Abstract
Wild-type Saccharomyces cerevisiae tubulin does not bind the anti-mitotic microtubule stabilizing agent paclitaxel. Previously, we introduced mutations into the S. cerevisiae gene for β-tubulin that imparted paclitaxel binding to the protein, but the mutant strain was not sensitive to paclitaxel and other microtubule-stabilizing agents, due to the multiple ABC transporters in the membranes of budding yeast. Here, we introduced the mutated β-tubulin gene into a S. cerevisiae strain with diminished transporter activity and developed the first paclitaxel-sensitive budding yeast strain. In the presence of paclitaxel, cytoplasmic microtubules were stable to cold depolymerization. Paclitaxel-treated cells showed evidence of a mitotic block, with an increase in large-budded cells and cells with a 2N DNA content and DNA fragmentation, identified by FACS analysis and the TUNEL assay. In the presence of paclitaxel, the number of dead cells in cultures increased three-fold and cells containing reactive oxygen species were present. We conclude that paclitaxel blocks mitosis in this strain, leading to an apoptotic-like cell death. This strain will also be useful in further studies of the effect of microtubule dynamics on various cellular processes in S. cerevisiae.
Keywords: paclitaxel, tubulin, microtubules, yeast, apoptosis
Introduction
Paclitaxel (Taxol™) is an anti-tumour agent that has proved to be effective against a number of cancers. Its primary mechanism of action in cells is to cause a mitotic block by stabilizing microtubules, thereby decreasing the dynamic nature of these cytoskeletal structures (Jordan, 2002). Prolonged exposure of mammalian cells to paclitaxel leads to apoptotic cell death (Woods et al., 1995). Microtubules are composed of the protein tubulin, a heterodimer consisting of α- and β-subunits. Paclitaxel binds to the β-subunit of tubulin in a 1 : 1 stoichiometry (Parness and Horwitz, 1981). It is essential that the binding interactions between the drug and tubulin be defined in detail so that rational drug design can be used to create more efficient paclitaxel derivatives. Although a high-resolution X-ray crystallographic structure of the tubulin–paclitaxel complex is lacking, information from a 3.7 Å resolution electron microscopic crystal structure (Nogales et al., 1998), photoaffinity labelling experiments (Combeau et al., 1994; Dasgupta et al., 1994; Rao et al., 1994, 1995, 1999), studies using NMR, fluorescence and molecular modelling (He et al., 2000; Li et al., 2000; Snyder et al., 2001) and paclitaxel-resistant cell lines (Giannakakou et al., 1997, 2000; Gonzalez-Garay et al., 1999) have been used to identify the significance of specific amino acid residues in paclitaxel binding.
Growth of Saccharomyces cerevisiae (budding yeast) is not inhibited by paclitaxel and purified yeast tubulin does not interact with paclitaxel (Kilmartin, 1981; Barnes et al., 1992; Bode et al., 2002). In an investigation into the lack of paclitaxel binding to yeast tubulin, we identified five residues implicated in paclitaxel binding to mammalian tubulin that differ between yeast and mammalian tubulin (Gupta et al., 2003). We provided experimental evidence for the importance of these five residues by mutating the residues in S. cerevisiae tubulin to those that occur in brain tubulin and showing that the mutated tubulin was capable of binding paclitaxel in vitro (Gupta et al., 2003). Because creating mutations in yeast is straightforward, we can now use our mutated yeast tubulin to probe more deeply into the interactions between tubulin and paclitaxel, e.g. we can determine the relative importance of the five amino acids already mutated and the role of other amino acids in the binding interactions.
Structure–activity relationship studies incorporating large numbers of paclitaxel analogues in conjunction with modified paclitaxel binding sites would be greatly aided by a simple, cell-based assay as a primary screen to assess tubulin-paclitaxel interactions. Unfortunately, proliferation of budding yeast containing the mutated tubulin is not inhibited by paclitaxel, most likely because yeast contain multiple ABC transporters that prevent a number of xenobiotics from accumulating in the cell (Decottignies and Goffeau, 1997). As described in this article, we have solved this problem by placing the mutated β-tubulin gene into a strain with diminished ABC-mediated transport (Decottignies et al., 1998), producing a strain that arrests in mitosis and undergoes apoptotic-like cell death in the presence of paclitaxel. This strain will be a valuable tool for the development of cell-based assays to assess tubulin–paclitaxel interactions and studies of microtubule dynamics on a variety of yeast cellular functions.
Materials and methods
Yeast strains and media
Strain MGY1-tax (genotype: MATa, leu2Δ1, trpΔ63, his4-917, URA3/ura3-52, tub2-His6-A19K-T23V-G26D-N227H-Y270F) contains five mutations in the β-tubulin gene that impart paclitaxel binding activity by tubulin and was derived from strain MGY1 (genotype: MATa, leu2Δ1, trpΔ63, his4-917, URA3/ura3-52, tub2-His6; Gupta et al., 2003). Strain AD 12 345 678 (AD1-8), in which the genes for seven ABC transporters and a transporter transcription factor have been inactivated, was obtained from André Goffeau, Université Cathlique de Louvain, Louvain-la-Neuve, Belgium, and has the genotype MATα, PDR1-3, ura3, his1, Δyor1∷hisG, Δsnq2∷hisG, Δpdr5∷hisG, Δpdr10∷hisG, Δpdr11∷hisG, Δycf1∷hisG, Δpdr3∷hisG, Δpdr15∷hisG (Decottignies et al, 1998). Strain AD1-8 was transformed with a DNA fragment containing the tub2-His6-A19K-T23V-G26D-N227H-Y227F gene to produce strain AD1-8-tax using procedures described previously (Gupta et al., 2001). The yeast strains were grown in YPD medium (1% yeast extract, 2% peptone, 2% glucose) at 30 °C. The transformant AD1-8-tax was selected in SD medium (0.67% yeast nitrogen base without amino acids, 2% glucose and supplemented with histidine).
Proliferation assays
The cell proliferation assays were performed on agar plates and in liquid medium. Yeast strains MGY1, MGY1-tax, AD1-8 and AD1-8-tax were incubated overnight in YPD medium at 30 °C to stationary phase. A sample of each culture was serially diluted in 10-fold steps up to a 106 dilution. Each dilution was spotted on YPD-agar plates containing 0, 15 and 25 µm paclitaxel using a Frogger (Dan-Kar Corp., Reading, MA) and the plates were incubated at 30 °C for 3 days.
The proliferation assays in liquid medium to determine IC50 values were performed in a final volume of 120 µl in sterile 96-well plates. Each well, containing 100 µl YPD supplemented with 100 U penicillin, 100 µg streptomycin, and paclitaxel in the range 0–25 µm, was inoculated with 2000 cells in a 20 µl volume from an overnight culture. The plates were placed in a humidified chamber on a shaker and incubated at 30 °C for 48 h. The plates were then agitated on a vortex plate shaker to ensure that yeast cells were completely suspended in solution and the optical density was read at 610 nm in a 96-well plate reader (Bio-tek Instruments, Inc.). Experiments were done in triplicate.
FACS analysis
Asynchronous log-phase cultures of MGY-1-tax, AD1-8 and AD1-8-tax were incubated either in the absence of paclitaxel or in the presence of 25 µm paclitaxel. Each culture was fixed and stained with propidium iodide using a procedure similar to one described by Hutter and Eipel (1978). Flow cytometry was performed on a Becton Dickinson FACScan and analysed using Cell Quest software. For each experiment at least 10 000 cells were analysed.
Bud morphology
To determine bud morphology distributions, log-phase cells were grown for 6 h with and without 25 µm paclitaxel. The cultures were sonicated briefly and 10 µl of each culture was mounted on a microscope slide. At least 200 cells in each culture were classified as non-budded, small-budded or large-budded (>50% of mother cell diameter).
Microscopy
To visualize microtubules, log-phase cultures were incubated at 30 °C in the presence of 25 µm paclitaxel for 6 h. A portion of the cells from the cultures was fixed using the method of Pringle and Hartwell (1981). The remaining cells were incubated at 4 °C for 20 h before fixation. The primary antibody used was the anti-α-tubulin antibody YOL1/34 and the secondary antibody was a fluorescein-conjugated goat anti-rat antibody; both were purchased from Accurate Chemical and Scientific Corp., Westbury, NY, and were used at a 1 : 250 dilution. The cells were visualized using a 63×/1.4NA planapochromat objective lens on a Ziess Axioplan 2ie microscope equipped for epifluorescence and transmitted light DIC microscopy. A Hamamatsu Orca-ER CCD camera linked to Open-lab software was used to capture images.
Programmed cell death assays
Log-phase cultures of AD1-8 and AD1-8-tax were analysed for reactive oxygen species (ROS) production, using the fluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) (Molecular Probes, Eugene, OR) and for dead cells using phloxine B staining, as described by Severin and Hyman (2002). A modified form of the TUNEL assay, described by Roche Applied Sciences, Indianapolis, IN, was used to determine DNA degradation. In this modified assay the secondary anti-fluorescein antibody was not used and fluorescence microscopy was used to detect the fluorescein-labelled DNA directly (Pozniakovsky et al., 2005). For the TUNEL assay the cells were treated as described by Kilmartin and Adams (1984) before the addition of the TUNEL reagents.
Results
Inhibition of cell proliferation by paclitaxel
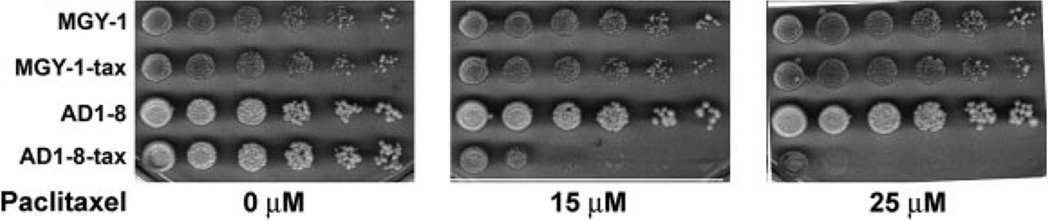
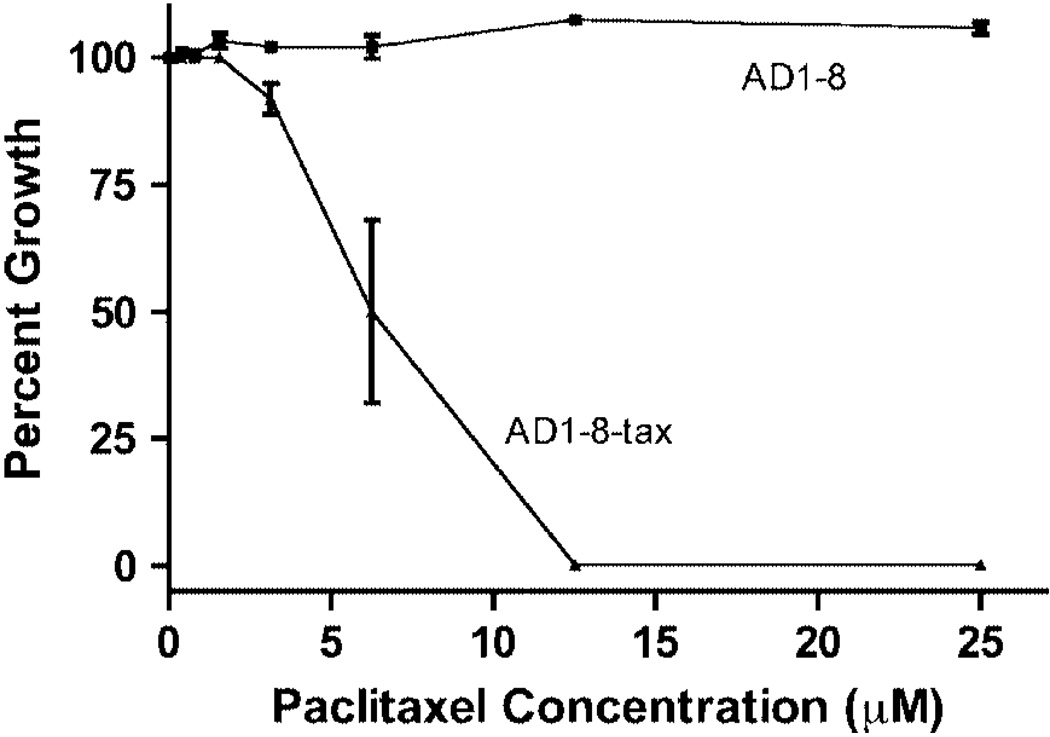
Because MGY1-tax, the strain containing the mutated β-tubulin gene, is not sensitive to paclitaxel, we introduced the mutated gene into strain AD1-8 (Decottignies et al., 1998), in which the genes for seven ABC transporters and a transporter transcription factor have been inactivated. We tested the paclitaxel sensitivity of the new strain, AD1-8-tax, using two different assays. In one assay, cells at increasing dilutions were spotted onto agar plates with and without the drug (Figure 1). The proliferation of MGY1, MGY1-tax and AD1-8 is not affected by up to 25 µm paclitaxel, but paclitaxel clearly inhibited proliferation of the AD1-8-tax strain. When the cells were assayed in liquid medium, the IC50 value was found to be 6 µm (Figure 2).
Figure 1.
Effect of paclitaxel on the growth of four yeast strains on agar plates. For experimental details, see Materials and methods
Figure 2.
Sensitivity of strains AD1-8 and AD1-8-tax to paclitaxel. Both strains were grown in liquid medium in the presence of increasing concentrations of paclitaxel, as described in Materials and methods. Percentage growth is shown relative to a culture without paclitaxel
Paclitaxel-induced microtubule stability
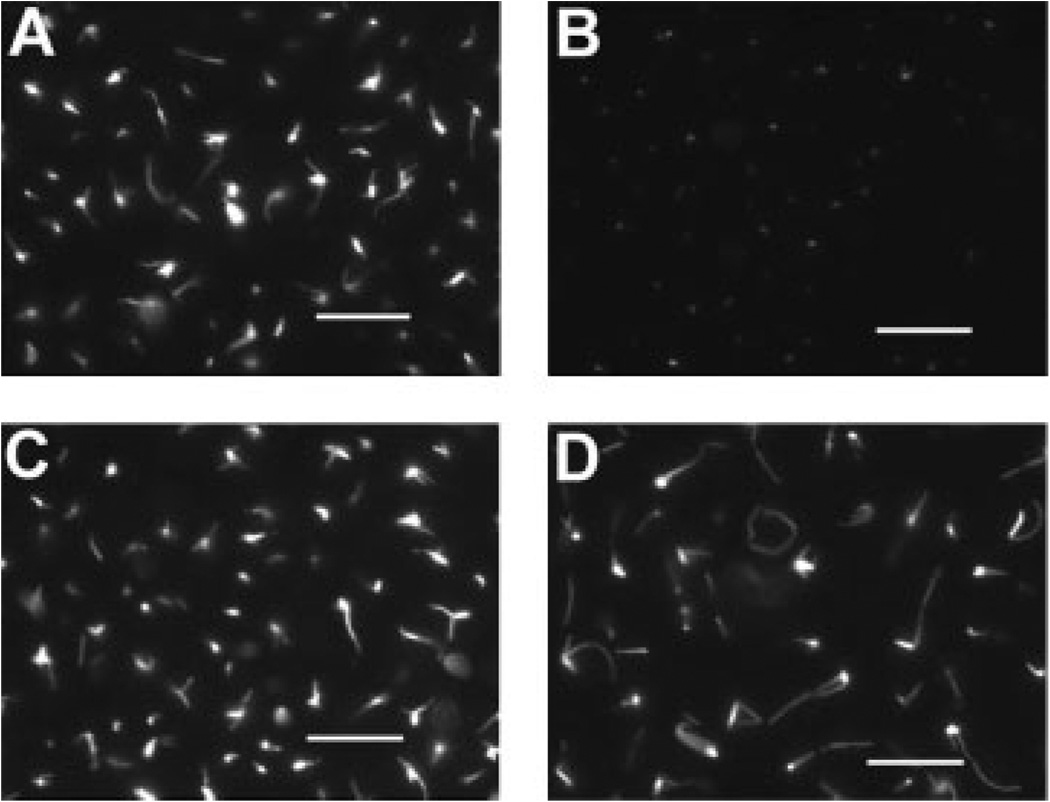
Cell proliferation of strain AD1-8, with diminished ABC transporter activity, was not inhibited by paclitaxel but, after mutating the gene for β-tubulin in this strain to create paclitaxel binding, the drug inhibited cell proliferation. Therefore, proliferation inhibition is most likely a result of paclitaxel-induced microtubule stabilization. To test this hypothesis, cells were incubated with 25 µm paclitaxel for 20 h at 4 °C and examined for microtubules using immunofluorescence microscopy (Figure 3). The 4 °C treatment caused the disappearance of microtubules in the AD1-8 strain, but in the AD1-8-tax strain, microtubules were still present. The mean length of the cytoplasmic microtubules after 20 h at 4 °C was twice that for microtubules in cells not incubated in the cold (n = 220).
Figure 3.
Stability of microtubules in the presence of paclitaxel. Cultures of AD1-8 and AD1-8-tax were incubated with 25 µm paclitaxel at 30 °C for 6 h. Samples were taken for fixation and immunostaining. The remainder of the cultures were placed at 4 °C for 20 h before fixation and immunostaining. A and B, AD1-8 at 30 °C and 4°C, respectively; C and D, AD1-8-tax at 30 °C and 4°C, respectively. Bars = 10 µm
Paclitaxel-induced mitotic block
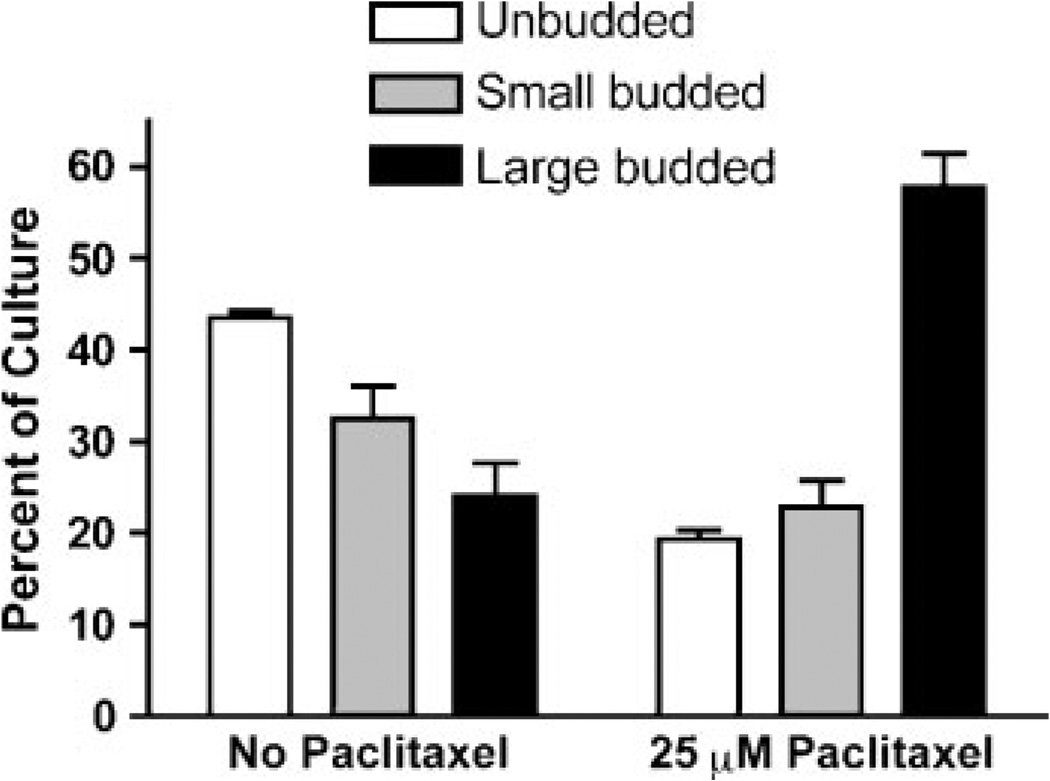
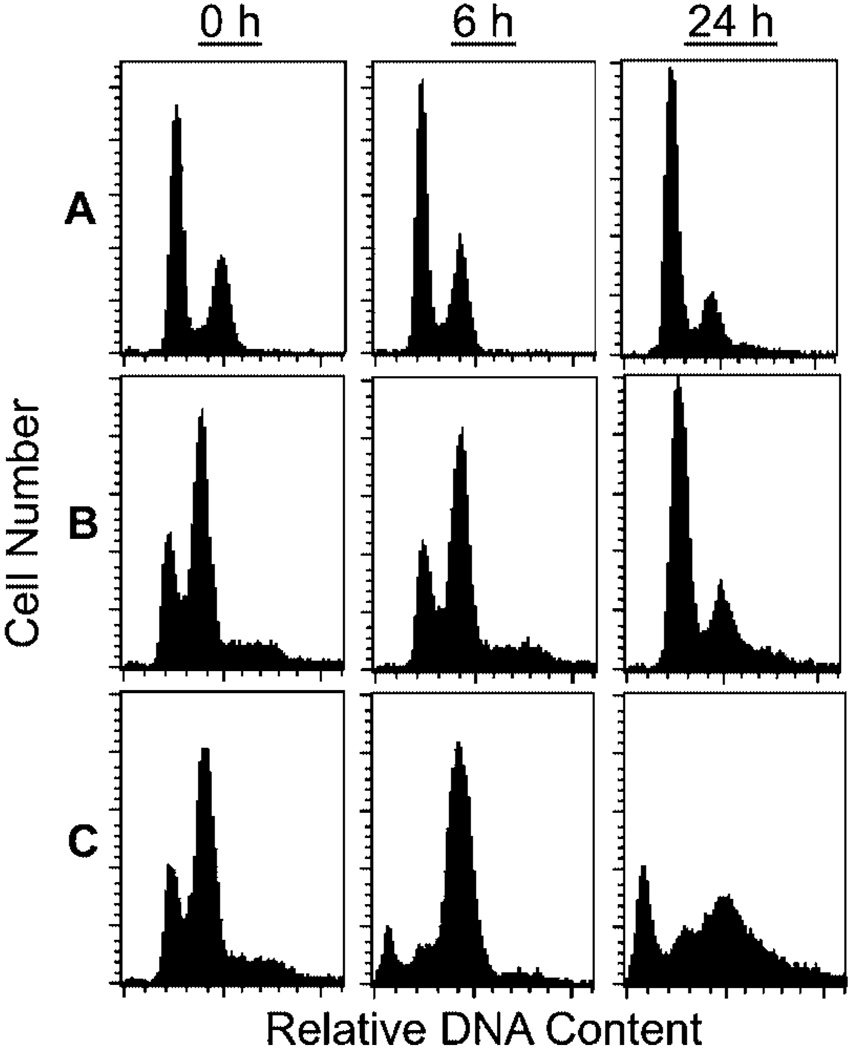
As an anti-mitotic agent, paclitaxel would be expected to inhibit cell proliferation at the G2/M phase of the cell cycle, leading to the build-up of cells having a 2N DNA content (the AD1-8-tax strain is haploid) and an accumulation of large-budded cells. S. cerevisiae cells are classified by bud morphology as unbudded (G1 phase), small-budded (S phase) and large-budded (G2/M phase). As shown in Figure 4, after 6 h of growth in the presence of 25 µm paclitaxel, the proportion of large-budded cells increased from 24% (without paclitaxel) to 58% (with paclitaxel) of the population. Unbudded cells decreased from 44% to 19% in the presence of paclitaxel. FACS analysis of the DNA content after treatment with paclitaxel for 6 h showed that, in the AD1-8-tax culture, the percentage of 2N DNA cells increased at the expense of 1N cells (Figure 5). Paclitaxel had no effect on the distribution of 1N and 2N cells in the AD1-8 and MGY1-tax cultures. Paclitaxel also caused the appearance of a sub-1N peak in the FACS profile of the AD1-8-tax cultures, indicative of DNA fragmentation in some cells. After 24 h in the presence of paclitaxel, the cultures were approaching stationary phase and 1N cells were the dominant species in the MGY1-tax and AD1-8 cultures. In the AD 1-8-tax culture 2N, and sub-1N DNA content predominated. The presence of fragmented DNA suggests that cells are undergoing an apoptotic-like death.
Figure 4.
Effect of paclitaxel on bud morphology in strain AD1-8-tax. Cultures were incubated in the absence and presence of 25 µm paclitaxel for 6 h before being examined for bud morphology. The results are from three separate experiments, with n = 220 in each case
Figure 5.
FACS analysis of DNA content. Log phase cells were incubated with 25 µm paclitaxel for 6 h and 24 h before being prepared for FACS analysis, as described in Materials and methods. A, strain MGY1; B, strain AD1-8; C, strain AD1-8-tax
Paclitaxel-induced apoptosis
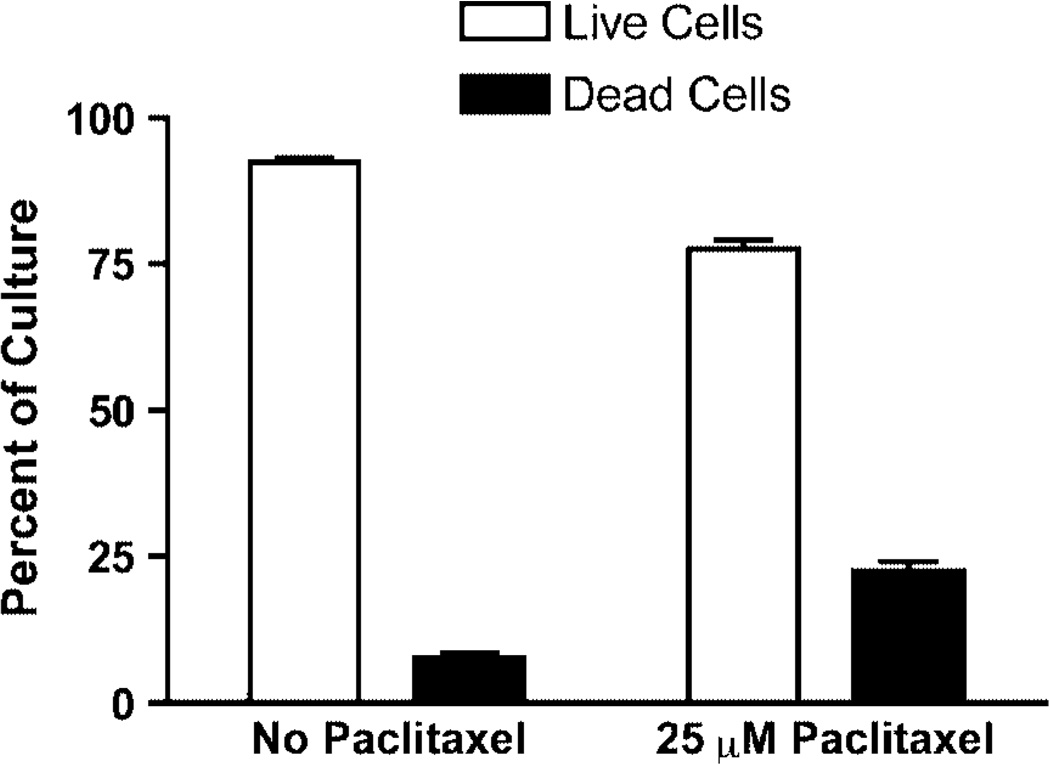
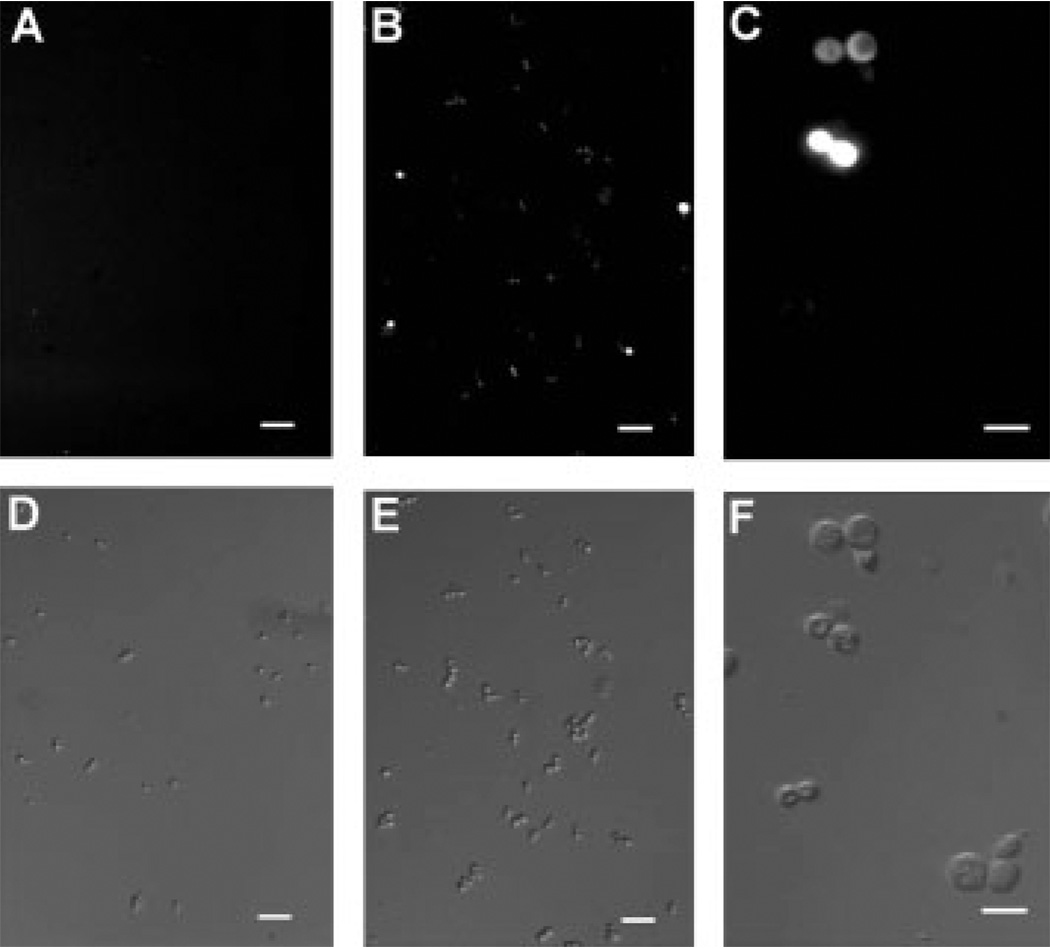
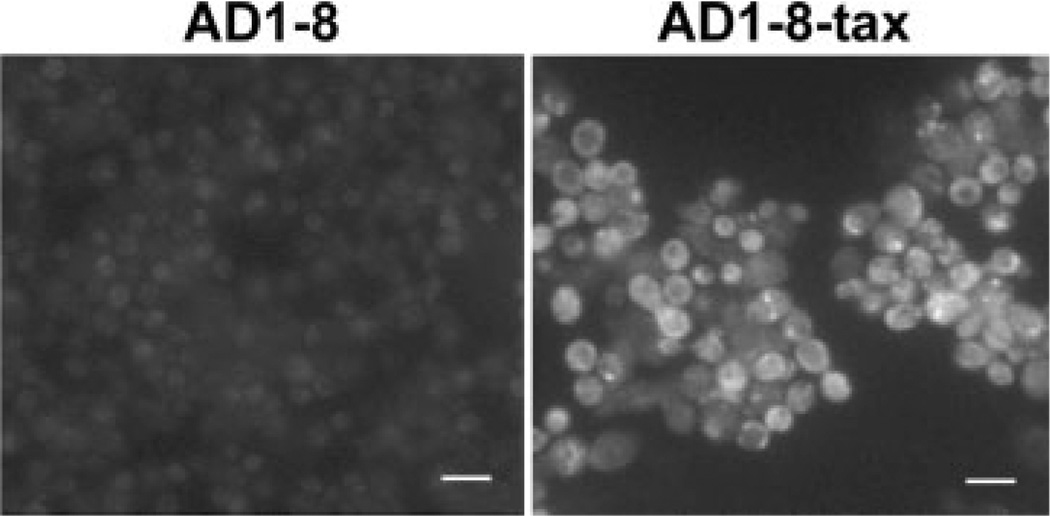
The appearance of cells with a DNA content less than 1N in the FACS analysis suggests that paclitaxel induced apoptosis. We therefore examined the cultures for indicators of programmed cell death using phloxine B staining for cell death, the TUNEL assay for DNA fragmentation, and staining for the presence of reactive oxygen species (ROS). Phloxine B is accumulated by dead but not by live cells. The results in Figure 6 show that, after 6 h in the presence of 25 µm paclitaxel, the percentage of AD1-8-tax cells stained by phloxine B increased by three-fold. The presence of ROS, an early event in apoptosis, in AD1-8-tax cells incubated with paclitaxel, was indicated with the use of H2DF-DA (Figure 7). AD1-8 cells treated the same way did not show the presence of ROS (data not shown). The TUNEL assay involves end-labelling of fragmented DNA with fluorescein-labelled nucleotides. This assay detected fragmentation of DNA in AD1-8-tax cells treated with paclitaxel, but not in AD1-8 cells (Figure 8).
Figure 6.
Cell death produced by paclitaxel. Strain AD1-8-tax was incubated at 30 °C for 6 h in the absence and presence of 25 µm paclitaxel before being stained by phloxine B. The results are from three separate experiments, with n = 200 in each case
Figure 7.
ROS production after incubation with paclitaxel. Cultures of AD1-8-tax cells were incubated for 6 h at 30 °C in the absence (A, D) and presence (B, C, E, F) of 25 µm paclitaxel. DIC microscopy was used in (D–F.). ROS was detected with the compound H2DF-DA using fluorescence microscopy (A–C). In (A, B, D, E) a 10× objective was used and bars = 30 µm; in (C, F) a 40× objective was used and bars = 10 µm
Figure 8.
Fragmented DNA determined by the TUNEL assay. Strains AD1-8 and AD1-8-taxwere incubated at 30 °C for 24 h in the presence of 25 µm paclitaxel. AD1-8-tax cells, but not AD1-8 cells, display fluorescence due to DNA labelled with fluorescein. Bars = 10 µm
Discussion
Recently, we were able to convert the non-paclitaxel binding tubulin in S. cerevisiae to a form that binds the drug efficiently by introducing five mutations into β-tubulin (Gupta et al., 2003). To facilitate further studies of the effects of β-tubulin mutations on the binding efficiency of paclitaxel and its analogues, we desired a cell-based assay for the purpose of initial screening. Unfortunately, yeast cells that contain the paclitaxel-binding tubulin (MGY1-tax) are not sensitive to paclitaxel, presumably because of the multiple ABC transporters in yeast (Decottignies and Goffeau, 1997). Therefore, we introduced the mutated β-tubulin gene into a strain (AD1-8) that lacks the activities of seven of these transporters and one transporter transcription factor. Introduction of the mutated β-tubulin produced a strain (AD1-8-tax) that is sensitive to paclitaxel, albeit at relatively high concentrations compared to mammalian cells. The IC50 value for paclitaxel in mammalian cells is in the low nm range, about 0.1% of the value we found for S. cerevisiae strain AD1-8-tax. However, the 6 µm IC50 value is lower than we find for benomyl (~50 µm), a common anti-mitotic agent that inhibits yeast proliferation. Possibly, one or more of the ABC transporters remaining in the AD1-8-tax strain is capable of effluxing paclitaxel to a certain extent. It is also possible that the rather high IC50 value is due to the low sensitivity of S. cerevisiae proliferation to decreased microtubule dynamics. For example, we found that a β-tubulin C354S mutant proliferated at a rate equivalent to that of the wild-type strain, even though microtubule dynamics in the mutant were decreased by 73% to 98%, depending on the stage in the cell cycle (Gupta et al., 2002). Thus, to affect cell proliferation, the paclitaxel concentration in the cell might have to be increased to such a level as to completely shut down microtubule dynamics.
Apoptosis, a natural process of programmed cell death that is required for the proper development of multicellular organisms, is induced by a number of stress-producing treatments, including anti-mitotic agents. Apoptosis has been studied in mammalian cells for several decades, but only recently has it been shown that apoptosis occurs in budding yeast (Madeo et al., 1997, 2002). Programmed cell death in budding yeast is triggered by a diverse group of treatments or insults that include DNA damage (Burhans et al., 2003), oxygen stress (Madeo et al., 1999), NaCl (Wadskog et al., 2004), cell ageing (Laun et al., 2001; Fabrizio et al., 2004; Herker et al., 2004), acetic acid (Ludovico et al., 2002), pheromones (Severin and Hyman, 2002; Pozniakovsky et al., 2005) and viral killer toxins (Reiter et al., 2005). To this list we can now add microtubule-stabilizing agents. It has been proposed that apoptosis in yeast can serve as a model system for the process in higher eukaryotes, especially because of the ease of genetic manipulations in yeast (Madeo et al., 2002). Yeast can now be used as a model system for studying apoptosis induced by antimicrotubule agents. The strain AD1-8-tax can also serve another useful purpose. Until now, drugs capable of stabilizing yeast microtubules in vivo have been lacking, and all effective microtubule poisons function as depolymerizing agents (e.g. nocodazole, benomyl). With strain AD1-8-tax and the use of paclitaxel, it is now possible to study the effect of dampening microtubule dynamics on a variety of biological processes in S. cerevisiae.
Acknowledgements
This work was supported by NIH grant R01 CA105305 to R.H.H. We thank Dr. André Goffeau for supplying us with a culture of strain AD1-8.
References
- Barnes G, Louie KA, Botstein D. Yeast proteins associated with microtubules in vitro and in vivo. Mol Biol Cell. 1992;3:29–47. doi: 10.1091/mbc.3.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode CJ, Gupta ML, Jr, Reiff EA, et al. Epothilone and paclitaxel: unexpected differences in promoting the assembly and stabilization of yeast microtubules. Biochemistry. 2002;41:3870–3874. doi: 10.1021/bi0121611. [DOI] [PubMed] [Google Scholar]
- Burhans WC, Weinberger M, Marchetti MA, et al. Apoptosis-like yeast cell death in response to DNA damage and replication defects. Mutat Res. 2003;532:227–243. doi: 10.1016/j.mrfmmm.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Combeau C, Commerçon A, Mioskowski C, et al. Predominant labelling of β- over α-tubulin from porcine brain by a photoactivatable taxoid derivative. Biochemistry. 1994;33:6676–6683. doi: 10.1021/bi00187a038. [DOI] [PubMed] [Google Scholar]
- Dasgupta D, Park H, Harriman GC, Georg GI, Himes RH. Synthesis of a photoaffinity Taxol analogue and its use in labelling tubulin. J Med Chem. 1994;37:2976–2980. doi: 10.1021/jm00044a019. [DOI] [PubMed] [Google Scholar]
- Decottignies A, Goffeau A. Complete inventory of the yeast ABC proteins. Nat Genet. 1997;15:137–145. doi: 10.1038/ng0297-137. [DOI] [PubMed] [Google Scholar]
- Decottignies A, Grant AM, Nichols JW, et al. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem. 1998;273:12612–12622. doi: 10.1074/jbc.273.20.12612. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Battistella L, Vardavas R, et al. Superoxide is a mediator of an altruistic ageing program in Saccharomyces cerevisiae. J Cell Biol. 2004;166:1055–1067. doi: 10.1083/jcb.200404002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakakou P, Sackett DL, Kang YK, et al. Paclitaxel-resistant human ovarian cancer cells have mutant β-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem. 1997;272:17118–17125. doi: 10.1074/jbc.272.27.17118. [DOI] [PubMed] [Google Scholar]
- Giannakakou P, Gussio R, Nogales E, et al. A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc Natl Acad Sci USA. 2000;97:2904–2909. doi: 10.1073/pnas.040546297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Garay ML, Chang L, Blade K, Menick DR, Cabral F. A β-tubulin leucine cluster involved in microtubule assembly and paclitaxel resistance. J Biol Chem. 1999;274:23875–23882. doi: 10.1074/jbc.274.34.23875. [DOI] [PubMed] [Google Scholar]
- Gupta ML, Jr, Bode CJ, Dougherty CA, Marquez RT, Himes RH. Mutagenesis of β-tubulin cysteine residues in Saccharomyces cerevisiae: mutation of cysteine 354 results in cold-stable microtubules. Cell Motil Cytoskeleton. 2001;49:67–77. doi: 10.1002/cm.1021. [DOI] [PubMed] [Google Scholar]
- Gupta ML, Jr, Bode CJ, Thrower DA, et al. β-Tubulin C354 mutations that severely decrease microtubule dynamics do not prevent nuclear migration in yeast. Mol Biol Cell. 2002;13:2919–2932. doi: 10.1091/mbc.E02-01-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta ML, Jr, Bode CJ, Georg GI, Himes RH. Understanding tubulin–axol interactions: mutations that impart Taxol binding to yeast tubulin. Proc Natl Acad Sci USA. 2003;100:6394–6397. doi: 10.1073/pnas.1131967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Jagtap PG, Kingston DGI, et al. A common pharmacophore for Taxol and the epothilones based on the biological activity of a taxane molecule lacking a C-13 side chain. Biochemistry. 2000;39:3972–3978. doi: 10.1021/bi992518p. [DOI] [PubMed] [Google Scholar]
- Herker E, Jungwirth H, Lehmann KA, et al. Chronological ageing leads to apoptosis in yeast. J Cell Biol. 2004;164:501–507. doi: 10.1083/jcb.200310014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter KJ, Eipel HE. Flow cytometric determinations of cellular substances in algae, bacteria, moulds and yeasts. Antonie Van Leeuwenhoek. 1978;44:269–282. doi: 10.1007/BF00394305. [DOI] [PubMed] [Google Scholar]
- Jordan MA. Mechanism of action of antitumour drugs that interact with microtubules and tubulin. Curr Med Chem Anti-Cancer Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- Kilmartin JV. Purification of yeast tubulin by self-assembly in vitro. Biochemistry. 1981;20:3629–3633. doi: 10.1021/bi00515a050. [DOI] [PubMed] [Google Scholar]
- Kilmartin JV, Adams AE. Structural rearrangements of tubulin and actin during the cell cycle of the yeast Saccharomyces. J Cell Biol. 1984;98:922–933. doi: 10.1083/jcb.98.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laun P, Pichova A, Madeo F, et al. Aged mother cells of Saccharomyces cerevisiae show markers of oxidative stress and apoptosis. Mol Microbiol. 2001;39:1166–1173. [PubMed] [Google Scholar]
- Li Y, Poliks B, Cegelski L, et al. Conformation of microtubule-bound paclitaxel determined by fluorescence spectroscopy and REDOR NMR. Biochemistry. 2000;39:281–291. doi: 10.1021/bi991936r. [DOI] [PubMed] [Google Scholar]
- Ludovico P, Rodrigues F, Almeida A, et al. Cytochrome c release and mitochondria involvement in programmed cell death induced by acetic acid in Saccharomyces cerevisiae. Mol Biol Cell. 2002;13:2598–2606. doi: 10.1091/mbc.E01-12-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Frohlich E, Frohlich KU. A yeast mutant showing diagnostic markers of early and late apoptosis. J Cell Biol. 1997;139:729–734. doi: 10.1083/jcb.139.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Frohlich E, Ligr M, et al. Oxygen stress: a regulator of apoptosis in yeast. J Cell Biol. 1999;145:757–767. doi: 10.1083/jcb.145.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeo F, Engelhardt S, Herker E, et al. Apoptosis in yeast: a new model system with applications in cell biology and medicine. Curr Genet. 2002;41:208–216. doi: 10.1007/s00294-002-0310-2. [DOI] [PubMed] [Google Scholar]
- Nogales E, Wolf SG, Downing KH. Structure of the αβ-tubulin dimer by electron crystallography. Nature. 1998;391:199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- Parness J, Horwitz SB. Taxol binds to polymerized tubulin in vitro. J Cell Biol. 1981;91:479–487. doi: 10.1083/jcb.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozniakovsky AI, Knorre DA, Markova OV, et al. Role of mitochondria in the pheromone- and amiodarone-induced programmed death of yeast. J Cell Biol. 2005;168:257–269. doi: 10.1083/jcb.200408145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JR, Hartwell LH. The Saccharomyces cerevisiae cell cycle. In: Strathern JN, Jones EW, Broach JR, editors. The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1981. pp. 97–142. [Google Scholar]
- Rao S, Krauss NE, Heerding JM, et al. 3′-(p-Azidobenzamido)Taxol photolabels the N-terminal 31 amino acids of β-tubulin. J Biol Chem. 1994;269:3132–3134. [PubMed] [Google Scholar]
- Rao S, Orr GA, Chaudhary AG, Kingston DGI, Horwitz SB. Characterization of the Taxol binding site on the microtubule. 2-(m-Azidobenzoyl)Taxol photolabels a peptide (amino acids 217–231) of β-tubulin. J Biol Chem. 1995;270:20235–20238. doi: 10.1074/jbc.270.35.20235. [DOI] [PubMed] [Google Scholar]
- Rao S, He L, Chakravarty S, et al. Characterization of the Taxol binding site on the microtubule. Identification of Arg(282) in β-tubulin as the site of photoincorporation of a 7-benzophenone analogue of Taxol. J Biol Chem. 1999;274:37990–37994. doi: 10.1074/jbc.274.53.37990. [DOI] [PubMed] [Google Scholar]
- Reiter J, Herker E, Madeo F, Schmitt MJ. Viral killer toxins induce caspase-mediated apoptosis in yeast. J Cell Biol. 2005;168:353–358. doi: 10.1083/jcb.200408071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severin FF, Hyman AA. Pheromone induces programmed cell death in S. cerevisiae. Curr Biol. 2002;12:R233–R235. doi: 10.1016/s0960-9822(02)00776-5. [DOI] [PubMed] [Google Scholar]
- Snyder JP, Nettles JH, Cornett B, Downing KH, Nogales E. The binding conformation of Taxol in β-tubulin: a model based on electron crystallographic density. Proc Natl Acad Sci USA. 2001;98:5312–5316. doi: 10.1073/pnas.051309398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadskog I, Maldener C, Proksch A, Madeo F, Adler L. Yeast lacking the SRO7/SOP1-encoded tumour suppressor homologue show increased susceptibility to apoptosis-like cell death on exposure to NaCl stress. Mol Biol Cell. 2004;15:1436–1444. doi: 10.1091/mbc.E03-02-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods CM, Zhu J, McQueney PA, Bollag D, Lazarides E. Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol Med. 1995;1:506–526. [PMC free article] [PubMed] [Google Scholar]