Abstract
The control of force production in vascular smooth muscle is critical to the normal regulation of blood flow and pressure, and altered regulation is common to diseases such as hypertension, heart failure, and ischemia. A great deal has been learned about imbalances in vasoconstrictor and vasodilator signals, e.g., angiotensin, endothelin, norepinephrine, and nitric oxide, that regulate vascular tone in normal and disease contexts. In contrast there has been limited study of how the phenotypic state of the vascular smooth muscle cell may influence the contractile response to these signaling pathways dependent upon the developmental, tissue-specific (vascular bed) or disease context. Smooth, skeletal, and cardiac muscle lineages are traditionally classified into fast or slow sublineages based on rates of contraction and relaxation, recognizing that this simple dichotomy vastly underrepresents muscle phenotypic diversity. A great deal has been learned about developmental specification of the striated muscle sublineages and their phenotypic interconversions in the mature animal under the control of mechanical load, neural input, and hormones. In contrast there has been relatively limited study of smooth muscle contractile phenotypic diversity. This is surprising given the number of diseases in which smooth muscle contractile dysfunction plays a key role. This review focuses on smooth muscle contractile phenotypic diversity in the vascular system, how it is generated, and how it may determine vascular function in developmental and disease contexts.
Keywords: phasic, tonic, nitric oxide, vasomotion, myosin, phosphatase
smooth muscle may be classified on embryologic, anatomic, physiologic, or molecular bases; given the great diversity in smooth muscle, all the classification schemes are imperfect, substantially underrepresenting this diversity, but serve as essential frameworks for the study of smooth muscle function within a given organ system. Perhaps the most useful classification scheme, and the one that will be used here due to the emphasis on smooth muscle function, is the dichotomy of phasic vs. tonic contracting smooth muscle. Phasic smooth muscle is characteristic of the gastrointestinal and urogenital systems and, as the name implies, displays rhythmic contractile activity. Tonic smooth muscle is characteristic of the large arteries and veins and is continuously contracted. Phasic and tonic smooth muscle each express a unique repertoire of contractile protein isoforms that are referred to as fast and slow isoforms, respectively, in analogy to the fast vs. slow dichotomy of striated muscle. Smooth muscles were originally classified as single unit vs. multiunit based on their innervation and evoked responses (127). The phasic smooth muscles behave as unitary muscles with action potentials spreading from cell to cell, resulting in a coordinated phasic contraction, as for example in gut peristalsis. The phasic contractions may be initiated by pacemaker cells[interstitial cells of Cajal (ICCs)] residing within the tissue (185), though this is controversial (see Ref. 70). The tonic smooth muscle behaves as multiple independent units, or motor units, with graded changes in membrane potential of each unit, i.e., without propagation of action potentials, resulting in graded changes in force (tone). A somewhat different early classification of smooth muscle was based on force activation by electromechanical vs. pharmacomechanical coupling (200). In electromechanical coupling force is activated by a change in membrane potential, while in pharmacomechanical coupling force is activated by receptor signaling, which may or may not include a change in membrane potential. As it is now clear that all smooth muscle may be activated by either mechanism this distinction is less useful for the understanding of phenotypic diversity but crucial in the study of smooth muscle function in situ.
The classification of smooth muscle into tonic vs. phasic subtypes is analogous to striated muscle fast vs. slow subtypes, but there are a number of key functional differences between these muscle types. The fastest smooth muscle has a maximum velocity of shortening that is still more than an order of magnitude slower than even slow striated muscle. Tonic smooth muscle has a force maintenance phase with very low energy expenditures, referred to as the “latch phase” based on analogy to catch muscles of invertebrates, e.g., the adductor muscles of bivalve mollusks (142). While mature striated muscle is terminally differentiated with very limited proliferative potential, mature smooth muscle cells (SMCs) may undergo hyperplastic (or hypertrophic) growth in disease. The study of smooth muscle phenotype has been dominated by the control of vascular smooth muscle (VSM) proliferation and differentiation (reviewed in Ref. 160), reflecting the epidemic of atherosclerosis in Western societies in which proliferation of large vessel smooth muscle plays a key pathogenic role.
VSM PHENOTYPIC DIVERSITY
Overview
VSM may be classified 1) anatomically as arterial vs. venous vs. lymphatic, systemic vs. pulmonic, and macro- vs. microvascular; 2) based on its embryonic derivation from mesothelium, mesenchyme, neural crest, etc. (reviewed in Ref. 123); 3) based on its contractile properties as phasic vs. tonic. This review focuses on smooth muscle contractile diversity and its role in vascular function. Visceral smooth muscle is generally thought of as phasic, single unit and VSM as tonic, multiunit, yet there is considerable phenotypic diversity in smooth muscle of both the visceral (133, 206, 207) and vascular systems. It is interesting to note from a historical perspective that the original description of pharmacomechanical coupling eliciting action potentials and phasic contractile activity vs. electromechanical coupling with graded depolarizations and contractions relied solely on different (dog or rabbit) vascular preparations: mesenteric vein for the former and pulmonary artery and umbilical vein for the latter (200). Portal venous smooth muscle is unique within the vascular system as a prototypical phasic smooth muscle exhibiting fast contraction and relaxation kinetics, spontaneous action potentials and phasic contractile activity, and expression of a pure fast gene program (6, 31, 141). The portal vein (PV) may thus serve as a prototype for the study of specification of the phasic smooth muscle phenotype in the vascular system and its modulation in disease, the only caveat being the extent to which findings may be generalized to other vascular tissues. However, the role of the PV in the regulation of blood flow in the splanchnic circulation is uncertain, making it more difficult to hypothesize a relation between the smooth muscle phenotype and vascular function.
Due to the steep inverse relationship between vessel radius and vascular resistance (r4) according to Poiseuille's Law, vascular function is determined, i.e., pressure and flow are predominately regulated, at the level of the small resistance arteries (SRAs, 50–300 μm diameter). It has been appreciated for many years that the SRAs do not simply represent smaller versions of the large arteries (25, 28, 47, 210). To what extent the differences between the function of the large and small arteries are intrinsic to the smooth muscle has received limited investigation, no doubt reflecting the difficulty in isolating and characterizing small vessel smooth muscle. Small arteries in vivo exhibit a mixture of tonic contractions and phasic contractile activity termed vasomotion (reviewed in Refs. 72, 150, 166, 171) and conducted vasomotor responses (57). Vasomotion was first described 150 years ago in observations of veins of the bat wing, yet neither the mechanisms nor significance of vasomotion is understood today (reviewed in Ref. 150). Consistent with the mixed contractile properties the microcirculatory smooth muscle expresses a mixture of fast and slow contractile protein isoforms (Table 1). This review will focus on the role of this gene program in determining the unique functional properties of micro-VSM in the developing and mature organism and its modulation in disease contexts. The hypothesis will be developed that the expression of the fast gene program subserves the phasic contractile activity termed vasomotion.
Table 1.
Contractile protein isoforms and smooth muscle phenotype
| Gene(s) (HUGO) | Gene Products | Generation | Expression | Function of Isoforms |
|---|---|---|---|---|
| MHC | A, B | S | T vs. P | velocity of shortening |
| (MYH11) | 1, 2 | S | ?filament assembly | |
| MLC17 | A, B | S | T vs. P | velocity of shortening |
| (MYL6) | ||||
| Actins | α, β, γ | G | U vs. D | unknown |
| (ACT:A2,G1,B) | T vs. P | |||
| Calponins | basic (h1), neutral (h2), acidic (h3) | G | U vs. D | unknown |
| (CNN:1,2,3) | ||||
| Caldesmon | high vs. low MW | T,S | U vs. D | unknown |
| (CALD1) | ||||
| Tropomyosin | α, β + | T,S | U vs. D | ?calcium sensitivity |
| (TPM:1,2) | ||||
| α-Actinin | S | U vs. D | unknown | |
| (ACTN1) | ||||
| MLCK | smMLCK | T | TS | calcium activation |
| (MYLK1) | nmMLCK | T vs. P | unknown | |
| telokin | ||||
| MYPT | MYPT1 LZ+/− | G,T,S | T vs. P | calcium desensitization |
| (PP1R:12A,B;13) | M21 | TS | ||
| p85 | ||||
| PPI | CPI-17 | G,T | T vs. P | calcium sensitization |
| (PPP1R14:A–D) | PHI-1,2 | O | ||
| KEPI | ||||
| GBPI | ||||
| BKCa | BKCa | G,S | TS, H | signals that regulate potassium currents |
| (KCNM:A1;B1–4) | ||||
| Kir | Kir2.1 | G,T | TS | EDHF in small arteries |
| (KCNJ2) | ||||
| KATP (Kir6.1/SUR2B) | KATP | G,S | TS | unknown |
| (KCNJ8/ABCC9) | ||||
| Kv | Kv | G | TS | unknown |
| (KCNx) | ||||
| LTCC | Cav1.2 | S | TS | voltage-dependent activation |
| (CACNA1C) |
S, splicing; G, gene; T, transcription; Tvs P, tonic versus phasic; Uvs D, undifferentiated versus differentiated; TS, tissue-specific; O, other cell types; H, hormonal.
Basal Contractile Apparatus
The interaction of myosin with actin is the primary determinant of force production in all muscle tissues (Fig. 1). Isoforms of smooth muscle myosin heavy chain (MHC) (MYH11) are generated by alternative splicing of exons in the head (SM-A,B) and tail (SM1,2) of the motor protein (reviewed in Ref. 119). The inclusion of a 21 nt alternative exon (E8) in the head of the myosin (SM-B) increases myosin ATPase activity several-fold correlating with several-fold higher maximum velocity of shortening of the phasic muscle (99). The tonic smooth muscle of the large arteries and veins express almost exclusively the slow isoform of MHC (E8 skipped). The fast isoform of myosin heavy chain (E8 included, MHC-B) is expressed in the SRAs of the heart (234), lung (120), muscular femoral artery (41), small mesenteric arteries (8), and renal afferent but not efferent arteriole (168, 193) of rodents and rabbits. There is good correlation between increasing expression of the fast isoforms of myosin heavy (and light chains) in smaller arteries and faster rates of contraction (8, 41). That expression of MHC A,B isoforms substantially determines velocity of shortening is suggested by genetic manipulation experiments. Forced expression of the SM-A isoform by germ-line inactivation of the alternative exon reduced the velocity of shortening of mesenteric artery and bladder by two- to threefold (7, 97). However, the velocity of contraction of the renal afferent arteriole, which also normally expresses significant amounts of the fast isoform, was unaffected (168). These disparate findings could reflect differences in how the muscle was activated, e.g., loaded vs. unloaded, in adaptations to the germ-line manipulation of the MHC gene, or to true differences in contractile performance between renal afferent arteriole and mesenteric arterial smooth muscle. Complete isoform substitutions as performed in striated muscle (105, 139) have yet to be accomplished in smooth muscle. The role of the expression of the fast isoform of MHC in the micro-VSM with respect to the regulation of blood flow is not known.
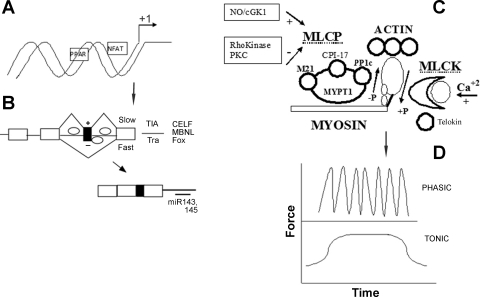
Fig. 1.
Control of gene expression, vascular smooth muscle contraction, and functional diversity. A: different sets of genes are transcribed in phasic vs. tonic smooth muscle. Genes are specifically transcribed in slow (striated) muscle under the control of nuclear factor of activated T cells (NFAT) and peroxisome-proliferator-activated receptor (PPAR). Diversity is also generated by multiple transcription start sites within each gene. B: additional diversity is generated by the alternative splicing of exons (filled box); only one of many types of alternative splicing is shown. In limited studies TIA proteins are proposed to mediate slow splicing and Tra proteins fast splicing, while other factors that may play a role in tissue-specific splicing of exons have not been studied in this context. microRNAs (miR143, 145) regulate gene expression by binding to 3′-untranslated region and destabilizing the message or blocking its translation. C: the basic components of the contractile apparatus are depicted. Myosin binding to actin generates force and displacement. Myosin is activated by phosphorylation by myosin light chain kinase (MLCK) and deactivated by dephosphorylation by myosin light-chain phosphatase (MLCP aka myosin phosphatase or MP). MLCK activity is regulated by calcium, while MLCP is both positively and negatively regulated by a number of signaling pathways. D: smooth muscle may produce force in a tonic or phasic pattern. In the vasculature phasic force production is termed vasomotion. Abbreviations are defined in the text.
The expression of the MHC isoforms generated by alternative splicing of the alternative exon in the tail (SM1,2) is not tissue-specific and the functional significance uncertain, though their position in the myosin tail and preliminary experiments suggests that they may influence filament assembly (reviewed in Ref. 119). Ablation of SM2 through a similar exon inactivation approach also alters smooth muscle function and is lethal to the mouse but the molecular basis of these effects is not clear (27).
Isoforms of the 17 kDa essential myosin light chain (MYL6) are generated by alternative splicing of a 39 nt exon near the carboxy-terminus (E6) (78). This exon is predominately included in tonic smooth muscle and skipped in phasic smooth muscle, a pattern opposite to MHC E8. The pattern of expression of fast vs. slow MLC17 isoforms is similar to that of MHC though it has not been as thoroughly investigated (78). The MLC17 isoforms are also proposed to influence myosin ATPase activity and velocity of shortening (60, 75, 126), though this has yet to be tested through isoform substitutions in vivo. It is also unknown as to whether there is interaction between the myosin heavy and light chain isoforms in determining contractile function.
Nonmuscle myosin heavy (MYH15) and light chains (MYL6B) are generated from different genes and also undergo alternative splicing (reviewed in Refs. 32, 102). It has recently been suggested that these myosins may also play significant roles in force production in smooth muscle, particularly in force maintenance in tonic smooth muscle (6, 140, 156, 184). This illustrates the danger of assuming the functional role of a protein based on its name or pattern of expression, particularly when examined in cultured or “nonmuscle” cells. Whether the nonmuscle myosins are differentially expressed in tonic vs. phasic smooth muscle and impart functional differences to these tissues has not been examined.
Differentiated smooth muscle express α-(ACTA2) and γ-(ACTG1) actin isoforms, while the β-actin (ACTB) is the nonmuscle isoform (64, 161). Each actin is a separate gene product. Mature fully differentiated large VSM predominantly expresses α-actin, while γ-actin is more highly expressed in the visceral (phasic) smooth muscle (54). The expression of actin isoforms throughout the vascular system has not been systematically examined, though interestingly the swine renal vein expresses a nearly equal mixture of α- and γ-actin isoforms (54). The two isoforms differ only by four residues toward the NH2 terminus, and the evidence to date is that there is no functional difference between them (45), leaving open the question of the significance of these isoforms.
Other Contractile Proteins
Calponin is a thin filament-associated protein with homology to striated muscle troponin. Calponin inhibits actin-activated myosin ATPase activity, but its exact role in smooth muscle contraction has yet to be defined (reviewed in Ref. 239). Three calponin isoforms are generated from three different genes and designated h1 (basic) (CNN1), h2 (neutral) (CNN2), and h3 (acidic) (CNN3). There is some evidence for differential expression of these isoforms in smooth muscle and nonmuscle tissues. Caldesmon and tropomyosin are additional thin filament proteins whose function in smooth muscle is thought to be in regulation of calcium activation of the myofilaments though the exact mechanism is still debated. A single caldesmon gene (CALD1) gives rise to multiple isoforms through 1) alternative splicing by competing 5′-splice sites in exon 3 and 2) alternative promoters (reviewed in Ref. 198). The resultant protein products are described as high (h) vs. low (l) molecular weight forms. The h-CaD is more highly expressed in differentiated SMCs. Two tropomyosin genes (α,β = TPM1,2) are expressed in SMCs (and in other cell types) and give rise to an array of gene products through alternative splicing of exons. Smooth muscle α-tropomyosin is produced by splicing in of exons 2b and 9d and smooth muscle β-tropomyosin by splicing in of exons 6b and 9a; other exons at these loci are specifically spliced in striated muscle or nonmuscle cells generating 20–40 isoforms in birds and mammals (reviewed in Ref. 226). It has been proposed that the different tropomyosin isoforms may have effects on the calcium sensitivity of the myofilaments (reviewed in Refs. 128, 129). Like tropomyosin, the alternative splicing of two mutually exclusive exons of the actin binding protein α-actinin (ACTN1) gives rise to either a smooth muscle-specific or a nonmuscle isoform (165, 227). Whether the expression of any of these proteins' isoforms segregates according to vessel type or smooth muscle contractile phenotype, or influences calcium activation of the myofilaments of VSM, is not known.
Contractile Regulatory Enzymes
Phosphorylation of the regulatory light chain of myosin (MLC20) activates the smooth muscle myosin ATPase activity resulting in force production. Smooth muscle force is thus predominately determined by the balance between the activities of the calcium/calmodulin-activated myosin light chain kinase (MLCK) and the myosin phosphatase (MP, also known as MLCP or myosin light-chain phosphatase), and it is on to these enzymes that signals that regulate vascular tone ultimately converge. Vasoconstrictor signals activate smooth muscle force through 1) calcium flux activating MLCK and 2) second messengers that inhibit MP, thereby sensitizing the myofilaments to calcium activation (reviewed in Ref. 91). Vasodilators do the opposite, relaxing smooth muscle through inhibition of calcium flux and activation of MP, thereby desensitizing the myofilaments to calcium. This section reviews how regulated expression of these enzymes may influence VSM responses to vasoconstrictor and vasodilator signals.
MLCK.
MYLK1 (MYLK2 is restricted to striated muscle) gives rise to three distinct primary transcripts generated from three separate promoters. The large (∼220 kDa) protein is termed the nonmuscle MLCK, though the negative descriptor is of limited utility and potentially misleading; for example this MLCK is expressed at high levels in embryonic smooth muscle (17, 59, 79). The intermediate (∼130 kDa) protein is highly expressed in and thus termed the smooth muscle (sm) MLCK though it is also more widely expressed. The smallest product is termed telokin, a 17 kDa protein generated from a promoter within intron 28 of MLCK (90). smMLCK, like smooth muscle myosin light-chain phosphatase (MP), is expressed at several-fold higher levels in phasic vs. tonic smooth muscles correlating with several-fold higher enzymatic activities in these tissues (69). The higher MLCK and MLCP activities likely also contribute to the several-fold faster rates of contraction and relaxation in the phasic tissues though this has not been formally proven. Limited investigation suggests that smMLCK and smMLCP are also expressed at higher levels in the SRAs vs. large vessel smooth muscle (246).
Of the three transcripts telokin is most highly differentially expressed in the mature animal, being much more abundant in phasic vs. tonic smooth muscle (65, 80, 240). The expression of telokin in the vascular system has received limited investigation. One study of cats found it to be undetectable in cerebral artery (tonic) smooth muscle, consistent with prior studies (reviewed in Ref. 79), and more abundant in small vs. large pulmonary artery smooth muscle (122). The precise role of telokin in the regulation of smooth muscle contractility has not been defined. Telokin is phosphorylated by cGMP kinase (cGK) (104, 228), and since cGK relaxes smooth muscle through calcium desensitization, it is presumed that this is the function of telokin. A specific molecular mechanism has not been defined; it is proposed to function through activation of MP (101), though how this fits with telokin being a derivative of MLCK is problematic. A telokin-specific knockout mouse with preserved expression of MLCK was generated through insertion of a flox-Neo cassette disrupting the telokin promoter in intron 28 of MLCK (101). The ileal (phasic) smooth muscle of these mice had ∼50% reduction in sensitivity to cGMP-mediated activation of MP and calcium desensitization of force production. There was no effect in the aortic (tonic) smooth muscle, consistent with the tissue-specific expression of telokin and suggesting phenotype-specific responses to cGMP, discussed further below.
MLCP.
MLCP purifies as a hetero-trimeric protein composed of catalytic (PP1c), targeting (MYPT1), and 21 kDa subunits (1) (reviewed in Ref. 91) (Fig. 2). The ∼130 kDa MYPT1 subunit (also described as MBS) (PPP1R12A) targets PP1c to myosin and is thus critical for its activity and also functions as a regulatory subunit. The function of the 21 kDa subunit (M21) is unknown. The MYPT2 (PPP1R12B) gene is expressed in striated muscle, while a third MYPT gene (PPP1R13B) gives rise to the p85 (85 kDa) product that is ubiquitously expressed. The M21 subunit is generated from a transcriptional start site within intron 13 of MYPT2 (Ref. 5 and unpublished data).
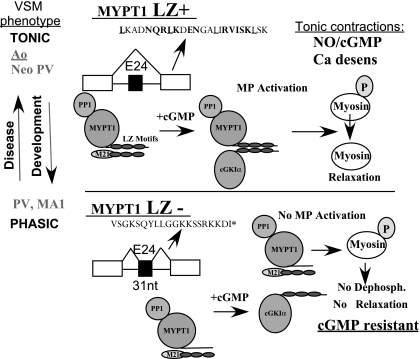
Fig. 2.
MP isoforms. A 31 nt exon near the 3′ end of the gene is skipped in tonic and included in phasic smooth muscle. Skipping of the alternative exon codes for a COOH-terminal leucine zipper motif (LZ+) that mediates the heterodimerization of cGMP kinase (cGK1α) with MYPT1. This dimerization is proposed to be required for cGMP activation of myosin phosphatase (MP) and calcium desensitization of force production. Inclusion of the 31 nt exon in phasic smooth muscle codes for the LZ− isoform, which does not dimerize with cGK and thus cGMP does not activate MP.
The activity of the vascular MLCP is highly regulated. A number of constrictor signaling pathways using kinases such as PKC, Rho kinase, Zip kinase, and integrin-linked kinase inhibit MP, resulting in increased force production to activating calcium (reviewed in Ref. 91). These kinases may inhibit MP through phosphorylation of the regulatory MYPT1 subunit and/or the CPI-17 subunit (PPP1R14A), which derives its name as a 17 kDa PKC-potentiated inhibitor of MP (51). Phosphorylation of CPI-17 at Thr38 activates CPI-17 1,000-fold, resulting in potent inhibition of MP activity (IC50 ∼1 nM, reviewed in Ref. 52). CPI-17 is expressed at ∼10-fold higher levels in tonic smooth muscle such as the aorta compared with phasic smooth muscle such as ileum, bladder (236), and PV (169). Given the threefold higher MP expression and activity in phasic vs. tonic smooth muscle, the stoichiometry of CPI-17 to MP is on the order of 30-fold higher in tonic vs. phasic smooth muscle correlating with greater PKC-mediated calcium sensitization of tonic smooth muscle, though the magnitude of the difference is considerably less, about threefold (236). Our unpublished data suggest that CPI-17 is expressed at lower levels in mesenteric SRAs compared with the tonic smooth muscle of the large arteries and veins. How this may influence sensitivity to contractile agonists is not known and will require gene inactivation or mutagenesis studies in vivo, e.g., alanine substitutions of phosphorylatable residues, as performed in vitro (77). Based on sequence similarity three homologs of CPI-17 are present in mammalian genomes (PPP1R14B-D): PHI (phosphatase inhibitor-1, 2), KEPI, and GBPI (reviewed in Ref. 52). Whether these may function in VSM in agonist-mediated inhibition of MP and whether their expression and activity may be phenotype-dependent remains to be determined.
Isoforms of the MP regulatory subunit MYPT1 are generated by the alternative splicing of exons. Exons 13 and 14 in mammals and the immediately upstream exon 12 in birds are alternatively spliced (40, 91). These exons are just upstream of the Thr656, which has been proposed as a Rho kinase phosphorylation site, mediating inhibition of MP and calcium desensitization (91); whether there is differential response of the isoforms to this signal is not known. The alternative splicing of these exons is not evolutionarily conserved, is tissue-specific in birds, but is less so in mammals (40), and the functional significance of these splice variants is neither known nor hypothesized.
MYPT1 exon 24 (of 26 total exons) is alternatively spliced in mammals and birds in a highly tissue specific and developmentally regulated fashion. Skipping of the 3′ 31 nt alternative exon codes for a COOH-terminal leucine zipper motif (LZ+). Inclusion of this exon alters the reading frame, resulting in a premature stop codon and coding for a MYPT1 subunit with a completely different COOH-terminal sequence. Based on in vitro studies the COOH-terminal LZ motif of MYPT1 and adjacent coiled-coil domain is proposed to mediate its hetero-dimerization with the NH2-terminal LZ motif of cGK1α (67, 191, 205). The hetero-dimerization of cGK1 and MYPT1 is thought to be required for NO/cGMP-mediated activation of MP and desensitization of smooth muscle to calcium (100, 205), though the precise mechanism is not established (145, 237). In tonic smooth muscle of the large arteries and veins the 31 nt alt exon is skipped, coding for the LZ+ isoform. In contrast, in the phasic smooth muscle of the PV and intestines the 31 nt exon is included coding for the LZ− isoform (100, 170). The first order mesenteric resistance arteries express predominately (80%) the MYPT1 E23-included/LZ− (fast) isoform (246), and in general there is a direct relationship between artery size and relative expression of the MYPT1 LZ+ (slow) isoform (for example Ref. 98), similar to myosin isoforms, though this is incompletely characterized.
Across all smooth muscle tissues there is a good correlation between expression of MYPT1 LZ+ isoform and sensitivity to cGMP-mediated calcium desensitization, i.e., activation of MP, supporting the model as originally proposed. For example, the avian gizzard and rat PV each express exclusively the MYPT1 LZ− isoform as part of a pure fast gene program, and in neither does cGMP cause calcium desensitization of force production, i.e., MP activation (100, 169). That these smooth muscle tissues are completely or relatively resistant to NO/cGMP-mediated relaxation has been known for some time (55, 173) and more recently demonstrated in vivo for the rat portal venous circulation (162). This may depend upon how the muscle is activated, with cGMP able to reverse calcium sensitization induced by contractile agonists but not when force is activated by calcium alone (see, e.g., Refs. 19, 187), a topic that requires further investigation. Preliminary studies also support a correlation between expression of MYPT1 LZ isoforms throughout the vasculature and sensitivity to cGMP-mediated calcium desensitization, with the smaller vessels that express more of the LZ− isoform being less sensitive. This is consistent with older physiological studies showing that the role of endothelium-derived relaxing factor [nitric oxide (NO)] relative to endothelium-derived hyperpolarizing factor(s) (EDHF) in endothelium-mediated vasodilation decreases as vessel size decreases in the mesenteric, coronary, and other circulations (30, 66, 88, 106, 143, 152, 189, 192). Even two closely related arteries, such as the superior mesenteric artery vs. its first subbranch, the MA1, show substantial differences in endothelial mediated vasodilation (88). Whether these differences truly reflect segmental properties of the vascular system, perhaps generated by a Homeobox code, requires further study.
Further support for this concept of differential control of vascular function comes from studies of cGK1-inactivated mice, in which (ACh) endothelium-dependent relaxations were abolished in the aorta but not the cremasteric muscle arterioles (103, 172), suggesting that the latter may be independent of NO/cGMP signaling. However, in another study leucine-to-alanine mutation of cGK1α LZ motifs markedly reduced endothelium-dependent vasodilator responses in aorta and small cerebral (pial) arteries (138), thereby demonstrating the dependence of the endothelium-mediated vasodilatation on cGK1α and specifically the LZ motif. The putatively different control mechanisms could reflect differences in the strength of the signal and/or the smooth muscle response in organ and vessel-specific control of blood flow, an issue that is best explored through gene based approaches.
NO/cGMP and other signals may relax VSM through reductions in calcium sensitivity or calcium flux (114). Interestingly in closely related smooth muscle tissues, segments of mouse intestinal smooth muscle, cGMP predominately reduces calcium sensitivity in one (jejunum) while predominately reducing calcium flux in the other (colon) (63). The molecular basis for these differences was not defined. Phenotype-specific regulation of vascular tone through tissue-specific expression of ion channels and calcium cycling proteins, and their response to vasoactive signals, is addressed in the next section. Studies to date have predominately examined tonic contractions, and it is quite possible that the regulation of force and role of NO/cGMP signaling may be different in phasically contracting VSM, i.e., those exhibiting vasomotion (231). Studies are conflicting as to whether NO and cGMP activate or inhibit vasomotion, i.e., force development (reviewed in Refs. 72, 150). A recent study suggests that cGMP activates vasomotion within a defined window of agonist-induced preactivation of force in the resistance arteries (190). The only putative vasomotion gene identified to date is bestrophin-3 (130), a cGMP-dependent calcium-sensitive chloride channel, but its expression is not restricted to phasic smooth muscle. That NO and cGMP may relax tonic VSM and induce force (vasomotion) in phasic smooth muscle argues for smooth muscle phenotype as a strong determinant of NO/cGMP signaling.
Despite its discovery over 150 years ago the function of vasomotion in the regulation of blood flow remains mysterious. Modeling suggests vasomotion may enhance blood flow or tissue oxygenation (135, 178). In the surgical literature it has been proposed that pulsatile flow provides for better organ recovery during cardiopulmonary bypass, chronic mechanical cardiac support, and organ transplantation than does nonpulsatile flow (73, 216, 219, 220), though this remains controversial, as e.g.,. in patients with heart failure receiving left ventricular assist devices providing pulsatile vs. steady flow (reviewed in Ref. 113). It also must be recognized that pulsatile flow has multiple components including cardiac ejection and local vasomotion.
In summary, the patterns of expression and functional studies suggest that the activity of the MP in the tonic smooth muscle of the large arteries and veins is more highly regulated by signaling pathways than the MP in the phasic smooth muscle of the intestines, PV, and small arteries. It is proposed that this is due to tissue-specific expression of MP regulatory subunits including CPI-17 and MYPT1, while the role of tissue-specific expression of telokin is less clear. At this point these must be considered hypotheses that must be tested through genetic manipulations. How activating and inhibiting signals are integrated at the MP and the influence of tissue-specific expression of the subunits and isoforms in the context of muscle phenotype and vascular function is deserving of further investigation.
Ion Channels and Calcium Cycling
Force development in smooth muscle is a function of the calcium sensitivity of the myofilaments, reviewed above, and calcium flux. Calcium flux into SMCs is predominately through plasmalemmal L-type calcium channels (LTCCs), the target of widely used calcium channel blocking drugs. The LTCCs may open in response to membrane depolarization (voltage-dependent calcium channels), controlled by a diverse array of potassium channels and sodium and nonselective ion channels (TRPs), or in response to receptor signaling (receptor-operated calcium channels). Some calcium fluxes through smooth muscle sarcoplasmic reticulum (SR) Ip3R/RyR calcium release channels, but in stark contrast to striated muscle, there is not a simple relationship between SR calcium release and force development. SR calcium release may activate or inhibit contraction, the latter through localized calcium spark-mediated induction of spontaneous transient outward current reducing membrane excitability (149). Ion fluxes in the control of smooth muscle tone was recently and thoroughly reviewed (212, 238); this review will focus on how regulated expression of the component gene products may determine vessel-specific function. To the best of my knowledge there are no data available regarding the expression of these genes in phasic vs. tonic smooth muscle and limited data on differential expression across vascular tissues.
Potassium channels and membrane potential.
A diverse array of potassium channels are expressed in smooth muscle such that SMC membrane potential may be controlled by many inputs, including voltage (Kv), calcium (KCa), ATP (KATP), and inwardly rectifying (Kir), and second messengers such as PKC, cAMP, and cGMP (reviewed in Ref. 92).
BKCA CHANNEL.
Of the three (large, intermediate, and small conducting) calcium-activated potassium channels, the large conductance channel (BKCa, also known as MaxiK) plays a dominant role in setting membrane potential, and mice in which this protein is inactivated are hypertensive (reviewed in Ref. 83a). The BKCa channel is composed of four pore-forming α-subunits generated from a single gene (KCNMA1) and an equal number of regulatory β-subunits that may be generated from four different genes, with B1 (KCNMB1) predominant in VSM. One study observed less BKCa expression and activity, and a reduced β1:α subunit ratio in rat cremaster muscle arteriole compared with middle cerebral artery (244), suggesting differing mechanisms of vascular control that is either vascular bed or vessel type specific. Isoforms of BKCa α-subunit are generated by alternative splicing of exons that phylogenetically tend to cluster at identical regions of the channel, while the alternative sequences are not conserved (reviewed in Ref. 61). Splicing of one of the mammalian 13 alternative exons, termed STREX (stress axis-regulated exon) is regulated by stress and gestational hormones (242), calcium (through Cam kinase IV) (241), and other signals and converts the response of the channel to cAMP from stimulatory to inhibitory (215). BKCa activity is regulated by a host of other signals including cGK and PKC, with a necessary role for LZ motifs in the COOH terminus (214), analogous to regulation of MP activity discussed above. Differential expression of BKCa splice variants has been demonstrated between neural and vascular tissues (179) but not between different vascular beds tissues or with respect to VSM phenotype. Interestingly changes in BKCa splice variants has been shown in myometrial smooth muscle in pregnancy and proposed to regulate the transition from contractile quiescence to activity (248).
Kir CHANNELS.
There are seven subfamilies of the widely expressed inward rectifying potassium channels (Kir) (reviewed in Ref. 81). The Kir2 subfamily is predominant in VSM with multiple isoforms generated by different genes. VSM predominately or solely expresses the Kir2.1 isoform (KCNJ2), as evidenced by the hypertension and absence of Kir activity in mice in which this gene is inactivated (245). There are no reports of Kir isoform expression in VSM that could affect function. However, increased density of inward rectifier potassium currents in the smooth muscle of the small vs. large cerebral and coronary arteries has been reported (48, 182), consistent with the increased role of EDHF in the resistance arteries. (This pattern is opposite to that of BKCa, which predominates in the larger arteries). A recent study observed no difference in Kir 2.1 expression between rat kidney afferent and efferent arteriolar smooth muscle, yet only afferent arteriole tone appeared to be sensitive to Kir (29), raising the possibility that the functional differences are due to differential expression of other Kir subunits or differential regulation by signaling pathways (reviewed in Ref. 163). The KATP channels are octomers of four Kir6.x subunits and four auxiliary subunits, the sulfonylurea receptors (SURx), the target of the diabetic sulfonylurea drugs (reviewed in Refs. 81, 163). Kir6.1 (KCNJ8)/SUR2B (ABCC9) subunits form the KATP channels in most all VSM, with possibly the exception of coronary vessels where SUR2A expression was detected (177). SUR2a and b are splice variants with alternative splicing of exons 39 and 40 resulting in differing carboxy-terminal sequences. Germ-line inactivation of SUR2 or Kir 6.1 results in coronary artery spasm (93), consistent with higher KATP subunit expression in this vascular tissue (177), though SUR2 transgenic rescue experiments did not support this being a primary VSM defect (93). The use of KATP channel openers (Pinacidil, Nicroandil, Diazoxide, Minoxidil) for the treatment of hypertension and nonvascular disorders (reviewed in Ref. 81) provides a strong argument for identification of isoform-specific expression, activity, and pharmacologic inhibition.
KV CHANNELS.
The voltage-dependent potassium channels (previously termed delayed rectifiers) comprises 12 families of KCNx genes, a review of which would not be possible here (see IUPHAR compendium). There is some evidence for differential expression of Kv subunits, with evidence that Kv are more highly expressed in both the systemic (62) and pulmonic (18) resistance arteries compared with the respective conduit arteries, as is true of Kir. The Kv current is also greater in these resistance vs. conduit arteries, though the magnitude of the difference is much smaller. Overall the physiological significance of the great diversity in the KCN gene family with respect to vascular function is not appreciated.
SR and calcium cycling proteins.
Phasic smooth muscle has lesser amounts (∼2% cell volume) of more peripherally located SR, e.g., PV and mesenteric artery and vein, compared with centrally located SR in tonic aorta (∼5% cell volume)(reviewed in Ref. 238). The functional significance of these differences is not known, though it may be that this allows a greater amount of calcium to be released from SR stores in the tonic smooth muscle. As for most gene families, an array of isoforms of the major calcium handling proteins, including those for SR calcium release (IP3R and RyR) and uptake (SR calcium ATPase) are generated via multiple genes and alternative splicing of exons. There is some evidence for differential expression of the gene products in phasic vs. tonic smooth muscle, but the physiological significance has not been established (reviewed in Ref. 238) and so will not be reviewed here. Of note, the cGK target the IP3 receptor-associated gated protein (IRAG = MRVI1) has not been reported to be differentially expressed across smooth muscle tissues (86). Plasmalemmal calcium channels (L, T, and P/Q type), the targets of calcium channel blocking drugs used to treat hypertension and angina, may also be differentially expressed, particularly in renal vessels (3, 74). The LTCC is composed of pore-forming α-subunit and auxiliary β-, δ-, or γ-subunits. Four genes encode the LTCC (1.1–1.4) of which Cav1.2 (CACNA1C) forms the major LTCC in cardiac and VSM (reviewed in Ref. 111). Of the 55 exons in the mammalian Cav1.2 gene, 19 may be alternatively spliced, leading to potentially 219 isoforms (209). The best characterized isoforms display variability at the NH2 terminus (Exons1, a-c), exons 8–9 (I-II cytoplasmic loop), exons 31–32 (transmembrane domain IV), and exon 45 in the regulatory cytoplasmic COOH terminus (reviewed in Ref. 111). These isoforms are proposed to determine LTCC voltage-dependent activation and inactivation, and differential response to CCBs, in cardiac vs. VSM (26, 112, 153). Mutations in exon 8/8a are proposed to cause Timothy syndrome, characterized by long QT syndrome and arrhythmias and autism amongst other defects (203). There are no data directly comparing expression of these splice variants in phenotypically diverse smooth muscle.
BLOOD VESSEL DEVELOPMENT
Differentiation of the prototypical slow smooth muscle of the large arteries and veins begins as early as embryonic day (E) 10.5 in the mouse. There are regional and vessel-specific differences in both the source of smooth muscle progenitors and timing of their differentiation (reviewed in Refs. 123, 159). Little is known regarding when or how VSM contractile phenotypic diversity is generated during the development and maturation of the vascular system. That there may be developmental VSM phenotypic transitions is suggested by organ-specific changes in vascular function in the transition from the in utero to mature circulation though this has received limited investigation (4, 43, 49). In the pig mesenteric circulation the vascular resistance increases and flow-mediated dilation/NO responses decrease in the neonatal transition from the fetal to adult circulation (146, 147, 183). The pulmonary circulation after birth changes from a high resistance to low resistance circulation dependent upon increases in vascular relaxant responses to endothelium-dependent (ACh) and endothelium-independent (NO donor) signals (44, 58, 76, 115). Some of these changes may be attributed to structural remodeling that alters vascular resistance or to changes in the neurohumoral control mechanisms. Whether these may in part reflect adoption of different smooth muscle contractile phenotypes has not been investigated at least in part due to the small size of the resistance arteries prior to maturation. This limitation may be overcome with the use of large animal models, and indeed the sheep is a commonly used model, with a particular need for studies of humans. In the large arteries of rodent and birds the slow gene program is expressed from the early stages of development, with minimal expression of fast contractile protein isoforms (100, 169). This suggests that fast-to-slow switching does not occur in the development of the large vessel smooth muscle fated to the tonic phenotype. That SMCs in vitro also express the slow gene program is also consistent with the tonic phenotype being the developmental default.
The PV as a unique and prototypical phasic smooth muscle may serve as a useful model for the study of developmental specification of the phasic smooth muscle phenotype in the vascular system given its larger size and accessibility, the only caveat being the question of the commonality of the observations. Rat portal venous smooth muscle differentiation occurs within the first 3 wk of the neonatal period, and by 5 wk of age the rat PV displays characteristic phasic contractile activity (116, 217). In species that are more mature at birth such as cat, guinea pig, and human this process may begin prenatally but extends well into the postnatal period (31, 134). The expression of contractile protein isoforms during this period of rat PV development is consistent with a tonic-to-phasic phenotypic transition. Between postnatal days 3 and 12 there is complete switching from the slow to fast isoforms of myosin heavy chain (MHC E8) and myosin phosphatase (MYPT1 E24) (169). The switch to the MYPT1 E24+/LZ−/phasic isoform between postnatal days 6 and 12 correlates with a switch from complete sensitivity to cGMP-mediated relaxation to minimal relaxation (20%) of PVs contracted by KCL depolarization, supporting the model described above. It has also been reported that the neonatal PV [postnatal day (D) 3–6] is more sensitive to the calcium-sensitizing effect of contractile agonists compared with mature PV (22). Whether this may be due to regulated expression of CPI-17 or some other component of the slow gene program requires further study.
We and others have observed the same phenotypic and functional switching in a second prototypical phasic smooth muscle, the chicken gizzard, though the timing is slightly different, occurring prior to and around the time of hatching (40, 60, 100, 157). This suggests that switching from tonic to phasic phenotypes during developmental smooth muscle specification may be a generalized phenomenon, though more complete molecular characterization of these tissues is needed to strengthen this paradigm. There is currently no in vitro model for slow-to-fast phenotypic conversion or maintenance of phasic SMC phenotypes in vitro; such a model would expedite research in this field.
Maturational differences in the calcium sensitization and desensitization pathways, as well as calcium handling, have been reported for other smooth muscle tissues, e.g., gallbladder (24). It has also been reported that ovine fetal cerebral arteries exhibit greater calcium sensitivity and desensitization than do the mature arteries (89, 148, 186), though the mechanism has yet to be identified. In summary, there is evidence for slow-to-fast transitions in smooth muscle during the developmental maturation of the vascular system. It will be critical to determine if this occurs in pulmonary and regional systemic circulations, its molecular basis, and how this may affect control of vascular function and drug responses.
VASCULAR DISEASE
All mature muscle cells exhibit phenotypic plasticity to varying degrees. In the smooth muscle field this question has been dominated by the study of the proliferation of the smooth muscle of the large arteries and veins and their modulation from a contractile to a synthetic phenotype. As the large artery and vein smooth muscle expresses exclusively the tonic gene program and this appears to be the default, it would not be anticipated that there would be switching of the muscle-specific contractile gene program in large vessel disease. There is induction of nonmuscle isoforms in these SMCs and preliminary evidence that these isoforms may influence smooth muscle contractile properties (156, 184), a subject requiring further study. The migration of SMCs into the neo-intima to form an atherosclerotic plaque may be life threatening if the plaque ruptures. However, the atherosclerotic plaques in the large arteries have little effect on vascular function until very late in the disease process due to the large radius of the vessels and flow reserve in the microcirculation.
Disease Models
Microvascular dysfunction is described in a number of pathological conditions, including distal to a chronic coronary artery occlusion in humans and in animal models (87, 202, 232) and in obesity, diabetes, and hypertension (204), all conditions also associated with large vessel disease. The dysfunction could result from a change in the signal or the VSM function and response. That there is vascular remodeling in these conditions as well as some preliminary data suggest that there may be intrinsic changes in VSM contractility in these contexts. Whether this reflects phenotypic modulation has not been examined, and in general SMCs with a phasic or intermediate contractile phenotype have been much less studied in disease models.
Flow-induced Remodeling
An elegant and robust model to examine smooth muscle phenotypic modulation in the microcirculation is that of flow-induced remodeling developed separately in the laboratories of De Mey (180) and Unthank (221). In this model, ligation of rat second order mesenteric arteries causes chronic low flow (LF) in the upstream MA1 (∼10% of normal) and chronic high flow (approximate doubling) in adjacent MA1s. Importantly, because of the pre-existing mesenteric collateral arcades, there is no tissue ischemia or necrosis that may confound the effects of altered blood flow on the vessel wall. De Mey and coworkers (23, 37) identified SMC death and proliferation in the remodeling of the MA1s. Using microarrays for unbiased measurements of mRNA in this model at 1–32 days they observed several-fold reductions in smooth muscle contractile mRNA and several-fold induction of a subset of nonmuscle mRNA (233). These data were interpreted as modulation to the nonmuscle phenotype though the limitations of this paradigm were noted in that smooth muscle genes were still substantially expressed and many nonmuscle genes were not induced. Furthermore, these MA1s exhibit functional properties typical of smooth muscle generating substantial amounts of force when activated.
We used this model to examine the question of smooth muscle contractile phenotypic modulation in the context of flow-induced vascular remodeling in the microcirculation. We observed significant and time-dependent changes in the expression of the contractile proteins and their isoforms during the 4 wk of flow dependent remodeling of the MA1 (246). In the LF MA1 there was nearly complete loss of the fast (E24 spliced/LZ−) isoform of MYPT1 by 28 days. There were also significant shifts from fast to slow isoforms (splice variants) of myosin heavy and light chains. The loss of fast gene expression in the chronic LF state suggests the hypothesis that pulsatile blood flow conditions the micro-VSM to express the fast (phasic) gene program. At intermediate time points (days 1–7) there was a partial shift in the MYPT1 isoforms, from 80:20 E24+ to ∼50:50 in both the high flow (HF) and LF MA1s. Whether this represents a complete switch in a subset of SMCs that have entered the cell cycle, or a partial shift in all of the SMCs, requires in situ approaches to identify the spatial distribution of the isoforms. By 28 days in the HF MA1 the MYPT1 and other isoforms had reverted to baseline expression, likely due to outward remodeling and normalization of blood flow. In this model there are changes in many aspects of gene expression, the regulation of which remains to be defined, including reduction of MYPT1 mRNA by 60–80%, and reduction of MYPT1 protein due to activation of the ubiquitin-proteasomal system, consistent with prior studies (199, 213). There was no change in the abundance of smooth muscle α-actin protein, another marker of smooth muscle differentiation, underscoring the complexity of the phenotypic modulation. High-throughput studies using microarrays, deep RNA sequencing, and proteomic approaches will more completely define time-dependent changes in smooth muscle phenotype in response to altered flow.
PV
The PV as a prototypic phasic smooth muscle provides an attractive alternative tissue for the study of phasic-to-tonic phenotypic transitions in disease models. Studies several decades ago showed that PV SMCs undergo hypertrophic growth in a PV ligature model of portal hypertension (124, 125, 222). Protein biochemical assays identified small shifts from α- to γ-actin and increases in the intermediate filaments desmin and vimentin with no change in myosin isoforms. The hypertrophied PV showed a marked reduction in the frequency of spontaneous phasic contractions and reductions in maximal shortening velocity in intact tissue, all consistent with a shift away from the phasic smooth muscle phenotype. We examined this same model more recently using molecular markers of phasic vs. tonic smooth muscle. At days 1–7 after PV ligature there was a near complete switch in MYPT1 from the phasic (E24+/LZ−) to tonic (E24-/LZ+) isoform along with a reduction in the protein subunit abundance by one-third (170). There was similar but less robust switching in myosin heavy and light chain isoforms, all consistent with modulation toward the tonic phenotype. There was also several-fold induction of β-actin, a marker of the nonmuscle gene program. In this model the upstream MA1 also shifted to tonic gene expression with MYPT1 switching from 80:20 to 50:50 E24+/LZ−. By 14 days both the PV and MA1 had reverted to the control phasic program of gene expression, likely due to the outward remodeling of the arteries and formation of portsystemic shunts resulting in the abatement of the inciting stimulus, again highlighting the phenotypic plasticity of VSM.
The changes that occur in the VSM in the PV ligature and MA HF/LF models are similar, suggesting that the phasic-to-tonic modulation could be a universal phenomenon. The induction of the slow gene program in the hypertensive PV follows the general rule of reversion of mature muscle to the fetal gene program under a growth stimulus. Given the substantial differences between regional circulations experimentation will be required to determine if similar phenomena occur in other circulations. Loss of smooth muscle phasic contractile properties has recently been described in disease models of other tissues, e.g., in chamydial infection of the oviduct (42) and mechanical obstruction of the intestines (14, 15, 117) and bladder (2). In the study of the oviducts the reductions in peristaltic activity were thought to be due to loss of the ICCs that may provide pacemaker activity for phasic smooth muscle tissues (reviewed in Ref. 185).
Vascular Function
In contrast to the well-described roles of endothelial dysfunction and neurohumoral activation in altered vasomotor tone in disease conditions, the role of the smooth muscle contractile gene program has received little investigation. The changes in gene expression in the HF/LF flow model described above generate testable hypotheses regarding microvascular function under conditions of chronic hypo- or hyperperfusion. In our studies the switch of the D28 LF MA1 to the MYPT1 LZ+ isoform/tonic gene program was associated with increased sensitivity to the NO donor SIN-1 and to the cGMP analog 8-Br-cGMP, and the dose responses were similar to that of the tonic smooth muscle of the aorta (246), as predicted from the model. Vessels were studied ex vivo under isometric conditions in a wire myograph system with preactivation of force with the α-adrenergic agonist phenylephrine. However, it has yet to be shown that the increased sensitivity to NO/cGMP was due to increased ability of cGMP to activate the slow/LZ+ isoform of MYPT1/MP. The LF day 28 MA1 was significantly less sensitive to phenylephrine, and whether this may be due to altered gene expression is not known. Pourageaud and De Mey (181), using the same model, studied pressurized vessels ex vivo under isobaric conditions measuring changes in diameter rather than force. They also noted in LF MA1 altered response to α-agonist NE, with a decrease in maximum force but no significant difference in sensitivity and no difference in constrictor responses to AVP, suggesting a specific defect in excitation-contraction coupling in response to α-agonist stimulation. In contrast to our study they observed several-fold reduced sensitivity of LF D28 MA1 to the NO donor SNP after maximal AVP-induced preconstriction and modestly reduced sensitivity to acetylcholine-mediated dilation after maximal NE constriction, with no change in the maximal responses. In contrast flow-mediated dilation was modestly increased in the D28 LF MA1. Why the two studies observed opposite responses to NO donor drugs is not certain but could result from differences in 1) experimental design, e.g., study of vessels under isometric vs. isobaric conditions, 2) agonists used to activate force, 3) NO donor drugs, each of which may have effects independent of the generation of cGMP. In a different model, that of l-NAME-induced hypertension of pregnancy, we also observed in the main uterine arteries a concordant shift toward MYPT1 LZ+ isoform and increased sensitivity to NO donor and cGMP-mediated calcium desensitization indicative of MP activation (121). Given the multiplicity of targets and complexity of NO signaling and control of vessel tone, more specific endpoints for NO/cGMP signaling must be studied to define specific molecular mechanisms that may be causative in the altered VSM sensitivity to contractile agonists/antagonists in these models.
The focus of this review on smooth muscle contractile phenotypic diversity, but there is no doubt that significant changes in gene expression or activity will fall outside of this paradigm. For example we observed induction of PDE5 after 4 days of high or low flow and proposed that this desensitizes the smooth muscle to the increased release of NO (247). Other investigators have shown altered expression or activity of other genes in the dilator and constrictor pathways, including guanylate cyclase and RGS proteins (reviewed in Refs. 53, 235). The interplay and cross talk between these signals, VSM phenotype, and microvascular function in disease is deserving of further study.
Collaterals
One key difference between VSM and cardiac muscle is the ability of the former to generate new tissue through the process of angiogenesis. The function and vasomotor control of the collateral vessels is substantially different from the innate vessels (see Ref. 202, and reviewed in Ref. 47). Whether this may in part be due to smooth muscle phenotypic differences has not been investigated. This question will be particularly important as delivery of stem cells is attempted to regenerate a normally functioning vascular supply in ischemic tissues.
INPUTS FOR SMOOTH MUSCLE DIVERSITY
Generation of Diversity
In striated muscle essentially all of the inputs, including innervation, mechanical load, and autocrine, paracrine, and endocrine signaling, may determine fast vs. slow contractile phenotype (reviewed in Ref. 12), while this has received scant attention in the smooth muscle field (Fig. 3).
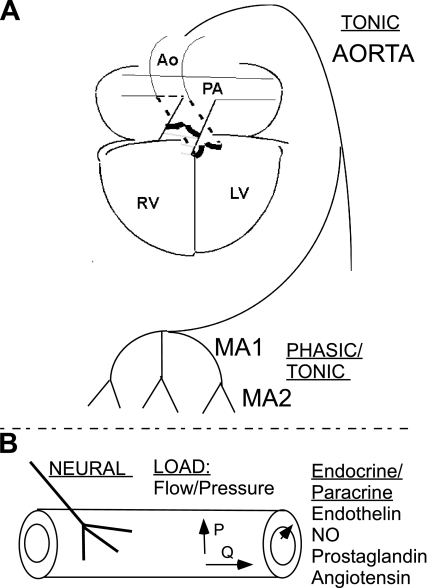
Fig. 3.
The vascular system and inputs that may control smooth muscle diversity. A: the vascular system may be categorized as pulmonic vs. systemic and macro- vs. microvascular. The large arteries and veins, e.g., aorta and inferior vena cava, contain smooth muscle of a pure tonic phenotype. The systemic microvascular (resistance artery) smooth muscle exhibits a mixed phasic/tonic phenotype. B: inputs that may control vascular smooth muscle phenotype include neural input, mechanical load, which is a function of pressure (stress/strain) and flow (shear), and local and distant hormonal signals.
Innervation
The innervation of smooth muscle throughout the vascular tree is highly variable. The more highly innervated vessels tend to express more of the fast gene program. Chemical sympathectomy of newborn rats with gaunethidine resulted in reduced expression of smooth muscle α-actin in the normally highly innervated femoral artery but not in the minimally innervated carotid artery (35). In this study coculture of sympathetic neurons with smooth muscle and endothelial cells increased smooth muscle α-actin and myosin expression several-fold. This study did not examine markers of the smooth muscle sublineages. The pure phasic smooth muscle of the (rat) PV is innervated by adrenergic and cGRP-containing neurons in the neonatal period (68, 134, 155, 211) when it implements the fast gene program (169). The phasic contractions of the rat PV are dramatically reduced by administration of 6-hydroxydopamine to the newborn, resulting in functional sympathectomy (116). These studies support a role for innervation in controlling VSM phenotype, but further studies are needed to determine if fast vs. slow gene programs are controlled by innervation as is the case in striated muscle (188).
Mechanical Load
The mechanical load on the blood vessel wall is a function of the interdependent variables of pressure and flow. Shear stress produced by blood flow is dissipated within the endothelial cell layer with minimal transmission to the adjacent smooth muscle. Thus it is most likely than any effect of flow (shear stress) on VSM phenotype would have to be through paracrine signaling from the endothelium, as demonstrated in large vessel remodeling (109) and discussed below. Changes in pressure are transmitted to the smooth muscle and produce wall stress and strain. Our observations on the loss of the phasic gene program in the MA1 in the absence of pulsatile flow (reviewed above) leads to a satisfying hypothesis that the pulsatile output of the heart conditions the phasic gene program in the resistance arteries, a hypothesis that is difficult to test in vivo.
An elegant series of studies by VanBavel and coworkers (9, 201) used an ex vivo system to study pressure, pressure oscillations, and flow in small vessel remodeling. They observed that exposure to oscillating pressures (1.5 Hz) suppressed the inward eutrophic remodeling that occurred in rat cremaster and coronary arterioles at 40–100 mmHg. The remodeling was also dependent upon the presence of vasomotion. The inward remodeling was inhibited when the vessels were also exposed to flow (176), consistent with in vivo studies of vascular remodeling, and this effect was NO dependent (but see below). The inward remodeling was reversed to outward remodeling when vessels were treated with the LTCC blocker Amlodipine, consistent with a proposed role of calcium signaling in muscle hypertrophy (discussed below); however, the mechanism for the salutary effect of the calcium channel blocker in this model was not identified. It should also be noted that different flow patterns in addition to the net forward flow markedly affect acute endothelial cell signaling to the VSM, but how these signals may chronically impact the smooth muscle is not known. The specific signals that mediate high and low flow-induced changes in microVSM phenotype remain to be defined (reviewed in Ref. 37).
Autocrine/paracrine/endocrine Signaling
A plethora of signals are used by the endothelium to communicate with adjacent smooth muscle. Flow-induced remodeling of the large vessels requires endothelium-derived NO, while both positive and negative results were obtained in tests of its role in small vessel remodeling (reviewed in Ref. 37). A recent in vitro coculture study suggests that flow (shear at 12 dyn/cm2)-mediated generation and release of prostacyclin (PGI2) by the endothelial cells activates peroxisome-proliferator-activated receptor (PPAR)-α or -δ in large VSMs and induces the contractile program of gene expression (218). Endothelial cells may also regulate smooth muscle differentiation through direct cell-to-cell contact using delta-notch signaling at least in developmental contexts (82, 83). Other factors that may regulate VSM growth and gene expression via autocrine and/or paracrine signaling include IGF, TGFβ, PDGF, and endothelin (ET) (reviewed in Refs. 13, 38). There are no data as to how these signals may influence VSM contractile phenotypic diversity other than one in vitro study showing that ET-1 may shift cultured embryonic SMC toward the tonic phenotype (60). Angiotensin II is a potent endocrine or para-/autocrine regulator of vascular function and smooth muscle hyperplastic or hypertrophic growth, but its role in determining SMC contractile tonic vs. phasic phenotype is not known. Endocrine signaling by thyroid hormone is a potent inducer of the fast phenotype in striated muscle and remains a useful model for studying both the gene regulatory mechanisms and physiological significance. A single study has shown that in guinea pigs made hyperthyroid for 12 days the fast isoform of MHC (E8 splice variant) is upregulated in both the slow aorta and fast intestinal smooth muscle; only in the latter was Vmax increased, by 20% (117). Whether this plays any role in the hyperdynamic circulation of hyperthyroidism has not been tested.
MOLECULAR PATHWAYS OF VSM DIVERSITY
Transcriptional Control
Sustained elevations of calcium activates the phosphatase calcineurin causing the dephosphorylation of nuclear factor of activated T cells (NFAT), which then translocates to the nucleus to activate the slow gene program in striated muscle (12). NFAT is also activated by calcium entry though voltage-gated calcium channels in SMCs in a process termed excitation-transcription coupling (reviewed in Refs. 10, 84, 229). Some of the genes controlled by NFAT in SMCs have been identified but only in the context of SMC differentiation; whether sustained elevations of calcium in phasic SMC would cause NFAT-dependent activation of slow genes in phasic or intermediate-type VSM, and whether this could be blocked by LTCC-blocking drugs used to treat vascular diseases, has not been examined. Calcium may also regulate gene expression though activation of Cam kinases and its phosphorylation and activation of CREB (cAMP response element binding protein). Whether this pathway when activated in phasic SMCs induces the slow gene program is also unknown. A second transcriptional regulatory family of considerable interest is the PPAR family and the PPAR-γ-coactivator-1 (PGC1) (46a). PPARδ and PGC-1 are key regulators of the metabolic gene program in the heart and skeletal muscle and mediate the downregulation of fatty acid metabolic machinery in the switch to glucose metabolism and the slow muscle phenotype. There are significant metabolic differences between phasic and tonic VSM that have not been well characterized; whether PPAR also regulates the metabolic phenotype of smooth muscle has not been investigated. PPARs are of particular interest in VSM given that prostaglandins may be PPAR ligands, the role of PGs in regulating VSM contractility and growth and the common use of cyclooxgenase inhibitors to reduce PG formation in vascular disease. It has also been proposed that PGs may induce smooth muscle differentiation through cAMP signaling and presumably activation of CREB, but not through PPAR (56). These in vitro studies were performed in uterine smooth muscle, in relation to pregnancy, a tissue with perhaps the most dynamic changes in contractile properties in the mature organism. The uterus develops phasic contractions at the end of pregnancy, but whether this involves turning on of the fast gene program is not known. A number of other more generalized transcription control mechanisms could also regulate smooth muscle contractile phenotype, including MAPK, NF-κB, and the constitutively active muscle-specific transcription factors MEF-2 and GATA4–6 (reviewed in Ref. 160). Myocardin, a member of the MRTF family, as a cofactor for the serum response factor (SRF), is a particularly potent transcriptional activator of the smooth muscle gene program (118, 136, 164, 175); how this might affect fast vs. slow gene programs has to my knowledge not been explored.
Far less is known about the control of the fast muscle gene program. The Six1+4 homeodomain proteins are necessary for the developmental activation of the fast striated muscle gene program (151). Whether they or homologs are expressed in and play a similar role in smooth muscle is unknown.
Posttranscriptional Control
Every step of gene expression, from transcription of pre-mRNA to the synthesis and degradation of the final protein product, is highly regulated. While for historic reasons the most progress has been made in transcriptional control, it is now clear that a vast amount of the regulation is posttranscriptional, e.g., at the level of the RNA. One of the first steps in the regulation of transcribed RNA is the splicing out of introns to form the mature mRNA. Across all vertebrate tissues a limited number of splicing factors have been identified that have restricted expression domains and may function as tissue-specific regulators of exon splicing (16, 132). These include Nova, Tra, Fox, CELF, MBNL, Hu, STAR/GSG, and TIA proteins. Additionally, these factors themselves undergo complex regulation through the use of alternative promoters, multiple splice variants, and nonsense-mediated decay of transcripts that may also provide specificity in their expression and activity. These modes of regulation seem to be more prominent than with transcription factors though this could reflect investigational bias (see for example Ref. 95).
There is limited information regarding the role of these factors in the phenotypic specification of the smooth (or any) muscle lineage. The Fox family of splicing factors were first identified in the worm Caenorhabditis elegans as the feminizing on X (fox) gene product. Subsequent studies identified three vertebrate homologs (Fox 1–3) that regulate alternative splicing by binding UGCAUG motifs (reviewed in Ref. 107). A diverse array of gene products are generated that are proposed to participate in tissue-specific splicing of exons in muscle, neuronal, and perhaps other cell types (34, 144). Their role in tissue-specific splicing of exons in smooth muscle has not been investigated; we did find a number of UGCAUG motifs in the introns surrounding the highly tissue-specific avian MYPT1 alternative exon E12 (39). The splicing of this exon is not evolutionarily conserved and so cannot serve as a model for control of exon splicing in mammals.
Transformer (Tra) proteins are the prototype for a master regulator of tissue-specific splicing. In Drosophila melanogaster the expression (splicing) of Tra in the female (XX) under the control of sxl (sex-lethal) is necessary and sufficient to determine all of the unique physical and behavioral characteristics of female flies (reviewed in Ref. 194). Tra with Tra-2 and classical SR proteins as cofactors regulate exon splicing of Doublesex (Dsx) and Fruitless (Fru), transcription factors that regulate the expression of the female- and male-specific gene programs. Vertebrate homologs of Tra-2 (Tra-2α and Tra2β) with ∼80% sequence conservation have been identified, shown to regulate RNA splicing, and to be functionally equivalent to the fly Tra-2 (36, 208) (reviewed in Refs. 16, 132). TIA proteins are named based on their original identification as T cell intracellular antigen (TIA-1) and TIA-related protein (TIA-R). They bind to U-rich sequence in RNAs and are proposed to regulate multiple aspects of RNA metabolism including exon splicing and RNA stability (reviewed in Refs. 16, 132). We have observed that Tra2β is expressed at up to 10-fold higher levels in rat phasic (PV, MA1) vs. tonic (large vessel) smooth muscle (195), while for TIA proteins it is just the opposite (196, 197). Furthermore, the expression of TIA and Tra proteins tracks with the tonic and phasic gene programs in multiple developmental and disease models described above. Tra2β can activate splicing of the MYPT1 E24 exon (fast isoform), while TIA proteins can activate splicing of the avian MYPT1 E12 exon (slow isoform). These preliminary functional characterizations suggest that Tra2β may activate the fast gene program and TIA the slow gene program in vascular and other smooth muscle tissues, a hypothesis that will require testing in vivo with gain-and-loss of function approaches.
The CELF (CUG-BP and ELAV-like factors) family of proteins (also described as Bruno-like proteins) constitute a family of six vertebrate genes that bind to RNA CUG repeats (reviewed in Ref. 11). Using a model gene approach, alternative splicing of cardiac TnT alt exon 5, it was proposed that CUGBP in competition with muscle-blind (MBNL) splicing suppressors may determine developmental specification of fast vs. slow cardiac muscle phenotype (85, 94, 108). MBNL is an evolutionarily conserved family of three genes and greater than 15 protein products generated by myriads of alternative splicing events in mammals that regulate alternative splicing (reviewed in Ref. 167). Preliminary analyses indicate that both CUG-BP and MBNL family members are expressed in somewhat tissue-restricted patterns and in smooth muscle tissues during development (21, 96, 167). Using a smooth-muscle specific alternative exon of α-actinin as a model, one group has proposed that CELF members ETR3 and CELF4 mediate the inclusion of this smooth muscle specific exon by overcoming the generalized repressive activity of the polypyrimidine tract binding protein (PTB) (71). These factors are of particular interest because of their proposed role in the pathogenesis of myotonic dystrophy (DM1+2). Sequestration of MBNL due to the expansion of CUG repeats in the DMPK 3′-untranslated region (UTR) is thought to be the mechanism for aberrant splicing of exons resulting in cardiac and skeletal muscle dystrophy (96, 158, 174). Smooth muscle involvement is suggested by symptoms of dysphagia and abdominal pain in humans with myotonic dystrophy, and low vascular resistance in a mouse model of overexpression of the DMPK (154). Whether CELF and MBNL family members determine smooth muscle contractile phenotype is not known. Interestingly the MBNL1 knockout mouse has increased splicing of MYPT1 E14 (46), though the splicing of this exon does not correlate with smooth muscle contractile phenotype.
MicroRNAs
Many regulatory steps control the subsequent steps of mRNA export, turnover, and translation into protein. The discovery of small (18–25 nt) highly conserved RNAs termed microRNAs (miRNAs) that turn off gene expression by blocking mRNA translation and/or increasing mRNA degradation has generated considerable excitement due to their therapeutic potential. The miRNAs bind to degenerate 6 nt sequence in the 3′-UTR of mRNAs, and because of this it is proposed that they will regulate expression of many genes or even entire gene programs (reviewed in Ref. 110). In striated muscle a series of miRNAs (−208a,b; −499) generated from within the MHC loci are proposed to determine expression of fast vs. slow gene programs, and stress or hypothyroid-induced gene switching, through their suppression of transcriptional repressors of slow myofiber genes (223–225). A role for miRNA in splicing dependent phenotypic specification is suggested by an in vitro (C2C12) model of skeletal myogenesis in which miR-133, -1, and -206 (all generated from the same precursor) block translation of the neural-specific isoform of the PTB splicing factor (20). In smooth muscle miR-143,-145 are generated from a bicistronic transcript under the control of SRF and myocardin and regulate the expression of the smooth muscle contractile genes, with different mechanisms having been proposed (33, 50, 243). There is no information on miRNAs control of smooth muscle fast vs. slow contractile gene program, but it is highly likely that they will play a significant role, particularly in vascular remodeling where there is substantial turnover of gene programs.
SUMMARY AND FUTURE DIRECTIONS
This review has examined smooth muscle phenotypic diversity in the vascular system in the context of phasic (fast) vs. slow (tonic) phenotypes. As a generalization the smaller arteries express components of the fast gene program that is hypothesized to be related to their phasic contractile activity termed vasomotion. In contrast the large arteries and veins express exclusively or nearly exclusively the slow gene program consistent with tonic force production. The portal venous smooth muscle is unique in the vascular system as a pure phasic smooth muscle and thus serves as an excellent model for the study of the regulated expression of the fast gene program in VSM. A number of topics in this field are ripe for further investigation:
1) The regulated expression of isoforms of the contractile regulatory machinery, including MP and kinase, and various ion channels is proposed to determine phenotype-specific response of VSM to constrictor and dilator signals. This should be tested through genetic substitution (reverse physiology) experiments.
2) To what extent do VSM phenotypic differences underlie the differences in the regulation of blood flow between the different vascular beds, i.e., pulmonary and coronary, mesenteric, cerebral, renal, and skeletal? Answering this question will require tools to isolate and characterize gene expression in small artery smooth muscle from the various organs, perhaps best done using a fluorescent marker for tonic vs. phasic phenotype. To date the mesenteric and renal small arteries have been best characterized, but still only to a limited extent. Differences between the renal afferent and efferent arteriolar, with only the former expressing fast (phasic) isoforms (reviewed above), suggests that the small vs. large artery dichotomy will turn out to be an oversimplification. Thus more extensive characterization of VSM phenotype in all vessels of all vascular beds is required. A related question is how these gene programs might impact on other aspect of vascular biology, e.g., the propensity of phasic and small VSM to undergo hypertrophic growth while the tonic VSM undergoes hyperplastic growth.
3) To what extent are the unique VSM phenotypes a function of their distinct developmental origins, i.e., neural crest, mesothelium, mesoderm, etc.? Studies to date suggest that the tonic (slow) gene program is the developmental default. Limited studies have shown that altered load or innervation will cause mature VSM with phasic properties to revert toward a tonic phenotype. However, further experimentation is required to distinguish between two hypotheses: 1) that VSM is genetically programmed to assume tonic vs. phasic phenotypes, perhaps dependent upon its tissue of origin, and load or innervation are required for maintenance of these phenotypes, or 2) VSM phenotype is entirely plastic and driven by innervation, load or another external signal.
4) To what extent do the isoform shifts and VSM phenotypic modulation determine functional differences in the regulation of the immature circulation and in disease states, and how does this influence drug responses?
The advent of high-throughput tools to assay gene expression, i.e., microarrays and more recently deep RNA sequencing, makes it now feasible to completely characterize gene expression between tissues and in different contexts, e.g., see Ref. 131. A limiting factor will be the ability to obtain sufficient quantities of pure populations of the relevant VSM cells. The unique and defined patterns of gene expression in VSM at different developmental stages and in different disease conditions will provide testable hypotheses regarding VSM function in organ-specific regulation of blood flow and drug responses in these different contexts.
GRANTS
Supported by National Heart, Lung, and Blood Institute Grant R01 HL-66171.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Alessi D, Macdougall LK, Sola MM, Ikebe M, Cohen P. The control of protein phosphatase-1 by targeting subunits. Eur J Biochem 210: 1023–1035, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Andreasen D, Friis UG, Uhrenholt TR, Jensen BL, Skott O, Hansen PB. Coexpression of voltage-dependent calcium channels Cav1.2, 21a, and 21b in vascular myocytes. Hypertension 47: 735–741, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Anwar MA, Ju K, Docherty CC, Poston L, Nathanielsz PW. Developmental changes in reactivity of small femoral arteries in the fetal and postnatal baboon. Am J Obstet Gynecol 184: 707–712, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Arimura T, Suematsu N, Zhou YB, Nishimura J, Satoh S, Takeshita A, Kanaide H, Kimura A. Identification, characterization, and functional analysis of heart-specific myosin light chain phosphatase small subunit. J Biol Chem 276: 6073–6082, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Arner A, Lofgren M, Morano I. Smooth, slow and smart muscle motors. J Muscle Res Cell Motil 24: 165–173, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Babu GJ, Loukianov E, Loukianova T, Pyne GJ, Huke S, Osol G, Low RB, Paul RJ, Periasamy M. Loss of SM-B myosin affects muscle shortening velocity and maximal force development. Nat Cell Biol 3: 1025–1029, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Babu GJ, Pyne GJ, Zhou Y, Okwuchukuasanya C, Brayden JE, Osol G, Paul RJ, Low RB, Periasamy M. Isoform switching from SM-B to SM-A myosin results in decreased contractility and altered expression of thin filament regulatory proteins. Am J Physiol Cell Physiol 287: C723–C729, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Bakker ENTP, Sorop O, Spaan JAE, VanBavel E. Remodeling of resistance arteries in organoid culture is modulated by pressure and pressure pulsation and depends on vasomotion. Am J Physiol Heart Circ Physiol 286: H2052–H2056, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Barlow CA, Rose P, Pulver-Kaste RA, Lounsbury KM. Excitation-transcription coupling in smooth muscle. J Physiol 570: 59–64, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreau C, Paillard L, Mereau A, Osborne HB. Mammalian CELF/Bruno-like RNA-binding proteins: molecular characteristics and biological functions. Biochimie 88: 515–525, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75: 19–37, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Berk BC. Vascular smooth muscle growth: autocrine growth mechanisms. Physiol Rev 81: 999–1030, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Bertoni S, Ballabeni V, Flammini L, Gobbetti T, Impicciatore M, Barocelli E. Intestinal chronic obstruction affects motor responsiveness of rat hypertrophic longitudinal and circular muscles. Neurogastroenterol Motil 20: 1234–1242, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Bertoni S, Gabella G, Ballabeni V, Ghirardi A, Impicciatore M, Barocelli E. Plasticity of rat small intestine after removal of a chronic mechanical obstruction. Neurogastroenterol Motil 18: 862–872, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Black DL. Mechanisms of alternative pre-messenger RNA splicing. Ann Rev Biochem 72: 291–336, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Blue EK, Goeckeler ZM, Jin Y, Hou L, Dixon SA, Herring BP, Wysolmerski RB, Gallagher PJ. 220- and 130-kDa MLCKs have distinct tissue distributions and intracellular localization patterns. Am J Physiol Cell Physiol 282: C451–C460, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonnet S, Archer SL. Potassium channel diversity in the pulmonary arteries and pulmonary veins: implications for regulation of the pulmonary vasculature in health and during pulmonary hypertension. Pharmacol Therapeut 115: 56–69, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Bonnevier J, Arner A. Actions downstream of cyclic GMP/protein kinase G can reverse protein kinase C-mediated phosphorylation of CPI-17 and Ca2+ sensitization in smooth muscle. J Biol Chem 279: 28998–29003, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Boutz PL, Chawla G, Stoilov P, Black DL. MicroRNAs regulate the expression of the alternative splicing factor nPTB during muscle development. Genes Dev 21: 71–84, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brimacombe KR, Ladd AN. Cloning and embryonic expression patterns of the chicken CELF family. Dev Dyn 236: 2216–2224, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Bruce L, Nixon GF. Increased sensitization of the myofilaments in rat neonatal portal vein: a potential mechanism. Exp Physiol 82: 985–993, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Buus CL, Pourageaud F, Fazzi GE, Janssen G, Mulvany MJ, De Mey JGR. Smooth muscle cell changes during flow-related remodeling of rat mesenteric resistance arteries. Circ Res 89: 180–186, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Camello-Almaraz C, Macias B, Gomez-Pinilla PJ, Alcon S, Martin-Cano FE, Baba A, Matsuda T, Camello PJ, Pozo MJ. Developmental changes in Ca2+ homeostasis and contractility in gallbladder smooth muscle. Am J Physiol Cell Physiol 296: C783–C791, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 356: 830–840, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Cheng X, Liu J, Asuncion-Chin M, Blaskova E, Bannister JP, Dopico AM, Jaggar JH. A novel Ca(V)1.2 N terminus expressed in smooth muscle cells of resistance size arteries modifies channel regulation by auxiliary subunits. J Biol Chem 282: 29211–29221, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chi M, Zhou Y, Vedamoorthyrao S, Babu GJ, Periasamy M. Ablation of smooth muscle myosin heavy chain SM2 increases smooth muscle contraction and results in postnatal death in mice. Proc Natl Acad Sci USA 105: 18614–18618, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chilian WM. Coronary microcirculation in health and disease. Summary of an NHLBI workshop. Circulation 95: 522–528, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chilton L, Loutzenhiser K, Morales E, Breaks J, Kargacin GJ, Loutzenhiser R. Inward rectifier K+ currents Kir2.1 expression in renal afferent and efferent arterioles. J Am Soc Nephrol 19: 69–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu A, Murray JJ, Lin CC, Russell M, Hagen PO, Cobb FR. Preferential proximal coronary dilation by activators of guanylate cyclase in awake dogs. Am J Physiol Heart Circ Physiol 259: H340–H345, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Collardeau-Frachon S, Scoazec JY. Vascular development and differentiation during human liver organogenesis. Anat Rec (Hoboken) 291: 614–627, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci 121: 11–18, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460: 705–710, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damianov A, Black DL. Autoregulation of Fox protein expression to produce dominant negative splicing factors. RNA 16: 405–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damon DH. Sympathetic innervation promotes vascular smooth muscle differentiation. Am J Physiol Heart Circ Physiol 288: H2785–H2791, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Dauwalder B, Amaya-Manzanares F, Mattox W. A human homologue of the Drosophila sex determination factor transformer-2 has conserved splicing regulatory functions. Proc Natl Acad Sci USA 93: 9004–9009, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Mey JGR, Schiffers PM, Hilgers RHP, Sanders MMW. Toward functional genomics of flow-induced outward remodeling of resistance arteries. Am J Physiol Heart Circ Physiol 288: H1022–H1027, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Delafontaine P, Song YH, Li Y. Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 24: 435–444, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Dirksen WP, Mohamed SA, Fisher SA. Splicing of a myosin phosphatase targeting subunit 1 alternative exon is regulated by intronic cis-elements and a novel bipartite exonic enhancer/silencer element. J Biol Chem 278: 9722–9732, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Dirksen WP, Vladic F, Fisher SA. A myosin phosphatase targeting subunit isoform transition defines a smooth muscle developmental phenotypic switch. Am J Physiol Cell Physiol 278: C589–C600, 2000 [DOI] [PubMed] [Google Scholar]
- 41.DiSanto ME, Cox RH, Wang Z, Chacko S. NH2-terminal-inserted myosin II heavy chain is expressed in smooth muscle of small muscular arteries. Am J Physiol Cell Physiol 272: C1532–C1542, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Dixon RE, Hwang SJ, Hennig GW, Ramsey KH, Schripsema JH, Sanders KM, Ward SM. Chlamydia infection causes loss of pacemaker cells and inhibits oocyte transport in the mouse oviduct. Biol Reprod 80: 665–673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Docherty CC, Kalmar-Nagy J, Engelen M, Nathanielsz PW. Development of fetal vascular responses to endothelin-1 and acetylcholine in the sheep. Am J Physiol Regul Integr Comp Physiol 280: R554–R562, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Domkowski PW, Cockerham JT, Kot PA, Myers JL, Wallace RB, Hopkins RA. The role of N omega-nitro-l-arginine in modulation of pulmonary vascular tone in the maturing newborn pig. J Thorac Cardiovasc Surg 110: 1486–1492, 1995 [DOI] [PubMed] [Google Scholar]
- 45.Drew JS, Murphy RA. Actin isoform expression, cellular heterogeneity, and contractile function in smooth muscle. Can J Physiol Pharmacol 75: 869–877, 1997 [PubMed] [Google Scholar]
- 46.Du H, Cline MS, Osborne RJ, Tuttle DL, Clark TA, Donohue JP, Hall MP, Shiue L, Swanson MS, Thornton CA, Ares M. Aberrant alternative splicing and extracellular matrix gene expression in mouse models of myotonic dystrophy. Nat Struct Mol Biol 17: 187–193, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46a.Duan SZ, Usher MG, Mortensen RM. Peroxisome proliferator-activated receptor-gamma mediates effects in the vasculature. Circ Res 102: 283–294, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Duncker DJ, Bache RJ. Regulation of coronary blood flow during exercise. Physiol Rev 88: 1009–1086, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Edwards FR, Hirst GD, Silverberg GD. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. J Physiol 404: 455–466, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ehmke H. Developmental physiology of the cardiovascular system. Am J Physiol Regul Integr Comp Physiol 282: R331–R333, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MVG, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ 16: 1590–1598, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eto M, Senba S, Morita F, Yazawa M. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett 410: 356–360, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Eto M. Regulation of cellular protein phosphatase-1 (PP1) by phosphorylation of the CPI-17 family, C-kinase-activated PP1 inhibitors. J Biol Chem 284: 35273–35277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HHHW, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov 5: 755–768, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fatigati V, Murphy RA. Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem 259: 14383–14388, 1984 [PubMed] [Google Scholar]
- 55.Feletou M, Hoeffner U, Vanhoutte PM. Endothelium-dependent relaxing factors do not affect the smooth muscle of portal vein. Blood Vessels 26: 21–32, 1989 [PubMed] [Google Scholar]
- 56.Fetalvero KM, Zhang P, Shyu M, Young BT, Hwa J, Young RC, Martin KA. Prostacyclin primes pregnant human myometrium for an enhanced contractile response in parturition. J Clin Invest 118: 3966–3979, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 19: 277–284, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Fineman JR, Wong J, Morin FC, III, Wild LM, Soifer SJ. Chronic nitric oxide inhibition in utero produces persistent pulmonary hypertension in newborn lambs. J Clin Invest 93: 2675–2683, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisher SA, Ikebe M. Developmental and tissue distribution of expression of nonmuscle and smooth muscle isoforms of myosin light chain kinase. Biochem Biophys Res Commun 217: 696–703, 1995 [DOI] [PubMed] [Google Scholar]
- 60.Fisher SA, Ikebe M, Brozovich FV. Endothelin-1 alters the contractile phenotype of cultured embryonic smooth muscle cells. Circ Res 80: 885–893, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Fodor AA, Aldrich RW. Convergent evolution of alternative splices at domain boundaries of the BK channel. Annu Rev Physiol 71: 19–36, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Fountain SJ, Cheong A, Flemming R, Mair L, Sivaprasadarao A, Beech DJ. Functional up-regulation of KCNA gene family expression in murine mesenteric resistance artery smooth muscle. J Physiol 556: 29–42, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frei E, Huster M, Smital P, Schlossmann J, Hofmann F, Wegener JW. Calcium-dependent and Calcium-independent inhibition of contraction by cGMP/cGKI in intestinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 297: G834–G839, 2009 [DOI] [PubMed] [Google Scholar]
- 64.Gabella G. Development of visceral smooth muscle. Results Probl Cell Differ 38: 1–37, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Gallagher PJ, Herring BP, Griffin SA, Stull JT. Molecular characterization of a mammalian smooth muscle myosin light chain kinase. J Biol Chem 266: 23936–23944, 1991 [PMC free article] [PubMed] [Google Scholar]
- 66.Garland JG, McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol 105: 429–435, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Given AM, Ogut O, Brozovich FV. MYPT1 mutants demonstrate the importance of aa 888–928 for the interaction with PKGIα. Am J Physiol Cell Physiol 292: C432–C439, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Goehler LE, Sternini C. Calcitonin gene-related peptide innervation of the rat hepatobiliary system. Peptides 17: 209–217, 1996 [DOI] [PubMed] [Google Scholar]
- 69.Gong MC, Cohen P, Kitazawa T, Ikebe M, Masuo M, Somlyo AP, Somlyo AV. Myosin light chain phosphatase activities and the effects of phosphatase inhibitors in tonic and phasic smooth muscle. J Biol Chem 267: 14662–14668, 1992 [PubMed] [Google Scholar]
- 70.Goyal RK, Chaudhury A. Mounting evidence against the role of ICC in neurotransmission to smooth muscle in the gut. Am J Physiol Gastrointest Liver Physiol 298: G10–G13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gromak N, Rideau A, Southby J, Scadden ADJ, Gooding C, Huttelmaier S, Singer RH, Smith CWJ. The PTB interacting protein raver1 regulates alpha-tropomyosin alternative splicing. EMBO J 22: 6356–6364, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haddock RE, Hill CE. Rhythmicity in arterial smooth muscle. J Physiol 566: 645–656, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haines N, Wang S, Undar A, Alkan T, Akcevin A. Clinical outcomes of pulsatile and non-pulsatile mode of perfusion. J Extra Corpor Technol 41: 26–29, 2009 [PMC free article] [PubMed] [Google Scholar]
- 74.Hansen PB, Jensen BL, Andreasen D, Skott O. Differential expression of T- and L-type voltage-dependent calcium channels in renal resistance vessels. Circ Res 89: 630–638, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Hasegawa Y, Morita F. Role of 17-kDa essential light chain isoforms of aorta smooth muscle myosin. J Biochem (Tokyo) 111: 804–809, 1992 [DOI] [PubMed] [Google Scholar]
- 76.Haworth SG. Development of the normal and hypertensive pulmonary vasculature. Exp Physiol 80: 843–853, 1995 [DOI] [PubMed] [Google Scholar]
- 77.Hayashi Y, Senba S, Yazawa M, Brautigan DL, Eto M. Defining the structural determinants and a potential mechanism for inhibition of myosin phosphatase by the protein kinase C-potentiated inhibitor protein of 17 kDa. J Biol Chem 276: 39858–39863, 2001 [DOI] [PubMed] [Google Scholar]
- 78.Helper DJ, Lash JA, Hathaway DR. Distribution of isoelectric variants of the 17,000-dalton myosin light chain in mammalian smooth muscle. J Biol Chem 263: 15748–15753, 1988 [PubMed] [Google Scholar]
- 79.Herring BP, El Mounayri O, Gallagher PJ, Yin F, Zhou J. Regulation of myosin light chain kinase and telokin expression in smooth muscle tissues. Am J Physiol Cell Physiol 291: C817–C827, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herring BP, Lyons GE, Hoggatt AM, Gallagher PJ. Telokin expression is restricted to smooth muscle tissues during mouse development. Am J Physiol Cell Physiol 280: C12–C21, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev 90: 291–366, 2010 [DOI] [PubMed] [Google Scholar]
- 82.High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet 9: 49–61, 2008 [DOI] [PubMed] [Google Scholar]
- 83.High FA, Zhang M, Proweller A, Tu L, Parmacek MS, Pear WS, Epstein JA. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J Clin Invest 117: 353–363, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83a.Hill MA, Yang Y, Ella SR, Davis MJ, Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett 584: 2033–2042, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hill-Eubanks DC, Gomez MF, Stevenson AS, Nelson MT. NFAT regulation in smooth muscle. Trends Cardiovasc Med 13: 56–62, 2003 [DOI] [PubMed] [Google Scholar]
- 85.Ho TH, Charlet B, Poulos MG, Singh G, Swanson MS, Cooper TA. Muscleblind proteins regulate alternative splicing. EMBO J 23: 3103–3112, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hofmann F, Feil R, Kleppisch T, Schlossmann J. Function of cGMP-dependent protein kinases as revealed by gene deletion. Physiol Rev 86: 1–23, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Hong H, Aksenov S, Guan X, Fallon JT, Waters D, Chen C. Remodeling of small intramyocardial coronary arteries distal to a severe epicardial coronary artery stenosis. Arterioscler Thromb Vasc Biol 22: 2059–2065, 2002 [DOI] [PubMed] [Google Scholar]
- 88.Hwa JJ, Ghibaudi L, Williams P, Chatterjee M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol Heart Circ Physiol 266: H952–H958, 1994 [DOI] [PubMed] [Google Scholar]
- 89.Injeti ER, Sandoval RJ, Williams JM, Smolensky AV, Ford LE, Pearce WJ. Maximal stimulation-induced in situ myosin light chain kinase activity is upregulated in fetal compared with adult ovine carotid arteries. Am J Physiol Heart Circ Physiol 295: H2289–H2298, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ito M, Dabrowska R, Guerrerio V, Jr, Hartshorne DJ. Identification in turkey gizzard of an acidic protein related to the C-terminal portion of smooth muscle myosin light chain kinase. J Biol Chem 264: 13971–13974, 1989 [PubMed] [Google Scholar]
- 91.Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem 259: 197–209, 2004 [DOI] [PubMed] [Google Scholar]
- 92.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation 12: 113–127, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kakkar R, Ye B, Stoller DA, Smelley M, Shi NQ, Galles K, Hadhazy M, Makielski JC, McNally EM. Spontaneous coronary vasospasm in KATP mutant mice arises from a smooth muscle-extrinsic process. Circ Res 98: 682–689, 2006 [DOI] [PubMed] [Google Scholar]
- 94.Kalsotra A, Xiao X, Ward AJ, Castle JC, Johnson JM, Burge CB, Cooper TA. A postnatal switch of CELF and MBNL proteins reprograms alternative splicing in the developing heart. Proc Natl Acad Sci USA 105: 20333–20338, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kanadia RN, Cepko CL. Alternative splicing produces high levels of noncoding isoforms of bHLH transcription factors during development. Genes Dev 24: 229–234, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Kanadia RN, Johnstone KA, Mankodi A, Lungu C, Thornton CA, Esson D, Timmers AM, Hauswirth WW, Swanson MS. A muscleblind knockout model for myotonic dystrophy. Science 302: 1978–1980, 2003 [DOI] [PubMed] [Google Scholar]
- 97.Karagiannis P, Babu GJ, Periasamy M, Brozovich FV. Myosin heavy chain isoform expression regulates shortening velocity in smooth muscle: studies using an SMB KO mouse line. J Muscle Res Cell Motil 25: 149–158, 2004 [DOI] [PubMed] [Google Scholar]
- 98.Karim SM, Rhee AY, Given AM, Faulx MD, Hoit BD, Brozovich FV. Vascular reactivity in heart failure: role of myosin light chain phosphatase. Circ Res 95: 612–618, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Kelley CA, Takahashi M, Yu JH, Adelstein RS. An insert of seven amino acids confers functional differences between smooth muscle myosins from the intestines and vasculature. J Biol Chem 268: 12848–12854, 1993 [PubMed] [Google Scholar]
- 100.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem 276: 37250–37257, 2001 [DOI] [PubMed] [Google Scholar]
- 101.Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R, Owens GK, Somlyo AP, Somlyo AV. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc Natl Acad Sci USA 103: 2440–2445, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim KY, Kovacs M, Kawamoto S, Sellers JR, Adelstein RS. Disease-associated mutations and alternative splicing alter the enzymatic and motile activity of nonmuscle myosins II-B and II-C. J Biol Chem 280: 22769–22775, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Koeppen M, Feil R, Siegl D, Feil S, Hofmann F, Pohl U, de Wit C. cGMP-dependent protein kinase mediates NO- but not acetylcholine-induced dilations in resistance vessels in vivo. Hypertension 44: 952–955, 2004 [DOI] [PubMed] [Google Scholar]
- 104.Komatsu S, Miyazaki K, Tuft RA, Ikebe M. Translocation of telokin by cGMP signaling in smooth muscle cells. Am J Physiol Cell Physiol 283: C752–C761, 2002 [DOI] [PubMed] [Google Scholar]
- 105.Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, Osinska HE, Lorenz JN, Brosseau C, Federico A, Alpert NR, Warshaw DM, Perryman MB, Helmke SM, Robbins J. Analysis of myosin heavy chain functionality in the heart. J Biol Chem 278: 17466–17474, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Kuo L, Davis MJ, Chilian WM. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation 92: 518–525, 1995 [DOI] [PubMed] [Google Scholar]
- 107.Kuroyanagi H. Fox-1 family of RNA-binding proteins. Cell Mol Life Sci 66: 3895–3907, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ladd AN, Stenberg MG, Swanson MS, Cooper TA. Dynamic balance between activation and repression regulates pre-mRNA alternative splicing during heart development. Dev Dyn 233: 783–793, 2005 [DOI] [PubMed] [Google Scholar]
- 109.Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231: 405–407, 1986 [DOI] [PubMed] [Google Scholar]
- 110.Liang M. MicroRNA: a new entrance to the broad paradigm of systems molecular medicine. Physiol Genomics 38: 113–115, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liao P, Yong TF, Liang MC, Yue DT, Soong TW. Splicing for alternative structures of Cav1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovasc Res 68: 197–203, 2005 [DOI] [PubMed] [Google Scholar]
- 112.Liao P, Yu D, Li G, Yong TF, Soon JL, Chua YL, Soong TW. A smooth muscle Cav1.2 calcium channel splice variant underlies hyperpolarized window current and enhanced state-dependent inhibition by nifedipine. J Biol Chem 282: 35133–35142, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Lim KM, Kim IS, Choi SW, Min BG, Won YS, Kim HY, Shim EB. Computational analysis of the effect of the type of LVAD flow on coronary perfusion and ventricular afterload. J Physiol Sci 59: 307–316, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lincoln TM, Komalavilias P. Cyclic GMP-Mediated Signaling Mechanisms in Smooth Muscle. In: Nitric Oxide Biology and Pathobiology, edited by Ignarro LJ. San Diego, CA: Academic, 2000, p. 401–442 [Google Scholar]
- 115.Liu SF, Hislop AA, Haworth SG, Barnes PJ. Developmental changes in endothelium-dependent pulmonary vasodilatation in pigs. Br J Pharmacol 106: 324–330, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ljung B, Lundberg JM, Dahlstrom A, Kjellstedt A. Structural and functional ontogenetic development of the rat portal vein after neonatal 6-hydroxydopamine treatment. Acta Physiol Scand 106: 271–279, 1979 [DOI] [PubMed] [Google Scholar]
- 117.Lofgren M, Fagher K, Woodard G, Arner A. Effects of thyroxine on myosin isoform expression and mechanical properties in guinea-pig smooth muscle. J Physiol 543: 757–766, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Long X, Bell RD, Gerthoffer WT, Zlokovic BV, Miano JM. Myocardin is sufficient for a smooth muscle-like contractile phenotype. Arterioscler Thromb Vasc Biol 28: 1505–1510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Loukianov E, Loukianova T, Periasamy M. Myosin heavy chain isoforms in smooth muscle. Comp Biochem Physiol B 117: 13–18, 1997 [DOI] [PubMed] [Google Scholar]
- 120.Low RB, Mitchell J, Woodcock-Mitchell J, Rovner AS, White SL. Smooth-muscle myosin heavy-chain SM-B isoform expression in developing and adult rat lung. Am J Respir Cell Mol Biol 20: 651–657, 1999 [DOI] [PubMed] [Google Scholar]
- 121.Lu Y, Zhang H, Gokina N, Mandala M, Sato O, Ikebe M, Osol G, Fisher SA. Uterine artery myosin phosphatase isoform switching and increased sensitivity to SNP in a rat l-NAME model of hypertension of pregnancy. Am J Physiol Cell Physiol 294: C564–C571, 2008 [DOI] [PubMed] [Google Scholar]
- 122.Madden JA, Dantuma MW, Sorokina EA, Weihrauch D, Kleinman JG. Telokin expression and the effect of hypoxia on its phosphorylation status in smooth muscle cells from small and large pulmonary arteries. Am J Physiol Lung Cell Mol Physiol 294: L1166–L1173, 2008 [DOI] [PubMed] [Google Scholar]
- 123.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol 27: 1248–1258, 2007 [DOI] [PubMed] [Google Scholar]
- 124.Malmqvist U, Arner A. Contractile properties during development of hypertrophy of the smooth muscle in the rat portal vein. Acta Physiol Scand 133: 49–61, 1988 [DOI] [PubMed] [Google Scholar]
- 125.Malmqvist U, Arner A. Isoform distribution and tissue contents of contractile and cytoskeletal proteins in hypertrophied smooth muscle from rat portal vein. Circ Res 66: 832–845, 1990 [DOI] [PubMed] [Google Scholar]
- 126.Malmqvist U, Arner A. Correlation between isoform composition of the 17 kDa myosin light chain and maximal shortening velocity in smooth muscle. Pflügers Arch 418: 523–530, 1991. [DOI] [PubMed] [Google Scholar]
- 127.Marshall JM. Vertebrate smooth muscle. In: Medical Physiology, edited by Mountcastle VB. St. Louis, MO: C. V. Mosby, 1980, p. 120–148 [Google Scholar]
- 128.Marston S, El-Mezgueldi M. Role of tropomyosin in the regulation of contraction in smooth muscle. Adv Exp Med Biol 644: 110–123, 2008 [DOI] [PubMed] [Google Scholar]
- 129.Martin C, Gunning P. Isoform sorting of tropomyosins. Adv Exp Med Biol 644: 187–200, 2008 [DOI] [PubMed] [Google Scholar]
- 130.Matchkov VV, Larsen P, Bouzinova EV, Rojek A, Boedtkjer DMB, Golubinskaya V, Pedersen FS, Aalkjaer C, Nilsson H. Bestrophin-3 (vitelliform macular dystrophy 2-like 3 protein) is essential for the cGMP-dependent calcium-activated chloride conductance in vascular smooth muscle cells. Circ Res 103: 864–872, 2008 [DOI] [PubMed] [Google Scholar]
- 131.Matkovich SJ, Zhang Y, Van Booven DJ, Dorn GW. Deep mRNA sequencing for in vivo functional analysis of cardiac transcriptional regulators: application to Galphaq. Circ Res 106: 1459–1467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matlin AJ, Clark F, Smith CWJ. Understanding alternative splicing: toward a cellular code. Nat Rev Mol Cell Biol 6: 386–398, 2005 [DOI] [PubMed] [Google Scholar]
- 133.McHugh KM. Molecular analysis of gastrointestinal smooth muscle development. J Pediatr Gastroenterol Nutr 23: 379–394, 1996 [DOI] [PubMed] [Google Scholar]
- 134.McMurphy DS, Ljung B. Neuroeffector maturity of portal veins from newborn rats, rabbits, cats and guinea pigs. Acta Physiol Scand 102: 218–223, 1978 [DOI] [PubMed] [Google Scholar]
- 135.Meyer C, de Vries G, Davidge ST, Mayes DC. Reassessing the mathematical modeling of the contribution of vasomotion to vascular resistance. J Appl Physiol 92: 888–889, 2002 [DOI] [PubMed] [Google Scholar]
- 136.Miano JM, Long X, Fujiwara K. Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol 292: C70–C81, 2007 [DOI] [PubMed] [Google Scholar]
- 138.Michael SK, Surks HK, Wang Y, Zhu Y, Blanton R, Jamnongjit M, Aronovitz M, Baur W, Ohtani K, Wilkerson MK, Bonev AD, Nelson MT, Karas RH, Mendelsohn ME. High blood pressure arising from a defect in vascular function. Proc Natl Acad Sci USA 105: 6702–6707, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moga MA, Nakamura T, Robbins J. Genetic approaches for changing the heart and dissecting complex syndromes. J Mol Cell Cardiol 45: 148–155, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morano I. Tuning smooth muscle contraction by molecular motors. J Mol Med 81: 481–487, 2003 [DOI] [PubMed] [Google Scholar]
- 141.Moreau R, Lebrec D. Molecular and structural basis of portal hypertension. Clin Liver Dis 10: 445–457, 2006 [DOI] [PubMed] [Google Scholar]
- 142.Murphy RA, Rembold CM. The latch-bridge hypothesis of smooth muscle contraction. Can J Physiol Pharmacol 83: 857–864, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nagao T, Illiano S, Vanhoutte PM. Heterogeneous distribution of endothelium-dependent relaxations resistant to NG-nitro-l-arginine in rats. Am J Physiol Heart Circ Physiol 263: H1090–H1094, 1992 [DOI] [PubMed] [Google Scholar]
- 144.Nakahata S, Kawamoto S. Tissue-dependent isoforms of mammalian Fox-1 homologs are associated with tissue-specific splicing activities. Nucl Acids Res 33: 2078–2089, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res 101: 712–722, 2007 [DOI] [PubMed] [Google Scholar]
- 146.Nankervis CA, Dunaway DJ, Nowicki PT. Determinants of terminal mesenteric artery resistance during the first postnatal month. Am J Physiol Gastrointest Liver Physiol 280: G678–G686, 2001 [DOI] [PubMed] [Google Scholar]
- 147.Nankervis CA, Reber KM, Nowicki PT. Age-dependent changes in the postnatal intestinal microcirculation. Microcirculation 8: 377–387, 2001 [DOI] [PubMed] [Google Scholar]
- 148.Nauli SM, Zhang L, Pearce WJ. Maturation depresses cGMP-mediated decreases in [Ca2+]i and Ca2+ sensitivity in ovine cranial arteries. Am J Physiol Heart Circ Physiol 280: H1019–H1028, 2001 [DOI] [PubMed] [Google Scholar]
- 149.Nelson MT, Rubart M, Santana LF, Boney AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science 270: 633–637, 1995 [DOI] [PubMed] [Google Scholar]
- 150.Nilsson H, Aalkjaer C. Vasomotion: mechanisms and physiological importance. Mol Interv 3: 79–89, 2003 [DOI] [PubMed] [Google Scholar]
- 151.Niro C, Demignon J, Vincent S, Liu Y, Giordani J, Sgarioto N, Favier M, Guillet-Deniau I, Blais A, Maire P. Six1 and Six4 gene expression is necessary to activate the fast-type muscle gene program in the mouse primary myotome. Dev Biol 338: 168–182, 2010 [DOI] [PubMed] [Google Scholar]
- 152.Nishikawa Y, Stepp DW, Chilian WM. In vivo location and mechanism of EDHF-mediated vasodilation in canine coronary microcirculation. Am J Physiol Heart Circ Physiol 277: H1252–H1259, 1999 [DOI] [PubMed] [Google Scholar]
- 153.Nystoriak MA, Murakami K, Penar PL, Wellman GC. Cav1.2 splice variant with exon 9* is critical for regulation of cerebral artery diameter. Am J Physiol Heart Circ Physiol 297: H1820–H1828, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O'Cochlain DF, Perez-Terzic C, Reyes S, Kane GC, Behfar A, Hodgson DM, Strommen JA, Liu XK, van den Broek W, Wansink DG, Wieringa B, Terzic A. Transgenic overexpression of human DMPK accumulates into hypertrophic cardiomyopathy, myotonic myopathy and hypotension traits of myotonic dystrophy. Hum Mol Genet 13: 2505–2518, 2004 [DOI] [PubMed] [Google Scholar]
- 155.Ody M, Thievent A, Millet M, Connat JL. Postnatal development of the rat portal vein: correlation with occurrence of peptidergic innervation. Cell Tissue Res 272: 303–314, 1993 [DOI] [PubMed] [Google Scholar]
- 156.Ogut O, Yuen SL, Brozovich FV. Regulation of the smooth muscle contractile phenotype by nonmuscle myosin. J Muscle Res Cell Motil 28: 409–414, 2007 [DOI] [PubMed] [Google Scholar]
- 157.Ogut O, Brozovich FV. Determinants of the contractile properties in the embryonic chicken gizzard and aorta. Am J Physiol Cell Physiol 279: C1722–C1732, 2000 [DOI] [PubMed] [Google Scholar]
- 158.Orengo JP, Cooper TA. Alternative splicing in disease. Adv Exp Med Biol 623: 212–223, 2007 [DOI] [PubMed] [Google Scholar]
- 159.Owens GK. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev 75: 487–517, 1995 [DOI] [PubMed] [Google Scholar]
- 160.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004 [DOI] [PubMed] [Google Scholar]
- 161.Owens GK, Thompson MM. Developmental changes in isoactin expression in rat aortic smooth muscle cells in vivo. J Biol Chem 261: 13373–13380, 1986 [PubMed] [Google Scholar]
- 162.Pannen BH, Bauer M. Differential regulation of hepatic arterial and portal venous vascular resistance by nitric oxide and carbon monoxide in rats. Life Sci 62: 2025–2033, 1998 [DOI] [PubMed] [Google Scholar]
- 163.Park W, Han J, Earm Y. Physiological role of inward rectifier K+ channels in vascular smooth muscle cells. Pflügers Arch 457: 137–147, 2008 [DOI] [PubMed] [Google Scholar]
- 164.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res 100: 633–644, 2007 [DOI] [PubMed] [Google Scholar]
- 165.Parr T, Waites GT, Patel B, Millake DB, Critchley DR. A chick skeletal-muscle alpha-actinin gene gives rise to two alternatively spliced isoforms which differ in the EF-hand Ca2+-binding domain. Eur J Biochem 210: 801–809, 1992 [DOI] [PubMed] [Google Scholar]
- 166.Parthimos D, Haddock RE, Hill CE, Griffith TM. Dynamics of a three-variable nonlinear model of vasomotion: comparison of theory and experiment. Biophys J 93: 1534–1556, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pascual M, Vicente M, Monferrer L, Artero R. The Muscleblind family of proteins: an emerging class of regulators of developmentally programmed alternative splicing. Differentiation 74: 65–80, 2006 [DOI] [PubMed] [Google Scholar]
- 168.Patzak A, Petzhold D, Wronski T, Martinka P, Babu GJ, Periasamy M, Haase H, Morano I. Constriction velocities of renal afferent and efferent arterioles of mice are not related to SMB expression. Kidney Int 68: 2726–2734, 2005 [DOI] [PubMed] [Google Scholar]
- 169.Payne MC, Zhang HY, Prosdocimo T, Joyce KM, Koga Y, Ikebe M, Fisher SA. Myosin phosphatase isoform switching in vascular smooth muscle development. J Mol Cell Cardiol 40: 274–282, 2006 [DOI] [PubMed] [Google Scholar]
- 170.Payne MC, Zhang HY, Shirasawa Y, Koga Y, Ikebe M, Benoit JN, Fisher SA. Dynamic changes in expression of myosin phosphatase in a model of portal hypertension. Am J Physiol Heart Circ Physiol 286: H1801–H1810, 2004 [DOI] [PubMed] [Google Scholar]
- 171.Peng H, Matchkov V, Ivarsen A, Aalkjar C, Nilsson H. Hypothesis for the initiation of vasomotion. Circ Res 88: 810–815, 2001 [DOI] [PubMed] [Google Scholar]
- 172.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszodi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fassler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17: 3045–3051, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pfitzer G, Merkel L, Ruegg JC, Hofmann F. Cyclic GMP-dependent protein kinase relaxes skinned fibers from guinea pig taenia coli but not from chicken gizzard. Pflügers Arch 407: 87–91, 1986 [DOI] [PubMed] [Google Scholar]
- 174.Philips AV, Timchenko LT, Cooper TA. Disruption of splicing regulated by a CUG-binding protein in myotonic dystrophy. Science 280: 737–741, 1998 [DOI] [PubMed] [Google Scholar]
- 175.Pipes GCT, Creemers EE, Olson EN. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev 20: 1545–1556, 2006 [DOI] [PubMed] [Google Scholar]
- 176.Pistea A, Bakker EN, Spaan JA, VanBavel E. Flow inhibits inward remodeling in cannulated porcine small coronary arteries. Am J Physiol Heart Circ Physiol 289: H2632–H2640, 2005 [DOI] [PubMed] [Google Scholar]
- 177.Ploug KB, Baun M, Hay-Schmidt A, Olesen J, Jansen-Olesen I. Presence and vascular pharmacology of KATP channel subtypes in rat central and peripheral tissues. Eur J Pharmacol 637: 109–117, 2010 [DOI] [PubMed] [Google Scholar]
- 178.Popel AS, Goldman D, Vadapalli A. Modeling of oxygen diffusion from the blood vessels to intracellular organelles. Adv Exp Med Biol 530: 485–495, 2003 [DOI] [PubMed] [Google Scholar]
- 179.Poulsen AN, Wulf H, Hay-Schmidt A, Jansen-Olesen I, Olesen J, Klaerke DA. Differential expression of BK channel isoforms and beta-subunits in rat neuro-vascular tissues. Biochim Biophys Acta 1788: 380–389, 2009 [DOI] [PubMed] [Google Scholar]
- 180.Pourageaud F, De Mey JG. Structural properties of rat mesenteric small arteries after 4-wk exposure to elevated or reduced blood flow. Am J Physiol Heart Circ Physiol 273: H1699–H1706, 1997 [DOI] [PubMed] [Google Scholar]
- 181.Pourageaud F, De Mey JG. Vasomotor responses in chronically hyperperfused and hypoperfused rat mesenteric arteries. Am J Physiol Heart Circ Physiol 274: H1301–H1307, 1998 [DOI] [PubMed] [Google Scholar]
- 182.Quayle JM, Dart C, Standen NB. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth muscle. J Physiol 494: 715–726, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reber KM, Nankervis CA, Nowicki PT. Newborn intestinal circulation. Physiol Pathophysiol Clin Perinatol 29: 23–39, 2002 [DOI] [PubMed] [Google Scholar]
- 184.Rhee AY, Ogut O, Brozovich FV. Nonmuscle myosin, force maintenance, and the tonic contractile phenotype in smooth muscle. Pflügers Arch 452: 766–774, 2006 [DOI] [PubMed] [Google Scholar]
- 185.Sanders KM, Ward SM. Interstitial cells of Cajal: a new perspective on smooth muscle function. J Physiol 576: 721–726, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sandoval RJ, Injeti ER, Gerthoffer WT, Pearce WJ. Postnatal maturation modulates relationships among cytosolic Ca2+, myosin light chain phosphorylation, and contractile tone in ovine cerebral arteries. Am J Physiol Heart Circ Physiol 293: H2183–H2192, 2007 [DOI] [PubMed] [Google Scholar]
- 187.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem 275: 21722–21729, 2000 [DOI] [PubMed] [Google Scholar]
- 188.Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 22: 269–278, 2007 [DOI] [PubMed] [Google Scholar]
- 189.Sellke FW, Myers PR, Bates JN, Harrison DG. Influence of vessel size on the sensitivity of porcine coronary microvessels to nitroglycerin. Am J Physiol Heart Circ Physiol 258: H515–H520, 1990 [DOI] [PubMed] [Google Scholar]
- 190.Seppey D, Sauser R, Koenigsberger M, Beny JL, Meister JJ. Does the endothelium abolish or promote arterial vasomotion in rat mesenteric arteries? Explanations for the seemingly contradictory effects. J Vasc Res 45: 416–426, 2008 [DOI] [PubMed] [Google Scholar]
- 191.Sharma AK, Zhou GP, Kupferman J, Surks HK, Christensen EN, Chou JJ, Mendelsohn ME, Rigby AC. Probing the interaction between the coiled coil leucine zipper of cGMP-dependent protein kinase Ialpha and the C terminus of the myosin binding subunit of the myosin light chain phosphatase. J Biol Chem 283: 32860–32869, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 28: 703–711, 1996 [DOI] [PubMed] [Google Scholar]
- 193.Shiraishi M, Wang X, Walsh MP, Kargacin G, Loutzenhiser K, Loutzenhiser R. Myosin heavy chain expression in renal afferent and efferent arterioles: relationship to contractile kinetics and function. FASEB J 17: 2284–2286, 2003 [DOI] [PubMed] [Google Scholar]
- 194.Shirangi TR, McKeown M. Sex in flies: what ‘body–mind’ dichotomy? Dev Biol 306: 10–19, 2007 [DOI] [PubMed] [Google Scholar]
- 195.Shukla S, Fisher SA. Tra2beta as a novel mediator of vascular smooth muscle diversification. Circ Res 103: 485–492, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shukla S, Gatto-Konczak F, Breathnach R, Fisher SA. Competition of PTB with TIA proteins for binding to a U-rich cis-element determines tissue-specific splicing of the myosin phosphatase targeting subunit 1. RNA 11: 1725–1736, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Shukla S, Dirksen WP, Joyce KM, Guiner-Blanvillain CL, Breathnach R, Fisher SA. TIA proteins are necessary but not sufficient for the tissue-specific splicing of the myosin phosphatase targeting subunit 1. J Biol Chem 279: 13668–13676, 2004 [DOI] [PubMed] [Google Scholar]
- 198.Sobue K, Hayashi K, Nishida W. Expressional regulation of smooth muscle cell-specific genes in association with phenotypic modulation. Mol Cell Biochem 190: 105–118, 1999 [PubMed] [Google Scholar]
- 199.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271: 26690–26697, 1996 [DOI] [PubMed] [Google Scholar]
- 200.Somlyo AV, Somlyo AP. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J Pharmacol Exper Therapeut 159: 129–145, 1968 [PubMed] [Google Scholar]
- 201.Sorop O, Bakker ENTP, Pistea A, Spaan JAE, VanBavel E. Calcium channel blockade prevents pressure-dependent inward remodeling in isolated subendocardial resistance vessels. Am J Physiol Heart Circ Physiol 291: H1236–H1245, 2006 [DOI] [PubMed] [Google Scholar]
- 202.Sorop O, Merkus D, de Beer VJ, Houweling B, Pistea A, McFalls EO, Boomsma F, van Beusekom HM, van der Giessen WJ, VanBavel E, Duncker DJ. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res 102: 795–803, 2008 [DOI] [PubMed] [Google Scholar]
- 203.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119: 19–31, 2004 [DOI] [PubMed] [Google Scholar]
- 204.Stepp DW, Belin de Chantemele EJ. Structural remodeling in the limb circulation: impact of obesity and diabetes. Microcirculation 14: 311–316, 2007 [DOI] [PubMed] [Google Scholar]
- 205.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP-dependent protein kinase Ialpha. Science 286: 1583–1587, 1999 [DOI] [PubMed] [Google Scholar]
- 206.Szymanski PT, Szymanska G, Goyal RK. Differences in calmodulin and calmodulin-binding proteins in phasic and tonic smooth muscles. Am J Physiol Cell Physiol 282: C94–C104, 2002 [DOI] [PubMed] [Google Scholar]
- 207.Szymanski PT, Chacko TK, Rovner AS, Goyal RK. Differences in contractile protein content and isoforms in phasic and tonic smooth muscles. Am J Physiol Cell Physiol 275: C684–C692, 1998 [DOI] [PubMed] [Google Scholar]
- 208.Tacke R, Tohyama M, Ogawa S, Manley JL. Human Tra2 proteins are sequence-specific activators of pre-mRNA splicing. Cell 93: 139–148, 1998 [DOI] [PubMed] [Google Scholar]
- 209.Tang ZZ, Liang MC, Lu S, Yu D, Yu CY, Yue DT, Soong TW. Transcript scanning reveals novel and extensive splice variations in human L-type voltage-gated calcium channel, Cav1.2 alpha1 subunit. J Biol Chem 279: 44335–44343, 2004 [DOI] [PubMed] [Google Scholar]
- 210.Tanko LB, Matrougui K. Can we apply results from large to small arteries? Circ Res 90: 68e, 2002 [DOI] [PubMed] [Google Scholar]
- 211.Thievent A, Connat JL. Cytoskeletal features in longitudinal and circular smooth muscles during development of the rat portal vein. Cell Tissue Res 279: 199–208, 1995 [DOI] [PubMed] [Google Scholar]
- 212.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol 83: 215–242, 2005 [DOI] [PubMed] [Google Scholar]
- 213.Thyberg J, Blomgren K. Effects of proteasome and calpain inhibitors on the structural reorganization and proliferation of vascular smooth muscle cells in primary culture. Lab Invest 79: 1077–1088, 1999 [PubMed] [Google Scholar]
- 214.Tian L, Coghill LS, MacDonald SH, Armstrong DL, Shipston MJ. Leucine zipper domain targets cAMP-dependent protein kinase to mammalian BK channels. J Biol Chem 278: 8669–8677, 2003 [DOI] [PubMed] [Google Scholar]
- 215.Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem 276: 7717–7720, 2001 [DOI] [PubMed] [Google Scholar]
- 216.Travis AR, Giridharan GA, Pantalos GM, Dowling RD, Prabhu SD, Slaughter MS, Sobieski M, Undar A, Farrar DJ, Koenig SC. Vascular pulsatility in patients with a pulsatile- or continuous-flow ventricular assist device. J Thorac Cardiovasc Surg 133: 517–524, 2007 [DOI] [PubMed] [Google Scholar]
- 217.Ts'ao CH, Glagov S, Kelsey BF. Structure of mammalian portal vein: postnatal establishment of two mutually perpendicular medial muscle zones in the rat. Anat Rec 171: 457–470, 1971 [DOI] [PubMed] [Google Scholar]
- 218.Tsai MC, Chen L, Zhou J, Tang Z, Hsu TF, Wang Y, Shih YT, Peng HH, Wang N, Guan Y, Chien S, Chiu JJ. Shear stress induces synthetic-to-contractile phenotypic modulation in smooth muscle cells via peroxisome proliferator-activated receptor alpha/delta activations by prostacyclin released by sheared endothelial cells. Circ Res 105: 471–480, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Undar A. Myths and truths of pulsatile and nonpulsatile perfusion during acute and chronic cardiac support. Artif Organs 28: 439–443, 2004 [DOI] [PubMed] [Google Scholar]
- 220.Undar A. Benefits of pulsatile flow during and after cardiopulmonary bypass procedures. Artif Organs 29: 688–690, 2005 [DOI] [PubMed] [Google Scholar]
- 221.Unthank JL, Nixon JC, Burkhart HM, Fath SW, Dalsing MC. Early collateral and microvascular adaptations to intestinal artery occlusion in rat. Am J Physiol Heart Circ Physiol 271: H914–H923, 1996 [DOI] [PubMed] [Google Scholar]
- 222.Uvelius B, Arner A, Johansson B. Structural and mechanical alterations in hypertrophic venous smooth muscle. Acta Physiol Scand 112: 463–471, 1981 [DOI] [PubMed] [Google Scholar]
- 223.Van Rooij E, Olson EN. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J Clin Invest 117: 2369–2376, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr, Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17: 662–673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316: 575–579, 2007 [DOI] [PubMed] [Google Scholar]
- 226.Vrhovski B, Theze N, Thiebaud P. Structure and evolution of tropomyosin genes. Adv Exp Med Biol 644: 6–26, 2008 [DOI] [PubMed] [Google Scholar]
- 227.Waites GT, Graham IR, Jackson P, Millake DB, Patel B, Blanchard AD, Weller PA, Eperon IC, Critchley DR. Mutually exclusive splicing of calcium-binding domain exons in chick alpha-actinin. J Biol Chem 267: 6263–6271, 1992 [PubMed] [Google Scholar]
- 228.Walker LA, MacDonald JA, Liu X, Nakamoto RK, Haystead TAJ, Somlyo AV, Somlyo AP. Site-specific phosphorylation and point mutations of telokin modulate its Ca2+-desensitizing effect in smooth muscle. J Biol Chem 276: 24519–24524, 2001 [DOI] [PubMed] [Google Scholar]
- 229.Wamhoff BR, Bowles DK, Owens GK. Excitation-transcription coupling in arterial smooth muscle. Circ Res 98: 868–878, 2006 [DOI] [PubMed] [Google Scholar]
- 231.Wang W, Nepiyushchikh Z, Zawieja DC, Chakraborty S, Zawieja SD, Gashev AA, Davis MJ, Muthuchamy M. Inhibition of myosin light chain phosphorylation decreases rat mesenteric lymphatic contractile activity. Am J Physiol Heart Circ Physiol 297: H726–H734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Werner GS, Ferrari M, Richartz BM, Gastmann O, Figulla HR. Microvascular dysfunction in chronic total coronary occlusions. Circulation 104: 1129–1134, 2001 [DOI] [PubMed] [Google Scholar]
- 233.Wesselman JP, Kuijs R, Hermans JJ, Janssen GM, Fazzi GE, van Essen H, Evelo CT, Struijker-Boudier HA, De Mey JG. Role of the RhoA/Rho kinase system in flow-related remodeling of rat mesenteric small arteries in vivo. J Vasc Res 41: 277–290, 2004 [DOI] [PubMed] [Google Scholar]
- 234.Wetzel U, Lutsch G, Haase H, Ganten U, Morano I. Expression of smooth muscle myosin heavy chain B in cardiac vessels of normotensive and hypertensive rats. Circ Res 83: 204–209, 1998 [DOI] [PubMed] [Google Scholar]
- 235.Wieland T, Mittmann C. Regulators of G-protein signalling: multifunctional proteins with impact on signalling in the cardiovascular system. Pharmacol Ther 97: 95–115, 2003 [DOI] [PubMed] [Google Scholar]
- 236.Woodsome TP, Eto M, Everett A, Brautigan DL, Kitazawa T. Expression of CPI-17 and myosin phosphatase correlates with Ca2+ sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 535: 553–564, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TAJ. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of serine 695 in response to cyclic nucleotides. J Biol Chem 279: 34496–34504, 2004 [DOI] [PubMed] [Google Scholar]
- 238.Wray S, Burdyga T. Sarcoplasmic reticulum function in smooth muscle. Physiol Rev 90: 113–178, 2010 [DOI] [PubMed] [Google Scholar]
- 239.Wu KC, Jin JP. Calponin in non-muscle cells. Cell Biochem Biophys 52: 139–148, 2008 [DOI] [PubMed] [Google Scholar]
- 240.Wu X, Haystead TA, Nakamoto RK, Somlyo AV, Somlyo AP. Acceleration of myosin light chain dephosphorylation and relaxation of smooth muscle by telokin. Synergism with cyclic nucleotide-activated kinase. J Biol Chem 273: 11362–11369, 1998 [DOI] [PubMed] [Google Scholar]
- 241.Xie J, Black DL. A camk iv responsive RNA element mediates depolarization-induced alternative splicing of ion channels. Nature 410: 936–939, 2001 [DOI] [PubMed] [Google Scholar]
- 242.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science 280: 443–446, 1998 [DOI] [PubMed] [Google Scholar]
- 243.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 23: 2166–2178, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Braun AP, Peichun G, Korthuis RJ, Davis MJ, Hill MA. Heterogeneity in function of small artery smooth muscle BKCa: involvement of the α-1-subunit. J Physiol 587: 3025–3044, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir22 genes reveals the essential role of the inwardly rectifying K+ current in K+-mediated vasodilation. Circ Res 87: 160–166, 2000 [DOI] [PubMed] [Google Scholar]
- 246.Zhang H, Fisher SA. Conditioning effect of blood flow on resistance artery smooth muscle myosin phosphatase. Circ Res 100: 730–737, 2007 [DOI] [PubMed] [Google Scholar]
- 247.Zhang H, Pakeerappa P, Lee HJ, Fisher SA. Induction of PDE5 and de-sensitization to endogenous NO signaling in a systemic resistance artery under altered blood flow. J Mol Cell Cardiol 47: 57–65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L. Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Lett 579: 4856–4860, 2005 [DOI] [PubMed] [Google Scholar]