. 2B). Although the level of Ki-67 positive cells in the γT group was decreased in comparison with control group, there was no significant difference (p<0.06).
3.4 Comparison of levels of αT and γT in serum
Levels of αT and γT in sera were determined by HPLC analyses (Table 1). Serum levels of αT in the αT supplemented group were significantly increased in comparison with control (14.2 ± 1.7 versus 8.5 ± 1.3 μmol/L, p < 0.048). Although serum levels of αT in the combination αT + γT group was increased in comparison with control (12.5 ± 1.7 versus 8.5 ± 1.3 μmol/L) it was not a statistically significant difference. Serum levels of αT in the γT supplemented group was lower in comparison to the control group (5.7 ± 0.4 versus 8.5 ± 1.3 μmol/L), but it was not a statistically significant difference. Serum levels of γT were 1.3 ± 0.2 μmol/L in γT supplemented mice and were below detectable levels in the αT + γT supplemented group (Table 1). Levels of αT in the γT supplemented group were 4 fold higher than γT, while αT levels in the combination treated group increased from 5.7 ± 0.4 μmol/L to 12.5 ± 1.7 μmol/L, while γT levels decreased to undetectable levels (Table 1).
Table 1.
HPLC analyses of αT and γT levels in serum
| Serum Levelsa (μmol/L) | ||
|---|---|---|
| Group | αTb | γT |
| Control | 8.5 ±1.3 | NDc |
| αT | 14.2 ± 1.7 | ND |
| γT | 5.7 ± 0.4 | 1.3 ± 0.2 |
| αT + γT | 12.5 ± 1.7 | ND |
At euthanasia, blood was allowed to clot, serum collected, lipids extracted, and levels of αT and γT measured by an internal standard method using reverse-phase HPLC with fluorometric detection. The data are presented as mean ± S.E from 5 individual animals/group.
αT levels reflect all stereoisomeric forms
ND = not detected (below level of detection; i.e. 0.7 μmol/L)
3.5 Effect of vitamin E compounds on colony formation of MDA-MB-231-GFP human breast cancer cells
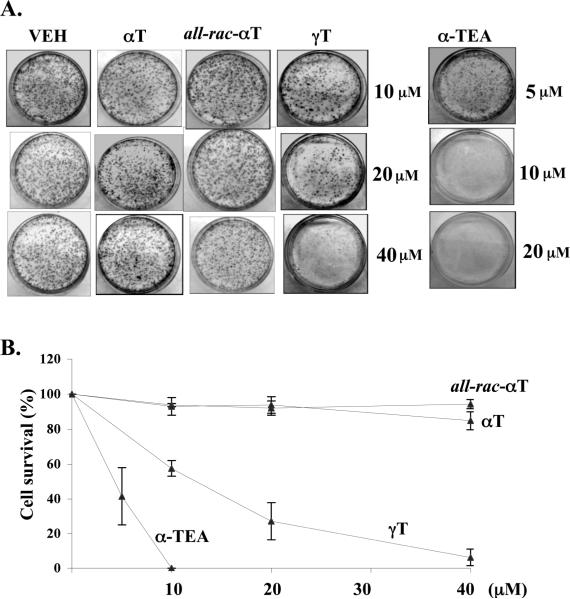
Vitamin E treatments for 10 days in concentrations ranging from 10–40 μM for αT, all-rac-αT and γT, and 5–15 μM for α-TEA were evaluated for ability to reduce colony formation of MDAMB-231-GFP cells. Representative photographs of colony assay from 100 mm plates are presented in Fig 3A. Cell survival (%) curves are given in Fig 3B. IC50 values calculated from data in Fig 3B are: 4.8 μM and 11.8 μM for α-TEA and γT, respectively. In the 10–40 μM range tested, there was no inhibitory effect of αT or all-rac-αT on colony formation (Fig 3 A&B).
Figure 3.
Colony formation assays. Cells were treated with a range of concentrations of different vitamin E compounds for 10 days. A. photo-picture. B. Colonies are expressed as cell survival (%), which was calculated as number of colonies in treatment/number of colonies in control × 100%. Data are mean ± SD of at least two separate experiments.
3.6 Evaluation of pro-apoptotic properties of vitamin E compounds
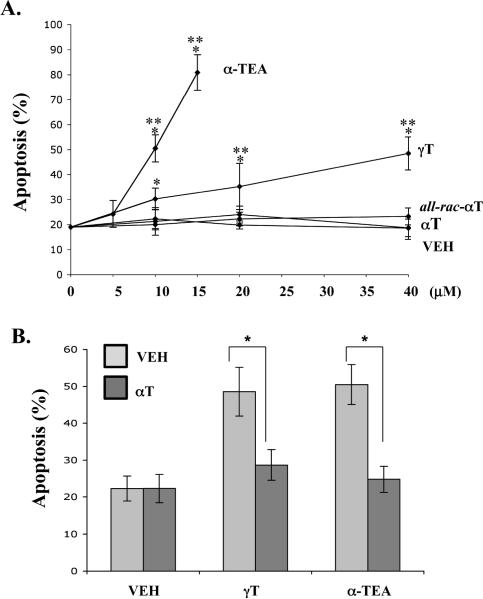
FACS analyses of phosphatidylserine translocation to the cell surface using PE-tagged Annexin V were used to quantify apoptosis. γT- and α-TEA, but not αT or all-rac-αT induced dose-dependent apoptosis in MDA-MB-231-GFP cells following 5 day treatments (Fig 4A). 10 μM αT blocked apoptosis induced by γT (40 μM) and α-TEA (10 μM) by 60% and 70%, respectively (Fig 4B).
Figure 4.
Evaluation of pro-apoptotic properties. Cells were treated with vitamin E compounds at the indicated concentrations for 5 days. (A) Quantification of percent apoptotic cells by FACS analyses of annexin V-PE positive cells treated with different doses of different vitamin E compounds. * Significant difference between control and treatments p<0.05, ** significant difference between higher dose and lower dose p<0.05. B. Quantification of percent apoptotic cells by FACS analyses of annexin V-PE positive cells treated with 40 μM γT and 10 μM α-TEA alone or plus 10 μM αT. The data are presented as mean ± S.D of at least three independent experiments.
3.7 Mechanisms of vitamin E-induced apoptosis
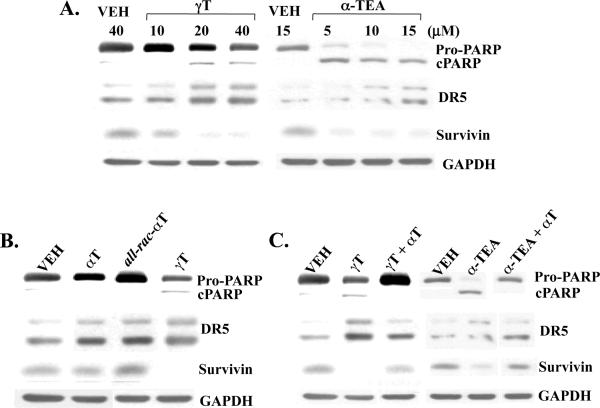
γT and α-TEA induced apoptosis in MDA-MB-231GFP cells to in a manner similar to MDAMB-435 cells as we previously reported [29, 31], namely, apoptosis involved upregulation of pro-apoptotic death receptor DR5 and downregulation of anti-apoptotic Survivin (Fig 5A). In comparison with γT, αT and all-rac-αT did not cause cleavage of PARP and did not downregulate Survivin. Interestingly, both αT and all-rac-αT induced an increase in DR5 protein expression (Fig 5B). Of importance, αT blocked γT- and α-TEA-induced cleavage of PARP, upregulation of DR5 and downregulation of Survivin (Fig 5C).
Figure 5.
Assessment of biomarkers of apoptosis. PARP cleavage and pro-apoptotic DR5 and anti-apoptotic survivin protein expression were detected by western blot analyses using the same samples in Fig 4A. Dose-response expression of the markers treated with γT and α-TEA. B. Comparison of the marker expression in different vitamin E compounds. C. The effect of αT on γT and α-TEA-induced apoptotic biomarker expression. Data are representative of two independent experiments.
4 Discussion
Data presented here show: 1) diet supplementation with γT, all-rac-αT and α-TEA, but not αT, suppressed the growth of estrogen non-responsive human MDA-MB-231 breast cancer cells in a xenograft model, 2) γT, all-rac-αT and α-TEA induced apoptosis and all-rac-αT and α-TEA inhibited tumor cell proliferation in tumor tissue, suggesting possible mechanisms for the anticancer action of these vitamin E forms in vivo, 3) αT reduced γT's ability to suppress in vivo tumor growth and induction of tumor cell apoptosis, 4) α-TEA and γT, but not αT or all-rac-αT inhibited colony formation and induced apoptosis in cell culture, 5) α-TEA- and γT-induced apoptosis was associated DR5 upregulation, and Survivin downregulation in culture, and 6) αT blocked in vitro anticancer actions of both γT and α-TEA.
Previously, we showed that γT, all-rac-αT and α-TEA formulated in liposomes and delivered by oral gavage suppressed tumor growth in a syngeneic mouse model using BALB/c 66cl-4-GFP mouse mammary cancer cells, while αT did not [29]. To confirm these findings and extend this study to a human breast cancer cell line we conducted the current study using MDAMB-231-GFP in Nu/Nu mouse (versus mouse cell line 66cl-4) and delivered the vitamin E compounds in the diet (versus by oral gavage). The data confirm that γT, all-rac-αT and α-TEA, but not αT possess anticancer actions in vivo. The data also confirm the data reported by Cameron, et al. [32] showing that all-rac-αT supplemented in the diet reduced tumor burden of human MDA-MB-231 breast cancer cells in a xenograft model and confirm the data reported by Hahn, et al. [33] generated in a syngenec 4T1 mouse model that dietary administration of α-TEA is an effective route of delivery. The data also support the results reported recently by Suh and coworkers [34] showing that adding mixed tocopherols (58% γT, 21% delta-tocopherol, 12% αT, 1.5% beta-tocopherol) at 0.1% to an AIN-76 diet significantly suppressed chemically induced mammary tumor growth in rats. Taken together, these studies suggest that various forms of vitamin E can be effective anticancer agents when fed in the diet in several pre-clinic animal models.
As we observed previously [29], γT and α-TEA, but not αT and all-rac-αT, inhibited colon formation and apoptosis in MDA-MB-231 human breast cancer cells in vitro. While we have some understanding of how VES and α-TEA may mediate their anticancer actions (8, 35) much remains to be done to better understand how γT and all-rac-αT mediate their anticancer actions. Previously, we reported that γT and α-TEA, but not αT and all-rac-αT, induced cleavage of caspase-8, 9 and PARP and downregulated cFLIP and Survivin in MDA-MB-435 human breast cancer cells [29], and activation of the DR5 pathway is involved in γT-induced apoptosis, leading to activation of a caspase-8- and mitochondria-dependent apoptotic cascade in MCF-7 and MDA-MD-435 human breast cancer cells [31]. In this study, we found that all four agents upregulated DR5 protein expression in MDA-MB-231 cells (Fig 5 B), but only γT and α-TEA induced increased level of apoptosis and Survivin downregulation. Thus, perhaps one reason why γT and α-TEA induce apoptosis is that they suppress anti-apoptotic Survivin, while αT and all-rac-αT which did not induce apoptosis did not. These data suggest that dual activation of pro-apoptotic pathways and suppression of anti-apoptotic pathways are needed in vitamin E-compound-induced apoptosis in vitro.
As previously reported [29], all-rac-αT exhibits anticancer actions in vivo but not in cell culture. In speculating what might account for this in vivo versus in vitro difference, there are several possibilities including: (i) all-rac-αT generates unique metabolites only in vivo that mediate its anticancer actions (ii) differences in tumor cell uptake in cell culture versus in vivo, and (iii) all-rac-αT may not directly act on tumor cells but rather generates indirect effects; such as, elimination of tumor supportive stroma cells or their pro-growth/pro-survival mediating factors or perhaps it enhances antiangiogenic effects directed to endothelial cells. The possibility that all-rac-αT may be mediating its anticancer actions in vivo via the immune response system is also a possibility because even though the immune response in the Balb/c Nu/Nu mice is compromised by a lack of a thymus, anticaner responses mediated by NK cells and T-independent B-cell functions are still operative.
The subject of bioavailability and tissue distribution among the different forms of vitamin E has been the focus of several studies and has been summarized in recent comprehensive reviews [36–38]. Briefly, all forms of orally administered vitamin E are absorbed from the gastrointestinal tract in chylomicrons in a comparable manner and can be taken up by tissues prior to chylomicron clearance by the liver. The α-tocopherol transfer protein (α-TTP) which is expressed in the liver plays an important role in the subsequent preferential release of RRR-αT from the liver into the plasma. Vitamin E forms that are not transported into the plasma are excreted into the bile or metabolized in the liver by cytochrome P450 enzymes [36–39]. Thus, RRR-αT is the predominant form of vitamin E in serum of fasted individuals. In addition to preferential binding of RRR-αT by α-TTP, RRR-αT activates P450 enzymes, leading to degradation and elimination of other vitamin E forms which also accounts for the marked presence of RRR-αT in serum and tissues [36–39]. Since αT does not appear to be playing a beneficial anticancer function, we hypothesized that it may be a bad actor blocking the antcancer actions of other vitamin E forms. Based on the well studied antagonistic effect of αT on reductions of plasma and tissue levels of γT (39–41), we examined whether αT could block γT anticancer actions in vivo. Here, we show that supplementation with γT produced antitumor outcomes which were lost when mice were supplemented with a combination of αT + γT. Data showing that γT supplementation produced detectable γT serum levels while the combination supplementation did not is in agreement with published literature showing that αT reduces serum levels of γT [36, 39–41]. Thus, one possible mechanism for αT to antagonize γT antitumor actions is to reduce γT bioavailability due to lower concentration of γT in serum. The antagonistic effect of αT on γT anticancer actions was confirmed by in vitro studies showing that αT blocked γT-induced apoptosis and involved inhibition of γT-induced upregulation of DR5 and downregulation of survival factor Survivin. These data suggest that αT blocks an upstream event in γT-induced apoptosis. In addition, cell culture data show that αT not only blocked γT anticancer actions, but also those of α-TEA, suggesting that αT's antagonistic effect(s) may apply to other vitamin E forms. Since all-rac-αT did not exhibit anticancer actions in vitro, we were unable to study if αT can block its anticancer actions. This will require future in vivo analyses.
Our data show that αT at a relevant supplemental dose blocked γT anticancer actions at a 1:1 ratio in vivo and blocked γT-induced apoptosis at a 1:4 ratio (αT at 10 μM to γT at 40 μM) in vitro. Thus it is interesting that Suh and coworkers [34] showed that adding mixed tocopherols (58% γT, 21% delta-tocopherol, 12% αT, 1.5% beta-tocopherol), at 0.1% to an AIN-76 diet significantly suppressed chemically induced mammary tumor growth in rats. This diet had a 1:9 ratio of αT to all other vitamin E forms (note delta-tocopherol has been reported to have antitumor action [42]. Taken together, this raises the question how can γT's potential for anticancer function be maximized by limiting αT? Future studies are needed to determine optimal ratio of αT:anticancer forms of vitamin E to minimize αT's antagonistic effects
In summary, these findings demonstrate that certain forms of vitamin E can function effectively as anticancer agents and others cannot. For the first time, αT has been shown to block γT's anticancer actions in vivo and in vitro. Studies are needed to better understand how the anticancer actions of these forms of vitamin E are antagonized by αT. Such information will provide important insights for better utilizing certain vitamin E forms as anticancer agents.
Acknowledgments
This work was supported by grants from the Public Health Service (CA59739), the Clayton Foundation for Research, the American Institute for Cancer Research (05B115) and the National Institute of Environmental Health Sciences (ES007784). Authors thank the staff of the Histology & Tissue Processing Facility Core at The University of Texas M.D. Anderson Cancer Center, Science Park Research Division for immunohistochemical staining of tumor samples, Dr. LuZhe Sun for providing the breast cancer cell line and Dr. Masa Hirose and Tama Biochemical Company for providing γT.
Abbreviations
- all-rac-αT
all-racemic-α-tocopherol
- all-rac-αTAc
all-racemic-α-tocopherol acetate
- αT
RRR-α-tocopherol
- α-TEA
RRR-α-tocopherol ether-linked acetic acid analog
- α-TTP
α-tocopherol transfer protein
- γT
RRR-γ-tocopherol
- PARP
poly (ADP-ribose) polymerase
- DR5
death receptor 5 (also called TRAIL-R2:TNFα-related apoptosis-inducing ligand-receptor 2)
Footnotes
Conflict of interest statement Bob G. Sanders, Kimberly Kline and Weiping Yu are inventors on a patent held by the Foundation for Research on anticancer actions of α-TEA (RRR-α-tocopherol ether-linked acetic acid analog).
References
- [1].Institute of Medicine, Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds . Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids. National Academy Press; Washington, D.C.: 2000. pp. 186–283. [PubMed] [Google Scholar]
- [2].Traber MG, Atkinson J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Azzi A. Molecular mechanism of alpha-tocopherol action. Free Radic. Biol. Med. 2007;43:16–21. doi: 10.1016/j.freeradbiomed.2007.03.013. [DOI] [PubMed] [Google Scholar]
- [4].Brigelius-Flohé R, Kelly FJ, Salonen JT, Neuzil J, et al. The European perspective on vitamin E: current knowledge and future research. Am. J. Clin. Nutr. 2002;76:703–716. doi: 10.1093/ajcn/76.4.703. [DOI] [PubMed] [Google Scholar]
- [5].Litwack G, editor. Vitam. Horm. Vol. 76. 2007. Vitamin E; pp. 1–588. [DOI] [PubMed] [Google Scholar]
- [6].Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitam. Horm. 2007;76:204–263. doi: 10.1016/S0083-6729(07)76008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sylvester PW. Vitamin E and apoptosis. Vitam. Horm. 2007;76:330–357. doi: 10.1016/S0083-6729(07)76012-0. [DOI] [PubMed] [Google Scholar]
- [8].Kline K, Lawson KA, Yu W, Sanders BG. Vitamin E and Cancer. Vitam. Horm. 2007;76:435–461. doi: 10.1016/S0083-6729(07)76017-X. [DOI] [PubMed] [Google Scholar]
- [9].Tomasetti M, Neuzil J. Vitamin E analogues and immune response in cancer treatment. Vitam. Horm. 2007;76:463–491. doi: 10.1016/S0083-6729(07)76018-1. [DOI] [PubMed] [Google Scholar]
- [10].Ni J, Yeh S. The roles of α-vitamin E and its analogues in prostate cancer. Vitam. Horm. 2007;76:494–519. doi: 10.1016/S0083-6729(07)76019-3. [DOI] [PubMed] [Google Scholar]
- [11].Wang XF, Dong LF, Zhano Y, Tomasetti M, et al. Vitamin E analogues as anticancer agents: lessons from studies with α-tocopheryl succinate. Mol. Nutr. Food Res. 2006;50:675–685. doi: 10.1002/mnfr.200500267. [DOI] [PubMed] [Google Scholar]
- [12].Constantinou C, Papas A, Constantinou AI. Vitamin E and cancer: An insight into the anticancer activities of vitamin E isomers and analogs. Int. J. Cancer. 2008;123:739–772. doi: 10.1002/ijc.23689. [DOI] [PubMed] [Google Scholar]
- [13].Schwenke DC. Does lack of tocopherols and tocotrienols put women at increased risk of breast cancer? J. Nutr. Biochem. 2002;13:2–20. doi: 10.1016/s0955-2863(01)00207-8. [DOI] [PubMed] [Google Scholar]
- [14].Kimmick GG, Bell RA, Bostick RM. Vitamin E and breast cancer: a review. Nutr. Cancer. 1997;27:109–117. doi: 10.1080/01635589709514511. [DOI] [PubMed] [Google Scholar]
- [15].Lippman SM, Klein EA, Goodman PJ, Lucia MS, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The selenium and vitamin E cancer prevention trial (SELECT) JAMA. 2009;301(1) doi: 10.1001/jama.2008.864. doi:10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wagner KH, Kamal-Eldin A, Elmadfa I. Gamma-tocopherol-an underestimated vitamin? Ann. Nutr. Metab. 2004;48:169–188. doi: 10.1159/000079555. [DOI] [PubMed] [Google Scholar]
- [17].Hensley K, Benaksas EJ, Bolli R, Comp P, et al. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic. Biol. Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- [18].Jiang Q, Christen S, Shigenaga MK, Ames BN. Gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am. J. Clin. Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- [19].Campbell S, Stone W, Whaley S, Krishnan K. Development of gamma (γ)-tocopherol as a colorectal cancer chemopreventive agent. Crit. Rev. Oncol. Hematol. 2003;47:249–259. doi: 10.1016/s1040-8428(03)00042-8. [DOI] [PubMed] [Google Scholar]
- [20].Stone WL, Krishnan K, Campbell SE, Qui M, et al. Tocopherols and the treatment of colon cancer. Ann. N. Y. Acad. Sci. 2004;1031:223–233. doi: 10.1196/annals.1331.022. [DOI] [PubMed] [Google Scholar]
- [21].Wright ME, Weinstein SJ, Lawson KA, Albanes D, et al. Supplemental and dietary vitamin E intakes and risk of prostate cancer in a large prospective study. Cancer Epidemiol. Biomarkers Prev. 2007;16:1128–1135. doi: 10.1158/1055-9965.EPI-06-1071. [DOI] [PubMed] [Google Scholar]
- [22].Jemal A, Siegel R, Ward E, Hao Y, et al. Cancer statistics, 2008. CA Cancer J. Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- [23].Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: A systematic review. J. Clin. Oncol. 2008;26:665–673. doi: 10.1200/JCO.2007.13.5905. [DOI] [PubMed] [Google Scholar]
- [24].Lawson KA, Anderson K, Simmons-Menchaca M, Atkinson J, et al. Comparison of vitamin E derivatives α-TEA and VES in reduction of mouse mammary tumor burden and metastasis. Exp. Biol. Med. 2004;229:954–963. doi: 10.1177/153537020422900913. [DOI] [PubMed] [Google Scholar]
- [25].National Research Council . Nutrient requirements of laboratory animals. National Academy Press, Washington DC; Washington, DC: 1995. p. 82. [Google Scholar]
- [26].Derelanko MJ, editor. Toxicologist's Pocket Handbook. CRC Press; Boca Raton: 2000. p. 16. [Google Scholar]
- [27].Zhang S, Lawson KA, Simmons-Menchaca M, Sun LZ, et al. Vitamin E analog α-TEA and celecoxib alone and together reduce human MDA-MB-435-FL-GFP breast cancer burden and metastasis in nude mice. Breast Cancer Res. Treat. 2004;87:111–121. doi: 10.1023/B:BREA.0000041593.69178.57. [DOI] [PubMed] [Google Scholar]
- [28].Tirmenstein MA, Watson BW, Haar NC, Fariss MW. Sensitive method for measuring tissue alpha-tocopherol and alpha-tocopheryloxybutyric acid by high-performance liquid chromatography with fluorometric detection. J. Chromatogr. B Biomed. Sci. Appl. 1998;707:308–311. doi: 10.1016/s0378-4347(97)00592-6. [DOI] [PubMed] [Google Scholar]
- [29].Yu W, Jia L, Wang P, Lawson KA, et al. In vitro and in vivo evaluation of anticancer actions of natural and synthetic vitamin E forms. Mol. Nutr. Food Res. 2008;52:447–456. doi: 10.1002/mnfr.200700254. [DOI] [PubMed] [Google Scholar]
- [30].Piao YS, Du YC, Oshima H, Jin JC, Nomura M, Yoshimoto T, Oshima M. Platelet-type 12-lipoxygenase accelerates tumor promotion of mouse epidermal cells through enhancement of cloning efficiency. Carcinogenesis. 2008;29(2):440–447. doi: 10.1093/carcin/bgm274. [DOI] [PubMed] [Google Scholar]
- [31].Yu W, Park SK, Jia L, Tiwary R, Scott WW, Li J, Wang P, Simmons-Menchaca M, Sanders BG, Kline K. RRR-gamma-tocopherol induces human breast cancer cells to undergo apoptosis via death receptor 5 (DR5)-mediated apoptotic signaling. Cancer Lett. 2008;259(2):165–176. doi: 10.1016/j.canlet.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cameron IL, Munoz J, Barnes CJ, Hardman WE. High dietary level of synthetic vitamin E on lipid peroxidation, membrane fatty acid composition and cytotoxicity in breast cancer xenograft and in mouse host tissue. Cancer Cell Internatl. 2003;3:3–10. doi: 10.1186/1475-2867-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hahn T, Szabo L, Gold M, Ramanathapuram L, et al. Dietary administration of the proapoptotic vitamin E analogue α-tocopheryloxyacetic acid inhibits metastatic murine breast cancer. Cancer Res. 2006;66:9374–9378. doi: 10.1158/0008-5472.CAN-06-2403. [DOI] [PubMed] [Google Scholar]
- [34].Suh N, Paul S, Lee HJ, Ji Y, et al. Mixed tocopherols inhibit N-methyl-N-ntirosourea-induced mammary tumor growth in rats. Nutr. Cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- [35].Wang XF, Dong L, Zhao Y, Tomasetti M, Wu K, Neuzil J. Vitamin E analogues as anticancer agents: lessons from studies with alpha-tocopheryl succinate. Mol Nutr Food Res. 2006;50(8):675–685. doi: 10.1002/mnfr.200500267. [DOI] [PubMed] [Google Scholar]
- [36].Blatt DH, Leonard SW, Traber MG. Vitamin E kinetics and the function of tocopherol regulatory proteins. Nutrition. 2001;17:799–805. doi: 10.1016/s0899-9007(01)00637-2. [DOI] [PubMed] [Google Scholar]
- [37].Sylvester PW, Shah SJ. Mechanisms mediating the antiproliferative and apoptotic effects of vitamin E in mammary cancer cells. Front. Biosci. 2005;10:699–709. doi: 10.2741/1565. [DOI] [PubMed] [Google Scholar]
- [38].Traber MG. Vitamin E regulatory mechanisms. Ann. Rev. Nutr. 2007;27:347–362. doi: 10.1146/annurev.nutr.27.061406.093819. [DOI] [PubMed] [Google Scholar]
- [39].Huang HY, Appel LJ. Supplementation of diets with α-tocopherol reduces serum concentrations of γ- and δ-tocopherol in humans. J. Nutr. 2003;133:3137–3140. doi: 10.1093/jn/133.10.3137. [DOI] [PubMed] [Google Scholar]
- [40].Traber MG, Siddens LK, Leonard SW, Schock B, et al. α-tocopherol modulates Cyp3a expression, increases γ-CEHC production and limits tissue γ-tocopherol accumulation in mice fed high γ-tocopherol diets. Free Radic. Biol. Med. 2005;38:773–785. doi: 10.1016/j.freeradbiomed.2004.11.027. [DOI] [PubMed] [Google Scholar]
- [41].Wolf G. How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr. Rev. 2006;64:295–299. doi: 10.1111/j.1753-4887.2006.tb00213.x. [DOI] [PubMed] [Google Scholar]
- [42].Yu W, Simmons-Menchaca M, Gapor A, Sanders BG, Kline K. Induction of apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutr Cancer. 1999;33(1):26–32. doi: 10.1080/01635589909514744. [DOI] [PubMed] [Google Scholar]