Abstract
Over fifty years ago the discovery that rats would work to electrically stimulate their brains suggested the intriguing possibility that bliss could be achieved through the use of ‘pleasure electrodes’ implanted deep within the brain. Subsequent research has failed to bring about this brave new world of boundless pleasure, but more recent findings have started to throw new light on the intriguing links between brain mechanisms of pleasure and happiness. We discuss these findings of the underlying neural mechanisms and functional neuroanatomy of pleasure in the brain. In particular we address how they may come to shed light on our understanding of the brain basis of happiness. Beyond sensory pleasures, we examine how higher pleasures may be related to the brain’s default networks, especially in orchestrating cognitive aspects of the meaningfulness important to happiness. We also address how understanding of the hedonic brain might help alleviate the suffering caused by the lack of pleasure, anhedonia, which is a central feature of affective disorders such as depression and chronic pain.
Introduction
Just over fifty years ago, psychologists James Olds and Peter Milner, working at McGill University in Canada, carried out their pioneering experiments which discovered that rats would repeatedly press levers to receive tiny jolts of current injected through electrodes implanted deep within their brains (Olds and Milner, 1954). Especially when this brain stimulation was targeted at certain areas of the brain in the region of the septum and nucleus accumbens, the rats would repeatedly press the lever -- even up to 2000 times per hour (Olds, 1956).
These powerful findings seemed to suggest that Olds and Milner had discovered the pleasure center in the brain. Research in the next two decades established that dopamine is one of the main chemicals aiding neural signaling in these regions, and for many years dopamine was suggested to be the brain’s “pleasure chemical.” The results seemed to promise an easy fix to the unhappiness and suffering which is the traveling companion of far too many people. They certainly emboldened writers to envisage brave new worlds where drugs and electrical stimulation could induce bliss for the masses.
But is the high road to happiness really that simple? Subsequent human experiments suggest otherwise. Around the same time in the 1950s and 1960s, American psychiatrist Robert Heath at Tulane University took it upon himself to further these findings in some ethically questionable experiments on mentally ill human patients (Baumeister, 2000). Infamously, in one case he even implanted electrodes to try to cure homosexuality (Heath, 1972). This line of research was eventually stopped. Most substantively, however, the pleasure electrodes may never have lived up to their name. Although the researchers also found compulsive lever pressing in some patients, it was never clear from these patients’ subjective reports that the electrodes did indeed cause real pleasure. Some researchers today suggest that the electrodes never caused intense pleasure or ‘liking’ after all, but only a form of ‘wanting’ or motivation to obtain the stimulation (see discussion in Green et al., 2010; Smith et al., 2010).
Pleasure and happiness are linked, however, but in much more complex ways than simple pleasure electrodes would suggest, even if such electrodes exist. In this review we map out some of the intricate links between them to show how they are at the heart of affective neuroscience and the psychology of well-being. We will synthesize the results of last fifty years of careful study of reward and affective processing in the brain. Our main contention is that a better understanding of the pleasures of the brain may offer a more general insight into happiness, into how brains work to produce it in daily life for the fortunate, how brains fail in the less fortunate, and hopefully into better ways to enhance the quality of life.
A Science of Pleasure
The scientific study of pleasure and affect was pioneered by the ideas of Charles Darwin, who examined the evolution of emotions and affective expressions, and suggested that affects are adaptive responses to environmental situations. Prominent affective reactions such as pleasure ‘liking’ and displeasure reactions can be found in the behavior and brains of all mammals (Steiner et al., 2001), and likely have important evolutionary functions (Kringelbach, 2009). Both positive affect and negative affect have been proposed to have adaptive functions (Nesse, 2004) and it is clear that the neural mechanisms for generating affective reactions are present and similar in most mammalian brains, and as such appear to have been long ago evolutionarily selected for and conserved across species from humans to rodents (Kringelbach, 2010).
The progress in affective neuroscience in recent years has been made possible by identifying objective aspects of pleasure-elicited reactions and triangulating toward underlying brain substrates. This scientific strategy divides the concept of affect into two parts: the affective state, which has objective aspects in behavioral, physiological, and neural reactions; and conscious affective feelings, seen as the subjective experience of emotion (Kringelbach, 2004a). This definition allows conscious feelings to play a central role in hedonic experiences, but holds that the affective essence of a pleasure reaction is not limited to this conscious feeling. It means that objective affective state can be measured in other animals, regardless of the availability or accuracy of corresponding subjective reports, and as such is especially tractable to neuroscience investigations that involve brain manipulations.
The available evidence suggests that brain mechanisms involved in fundamental pleasures (food and sexual pleasures) overlap with those for higher-order pleasures (for example, monetary, artistic, musical, altruistic, and transcendent pleasures) (Kringelbach, 2010).
It is an important hedonic principle that the rewarding properties for all pleasures are likely to be generated by hedonic brain circuits that are distinct from the mediation of other features of the same events (e.g., sensory, cognitive) (Kringelbach, 2005). Thus pleasure is never merely a sensation or a thought, but is instead an additional hedonic gloss generated by the brain via dedicated systems (Frijda, 2010).
All pleasures from sensory pleasures and drugs of abuse to monetary, aesthetic, and musical delights would seem to involve the same fundamental hedonic brain systems. Pleasures important to happiness, such as socializing with friends, and related traits of positive hedonic mood, are thus all likely to draw upon the same neurobiological roots that evolved for sensory pleasures. The neural overlap may offer a way to generalize from fundamental pleasures that are best understood and so infer larger hedonic brain principles likely to contribute to happiness.
The Neuroanatomy of Pleasure
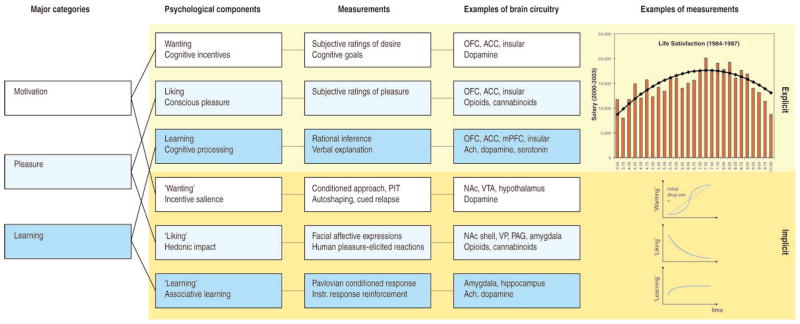
Pleasure is a complex psychological concept with many different sub-components which include ‘liking,’ ‘wanting,’ and ‘learning’ components (Berridge and Kringelbach, 2008; Smith et al., 2010). Each component has both conscious and non-conscious elements that can be studied in humans -- and at least the latter can also be probed in other animals (Figure 1).
Figure 1.
A scientific program for the study of pleasure. Pleasure is a complex psychological concept with at least three major subcomponents of motivation or wanting (white), pleasure liking or affect (light blue), and learning (blue). Each of these contains explicit (top rows, light yellow) and implicit (bottom rows, yellow) psychological components (second column) that constantly interact and require careful scientific experimentation to tease apart. Explicit processes are consciously experienced (e.g., explicit pleasure and happiness, desire, or expectation), whereas implicit psychological processes are potentially unconscious in the sense that they can operate at a level not always directly accessible to conscious experience (implicit incentive salience, habits, and ‘liking’ reactions), and must be further translated by other mechanisms into subjective feelings. Measurements or behavioral procedures that are especially sensitive markers of the each of the processes are listed (third column). Examples of some of the brain regions and neurotransmitters are listed (fourth column), as well as specific examples of measurements (fifth column), such as an example of how highest subjective life satisfaction does not lead to the highest salaries (top) (Haisken-De New and Frick, 2005). Another example shows the incentive-sensitization model of addiction and how ‘wanting’ to take drugs may grow over time independently of ‘liking’ and ‘learning’ drug pleasure as an individual becomes an addict (bottom) (Robinson and Berridge, 1993).
Hedonic hotspots
The brain has an extensive distribution of reward-related circuitry with some hedonic mechanisms found deep in the brain (nucleus accumbens, ventral pallidum, brainstem) and other candidates are in the cortex (orbitofrontal, cingulate, medial prefrontal, and insular cortices) (Figure 2). Pleasure coding brain networks are widespread and provide evidence for highly distributed brain coding of hedonic states. Yet, pleasure causation, which can be detected as increases in ‘liking’ reactions consequent to brain manipulation, has so far been found for only a few hedonic hotspots in the subcortical structures. Each hedonic hotspot is merely a cubic-millimeter or so in volume in the rodent brain (and should be a cubic-centimeter or so in humans, if proportional to whole brain volume). Hotspots are capable of generating enhancements of ‘liking’ reactions to a sensory pleasure such as sweetness, when stimulated with opioid, endocannabinoid, or other neurochemical modulators (Smith et al., 2010).
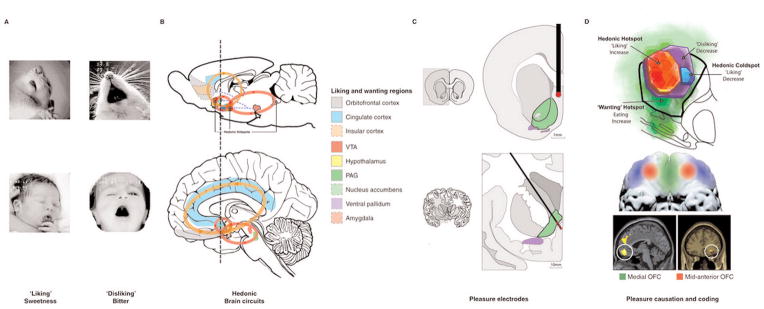
Figure 2.
Hedonic brain circuitry in humans and other animals. Pleasure-elicited reactions allow us to investigate the brain regions involved in pleasure in rodents and humans. (a) Facial ‘liking’ and ‘disliking’ expressions elicited by sweet and bitter taste are similar in rodents and human infants. (b, d) Pleasure causation has been identified in rodents as arising from interlinked subcortical hedonic hotspots, such as in nucleus accumbens and ventral pallidum, where neural activation may increase ‘liking’ expressions to sweetness. Similar pleasure coding and incentive salience networks have also been identified in humans. (c) The so-called ‘pleasure’ electrodes in rodents and humans are unlikely to have elicited true pleasure but perhaps only incentive salience or ‘wanting.’ (d) The cortical localization of pleasure coding may reach an apex in various regions of the orbitofrontal cortex, which differentiate subjective pleasantness from valence processing of aspects of the same stimulus, such as a pleasant food.
Hotspots have been found in nucleus accumbens shell and ventral pallidum, and possibly other forebrain and limbic cortical regions, and also in deep brainstem regions including the parabrachial nucleus in the pons (Figure 2D). The pleasure-generating capacity of these hotspots has been revealed in part by studies in which microinjections of drugs stimulated neurochemical receptors on neurons within a hotspot, and caused a doubling or tripling of the number of hedonic ‘liking’ reactions normally elicited by a pleasant sucrose taste. Analogous to scattered islands that form a single archipelago, hedonic hotspots are anatomically distributed but interact to form a functional integrated circuit. The circuit obeys control rules that are largely hierarchical and organized into brain levels. Top levels function together as a cooperative heterarchy, so that, for example, multiple unanimous ‘votes’ in favor from simultaneously-participating hotspots in the nucleus accumbens and ventral pallidum are required for opioid stimulation in either forebrain site to enhance ‘liking’ above normal.
In addition, as mentioned above, pleasure is translated into motivational processes in part by activating a second component of reward termed ‘wanting’ or incentive salience, which makes stimuli attractive when attributed to them by mesolimbic brain systems (Berridge and Robinson, 2003). Incentive salience depends in particular on mesolimbic dopamine neurotransmission (though other neurotransmitters and structures also are involved).
Importantly, incentive salience is not hedonic impact or pleasure ‘liking’ (Berridge, 2007). This is why an individual can ‘want’ a reward without necessarily ‘liking’ the same reward. Irrational ‘wanting’ without liking can occur especially in addiction via incentive-sensitization of the mesolimbic dopamine system and connected structures. At extreme, the addict may come to ‘want’ what is neither ‘liked’ nor expected to be liked, a dissociation possible because ‘wanting’ mechanisms are largely subcortical and separable from cortically-mediated declarative expectation and conscious planning. This is a reason why addicts may compulsively ‘want’ to take drugs even if, at a more cognitive and conscious level, they do not want to do so. That is surely a recipe for great unhappiness (Figure 2, bottom right).
Cortical pleasure
Hedonic evaluation of pleasure valence is separable from precursor operations such as sensory computations, suggesting existence of a hedonic cortex proper (Figure 2). Hedonic cortex involves regions such as the orbitofrontal, insula, medial prefrontal, and cingulate cortices, which, shown by a wealth of human neuroimaging studies, code for hedonic evaluations (including anticipation, appraisal, experience, and memory of pleasurable stimuli) and have close anatomical links to subcortical hedonic hotspots. It is important, however, to again make a distinction between brain activity coding and causing pleasure. Neural coding is inferred in practice by measuring brain activity correlated to a pleasant stimulus, using human neuroimaging techniques, or electrophysiological or neurochemical activation measures in animals (Aldridge and Berridge, 2010). Causation is generally inferred on the basis of a change in pleasure as a consequence of a brain manipulation such as a lesion or stimulation. Coding and causation often go together for the same substrate, but they may diverge so that coding occurs alone.
In humans, pleasure encoding may reach an apex of cortical localization in a subregion that is mid-anterior and roughly mid-lateral within the orbitofrontal cortex of the prefrontal lobe, where neuroimaging activity correlates strongly to subjective pleasantness ratings of food varieties - and to other pleasures such as sexual orgasms, drugs, chocolate, and music. Most importantly, activity in this special mid-anterior zone of orbitofrontal cortex tracks changes in subjective pleasure, such as a decline in palatability when the reward value of one food was reduced by eating it to satiety (while remaining high to another food). The mid-anterior subregion of orbitofrontal cortex is thus a prime candidate for the coding of subjective experience of pleasure (Kringelbach, 2005).
Another potential coding site for positive hedonics in orbitofrontal cortex is along its medial edge that has activity related to the positive and negative valence of affective events (Kringelbach and Rolls, 2004), contrasted to lateral portions that have been suggested to code unpleasant events (although lateral activity may reflect a signal to escape the situation, rather than displeasure per se) (Kringelbach, 2004b; O’Doherty et al., 2001). This medial-lateral hedonic gradient interacts with an abstraction-concreteness gradient in the posterior-anterior dimension, so that more complex or abstract reinforcers (such as monetary gain and loss) are represented more anteriorly in the orbitofrontal cortex than less complex sensory rewards (such as taste). The medial region that codes pleasant sensations does not, however, appear to change its activity with reinforcer devaluation, and so may not reflect the full dynamics of pleasure.
Still other cortical regions have been implicated by some studies in coding for pleasant stimuli, including parts of the mid-insular cortex that is buried deep within the lateral surface of the brain as well as parts of the anterior cingulate cortices on the medial surface of the cortex (Kringelbach, 2005). As yet, however, pleasure coding is not as clear for those regions as for the orbitofrontal cortex, and it remains uncertain whether insular or anterior cingulate cortices specifically code pleasure or only emotion more generally.
It remains still unknown, however, if even the mid-anterior pleasure-coding site of orbitofrontal cortex or medial orbitofrontal cortex or any other cortical region actually causes a positive pleasure state. Clearly, damage to orbitofrontal cortex does impair pleasure-related decisions, including choices and context-related cognitions in humans, monkeys, and rats (Anderson et al., 1999; Nauta, 1971). But some caution regarding whether cortex generates positive affect states per se is indicated by the consideration that patients with lesions to the orbitofrontal cortex do still react normally to many pleasures, although sometimes showing inappropriate emotions. Hedonic capacity after prefrontal damage has not, however, yet been studied in careful enough detail to draw firm conclusions about cortical causation (e.g., using selective satiation paradigms), and it would be useful to have more information on the role of orbitofrontal cortex, insular cortex, and cingulate cortex in generating and modulating hedonic states.
Pleasure causation has been so far rather difficult to assess in humans given the limits of information from lesion studies, and the correlative nature of neuroimaging studies. A promising tool, however, is deep brain stimulation (DBS) which is a versatile and reversible technique that directly alters brain activity in a brain target and where the ensuing whole-brain activity can be measured with MEG (Kringelbach et al., 2007). Pertinent to a view of happiness as freedom from distress, at least pain relief can be obtained from DBS of periaqueductal grey in the brainstem in humans, where specific neural signatures of pain have been found (Green et al., 2009), and where the pain relief is associated with activity in the mid-anterior orbitofrontal cortex, perhaps involving endogenous opioid release. Similarly, DBS may alleviate some unpleasant symptoms of depression, though without actually producing positive affect.
Linking Pleasure and Happiness
Pleasure can thus be seen to drive life, as most animals know it by the rewards associated with fulfilling ancient evolutionary imperatives of survival and procreation. Humans of course are able to consciously experience these pleasures and, perhaps uniquely, even contemplate the elusive prospect of happiness.
The advanced human ability to consciously predict and anticipate the outcome of choices and actions confers our species with an evolutionary advantage, but human conscious planning is a double-edged sword as John Steinbeck pointed out as he wrote of “the tragic miracle of consciousness” and how our “species is not set, has not jelled, but is still in a state of becoming” (Steinbeck and Ricketts, 1941). While consciousness allows us to experience pleasures, desires, and perhaps even happiness, this is always accompanied by the certainty of the end; yet most people remain optimistic in the face of adversity.
Happiness is, however, a slippery concept (Gilbert, 2006; Bloom, 2010). One way to approach it is to follow the insight of Aristotle that happiness can usefully thought of as consisting of two fundamental aspects: hedonia (pleasure) and eudaimonia (a life well-lived). In contemporary psychology these aspects are usually referred to as pleasure and meaning, and positive psychologists have recently proposed to add a third meaning-related component of engagement involving feelings of commitment and participation in life (Seligman et al., 2005).
Using these definitions scientists have made substantial progress in defining and measuring happiness in the form of self-reports of subjective well-being, in identifying its distribution across people in the real world, and in identifying how well-being is influenced by various life factors ranging from income to other people (Kahneman, 1999). This research shows that while there is clearly a sharp conceptual distinction between pleasure versus engagement-meaning components, hedonic and eudaimonic aspects empirically cohere together in happy people (Diener et al., 2006; Kahneman, 1999; Seligman et al., 2005).
Surveys of happiness provide interesting indicators of mental well-being in societies, but offer little evidence of the underlying neurobiology of happiness. Supporting a hedonic approach to that question, it has been suggested that the best measure of subjective well-being may be simply to ask people how they hedonically feel right now -- again and again -- so as to track their hedonic accumulation across daily life (Kahneman, 1999). Such repeated self-reports of hedonic states could also be used to identify more stable neurobiological hedonic brain traits that dispose particular individuals toward happiness. Further, a hedonic approach might even offer a toehold into identifying eudaimonic brain signatures of happiness, due to the empirical convergence between the two categories, even if pleasant mood is only half the happiness story (Kringelbach and Berridge, 2009).
We have previously suggested that one possible toehold linking pleasure and happiness might be found in the close links between sensory pleasure networks and the brain’s default network (Kringelbach and Berridge, 2009) (Figure 3). We have proposed that the eudaimonic happiness may be linked to potential interactions of hedonic brain circuits with circuits that assess meaningful relationships of self to social others (Lou et al., 1999), internal modes of cognition (Buckner et al., 2008), and perhaps even states of consciousness (Laureys et al., 2004). The default network might deserve further consideration for a role in connecting eudaimonic and hedonic happiness. At least, key regions of the frontal default network overlap with the hedonic network, such as the anterior cingulate and orbitofrontal cortices, and have a relatively high density of opiate receptors. Similarly, activity changes in the frontal default network, such as in the subgenual cingulate and orbitofrontal cortices, correlate to pathological changes in subjective hedonic experience, such as in depressed patients (Drevets et al., 1997).
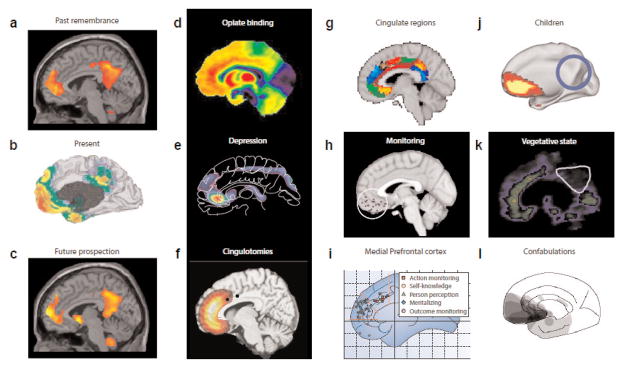
Figure 3.
A hypothesis of how pleasure and happiness are linked. Default networks are fundamental to human brain function and have been linked to self awareness, remembering the past and prospecting the future (a-c). It is clear that these networks are partly overlapping the pleasure networks. We have hypothesized that happiness might include a role for the default network, or for related neural circuits that contribute to computing relations between self and others, in evaluating eudaimonic meaning and interacting with hedonic circuits of positive affect. Some examples show (d) key regions of the default network such as the anterior cingulate and orbitofrontal cortices that have a high density of opiate receptors, (e) have been linked to depression, and (f) its surgical treatment. (g) Subregional localization of function may be indicated by connectivity analyses of cingulate cortex and related structures, (h) important in pleasure-related monitoring, learning, and memory, (i) as well as self-knowledge, person perception, and other cognitive functions. (j) The default network may change over early life in children and pre-term babies, (k) in pathological states including depression and vegetative states, (l) and after lesions to its medial orbitofrontal and subgenual cingulate cortices that disrupt reality monitoring and create spontaneous confabulations.
Pathological self-representations by the frontal default network could also contribute in unfortunate individuals to hedonic distortions of happiness that involve eudaimonic dissatisfaction, such as in cognitive rumination of depression. Conversely, mindfulness-based cognitive therapy for depression, which aims to disengage from dysphoria-activated depressogenic thinking might conceivably recruit default network circuitry to help mediate improvement in happiness via a linkage of eudaimonic to hedonic circuitry.
Conclusions
At first glance, pleasure electrodes once seemed to provide the prospect of happiness at the flick of a switch but careful scientific experimentation has shown that such electrodes are unlikely to truly cause pleasure, and are instead likely linked most closely to the psychological processes of ‘wanting’ -- with very little ‘liking’ involved (Berridge and Kringelbach, 2008).
Both pleasure and happiness are much more complex psychological states than the unitary words imply, with multiple sub-components within each; some of which are amenable to scientific investigation even now. In this article, we have shown the progress in building a science of pleasure and we have identified some of the mechanisms and regions important in the brain’s hedonic networks that generate basic pleasures. We have also speculated on potential interaction of hedonics with eudaimonic networks that may be important contributors to happiness. Yet, it is important to note that we have still not made substantial progress towards understanding the functional neuroanatomy of happiness.
While it remains unclear how pleasure and happiness are exactly linked, it may be safe to say at least that the pathological lack of pleasure, in anhedonia or dysphoria, amounts to a formidable obstacle to happiness. Exciting new insights have been gained while studying sensory pleasures, but many further challenges remain such as to understand how the brain networks underlying fundamental pleasure relate to higher pleasures such as music, dance, play, and flow to contribute to happiness.
Further, in social animals like humans, it is worth noting that cultural interactions with conspecifics are fundamental and central to enhancing the other pleasures. Humans are intensely social, and data indicate that one of the most important factors for happiness is relationships with other people. Social pleasures may still include vital sensory features such as visual faces, touch features of grooming and caress, as well as in humans more abstract and cognitive features of social reward and relationship evaluation. These may be especially important triggers for the brain’s hedonic networks in human beings.
In particular, adult pair bonds and attachment bonds between parents and infants are likely to be extremely important for the survival of the species (Kringelbach et al., 2008). The breakdown of these bonds is all too common and can lead to great unhappiness. And even bond formation can potentially disrupt happiness, such as in transient parental depression after birth of an infant - in over 10% of mothers and approximately 3% of fathers (Cooper and Murray, 1998). Progress in understanding the hedonics of social bonds could be useful in understanding happiness, and it will be important to map the developmental changes that occur over a lifespan. Fortunately, social neuroscience is beginning to unravel some of the complex dynamics of human social interactions and their relation to brain activations (Parsons et al., 2010).
Many future challenges remain before we will understand the functional neuroanatomy of happiness. We have previously proposed that hedonic happiness could be akin to ‘liking’ without ‘wanting;’ as a state of pleasure without disruptive desires -- a state of contentment (Kringelbach, 2009). Yet, alternatively happiness in daily life may rely on matching a proper balance ‘wanting’ and ‘liking’ to help facilitate engagement with the world. If the balance tips the wrong way, happiness becomes impossible. As an example too much ‘wanting’ can readily spiral into maladaptive patterns such as addiction, and is a certain recipe to great unhappiness. And of course, the eudaimonic components of meaning and engagement are crucial to happiness for human beings. Careful scientific experimentation will create a better scientific understanding of pleasure and happiness that may someday allow clinicians to make targeted interventions that will help to shift more among us into a better situation to enjoy daily events, to find life meaningful and worth living -- and perhaps even to achieve a degree of bliss.
Acknowledgments
Our research has been supported by grants from the TrygFonden Charitable Foundation to MLK and from the NIMH and NIDA to KCB.
Contributor Information
Morten L. Kringelbach, Department of Psychiatry, Warneford Hospital, University of Oxford, Oxford, United Kingdom and Centre for Functionally Integrative Neuroscience (CFIN), University of Aarhus, Aarhus, Denmark
Kent C. Berridge, Department of Psychology, University of Michigan, Ann Arbor, Michigan 48109, USA
References
- Aldridge JW, Berridge KC. Neural coding of pleasure: “rose-tinted glasses” of the ventral pallidum. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; New York, New York, USA: 2010. pp. 62–73. [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–7. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Baumeister AA. The Tulane Electrical Brain Stimulation Program a historical case study in medical ethics. J Hist Neurosci. 2000;9:262–78. doi: 10.1076/jhin.9.3.262.1787. [DOI] [PubMed] [Google Scholar]
- Berridge K. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology. 2008;199:457–80. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–13. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Bloom P. How Pleasure Works. W. W. Norton & Co; New York, New York, USA: 2010. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Murray L. Postnatal depression. BMJ. 1998;316:1884–6. doi: 10.1136/bmj.316.7148.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, Lucas RE, Scollon CN. Beyond the hedonic treadmill: revising the adaptation theory of well-being. Am Psychol. 2006;61:305–14. doi: 10.1037/0003-066X.61.4.305. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Frijda N. On the nature and function of pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; New York, New York, USA: 2010. pp. 99–112. [Google Scholar]
- Gilbert DT. Stumbling on Happiness. Knopf; New York, New York, USA: 2006. [Google Scholar]
- Green AL, Pereira EA, Aziz TZ. Deep brain stimulation and pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the brain. Oxford University Press; New York, New York, USA: 2010. pp. 302–319. [Google Scholar]
- Green AL, Wang S, Stein JF, Pereira EA, Kringelbach ML, Liu X, Brittain JS, Aziz TZ. Neural signatures in patients with neuropathic pain. Neurology. 2009;72:569–71. doi: 10.1212/01.wnl.0000342122.25498.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haisken-De New JP, Frick R. Desktop Companion to the German Socio-Economic Panel Study (GSOEP) German Institute for Economic Research (DIW); Berlin, Germany: 2005. [Google Scholar]
- Heath RG. Pleasure and brain activity in man. Deep and surface electroencephalograms during orgasm. J Nerv Ment Dis. 1972;154:3–18. doi: 10.1097/00005053-197201000-00002. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Objective happiness. In: Kahneman D, Diener E, Schwartz N, editors. Well-being: The Foundation of Hedonic Psychology. Russell Sage Foundation; New York, New York, USA: 1999. pp. 3–25. [Google Scholar]
- Kringelbach ML. Emotion. In: Gregory RL, editor. The Oxford Companion to the Mind, 2nd edition. Oxford University Press; Oxford, UK: 2004a. pp. 287–290. [Google Scholar]
- Kringelbach ML. Learning to change. PLoS Biol. 2004b;2:E140. doi: 10.1371/journal.pbio.0020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The Pleasure Center. Trust Your Animal Instincts. Oxford University Press; New York, New York, USA: 2009. [Google Scholar]
- Kringelbach ML. The hedonic brain: A functional neuroanatomy of human pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; Oxford, UK: 2010. pp. 202–221. [Google Scholar]
- Kringelbach ML, Berridge KC. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci. 2009;13:479–87. doi: 10.1016/j.tics.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Jenkinson N, Green AL, Owen SLF, Hansen PC, Cornelissen PL, Holliday IE, Stein J, Aziz TZ. Deep brain stimulation for chronic pain investigated with magnetoencephalography. Neuroreport. 2007;18:223–228. doi: 10.1097/WNR.0b013e328010dc3d. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Lehtonen A, Squire S, Harvey AG, Craske MG, Holliday IE, Green AL, Aziz TZ, Hansen PC, Cornelissen PL, Stein A. A specific and rapid neural signature for parental instinct. PLoS ONE. 2008;3:e1664. doi: 10.1371/journal.pone.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–46. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- Lou HC, Kjaer TW, Friberg L, Wildschiodtz G, Holm S, Nowak M. A 15O-H2O PET study of meditation and the resting state of normal consciousness. Hum Brain Mapp. 1999;7:98–105. doi: 10.1002/(SICI)1097-0193(1999)7:2<98::AID-HBM3>3.0.CO;2-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauta WJ. The problem of the frontal lobe: a reinterpretation. J Psychiatr Res. 1971;8:167–87. doi: 10.1016/0022-3956(71)90017-3. [DOI] [PubMed] [Google Scholar]
- Nesse RM. Natural selection and the elusiveness of happiness. Philos Trans R Soc Lond B Biol Sci. 2004;359:1333–47. doi: 10.1098/rstb.2004.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- Olds J. Pleasure centers in the brain. Sci Am. 1956;195:105–16. [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of the septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Murray L, Stein A, Kringelbach ML. The functional neuroanatomy of the evolving parent-infant relationship. Prog Neurobiol. 2010 doi: 10.1016/j.pneurobio.2010.03.001. in press. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Seligman ME, Steen TA, Park N, Peterson C. Positive psychology progress: empirical validation of interventions. Am Psychol. 2005;60:410–21. doi: 10.1037/0003-066X.60.5.410. [DOI] [PubMed] [Google Scholar]
- Smith KS, Mahler SV, Pecina S, Berridge KC. Hedonic hotspots: generating sensory pleasure in the brain. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. Oxford University Press; New York, New York, USA: 2010. pp. 27–49. [Google Scholar]
- Steinbeck J, Ricketts EF. The Log from the Sea of Cortez. Penguin; London, UK: 1941. [Google Scholar]
- Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]