Abstract
The primary transporter responsible for bile salt secretion is the bile salt export pump (BSEP, ABCB11), a member of the ATP-binding cassette (ABC) superfamily, which is located at the bile canalicular apical domain of hepatocytes. In humans, BSEP deficiency results in several different genetic forms of cholestasis, which include progressive familial intrahepatic cholestasis type 2 (PFIC2), benign recurrent intrahepatic cholestasis type 2 (BRIC2), as well as other acquired forms of cholestasis such as drug-induced cholestasis (DIC) and intrahepatic cholestasis of pregnancy (ICP). Because bile salts play a pivotal role in a wide range of physiologic and pathophysiologic processes, regulation of BSEP expression has been a subject of intense research. The authors briefly describe the molecular characteristics of BSEP and then summarize what is known about its role in the pathogenesis of genetic and acquired cholestatic disorders, emphasizing experimental observations from animal models and cell culture in vitro systems.
Keywords: Cholestasis, progressive familial intrahepatic cholestasis, bile salt export pump (BSEP) mutations, polymorphisms, ATP-binding cassette (ABC) transporters, trafficking, recycling, ubiquitination
One of the primary roles of the liver is to produce and secrete bile, a bile salt and phospholipid-containing fluid that is essential for solubilizing and absorbing dietary lipids. The primary transporter responsible for canalicular bile salt secretion is the bile salt export pump (BSEP, ABCB11). This membrane transporter belongs to the ATP-binding cassette (ABC) superfamily of proteins that make up one of the largest family of transporters within the human genome.1 BSEP plays a critical role in the physiologic maintenance of the enterohepatic circulation of bile acids. Impairment in bile flow as a result of genetic defects in this transporter leads to cholestatic liver disease, including progressive familial intrahepatic cholestasis type 2 (PFIC2), benign recurrent intrahepatic cholestasis type 2 (BRIC2), drug-induced cholestasis (DIC), and hormone-dependent intrahepatic cholestasis of pregnancy (ICP). Given the pivotal role that BSEP plays in liver function and disease, regulation of BSEP expression has been a subject of intense research. An emerging concept in this review is posttranslational regulation of BSEP and alterations in intracellular trafficking that lead to changes in functional BSEP expression on the liver canalicular membrane. This process partly involves endoplasmic reticulum and plasma membrane ubiquitin-proteasome mediated degradation. Additionally, short-term phosphorylation, oxidative stress, hormonal effects, membrane cholesterol content and drug-induction of BSEP may also be involved in regulating bile acid transport and trafficking processes. This review will update the range of post-translational molecular mechanisms that regulate BSEP expression, and where possible put them in context with human disease. Nevertheless, many details of BSEP-specific regulation and pathophysiologic aspects remain unknown and these are highlighted as areas for future study and discussion.
DISCOVERY OF THE CANALICULAR BILE SALT EXPORT PUMP
Bile salt transport across the bile canaliculus is the major driving force for bile flow and is the rate-limiting step in the hepatic clearance of bile salts from blood-to-bile. An electrochemical gradient of ~−35 mV exists across the hepatocyte canalicular membrane and was initially thought to drive the secretion of bile acids into bile.2,3 However, this electrochemical gradient was insufficient to account for the high concentration gradients for bile salts and organic solutes between the hepatocyte and bile, a process that was found to require ATP-dependent export pumps.4–7 Three lines of evidence eventually demonstrated that an ABC transporter was the canalicular bile salt efflux pump. First, in 1995, Childs and et al8 cloned a partial gene from a pig cDNA library using a probe sequence from the multidrug resistance gene, P-glycoprotein (also known as human MDR1, ABCB1) and named it sister of P-glycoprotein (Spgp). Tissue expression of Spgp mRNA was almost exclusively limited to the liver. Second, bile salt transport activity was demonstrated by Gerloff et al,9 when rat Spgp cRNA was injected into Xenopus laevis oocytes or in vesicles isolated from transfected Sf9 insect cells. Rat Spgp-mediated taurocholate transport in transfected Sf9 cells with similar affinity to its ATP-dependent transport across rat canalicular membranes. Third, a link between BSEP and progressive familial intrahepatic cholestasis (PFIC) was recognized when the gene was mapped to the disease locus on chromosome 2q24.10 Subsequently, BSEP mutations were found in several cholestatic children with elevated serum bile salts and impaired bile salt secretion,11 a disease now called PFIC2. These genetic findings allowed a specific diagnostic distinction to be made from two other genetic defects involving canalicular transporters that also caused progressive familial intrahepatic cholestasis. PFIC1 (Byler disease) results from mutations in FIC1 and PFIC3 occurs from mutations in MDR3.12–14 Collectively, these findings indicate that BSEP is the major, if not the only bile salt transporter at the mammalian canalicular membrane.
STRUCTURE AND FUNCTION OF BSEP
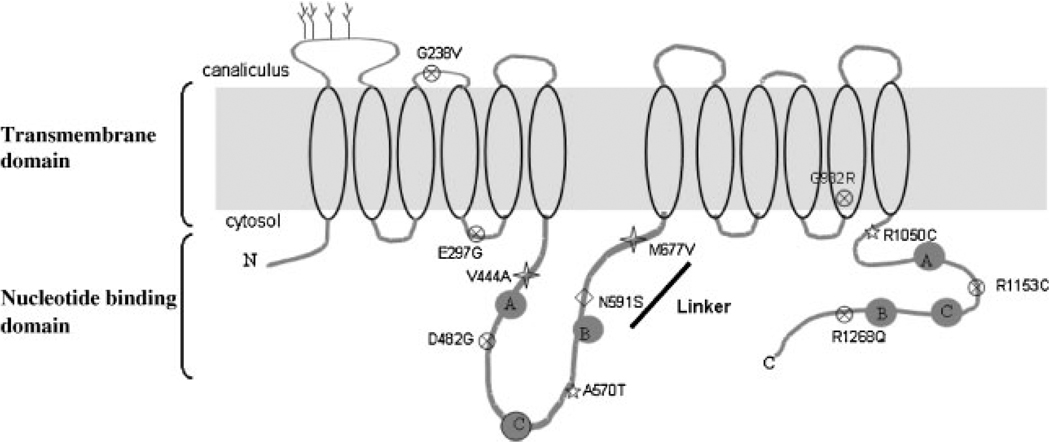
BSEP/ABCB11 consists of 1321 amino acids with a molecular mass of ~160 kDa. Like other full-length transporters of the ABC superfamily, the topology is predicted to be a tandemly duplicated structure with each half of the molecule consisting of six predicted transmembrane domains (TMD) and a large cytoplasmic nucleotide binding domain (NBD) in a TMD–NBD–TMD–NBD organization (see cartoon Fig. 1). The first extracellular loop is N-glycosylated, which facilitates BSEP stability, trafficking and membrane expression.15 The “linker” region of ~75 amino acids that connects the homologous halves interacts with a motor protein (myosin II regulatory light chain)16 and a cytoskeletal-associated protein (Hax-1)17 whose function in secretory and endocytic pathways is discussed below.
Figure 1.
Topology of bile salt export pump (BSEP) with 12 transmembrane domains (TMD) and two nucleotide binding domains (NBDs). The nucleotide-binding domains contains Walker A and B as well as a conserved signature C motif of ATP-binding cassette (ABC) superfamily of proteins. BSEP contains N-glycosylation sites (tree) on the first extracellular loop. The “linker” domain lies between the two homologous halves of the protein. A selective number of mutations have been studied and discussed in this review. progressive familial intrahepatic cholestasis type 2 (PFIC2) mutations (x), benign recurrent intrahepatic cholestasis type 2 (BRIC2; star), and intrahepatic cholestasis of pregnancy (ICP; diamond). Two single nucleotide polymorphisms (SNPs; V444A and M677V, cross) are also represented and have been characterized by functional studies (gray star).
BSEP mediates the excretion of monovalent conjugated bile acids and has low affinity for certain drugs that are also substrates for MDR1. However, whether human BSEP functions physiologically as a drug transporter is not clear.18,19 Human and rodent BSEP exhibit high affinity and selectivity toward conjugated bile salts in the order of taurochenodeoxycholate (TCDCA), taurocholate (TCA) >taurodeoxycholate (TDCA) >tauroursodeoxycholate (TUDCA) ~glycocholate (GCA).9,20–22 Conjugated bile salts not only serve as substrates for BSEP transport, but they can activate its ATPase activity.23 Substrate modulation of the functional ATPase domain appears to be a universal property of ABC transporters with pharmacologic implications. For example, hydrophobic peptides with high affinity interactions with the MDR1-ATPase have been tested in the modulation of chemotherapy resistance in cancer cells. Unfortunately, their high toxicity severely limits clinical use.24 In addition to modulating BSEP’s intrinsic ATPase activity, bile salts act as ligands for nuclear receptors that induce BSEP mRNA expression and as molecular signaling molecules for bile acid receptors that mediate the dynamic localization of BSEP within the hepatocyte (see below).
TRANSCRIPTIONAL REGULATION OF BSEP EXPRESSION
BSEP expression is highly regulated by the nuclear receptor, farnesoid X-receptor (FXR, NR1H4) in humans and rodents. Bile acids are ligands of FXR and, thus, serve to induce transcription of BSEP.25–27 Bile acid-activated FXR forms a heterodimer with the retinoid X receptor (RXR) and induces BSEP activity upon binding to a specific bile acid response cis-element in its promoter.28–30 Both the physiologic endogenous FXR agonist chenodeoxycholic acid (CDCA) and the more potent synthetic FXR agonists, GW4064 and 6-ethylchenodeoxycholic acid, induce BSEP mRNA and protein expression in many cell lines and in animal models. The physiologic role of FXR in BSEP regulation is demonstrated in Fxr−/− mice where basal levels of BSEP expression are markedly reduced and cannot be further induced by bile acids.31 Variants in the FXR gene have also been associated with some forms of intrahepatic cholestasis of pregnancy.32,33
Although FXR is an essential regulator of BSEP expression, other transcriptional factors may also be involved. For example, BSEP promoter activity is induced by the hepatocyte-specific liver receptor homolog-1 (LRH-1, NR5A2)34 and BSEP expression is decreased in livers of Lrh1−/− mice.35 In contrast, 1,25-dihydroxyvitamin D3 suppresses CDCA-FXR transactivation of BSEP promoter activity.36
Nrf2, a nuclear erythroid 2 p45-related factor 2 (nf-e2-related factor 2), is another positive transcriptional regulator of human BSEP expression.37 Nrf2 plays a major role in response to oxidative stress by binding to antioxidant-responsive elements that regulate many hepatic phase I and II enzymes as well as hepatic efflux transporters.38–40 Alpha-naphthyl isothiocyanate (ANIT)-induced BSEP expression is also abolished in Nrf2−/− mice.41
Taken together, these findings indicate that BSEP expression is regulated by a complex network of stimuli acting on the BSEP promoter as part of both physiologic and pathophysiologic adaptive responses.
BSEP MUTATIONS IN CHOLESTASIS
The essential physiologic function of BSEP in hepatobiliary bile salt secretion is apparent in several forms of cholestasis where BSEP gene mutations have been identified. These include gene mutations that cause progressive familial intrahepatic cholestasis type 2 (PFIC2) and the milder benign recurrent intrahepatic cholestasis type 2 (BRIC2), as well as mutations and polymorphisms that predispose to acquired forms of cholestasis, such as drug-induced cholestasis (DIC) and intrahepatic cholestasis of pregnancy (ICP). Independent and collaborative studies have identified more than 100 different BSEP variants worldwide and the more frequent mutations are grouped as missense, nonsense, deletions and insertions, and splice-site mutations. 11,42–47 A common result of these various gene mutations is the reduction or total loss of expression of the BSEP protein on the canalicular membrane.47 In addition, aberrant pre-mRNA splicing and reduced levels of BSEP mRNA can result from BSEP mutations and single nucleotide polymorphisms (SNPs) in the BSEP gene.48,49 Heterogeneity in clinical phenotypes from a single gene mutation (p.D482G) suggests that additional modifiers may influence the severity of the disease phenotype.47 To date, 86 polymorphisms in BSEP have been described in a population of Caucasians, Koreans, and African Americans.50 These polymorphisms are located in exons and introns, as well as in 5′-flanking regions, but no effect on the mRNA or protein has been determined. Two nonsynonymous SNPs, c.1331T>C (p.V444A) in exon 13 and c.2029A>G (p.M677V), have been consistently observed and patients with at least one c.1331T allele tended to have lower levels of BSEP expression.49,51 The V444A variant is also associated with ICP and drug-induced cholestasis,46,49,51–53 but functional activity is not affected.51 It should be noted that these polymorphisms in BSEP that have been associated with ICP and drug cholestasis will require further validation and functional analyses in a larger group of patients.
To more fully understand how changes in the BSEP gene may result in a particular clinical phenotype, in vitro studies have been conducted using a few of the most common gene mutations whose locations are illustrated in Fig. 1 (for a more complete list of mutations, see47). Similar to the results of immunofluorescence studies in liver tissue from PFIC2 patients,47 when PFIC2 human mutations were expressed in model mammalian cell lines (MDCK, HEK293, HepG2), the proteins failed to reach or be maintained at the cell surface.54–57 When BSEP mutations that cause PFIC2 (D482G, E297G), BRIC2 (A590T, R1050C), and ICP (N591S) were compared, the clinical severity of these mutations tended to correlate inversely with the amount of protein expressed on the cell surface. In addition, PFIC2 mutations significantly affected taurocholate transport activity, while BRIC2 and ICP mutations retained partial and normal activity, respectively.56 Mechanistically, the removal of misfolded Bsep proteins likely involves endoplasmic reticulum-associated degradation (ERAD). For example, the PFIC2 mutant D482G’s protein half-life is short compared with the wild-type and is shortened further after ubiquitylation with E3 ubiquitin ligases.58 However, a small amount of this mutant protein can reach the plasma membrane where it is functional.58
Additional studies have shown that the resident time on the cell surface is greatly reduced with D482G and E297G mutant proteins as a result of accelerated internalization, reduced recycling, and/or targeting of endocytosed proteins for degradation.57,59 These studies suggest that the use of small molecules that modulate these pathways might be worthwhile therapeutic approaches in some of these cholestatic disorders. For example, 4-phenylbutyrate (4-PBA) can enhance cell surface expression of D482G and E297G proteins.60 Furthermore, administration of 4-PBA to normal rats enhances BSEP expression and bile salt secretion.60 Further studies are clearly needed in this area.
ANIMAL MOUSE MODELS OF PFIC2
The development of the BSEP knockout mice was expected to clarify the molecular mechanisms that result in PFIC2. However, despite the fact that bile acid secretion is completely abolished in PFIC2 patients, BSEP knockout mice do not develop severe cholestasis as expected.61 This is because the bile acid pool is replaced with more hydrophilic bile acids, including muricholic acid and atypical bile acid species (24-tetra hydroxy bile acids) that are not found in humans.61,62 Consequently, these animals do not have any histopathologic signs of liver injury unless fed cholic acid.63 Three lines of evidence suggest that canalicular Mdr1a/Mdr1b is also involved in this salvage pathway. First, Mdr1a/Mdr1b mRNA and protein expression are increased significantly in the canalicular membrane of these mice. Second, this P-glycoprotein exhibits ATP-dependent taurocholate transport, albeit with an affinity sixfold lower than that of Bsep.64 And finally, the triple knockout mice (bsep−/−/mdr1a−/−/mdr1b−/−) does result in reduced bile flow rate and severe cholestasis, which suggests that Mdr1 plays a compensatory role in the mouse in the absence of Bsep.65 It remains to be determined whether Mdr1 also plays a compensatory role in human cholestatic liver disease.
INTRACELLULAR TRAFFICKING OF BSEP
Bsep Recycling Pool
There is increasing evidence for the existence of a recycling pool for Bsep that can be mobilized rapidly to the plasma membrane for insertion upon stimulation (Fig. 2). These cytoplasmic pools may be rapidly targeted to the plasma membrane to increase the transport capacity when needed (e.g., for instance, during a meal when there is a high demand for biliary excretion of bile salts to aid in digestion/absorption).66 The intrahepatic reservoir is large, and is estimated to contain at least sixfold greater amounts of ABC transporters than in the canalicular membrane. This reservoir of synthesized and apical recycling protein could account for the long metabolic half-life of 5 days for Bsep in rat liver.
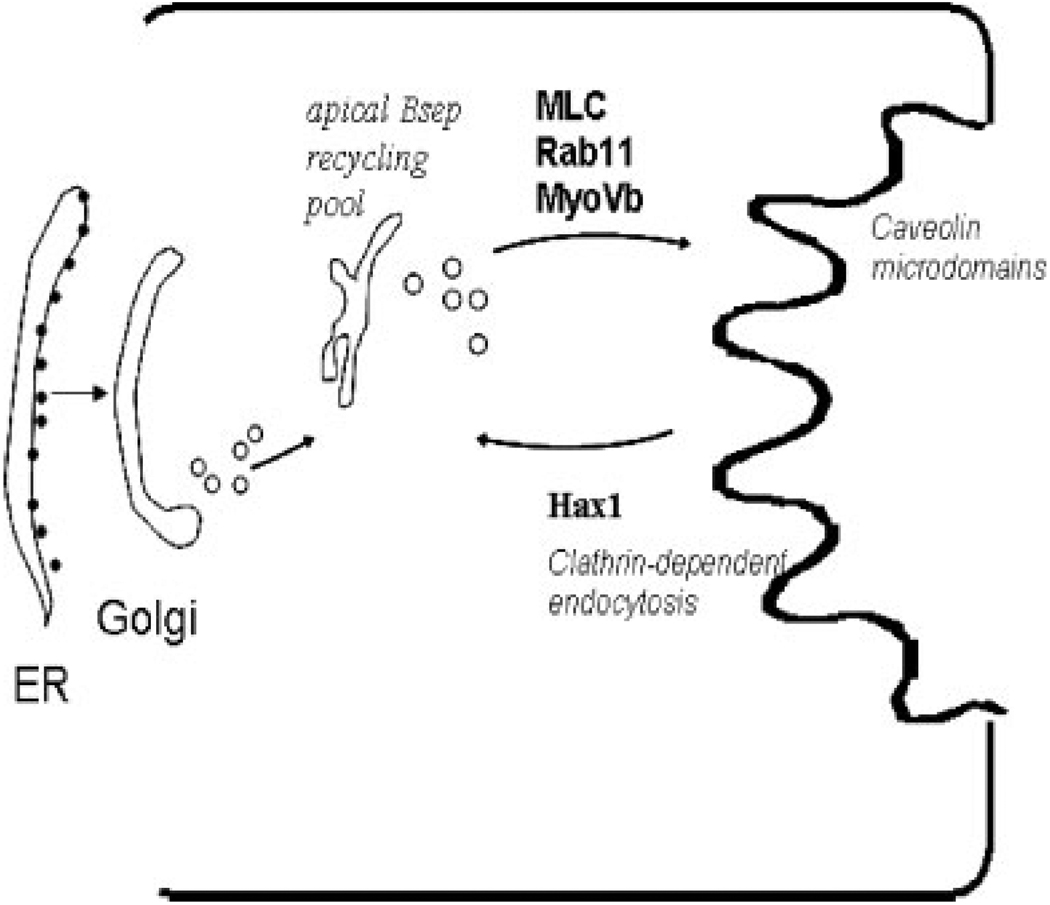
Figure 2.
Trafficking routes and molecular components involved in the exocytic and endocytic retrieval of bile salt export pump (BSEP). After biosynthesis in the endoplasmic reticulum (ER) and further posttranslational modifications in Golgi, BSEP is indirectly targeted first to a subapical endosomal compartment and then cycles to and from the canalicular membrane. This apical recycling pool is associated with Rab11a endosomes and exocytosis requires myosin light chain, Rab11a, and myosin Vb. BSEP resides within caveolin-containing microdomains along with MDR2 and MRP2 where they function to determine the formation of bile. BSEP endocytosis requires the clathrin-endocytic machinery that includes Hax-1, cortactin, EPS15, and other actin-binding proteins. Many components required for exocytosis and endocytosis are not yet identified.
In vivo pulse-chase experiments suggest that Bsep and Mdr1/Mdr2 are targeted directly from the Golgi to the canalicular membrane rather than indirectly via the basolateral membrane.67 However, although Mdr1/Mdr2 proteins are chased to the canalicular membrane in 30 minutes, Bsep requires an additional hour.67 During this time Bsep was found in a post-Golgi endosomal fraction, suggesting that significant amounts of Bsep are stored in intracellular pools before apical delivery.68 Earlier immunoelectron microscopic studies of rat hepatocytes also found Bsep in subapical vesicular compartments as well as in the canalicular membrane.9 When Bsep was labeled with yellow fluorescent protein (YFP) and transfected into WIFB9 cells, direct visualization of recycling was observed between the canalicular membrane and Rab11a-containing recycling endosomes. 69 In addition, depleting Rab11a or overexpressing dominant negative form of myosin Vb affected formation of the bile canalicular membrane and polarization in this cell line.70 It remains to be determined what other polarity cues are involved in hepatocyte polarization and if BSEP constitutively cycles to and from the canalicular membrane through regulation of exocytosis and endocytosis. Also, it is not known if the protein can be rerouted from degradation by such a rapid recycling process and, if so, what molecular mechanisms might be involved.
POSTTRANSCRIPTIONAL REGULATION OF BSEP TRAFFICKING AND LOCALIZATION
Glycosylation, Phosphorylation, and Ubiquitination
Loss of Bsep protein from the canalicular membrane is characteristic of some forms of experimental cholestatic liver injury, as well as human cholestatic liver diseases. Cholestasis induced by estradiol-17β-D-glucuronide, taurolithocholic acid, and cyclosporine A result in redistribution of Bsep to the subapical cytoplasm.71–74 On the other hand, the anticholestatic drugs ursodeoxycholic acid and silymarin are able to prevent or reverse defective canalicular membrane insertion of transporters.75,76 Membrane localization of Bsep can also be regulated through short-term posttranslational changes in protein expression, including phosphorylation, glycosylation, and ubiquitination. The following experimental studies have led to a better understanding of the regulation of BSEP in the normal hepatocyte and provide potential insights into the pathogenesis and treatment of human cholestatic diseases.
ROLE OF GLYCOSYLATION
The first extracellular loop of BSEP is N-glycosylated at four asparagine residues in rat Bsep.15 Similar asparagine residues are present in human BSEP, but their function has not been assessed. Protein glycosylation is important for proper folding and stabilization of newly synthesized proteins and for influencing the charge and solubility of the protein.77,78 Proteins that are misfolded in the endoplasmic reticulum (ER) can result in ER-associated degradation (ERAD).79,80 Mochizuki et al15 have shown that rat Bsep requires at least two of its four N-linked glycans for proper protein stability, intracellular trafficking, and functional activity. We have shown that a glycosylation-less mutant is heavily ubiquitinated and degraded by proteasomes.58 Based on these studies, it is predicted that human BSEP mutations that result in glycosylation defects will produce aberrant proteins that are trapped in the ER and directed to the proteasomes instead of the canalicular membrane.
ROLE OF PHOSPHORYLATION
Phosphorylation is another posttranslational modification that can affect BSEP localization. Treatment of rat hepatocytes and HepG2 cells with tauroursodeoxycholic acid (TUDCA) was shown to activate mitogen-activated proteins (MAPKs), such as extracellular signal-regulated kinase (Erk) and p38MAPK, and stimulate the trafficking of Bsep from the Golgi complex to the canalicular membrane.81 In addition, estradiol-17β-D-glucuronide treatment stimulates the recruitment of PKCα to the canalicular membrane with a concomitant decrease in the expression of canalicular Bsep and Mrp2.74 Direct phosphorylation of mouse Bsep with PKCα, but not with PKCδ or PKCε isoforms, was demonstrated in Sf9 insect cells; an effect further enhanced by treatment with the PKC activator, 12-O-tetradecanolphorbol 13-acetate. 23 The intracellular targets of PKCα activation are unknown, but phosphorylation may affect BSEP’s intrinsic ATPase activity. Serine residues in MDR1 have been identified as phosphorylation sites for PKC and have a positive regulatory effect on its ATPase activity. 82,83 Phosphorylation of nuclear receptors such as FXR and RXRα can also modulate the ability of their ligands to induce gene transcription and malfunction of phosphorylation by kinase signaling pathways has been linked to development of hepatocellular carcinoma (HCC).84 Patients with PFIC2 can also develop HCC.85 However, the biologic significance of phosphorylation defects associated with BSEP in human cholestasis or HCC remain to be elucidated.
ROLE OF UBIQUINATION
Recent studies by Hayashi and Sugiyama indicate that the surface resident time of Bsep is shortened after modification with short-chain ubiquitins after expression in MDCK II cells.59 Furthermore, they also showed that Bsep immunoprecipitated from the canalicular membrane is associated with short-chain ubiquitin. These observations suggest quality control involving ubiquitin-mediated degradation of misfolded or truncated BSEP proteins might be playing a role in surface expression of BSEP. Ubiquitylation is involved in the degradation of receptors, channels, and transporters from the endoplasmic reticulum and cell surface of yeast and higher eukaryotes.86–88 Wang et al, showed for the first time that specific E3 ubiquitin ligases are involved in Bsep degradation.58 Bsep mutants (p.G238V, p.D482G, p.G982R, p.R1153C, and p.R1268Q) were highly ubiquitinated following overexpression of different E3 ubiquitin ligases and were rapidly degraded by proteasomes resulting in shorter half-lives compared with the wild-type protein.58 This study suggests that stabilizing aberrant BSEP proteins by inactivating key E3 ubiquitin ligases might be a novel therapeutic approach, providing that global effects on proteasomal degradation can be avoided.
ROLE OF CAVEOLINS
Recent studies suggest that Bsep exists in caveolin-1 enriched microdomains within the canalicular membrane in rat liver (Fig. 2).89 The multidrug resistance protein, Mdr2, and the multidrug resistance-related protein, Mrp2, are also found within these “Lubrol WX-canalicular microdomains.” However, the roles of caveolin-1 and caveolae in canalicular membrane protein trafficking and signaling are not clearly understood. Sphingophospholipid- and cholesterol-rich domains serve as assembly platforms to facilitate trafficking and functional association of proteins with their adaptor proteins. The strategic localization of these proteins within such a membrane environment may act to stabilize and protect membrane proteins from the detergent effect of bile salts. Mice infected with recombinant caveolin-1 and caveolin-2 have significant increases in the taurocholate secretory maximum (×2.5) with no detectable changes in Bsep levels.90 These results suggest that caveolins alter the lipid (cholesterol) environment of plasma membrane domains and affect Bsep functional activity. Bsep activity is known to be critically dependent on canalicular membrane cholesterol content.91
ASSOCIATION OF BSEP WITH OTHER PROTEINS/COMPLEXES
Within the canalicular membrane, BSEP resides in specialized microdomains and its apical recycling to and from the apical membrane is highly regulated (Fig. 2). Although molecular components to control BSEP retrieval and targeting are not well known, clathrin-dependent endocytic machinery may participate.17 One of these clathrin-associated components is Hax-1, a protein that was identified as a potential partner for Bsep, Mdr1, or Mdr2 in yeast-two-hybrid screen.17 Hax-1 interacts with cortactin, an F-actin-binding protein involved in clathrin-dependent endocytosis. The same study also showed that depleting Hax-1 protein in MDCKII cells results in twice as much Bsep on the plasma membrane.17 In addition, disrupting the activities of other key clathrin accessory proteins, such as epidermal growth factor receptor (EGR) pathway substrate 15 (Eps15) and cortactin, by dominant negative constructs also enhances steady-state apical Bsep expression. These studies suggest that clathrin-dependent endocytosis may be involved in Bsep internalization and apical recycling.17
CONCLUSIONS
Considering the relatively recent (1998) discovery that BSEP is the primary bile salt export pump in the hepatic canalicular membrane, much has been learned about BSEP’s critical role in cholestatic liver disease. The association of specific gene mutations in BSEP with different forms of cholestasis has opened the door to understanding the pathophysiologic mechanisms in these liver diseases. However, much more information will be needed for clinical outcomes to improve. Further clarification of the physiologic mechanisms for Bsep regulation at the canalicular domain and the role of these processes in cholestasis have been frustrated by several technical problems. First, the bile canaliculus is inaccessible to microsampling, thus restricting studies of apical retrieval mechanisms. Second, site-directed mutagenesis experiments often lead to aberrant proteins that are degraded, precluding studies of the molecular motifs that are involved in trafficking pathways. Third, many of the studies on BSEP trafficking have been performed on model cell systems with heterologous expression of a tagged BSEP fusion protein. It will be important to make comparative studies in human hepatocytes to investigate how BSEP traffics within its native environment. There is increasing evidence that transporters interact directly with membrane components such as phospholipids and cholesterol and changes in membrane physicochemical properties can affect transporter activity. Also, we are beginning to understand the compartments through which BSEP traverses on its way to and from the apical membrane and the molecules that are important in regulating the entry and exit of BSEP between these compartments. It is becoming clear that altered trafficking that reduces cell surface transporters is a common feature of cholestasis. However, the underlying molecular mechanisms involved in BSEP trafficking are still not well understood. Derailed endocytosis of surface receptors underlies hallmarks of cancer and neurodegeneration disorders.92 These alterations include common platforms of endocytic accessory and adaptor proteins, ubiquitylation, phosphorylation, Rab family GTPases, as well as cytoskeletal elements. Thus, it will be important to identify the specific components of the BSEP-dependent trafficking machinery, and to modulate their expression or activity experimentally in appropriate polarized cell models and ultimately, within the organism.
ABBREVIATIONS
- ABC
ATP-binding cassette
- BRIC2
benign recurrent intrahepatic cholestasis type 2
- BSEP
bile salt export pump
- CDCA
chenodeoxycholic acid
- DIC
drug-induced cholestasis
- FXR
farnesoid X receptor
- HCC
hepatocellular carcinoma
- ICP
intrahepatic cholestasis of pregnancy
- MDR
multidrug resistance
- NBD
nucleotide binding domain
- PFIC2
progressive familial intrahepatic cholestasis type 2
- RXR
retinoid X receptor
- SNP
single nucleotide polymorphism
- Spgp
sister of P-glycoprotein
- TMD
transmembrane domain
- UDCA
ursodeoxycholic acid
REFERENCES
- 1.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11(7):1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 2.Meier PJ, Meier-Abt AS, Boyer JL. Properties of the canalicular bile acid transport system in rat liver. Biochem J. 1987;242(2):465–469. doi: 10.1042/bj2420465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinman SA, Graf J, Boyer JL. Voltage-driven, taurocholate-dependent secretion in isolated hepatocyte couplets. Am J Physiol. 1989;256(5 Pt 1):G826–G832. doi: 10.1152/ajpgi.1989.256.5.G826. [DOI] [PubMed] [Google Scholar]
- 4.Adachi Y, Kobayashi H, Kurumi Y, Shouji M, Kitano M, Yamamoto T. ATP-dependent taurocholate transport by rat liver canalicular membrane vesicles. Hepatology. 1991;14(4 Pt 1):655–659. doi: 10.1016/0270-9139(91)90053-x. [DOI] [PubMed] [Google Scholar]
- 5.Müller M, Ishikawa T, Berger U, et al. ATP-dependent transport of taurocholate across the hepatocyte canalicular membrane mediated by a 110-kDa glycoprotein binding ATP and bile salt. J Biol Chem. 1991;266(28):18920–18926. [PubMed] [Google Scholar]
- 6.Nishida T, Gatmaitan Z, Che M, Arias IM. Rat liver canalicular membrane vesicles contain an ATP-dependent bile acid transport system. Proc Natl Acad Sci U S A. 1991;88(15):6590–6594. doi: 10.1073/pnas.88.15.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stieger B, O’Neill B, Meier PJ. ATP-dependent bile-salt transport in canalicular rat liver plasma-membrane vesicles. Biochem J. 1992;284(Pt 1):67–74. doi: 10.1042/bj2840067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childs S, Yeh RL, Georges E, Ling V. Identification of a sister gene to P-glycoprotein. Cancer Res. 1995;55(10):2029–2034. [PubMed] [Google Scholar]
- 9.Gerloff T, Stieger B, Hagenbuch B, et al. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273(16):10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 10.Strautnieks SS, Kagalwalla AF, Tanner MS, et al. Identification of a locus for progressive familial intrahepatic cholestasis PFIC2 on chromosome 2q24. Am J Hum Genet. 1997;61(3):630–633. doi: 10.1086/515501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strautnieks SS, Bull LN, Knisely AS, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20(3):233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 12.Bull LN, van Eijk MJ, Pawlikowska L, et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18(3):219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 13.van Mil SW, Klomp LW, Bull LN, Houwen RH. FIC1 disease: a spectrum of intrahepatic cholestatic disorders. Semin Liver Dis. 2001;21(4):535–544. doi: 10.1055/s-2001-19034. [DOI] [PubMed] [Google Scholar]
- 14.de Vree JM, Jacquemin E, Sturm E, et al. Mutations in the MDR3 gene cause progressive familial intrahepatic cholestasis. Proc Natl Acad Sci U S A. 1998;95(1):282–287. doi: 10.1073/pnas.95.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mochizuki K, Kagawa T, Numari A, et al. Two N-linked glycans are required to maintain the transport activity of the bile salt export pump (ABCB11) in MDCK II cells. Am J Physiol Gastrointest Liver Physiol. 2007;292(3):G818–G828. doi: 10.1152/ajpgi.00415.2006. [DOI] [PubMed] [Google Scholar]
- 16.Chan W, Calderon G, Swift AL, et al. Myosin II regulatory light chain is required for trafficking of bile salt export protein to the apical membrane in Madin-Darby canine kidney cells. J Biol Chem. 2005;280(25):23741–23747. doi: 10.1074/jbc.M502767200. [DOI] [PubMed] [Google Scholar]
- 17.Ortiz DF, Moseley J, Calderon G, Swift AL, Li S, Arias IM. Identification of HAX-1 as a protein that binds bile salt export protein and regulates its abundance in the apical membrane of Madin-Darby canine kidney cells. J Biol Chem. 2004;279(31):32761–32770. doi: 10.1074/jbc.M404337200. [DOI] [PubMed] [Google Scholar]
- 18.Childs S, Yeh RL, Hui D, Ling V. Taxol resistance mediated by transfection of the liver-specific sister gene of P-glycoprotein. Cancer Res. 1998;58(18):4160–4167. [PubMed] [Google Scholar]
- 19.Lecureur V, Sun D, Hargrove P, et al. Cloning and expression of murine sister of P-glycoprotein reveals a more discriminating transporter than MDR1/P-glycoprotein. Mol Pharmacol. 2000;57(1):24–35. [PubMed] [Google Scholar]
- 20.Noé J, Stieger B, Meier PJ. Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology. 2002;123(5):1659–1666. doi: 10.1053/gast.2002.36587. [DOI] [PubMed] [Google Scholar]
- 21.Byrne JA, Strautnieks SS, Mieli-Vergani G, Higgins CF, Linton KJ, Thompson RJ. The human bile salt export pump: characterization of substrate specificity and identification of inhibitors. Gastroenterology. 2002;123(5):1649–1658. doi: 10.1053/gast.2002.36591. [DOI] [PubMed] [Google Scholar]
- 22.Green RM, Hoda F, Ward KL. Molecular cloning and characterization of the murine bile salt export pump. Gene. 2000;241(1):117–123. doi: 10.1016/s0378-1119(99)00460-6. [DOI] [PubMed] [Google Scholar]
- 23.Noe J, Hagenbuch B, Meier PJ, St-Pierre MV. Characterization of the mouse bile salt export pump overexpressed in the baculovirus system. Hepatology. 2001;33(5):1223–1231. doi: 10.1053/jhep.2001.24171. [DOI] [PubMed] [Google Scholar]
- 24.Anuchapreeda S, Leechanachai P, Smith MM, Ambudkar SV, Limtrakul PN. Modulation of P-glycoprotein expression and function by curcumin in multidrug-resistant human KB cells. Biochem Pharmacol. 2002;64(4):573–582. doi: 10.1016/s0006-2952(02)01224-8. [DOI] [PubMed] [Google Scholar]
- 25.Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. [see comments] [DOI] [PubMed] [Google Scholar]
- 26.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284(5418):1365–1368. doi: 10.1126/science.284.5418.1365. [see comments] [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3(5):543–553. doi: 10.1016/s1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 28.Ananthanarayanan M, Balasubramanian N, Makishima M, Mangelsdorf DJ, Suchy FJ. Human bile salt export pump promoter is transactivated by the farnesoid X receptor/bile acid receptor. J Biol Chem. 2001;276(31):28857–28865. doi: 10.1074/jbc.M011610200. [DOI] [PubMed] [Google Scholar]
- 29.Plass JR, Mol O, Heegsma J, et al. Farnesoid X receptor and bile salts are involved in transcriptional regulation of the gene encoding the human bile salt export pump. Hepatology. 2002;35(3):589–596. doi: 10.1053/jhep.2002.31724. [DOI] [PubMed] [Google Scholar]
- 30.Gerloff T, Geier A, Roots I, Meier PJ, Gartung C. Functional analysis of the rat bile salt export pump gene promoter. Eur J Biochem. 2002;269(14):3495–3503. doi: 10.1046/j.1432-1033.2002.03030.x. [DOI] [PubMed] [Google Scholar]
- 31.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 32.Van Mil SW, Milona A, Dixon PH, et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133(2):507–516. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Zimmer V, Müllenbach R, Simon E, Bartz C, Matern S, Lammert F. Combined functional variants of hepatobiliary transporters and FXR aggravate intrahepatic cholestasis of pregnancy. Liver Int. 2009;29(8):1286–1288. doi: 10.1111/j.1478-3231.2009.02026.x. [DOI] [PubMed] [Google Scholar]
- 34.Song X, Kaimal R, Yan B, Deng R. Liver receptor homolog 1 transcriptionally regulates human bile salt export pump expression. J Lipid Res. 2008;49(5):973–984. doi: 10.1194/jlr.M700417-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mataki C, Magnier BC, Houten SM, et al. Compromised intestinal lipid absorption in mice with a liver-specific deficiency of liver receptor homolog 1. Mol Cell Biol. 2007;27(23):8330–8339. doi: 10.1128/MCB.00852-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honjo Y, Sasaki S, Kobayashi Y, Misawa H, Nakamura H. 1,25-dihydroxyvitamin D3 and its receptor inhibit the chenodeoxycholic acid-dependent transactivation by farnesoid X receptor. J Endocrinol. 2006;188(3):635–643. doi: 10.1677/joe.1.06105. [DOI] [PubMed] [Google Scholar]
- 37.Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Nuclear factor erythroid 2-related factor 2 is a positive regulator of human bile salt export pump expression. Hepatology. 2009;50:1588–1596. doi: 10.1002/hep.23151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Enomoto A, Itoh K, Nagayoshi E, et al. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol Sci. 2001;59(1):169–177. doi: 10.1093/toxsci/59.1.169. [DOI] [PubMed] [Google Scholar]
- 39.Jowsey IR, Jiang Q, Itoh K, Yamamoto M, Hayes JD. Expression of the aflatoxin B1-8,9-epoxide-metabolizing murine glutathione S-transferase A3 subunit is regulated by the Nrf2 transcription factor through an antioxidant response element. Mol Pharmacol. 2003;64(5):1018–1028. doi: 10.1124/mol.64.5.1018. [DOI] [PubMed] [Google Scholar]
- 40.Okawa H, Motohashi H, Kobayashi A, Aburatani H, Kensler TW, Yamamoto M. Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. 2006;339(1):79–88. doi: 10.1016/j.bbrc.2005.10.185. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka Y, Aleksunes LM, Cui YJ, Klaassen CD. ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling. Toxicol Sci. 2009;108(2):247–257. doi: 10.1093/toxsci/kfp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen PL, Strautnieks SS, Jacquemin E, et al. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117(6):1370–1379. doi: 10.1016/s0016-5085(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 43.Scheimann AO, Strautnieks SS, Knisely AS, Byrne JA, Thompson RJ, Finegold MJ. Mutations in bile salt export pump (ABCB11) in two children with progressive familial intrahepatic cholestasis and cholangiocarcinoma. J Pediatr. 2007;150(5):556–559. doi: 10.1016/j.jpeds.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 44.van Mil SW, van der Woerd WL, van der Brugge G, et al. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004;127(2):379–384. doi: 10.1053/j.gastro.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 45.Lam CW, Cheung KM, Tsui MS, Yan MS, Lee CY, Tong SF. A patient with novel ABCB11 gene mutations with phenotypic transition between BRIC2 and PFIC2. J Hepatol. 2006;44(1):240–242. doi: 10.1016/j.jhep.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Keitel V, Vogt C, Häussinger D, Kubitz R. Combined mutations of canalicular transporter proteins cause severe intrahepatic cholestasis of pregnancy. Gastroenterology. 2006;131(2):624–629. doi: 10.1053/j.gastro.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Strautnieks SS, Byrne JA, Pawlikowska L, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134(4):1203–1214. doi: 10.1053/j.gastro.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 48.Byrne JA, Strautnieks SS, Ihrke G, et al. Missense mutations and single nucleotide polymorphisms in ABCB11 impair bile salt export pump processing and function or disrupt pre-messenger RNA splicing. Hepatology. 2009;49(2):553–567. doi: 10.1002/hep.22683. [DOI] [PubMed] [Google Scholar]
- 49.Ho RH, Leake BF, Kilkenny DM, et al. Polymorphic variants in the human bile salt export pump (BSEP; ABCB11): functional characterization and interindividual variability. Pharmacogenet Genomics. 2010;20(1):45–57. doi: 10.1097/FPC.0b013e3283349eb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lang T, Haberl M, Jung D, et al. Genetic variability, haplotype structures, and ethnic diversity of hepatic transporters MDR3 (ABCB4) and bile salt export pump (ABCB11) Drug Metab Dispos. 2006;34(9):1582–1599. doi: 10.1124/dmd.105.008854. [DOI] [PubMed] [Google Scholar]
- 51.Lang C, Meier Y, Stieger B, et al. Mutations and polymorphisms in the bile salt export pump and the multidrug resistance protein 3 associated with drug-induced liver injury. Pharmacogenet Genomics. 2007;17(1):47–60. doi: 10.1097/01.fpc.0000230418.28091.76. [DOI] [PubMed] [Google Scholar]
- 52.Meier Y, Zodan T, Lang C, et al. Increased susceptibility for intrahepatic cholestasis of pregnancy and contraceptive-induced cholestasis in carriers of the 1331T>C polymorphism in the bile salt export pump. World J Gastroenterol. 2008;14(1):38–45. doi: 10.3748/wjg.14.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dixon PH, van Mil SW, Chambers J, et al. Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut. 2009;58(4):537–544. doi: 10.1136/gut.2008.159541. [DOI] [PubMed] [Google Scholar]
- 54.Wang L, Soroka CJ, Boyer JL. The role of bile salt export pump mutations in progressive familial intrahepatic cholestasis type II. J Clin Invest. 2002;110(7):965–972. doi: 10.1172/JCI15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plass JR, Mol O, Heegsma J, et al. A progressive familial intrahepatic cholestasis type 2 mutation causes an unstable, temperature-sensitive bile salt export pump. J Hepatol. 2004;40(1):24–30. doi: 10.1016/s0168-8278(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 56.Lam P, Pearson CL, Soroka CJ, Xu S, Mennone A, Boyer JL. Levels of plasma membrane expression in progressive and benign mutations of the bile salt export pump (Bsep/Abcb11) correlate with severity of cholestatic diseases. Am J Physiol Cell Physiol. 2007;293(5):C1709–C1716. doi: 10.1152/ajpcell.00327.2007. [DOI] [PubMed] [Google Scholar]
- 57.Kagawa T, Watanabe N, Mochizuki K, et al. Phenotypic differences in PFIC2 and BRIC2 correlate with protein stability of mutant Bsep and impaired taurocholate secretion in MDCK II cells. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G58–G67. doi: 10.1152/ajpgi.00367.2007. [DOI] [PubMed] [Google Scholar]
- 58.Wang L, Dong H, Soroka CJ, Wei N, Boyer JL, Hochstrasser M. Degradation of the bile salt export pump at endoplasmic reticulum in progressive familial intrahepatic cholestasis type II. Hepatology. 2008;48(5):1558–1569. doi: 10.1002/hep.22499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi H, Sugiyama Y. Short-chain ubiquitination is associated with the degradation rate of a cell-surface-resident bile salt export pump (BSEP/ABCB11) Mol Pharmacol. 2009;75(1):143–150. doi: 10.1124/mol.108.049288. [DOI] [PubMed] [Google Scholar]
- 60.Hayashi H, Sugiyama Y. 4-phenylbutyrate enhances the cell surface expression and the transport capacity of wild-type and mutated bile salt export pumps. Hepatology. 2007;45(6):1506–1516. doi: 10.1002/hep.21630. [DOI] [PubMed] [Google Scholar]
- 61.Wang R, Salem M, Yousef IM, et al. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A. 2001;98(4):2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perwaiz S, Forrest D, Mignault D, et al. Appearance of atypical 3 alpha,6 beta,7 beta,12 alpha-tetrahydroxy-5 beta-cholan-24-oic acid in spgp knockout mice. J Lipid Res. 2003;44(3):494–502. doi: 10.1194/jlr.M200394-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Wang R, Lam P, Liu L, et al. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology. 2003;38(6):1489–1499. doi: 10.1016/j.hep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 64.Lam P, Wang R, Ling V. Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochemistry. 2005;44(37):12598–12605. doi: 10.1021/bi050943e. [DOI] [PubMed] [Google Scholar]
- 65.Wang R, Chen HL, Liu L, Sheps JA, Phillips MJ, Ling V. Compensatory role of P-glycoproteins in knockout mice lacking the bile salt export pump. Hepatology. 2009;50(3):948–956. doi: 10.1002/hep.23089. [DOI] [PubMed] [Google Scholar]
- 66.Kipp H, Arias IM. Intracellular trafficking and regulation of canalicular ATP-binding cassette transporters. Semin Liver Dis. 2000;20(3):339–351. doi: 10.1055/s-2000-9388. [DOI] [PubMed] [Google Scholar]
- 67.Kipp H, Arias IM. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. J Biol Chem. 2000;275(21):15917–15925. doi: 10.1074/jbc.M909875199. [DOI] [PubMed] [Google Scholar]
- 68.Kipp H, Pichetshote N, Arias IM. Transporters on demand: Intrahepatic pools of canalicular ATP-binding cassette transporters in rat liver. J Biol Chem. 2001;276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 69.Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Intracellular trafficking of bile salt export pump (ABCB11) in polarized hepatic cells: constitutive cycling between the canalicular membrane and rab11-positive endosomes. Mol Biol Cell. 2004;15(7):3485–3496. doi: 10.1091/mbc.E03-10-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wakabayashi Y, Dutt P, Lippincott-Schwartz J, Arias IM. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc Natl Acad Sci U S A. 2005;102(42):15087–15092. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crocenzi FA, Mottino AD, Cao J, et al. Estradiol-17beta-D-glucuronide induces endocytic internalization of Bsep in rats. Am J Physiol Gastrointest Liver Physiol. 2003;285(2):G449–G459. doi: 10.1152/ajpgi.00508.2002. [DOI] [PubMed] [Google Scholar]
- 72.Crocenzi FA, Mottino AD, Sánchez Pozzi EJ, et al. Impaired localisation and transport function of canalicular Bsep in taurolithocholate induced cholestasis in the rat. Gut. 2003;52(8):1170–1177. doi: 10.1136/gut.52.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Román ID, Fernández-Moreno MD, Fueyo JA, Roma MG, Coleman R. Cyclosporin A induced internalization of the bile salt export pump in isolated rat hepatocyte couplets. Toxicol Sci. 2003;71(2):276–281. doi: 10.1093/toxsci/71.2.276. [DOI] [PubMed] [Google Scholar]
- 74.Crocenzi FA, Sánchez Pozzi EJ, Ruiz ML, et al. Ca(2+)-dependent protein kinase C isoforms are critical to estradiol 17beta-D-glucuronide-induced cholestasis in the rat. Hepatology. 2008;48(6):1885–1895. doi: 10.1002/hep.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dombrowski F, Stieger B, Beuers U. Tauroursodeoxycholic acid inserts the bile salt export pump into canalicular membranes of cholestatic rat liver. Lab Invest. 2006;86(2):166–174. doi: 10.1038/labinvest.3700371. [DOI] [PubMed] [Google Scholar]
- 76.Crocenzi FA, Basiglio CL, Pérez LM, Portesio MS, Pozzi EJ, Roma MG. Silibinin prevents cholestasis-associated retrieval of the bile salt export pump, Bsep, in isolated rat hepatocyte couplets: possible involvement of cAMP. Biochem Pharmacol. 2005;69(7):1113–1120. doi: 10.1016/j.bcp.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 77.Paulson JC. Glycoproteins: what are the sugar chains for? Trends Biochem Sci. 1989;14(7):272–276. doi: 10.1016/0968-0004(89)90062-5. [DOI] [PubMed] [Google Scholar]
- 78.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klausner RD, Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- 80.Plemper RK, Wolf DH. Retrograde protein translocation: ERADication of secretory proteins in health and disease. Trends Biochem Sci. 1999;24(7):266–270. doi: 10.1016/s0968-0004(99)01420-6. [DOI] [PubMed] [Google Scholar]
- 81.Kubitz R, Sütfels G, Kühlkamp T, Kölling R, Häussinger D. Trafficking of the bile salt export pump from the Golgi to the canalicular membrane is regulated by the p38 MAP kinase. Gastroenterology. 2004;126(2):541–553. doi: 10.1053/j.gastro.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Sachs CW, Chambers TC, Fine RL. Differential phosphorylation of sites in the linker region of P-glycoprotein by protein kinase C isozymes alpha, betaI, betaII, gamma, delta, epsilon, eta, and zeta. Biochem Pharmacol. 1999;58(10):1587–1592. doi: 10.1016/s0006-2952(99)00240-3. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed M, Borsch CM, Taylor SS, Vázquez-Laslop N, Neyfakh AA. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J Biol Chem. 1994;269(45):28506–28513. [PubMed] [Google Scholar]
- 84.Matsushima-Nishiwaki R, Okuno M, Adachi S, et al. Phosphorylation of retinoid X receptor alpha at serine 260 impairs its metabolism and function in human hepatocellular carcinoma. Cancer Res. 2001;61(20):7675–7682. [PubMed] [Google Scholar]
- 85.Knisely AS, Strautnieks SS, Meier Y, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44(2):478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 86.Ward CL, Omura S, Kopito RR. Degradation of CFTR by the ubiquitin-proteasome pathway. Cell. 1995;83(1):121–127. doi: 10.1016/0092-8674(95)90240-6. [DOI] [PubMed] [Google Scholar]
- 87.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 88.Duprá S, Urban-Grimal D, Haguenauer-Tsapis R. Ubiquitin and endocytic internalization in yeast and animal cells. Biochim Biophys Acta. 2004;1695(1–3):89–111. doi: 10.1016/j.bbamcr.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 89.Ismair MG, Häusler S, Stuermer CA, et al. ABC-transporters are localized in caveolin-1-positive and reggie-1-negative and reggie-2-negative microdomains of the canalicular membrane in rat hepatocytes. Hepatology. 2009;49(5):1673–1682. doi: 10.1002/hep.22807. [DOI] [PubMed] [Google Scholar]
- 90.Moreno M, Molina H, Amigo L, et al. Hepatic overexpression of caveolins increases bile salt secretion in mice. Hepatology. 2003;38(6):1477–1488. doi: 10.1016/j.hep.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 91.Paulusma CC, de Waart DR, Kunne C, Mok KS, Elferink RP. Activity of the bile salt export pump (ABCB11) is critically dependent on canalicular membrane cholesterol content. J Biol Chem. 2009;284(15):9947–9954. doi: 10.1074/jbc.M808667200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8(11):835–850. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]