Abstract
Transcription regulatory genes are crucial modulators of cell physiology and metabolism whose intracellular levels are tightly controlled in response to extracellular stimuli. We previously reported a set of 29 transcription regulatory genes modulated by angiotensin II in H295R human adrenocortical cells and their roles in regulating the expression of the last and unique enzymes of the glucocorticoid and mineralocorticoid biosynthetic pathways, 11β-hydroxylase and aldosterone synthase, respectively, using gene expression reporter assays. To study the effect of this set of transcription regulatory genes on adrenal steroidogenesis, H295R cells were transfected by high-efficiency nucleofection and aldosterone and cortisol were measured in cell culture supernatants under basal and angiotensin II-stimulated conditions. BCL11B, BHLHB2, CITED2, ELL2, HMGA1, MAFF, NFIL3, PER1, SERTAD1, and VDR significantly stimulated aldosterone secretion, while EGR1, FOSB, and ZFP295 decreased aldosterone secretion. BTG2, HMGA1, MITF, NR4A1, and ZFP295 significantly increased cortisol secretion, while BCL11B, NFIL3, PER1, and SIX2 decreased cortisol secretion. We also report the effect of some of these regulators on the expression of endogenous aldosterone synthase and 11β-hydroxylase under basal and angiotensin II-stimulated conditions. In summary, this study reports for the first time the effects of a set of angiotensin II-modulated transcription regulatory genes on aldosterone and cortisol secretion and the expression levels of the last and unique enzymes of the mineralocorticoid and glucocorticoid biosynthetic pathways. Abnormal regulation of mineralocorticoid or glucocorticoid secretion is involved in several pathophysiological conditions. These transcription regulatory genes may be involved in adrenal steroidogenesis pathologies; thus they merit additional study as potential candidates for therapeutic intervention.
Keywords: adrenal cortex, transcription regulation, gene expression
one of the most basic levels in the regulation of cellular physiology is the level of control of gene transcription. Gene products with “Transcription Regulator Activity” are defined by the Gene Ontology Database as any gene products that play a role in regulating transcription, which may bind a promoter or enhancer DNA sequence or interact with a DNA-binding transcription factor (1). Transcription regulatory proteins are key molecules because any alteration in their level or activity generally results in the modification of multiple cellular processes. Adrenal cortical cells secrete mineralocorticoids and glucocorticoids under the stimulatory effect of a great variety of molecules, some of the most important being angiotensin II (ANG II), adrenocorticotropic hormone (ACTH), and potassium. Transcription regulators whose levels are modified by any of these secretagogues are expected to be important for normal adrenal cell physiology and, consequently, crucial to the maintenance of mineralocorticoid- and glucocorticoid-regulated homeostasis. Abnormal regulation or function of transcription regulatory factors would lead to alterations in adrenal gland development, morphology, and function, resulting in a wide range of pathophysiological conditions associated with adrenal steroid excess or deficiency (10, 13, 25, 27).
We previously reported (46) a high-throughput screening of the transcription regulatory genes modulated by ANG II, potassium, and forskolin in human H295R adrenocortical cells and their role in steroidogenic enzyme gene expression. We reported that ANG II modifies the expression level of 29 transcription regulatory genes, increasing and decreasing the expression of 25 and 4 genes, respectively. Forskolin, an adenylate cyclase activator that mimics ACTH-mediated responses in this adrenal cell line, and extracellular potassium also regulate many of these transcription regulatory genes, suggesting some degree of convergence in the intracellular signaling pathways triggered by these aldosterone secretagogues and ANG II. Reporter plasmids under the control of the human aldosterone synthase and 11β-hydroxylase promoters were used to analyze the effect of these ANG II-modulated transcription regulatory genes on the expression of the last and unique enzymes of the mineralo- and glucocorticoid biosynthetic pathways, aldosterone synthase and 11β-hydroxylase, in H295R cells. Most of the ANG II-upregulated transcription regulatory genes increase the expression of both steroidogenic enzymes. Even more, some of them (NFIL3, NR4A1, NR4A2, NR4A3) show a strong preference for upregulating the expression of aldosterone synthase compared with 11β-hydroxylase.
Since mineralo- and glucocorticoid biosynthesis are multistep pathways that not only involve a series of sequential enzymatic steps but also are regulated by many intracellular signaling pathways (9, 50), we wanted to study the effect of a set of ANG II-modulated transcription regulatory genes in the mineralo- and glucocorticoid biosynthetic pathway output, the secretion of aldosterone and cortisol. Using high-efficiency transfection by nucleofection, we analyzed the effect of 24 ANG II-modulated transcription regulatory genes on the secretion of aldosterone and cortisol under basal and ANG II-stimulated conditions in the human adrenocortical cell line H295R. We then selected five transcription regulatory genes and analyzed their effects on gene expression levels of endogenous aldosterone synthase and 11β-hydroxylase. In summary, we report for the first time the effect of a set of ANG II-modulated transcription regulatory genes on aldosterone and cortisol secretion by H295R human adrenocortical cells.
MATERIALS AND METHODS
Cell culture.
H295R human adrenocortical cells (6) were cultured in H295R complete medium containing DMEM-F-12 (1:1) supplemented with 2% Ultroser G (Biosepra, Villeneuve-la-Garenne, France), ITS-Plus (Discovery Labware, Bedford, MA), and an antibiotic-antimycotic mixture (Invitrogen, Carlsbad, CA), as previously described (43).
Transfection and steroid secretion.
H295R cells were transfected with Nucleofector technology (Amaxa Biosystems) as previously reported (48). Briefly, three million log phase cells were resuspended in 100 μl of Nucleofector Solution R, mixed with 3 μg of plasmid DNA, and electroporated with the proprietary program P-20. Cells were allowed to recover for 15 min in RPMI 1640 medium at 37°C and then plated in 24-well plates with 1 ml of complete medium per well. Cells were cultured for 16 h. The medium was then removed, and the cells were incubated with prewarmed medium with or without 10 nM ANG II (American Peptide, Sunnyvale, CA) for 24 h. At the end of the incubation period, cell culture supernatants were saved for aldosterone and cortisol determination by ELISA as previously reported (22, 47). Cells were lysed with M-PER lysis buffer (Pierce, Rockford, IL), and protein concentration was measured with the Coomassie Plus kit (Pierce). Steroid secretion was standardized by total cellular protein. Results are expressed as percentage compared with transfections with a control plasmid.
RNA extraction and real-time RT-PCR.
Total RNA was extracted, DNAase treated, quantified, and reverse transcribed as previously described (43). Aldosterone synthase mRNA expression was quantified with the Taqman Gene expression assay master mix (Applied Biosystems, Foster City, CA) and specific aldosterone synthase primers (Hs01597732_a1, Applied Biosystems) according to manufacturer-suggested protocols. 11β-Hydroxylase mRNA expression was quantified by Taqman technique as previously reported (23) with the following specific primers: forward 5′-GGCAGAGGCAGAGATGCTG-3′, reverse 5′-CTCTTGGGTTAGTGTCTCCACCT-3′, probe 5′-HEX-TGCTGCACCATGTGCTGAAACACCT-BH1–3′. GAPDH mRNA expression was quantified as previously reported (43). Real-time data were obtained during the extension phase, and threshold cycle values were obtained at the log phase of each gene amplification. PCR product quantification was performed by the relative quantification method (39) and standardized against GAPDH. The efficiency for each primer pair was assessed by using serial dilutions of RT product. Results are expressed as arbitrary units normalized against GAPDH mRNA expression.
Plasmids.
Mammalian expression plasmids expressing human or mouse genes under a cytomegalovirus promoter have been reported previously (46). All expression plasmids were obtained from Open Biosystems (Huntsville, AL) except for NR4A3, which was purchased from Origene Technologies (Rockville, MD). We previously reported (46) that this set of transcription regulatory protein-overexpressing plasmids effectively increased the expression of each of the genes as determined by RT-PCR.
Statistical analysis.
All results are expressed as means ± SE. Multiple groups were analyzed by two-way ANOVA followed by Student-Newman-Keuls multiple comparison test. Differences were considered statistically significant at P < 0.05. Statistical calculations were performed with SigmaPlot version 11 (Systat Software, San Jose, CA).
RESULTS
Angiotensin II-modulated transcription regulatory genes and steroidogenesis.
To determine the role of ANG II-modulated transcription regulatory genes in adrenal steroidogenesis, H295R human adrenocortical cells were transfected with plasmids overexpressing 24 different transcription regulatory genes (listed in Table 1) and incubated in the presence or absence of ANG II, and then aldosterone and cortisol secretion levels were quantified in cell culture supernatants.
Table 1.
Transcription regulatory genes
| Gene Symbol | Gene Name | Previous Symbols | Aliases |
|---|---|---|---|
| BCL11B | B-cell CLL/lymphoma 11B | CTIP-2, CTIP2, hRIT1-α | |
| BHLHB2 | Basic helix-loop-helix family, member 2 | STRA13 | DEC1, bHLHe40 |
| BTG2 | BTG family, member 2 | PC3, TIS21, MGC126063, MGC126064 | |
| CITED2 | Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2 | MRG1 | |
| EGR1 | Early growth response 1 | TIS8, G0S30, NGFI-A, KROX-24, ZIF-268, AT225, ZNF225 | |
| EGR2 | Early growth response 2 | KROX20 | |
| ELL2 | Elongation factor, RNA polymerase II, 2 | ||
| FOS | FBJ murine osteosarcoma viral oncogene homolog | c-fos, AP-1 | |
| FOSB | FBJ murine osteosarcoma viral oncogene homolog B | G0S3, GOSB, GOS3, AP-1, MGC42291, DKFZp686C0818 | |
| HDAC5 | Histone deacetylase 5 | KIAA0600, NY-CO-9, FLJ90614 | |
| HMGA1 | High-mobility group AT-hook 1 | HMGIY | |
| MAFF | v-maf musculoaponeurotic fibrosarcoma oncogene homolog F | hMafF | |
| MITF | Microphthalmia-associated transcription factor | WS2A, MI, bHLHe32 | |
| NFIL3 | Nuclear factor, interleukin 3 regulated | IL3BP1 | E4BP4, NFIL3A, NF-IL3A |
| NR4A1 | Nuclear receptor subfamily 4, group A, member 1 | HMR, GFRP1 | TR3, N10, NAK-1, NGFIB, NUR77 |
| NR4A2 | Nuclear receptor subfamily 4, group A, member 2 | NURR1 | TINUR, NOT, RNR1, HZF-3 |
| NR4A3 | Nuclear receptor subfamily 4, group A, member 3 | CSMF, CHN, NOR1, MINOR | |
| PER1 | Period homolog 1 | PER | RIGUI |
| RUNX1T1 | runt-related transcription factor 1; translocated to, 1 | AML1T1, CBFA2T1 | CDR, ETO, MTG8, ZMYND2 |
| SALL1 | sal-like 1 | TBS | Hsal1, ZNF794 |
| SERTAD1 | SERTA domain containing 1 | SEI1, TRIP-Br1 | |
| SIX2 | SIX homeobox 2 | ||
| VDR | Vitamin D (1,25-dihydroxyvitamin D3) receptor | NR1I1 | |
| ZNF295 | Zinc finger protein 295 | KIAA1227, ZBTB21 |
The genes under study were selected from our previous study in which we showed that these 24 transcription regulatory genes were modulated at the mRNA expression level by ANG II in H295R cells. Furthermore, most of these genes significantly regulated aldosterone synthase and/or 11β-hydroxylase reporter genes. High-efficiency transfection was performed by nucleofection technology, which has a transfection efficiency higher than 50% in H295R cells under the conditions described in materials and methods. This approach proved to be crucial to overcome the basal steroidogenesis of this cell line that otherwise would obscure the effect of specific genes if the transfection efficiency were as low as that achieved with traditional transfection methods. The transcription regulatory gene expression plasmids used in this study have been previously described and documented to increase the expression levels of each of the overexpressed genes (46). A submaximal stimulatory concentration of ANG II (10 nM) was chosen to avoid saturating the steroidogenic response of the adrenocortical cells. All transfections were performed with three different plasmid preparations, to avoid any plasmid DNA preparation-specific confounding effect. H295R cells is a widely used in vitro cell system in the study of adrenal cell physiology since its regulation and steroid secretion mimic those of freshly isolated adrenal cells (40, 41).
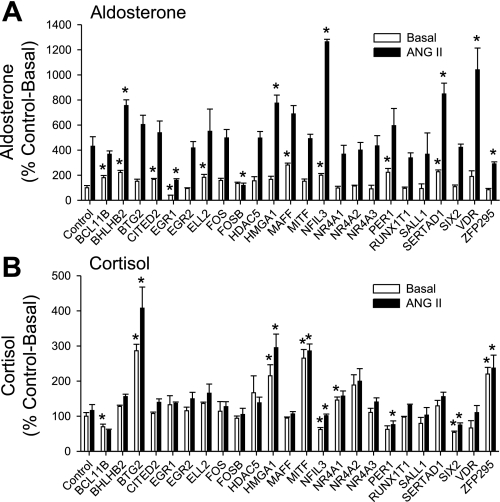
Figure 1 shows aldosterone (Fig. 1A) and cortisol (Fig. 1B) secretion by H295R cells as a percentage of control cells under basal and ANG II stimulatory conditions. We found that overexpression of nine transcription regulatory genes either increases (BCL11B, BHLHB2, CITED2, ELL2, MAFF, NFIL3, PER1, SERTAD1) or decreases (EGR1) aldosterone secretion under basal conditions. When H295R cells were treated with a submaximal dose of ANG II, overexpression of eight transcription regulatory genes was observed to either increase (BHLHB2, HMGA1, NFIL3, SERTAD1, VDR) or decrease (EGR1, FOSB, ZNF295) ANG II-mediated aldosterone secretion.
Fig. 1.
Effect of transcription regulatory genes on aldosterone (A) and cortisol (B) secretion. H295R cells were transfected with transcription regulatory gene-expressing plasmids or control plasmid. Cells were incubated under basal or angiotensin II (ANG II; 10 nM)-stimulated conditions for 24 h. Aldosterone and cortisol were measured by ELISA in cell culture supernatants. Data are expressed as fold induction compared with control plasmid. Transfection experiments were performed in duplicate with 3 independent plasmid DNA preparations at least 3 times. *P < 0.05 vs. Control.
We measured cortisol secretion in the same cell culture supernatant. We observed that overexpression of eight transcription regulatory genes either increased (BTG2, HMGA1, MITF, NR4A1, ZFP295) or decreased (BCL11B, NFIL3, SIX2) cortisol secretion under basal conditions. When H295R cells were treated with a submaximal dose of ANG II, overexpression of seven transcription regulatory genes was observed to either increase (BTG2, HMGA1, MITF, ZFP295) or decrease (NFIL3, PER1, SIX2) ANG II-mediated cortisol secretion.
Angiotensin II-modulated transcription regulatory genes and endogenous steroidogenic enzyme expression.
When we analyzed transcription regulatory gene-mediated aldosterone or cortisol secretion (see Fig. 1) and our previously reported data on the effect of these genes in reporter gene expression studies of aldosterone synthase or 11β-hydroxylase (46), we observed that there was not always agreement (see Table 2 and discussion). Furthermore, the effects were opposite for some genes. For example, BHLHB2 significantly decreased aldosterone synthase expression quantified with a reporter gene (46) but significantly increased basal and ANG II-mediated aldosterone secretion (Fig. 1). We selected five genes, four of them (BHLHB2, HMGA1, SERTAD1, and VDR) presenting contradictory results and NFIL3, which presented the most potent aldosterone synthase expression and aldosterone secretion effect, for further studies of their effects on endogenous aldosterone synthase and 11β-hydroxylase expression. We performed high-efficiency transfections with all five of these transcription regulatory genes using nucleofection technology and quantified endogenous aldosterone synthase and 11β-hydroxylase expression under basal and ANG II stimulatory conditions at 3 and 12 h after hormone treatment (Figs. 2 and 3). Incubation times were selected to reflect either 1) early regulated genes at 3 h of incubation or 2) maximal endogenous ANG II-stimulated aldosterone synthase expression at 12 h as we have previously reported (43). Figures 2 and 3 show, respectively, the endogenous aldosterone synthase and 11β-hydroxylase mRNA expression level for cells transfected with these five selected transcription regulatory genes or a control plasmid under basal or submaximal ANG II (10 nM) stimulatory conditions 3 and 12 h after hormone stimulation.
Table 2.
Summary of angiotensin II-modulated transcription regulatory genes' role in steroidogenic enzyme gene expression and steroid secretion
| Gene | ANG II/Control mRNA* | Aldosterone Synthase Reporter* | 11β-Hydroxylase Reporter* | Aldosterone Secretion |
Cortisol Secretion |
||
|---|---|---|---|---|---|---|---|
| Basal | ANG II | Basal | ANG II | ||||
| BCL11B | ↑ | ↓ | ↑ | ↓ | |||
| BHLHB2 | ↑ | ↓ | ↓ | ↑ | ↑ | ||
| BTG2 | ↑ | ↓ | ↓ | ↑ | ↑ | ||
| CITED2 | ↑ | ↑ | ↑ | ↑ | |||
| EGR1 | ↑ | ↑ | ↓ | ↓ | |||
| EGR2 | ↑ | ↑ | ↑ | ||||
| ELL2 | ↑ | ↑ | ↑ | ↑ | |||
| FOS | ↑ | ↑ | ↑ | ||||
| FOSB | ↑ | ↑ | ↑ | ↓ | |||
| HDAC5 | ↑ | ↑ | ↑ | ||||
| HMGA1 | ↑ | ↓ | ↑ | ↑ | ↑ | ||
| MAFF | ↑ | ↑ | ↑ | ↑ | |||
| MITF | ↑ | ↑ | ↑ | ↑ | ↑ | ||
| NFIL3 | ↑ | ↑ | ↑ | ↑ | ↑ | ↓ | ↓ |
| NR4A1 | ↑ | ↑ | ↑ | ↑ | |||
| NR4A2 | ↑ | ↑ | ↑ | ||||
| NR4A3 | ↑ | ↑ | ↑ | ||||
| PER1 | ↑ | ↑ | ↑ | ↑ | ↓ | ||
| RUNX1T1 | ↓ | ||||||
| SALL1 | ↑ | ↓ | ↓ | ||||
| SERTAD1 | ↑ | ↑ | ↑ | ||||
| SIX2 | ↓ | ↓ | ↓ | ||||
| VDR | ↑ | ↑ | ↑ | ↑ | |||
| ZNF295 | ↑ | ↓ | ↑ | ↑ | |||
Absence of an arrow indicates “no change.”
Data from Romero et al. (46).
Fig. 2.
Effect of transcription regulatory genes on endogenous aldosterone synthase expression. H295R cells were transfected with transcription regulatory gene-expressing plasmids or control plasmid and stimulated with ANG II (10 nM) for 3 or 12 h, and then aldosterone synthase expression was quantified by real-time RT-PCR. Transfection experiments were performed in triplicate with 3 independent plasmid DNA preparations at least 3 times. *P < 0.05 vs. Control. AU, arbitrary unit.
Fig. 3.
Effect of transcription regulatory genes on endogenous 11β-hydroxylase expression. H295R cells were transfected with transcription regulatory gene-expressing plasmids or control plasmid and stimulated with ANG II (10 nM) for 3 or 12 h, and then 11β-hydroxylase expression was quantified by real-time RT-PCR. Transfection experiments were performed in triplicate with 3 independent plasmid DNA preparations at least 3 times. *P < 0.05 vs. Control.
As expected, in H295R cells transfected with the control plasmid ANG II significantly increased endogenous aldosterone synthase mRNA expression 12 h after ANG II addition, while it did not significantly modify its expression after 3 h of treatment. BHLHB2 was the only transcription regulatory gene to cause a significant decrease in endogenous aldosterone synthase expression after 12 h of ANG II stimulation. The other four transcription regulatory genes (HMGA1, NFIL3, SERTAD1, and VDR) increased endogenous aldosterone synthase expression, although the degree of stimulation and the conditions (time and hormone stimulation status) differed for each gene. The most significant effect on endogenous aldosterone synthase mRNA expression was observed after 3 h of treatment with NFIL3, SERTAD1, and VDR under both basal and ANG II stimulatory conditions. HMGA1 only upregulated endogenous aldosterone synthase mRNA expression upon ANG II stimulation.
In H295R cells transfected with the control plasmid, ANG II significantly increased endogenous 11β-hydroxylase mRNA expression 3 and 12 h after ANG II addition, although to a much lower extent than the effect on aldosterone synthase. NFIL3 showed a significant stimulatory effect on endogenous 11β-hydroxylase mRNA expression after 12 of treatment under basal and ANG II stimulatory conditions. Neither BHLHB2 nor HMGA1 showed a significant regulatory effect on endogenous 11β-hydroxylase mRNA expression. Both SERTAD1 and VDR significantly decreased both basal and ANG II-stimulated endogenous 11β-hydroxylase mRNA expression.
DISCUSSION
We report for the first time a high-throughput functional screening of the steroidogenic activity of transcription regulatory genes modulated by ANG II with which we identified several new transcription regulatory genes that have not been previously known to regulate aldosterone and cortisol secretion. We also report the effects of five of these transcription regulatory genes on the expression levels of endogenous aldosterone synthase and 11β-hydroxylase under basal and ANG II-stimulated conditions.
Table 2 shows the effect (increase, decrease, or no change) of ANG II on the mRNA expression levels of all the transcription regulatory genes under study, their effect on the expression levels of aldosterone synthase and 11β-hydroxylase quantified with reporter genes, and their effect on aldosterone and cortisol secretion under basal and ANG II-stimulated conditions. The effect of ANG II on transcription regulatory gene expression levels and the effect of these transcription regulatory genes on steroidogenic enzyme reporter gene expression were previously reported (46).
Comparison of the effects of the ANG II-modulated transcription regulatory genes on aldosterone synthase reporter gene expression and on basal aldosterone secretion indicate that only nine genes (37.5%) show concordance in their effects. On the contrary, most of the genes (15/24) show either opposite or discordant (either increase or decrease in one variable and no effect in the other) effects. The nonconcordance is even more pronounced when 11β-hydroxylase reporter gene expression and cortisol secretion are compared. Only 6 of the 24 genes (25%) show similar effects on both variables. While this disagreement between the effect of these genes on the expression of the last and unique enzymes of the mineralo- and glucocorticoid biosynthetic pathways and steroid secretion does not invalidate reporter gene expression studies, it clearly demonstrates that extreme care should be taken in interpreting key steroidogenic enzyme reporter gene expression studies, as they do not always translate into increased secretion of the final biosynthetic product, either aldosterone or cortisol. A clear example of this point is the three NGFI-B nuclear orphan receptor superfamily members (NR4A1, NR4A2, and NR4A3), which significantly upregulated aldosterone synthase reporter gene expression but had no significant effect on aldosterone secretion by H295R cells.
The three members of the NGFI-B nuclear orphan receptor superfamily, NR4A1 (Nur77, NGFI-B), NR4A2 (Nurr1), and NR4A3 (Nor1) (20, 33), are highly expressed in the adrenal cortex (4, 11, 15, 56). We and others have reported that NGFI-B family members are upregulated by ANG II in H295R cells (4, 37, 43, 46, 51) and freshly isolated rat or bovine zona glomerulosa adrenal cells (36, 45, 51). NGFI-B family members have been reported to regulate the expression of several steroidogenic enzymes, including aldosterone synthase, 11β-hydroxylase, 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase, and 21-hydroxylase in the adrenal gland (3, 4, 15, 32, 46, 56, 57). However, all of these studies, with the exception of one reported by Bassett et al. (3) on the effect of NR4A1 on 3β-hydroxysteroid dehydrogenase expression, used reporter genes. Bassett et al. (3) reported that NR4A1 overexpression in human cultured fetal zone adrenal cells increases endogenous 3β-hydroxysteroid dehydrogenase expression and cortisol secretion, as we confirmed in the present report (see Fig. 1). All studies of aldosterone secretion regulation by NGFI-B family members, including our previous study (46), have been performed with reporter plasmids. In the present study we observed that NR4A1 and NR4A2 slightly increase cortisol secretion under basal conditions but none of the three NGFI-B family members significantly modified aldosterone secretion under either basal or ANG II stimulatory conditions. Similar results were observed when H295R cells were transduced with lentiviruses overexpressing any of the three NGFI-B family members (unpublished data). Recently, Nogueira et al. (37) reported that overexpression of a dominant-negative mutant of NR4A1 decreased endogenous aldosterone synthase expression and aldosterone secretion in ANG II-stimulated H295R cells. These latest results and those presented in this report may suggest that NGFI-B family members are indeed involved in ANG II-stimulated aldosterone secretion regulation. However, since NGFI-B family members have redundant roles and the expression of all of them is upregulated by ANG II stimulation, the overexpression of a single member may not significantly alter the final steroidogenic output of adrenal cells.
Ten transcription regulatory genes (BCL11B, BHLHB2, CITED2, ELL2, HMGA1, MAFF, NFIL3, PER1, SERTAD1, and VDR) were upregulated by ANG II and increased aldosterone secretion under basal, ANG II-stimulated, or both conditions. Of these, CITED2, HMGA1, and PER1 have already been studied in the adrenal gland, and a role for them in adrenal steroidogenesis has been suggested.
CITED2 (Cbp/p300-interacting transactivator, with Glu/Asp-rich carboxy-terminal domain, 2) is a transcriptional coregulator of the cAMP response element binding protein (CREB) binding protein (CBP) and p300 (5). CITED2 was originally implicated in adrenal development because CITED2-null embryos die during gestation with profound developmental abnormalities, including cardiac malformations, neural crest defects, and adrenal agenesis (2). CITED2 is expressed as early as 7 wk of gestation within the definitive zone of the human fetal adrenal gland (16, 24). CITED2 regulates adrenal gland morphogenesis by interacting with the transcription factor Wt1 to stimulate expression of the nuclear hormone receptor Sf-1 in the adrenogonadal primordium prior to the separation between gonad and adrenal cortex (53). In the adult adrenal gland, CITED2 is expressed in the zona glomerulosa and zona reticularis (24). Furthermore, CITED2 was found to be expressed in 75% of the adrenocortical carcinomas analyzed as well as in H295R cells (24). CITED2 expression is upregulated by basic fibroblast growth factor, forskolin, and overexpression of steroidogenic factor-1 (SF-1) in H295R cells (16, 24). Our results demonstrate that CITED2 increases aldosterone synthase expression (46) and basal levels of aldosterone secretion in H295R cells by almost 70%, suggesting a role of CITED2 not only in adrenal development but also in adulthood, in agreement with its expression in the zona glomerulosa of the adult adrenal gland.
HMGA1 belongs to the “high-mobility group” (HMGA) protein family. HMGA proteins are architectural transcription factors that both positively and negatively regulate the transcription of a variety of genes. They do not display direct transcriptional activation capacity but regulate gene expression by changing the DNA conformation by binding to AT-rich regions in the DNA and/or direct interaction with several transcription factors (8). HMGA1 is expressed in the fetal and adult human adrenal gland (19). Our results indicate that HMGA1 increases ANG II-stimulated endogenous aldosterone synthase expression after 3 h of stimulation and ANG II-stimulated aldosterone secretion. HMGA1 stimulatory effect on endogenous aldosterone synthase expression was only observed under ANG II stimulatory conditions, in agreement with our previous results (46), where HMGA1 did not modify aldosterone synthase reporter gene expression under basal conditions. Surprisingly, HMGA1 had a potent stimulatory effect on cortisol secretion under basal and ANG II-stimulated conditions despite significantly decreasing 11β-hydroxylase reporter gene expression (46).
PER1 (Period 1) is a transcription factor that belongs to the circadian clock gene family. PER1 is expressed in all cortical layers of the mouse and rat adrenal gland, and its levels of expression follow a daily circadian rhythm (7, 14, 21). A transgenic mouse expressing luciferase under the control of Per1 promoter nicely demonstrated that PER1 is increased during the light phase and that light-mediated Per1 induction is ACTH independent and maximal in the zona glomerulosa of the mouse adrenal gland (26). Furthermore, Per1 is expressed in Rhesus macaque adrenal gland and presents a daily cycle of expression peaking at early morning (30). Our results indicate that PER1 upregulates basal aldosterone secretion, suggesting that this transcription regulatory gene may be involved in the circadian cycle of aldosterone secretion.
Three transcription regulatory genes (EGR1, FOSB, and ZNF295) were upregulated by ANG II but decreased aldosterone secretion under basal, ANG II-stimulated, or both conditions. This is a very interesting set of genes that may exert negative feedback on ANG II-mediated aldosterone secretion as we have previously reported (44, 49) with two members of the regulators of G protein signaling (RGS) family, RGS2 and RGS4.
EGR1 (early growth response gene 1) is upregulated by ANG II in human H295R adrenocortical cells and primary cultures of rat and bovine glomerulosa cells (36, 43). Nogueira et al. (37) reported that EGR1 overexpression in H295R cells increases the reporter activity of CYP21A2 but significantly (50%) decreases the reporter activity of CYP11B2. Our results indicate that EGR1 decreases both basal and ANG II-stimulated aldosterone secretion, a decrease that may be mediated, at least in part, by a decrease in CYP11B2 expression. Although mainly known as a transcriptional activator, EGR1 also has potent transcriptional repressor activity mapped upstream of the zinc finger domain (17) in many experimental models (12, 31, 52). Furthermore, whole genome gene expression analysis of EGR1-null mouse embryonic fibroblasts (MEFs) indicates that of the 266 genes whose expression was regulated compared with wild-type MEFs, almost 60% of them were upregulated, suggesting that EGR1 may mainly function as a transcriptional repressor (28). These results may explain the inhibitory effect of EGR1 on aldosterone secretion and suggest a negative feedback role for EGR1 in mineralocorticoid secretion.
Fos family members (c-fos, FOSB) dimerize with Jun proteins to form the AP-1 transcription factor complex. ANG II and ACTH increase c-fos mRNA expression in bovine and ovine adrenal cells in vitro (42, 54). Furthermore, ACTH treatment in vivo increases both c-Fos and FOSB mRNA in rat adrenal zona glomerulosa and zona fasciculata (29), and a c-Fos reporter gene is activated by ANG II in H295R cells (55). Rincon Garriz et al. (42) reported that c-fos binds to the StAR protein proximal promoter forming a heterodimer with c-Jun. Furthermore, overexpression of a dominant-negative mutant of c-Fos decreased ANG II-mediated upregulation of StAR protein expression and aldosterone secretion. Under our experimental conditions, FOS did not modify either basal or ANG II-stimulated aldosterone secretion. The difference between our results and those of Rincon Garriz et al. may be due to the fact that in our experimental conditions we overexpressed a functional FOS that may have titrated JUN proteins and consequently fewer functional heterodimers would be able to bind, for example, to the StAR protein promoter and activate its transcription.
When we analyzed the effect of five transcription regulatory genes on endogenous aldosterone synthase expression, we found some surprising and puzzling results. For example, BHLHB2 decreased endogenous and reporter plasmid aldosterone synthase expression; however, it potently stimulated aldosterone secretion under basal and ANG II-stimulated conditions, suggesting that the net steroidogenic effect of a gene (i.e., BHLHB2) cannot easily be extrapolated from gene expression studies in a complex metabolic pathway such as that of steroidogenesis, even when studying key steroidogenic enzymes such as aldosterone synthase.
SERTAD1 was a strong inducer of endogenous aldosterone synthase expression and significantly increased basal and ANG II-mediated aldosterone secretion. The lack of effect of SERTAD1 on aldosterone synthase reporter gene expression highlights a very important point. It is very difficult to define the extent of a gene promoter since transcription regulatory regions may reside not only several kilobases upstream of the transcription initiation site but even downstream or in the coding region of the gene, as clearly observed in the ENCODE project (18).
NFIL3 and VDR showed a very good correlation at endogenous and reporter gene aldosterone synthase expression and aldosterone secretion, suggesting that expression levels of key biosynthetic enzymes may be a good first approach, but further validation at the metabolic level (i.e., aldosterone secretion) should be performed to study the effect of particular genes in adrenal gland steroidogenesis.
Five transcription regulatory genes (BTG2, HMGA1, MITF, NR4A1, and ZFP295) were upregulated by ANG II and increased cortisol secretion under basal, ANG II-stimulated, or both conditions. Three transcription regulatory genes (BCL11B, NFIL3, and PER1) were upregulated by ANG II and decreased cortisol secretion under basal, ANG II-stimulated, or both conditions. Further studies are required to analyze whether the slight inhibitory effect of NFIL3 on cortisol secretion is due to the diversion of substrate to the synthesis of aldosterone because of its strong stimulatory effect on aldosterone secretion.
Mukai et al. (34, 35) have reported that AP-1 is involved in 11β-hydroxylase gene expression and that cotransfection of c-fos or FOSB increases the expression of an 11β-hydroxylase reporter gene. In agreement with these studies, we previously reported (46) that FOS and FOSB increase the expression of an 11β-hydroxylase reporter gene. However, neither FOS nor FOSB significantly modified cortisol secretion under basal or ANG II stimulatory conditions, highlighting again that extreme care should be used when extrapolating reporter gene studies to the actual modulation of steroid secretion.
A similar lack of correlation between the effect of the five transcription regulatory genes on reporter gene expression and endogenous gene expression and steroid synthesis was observed with 11β-hydroxylase mRNA expression as with aldosterone synthase mRNA expression. BHLHB2 and HMGA1 both decreased 11β-hydroxylase reporter gene expression; however, neither significantly altered endogenous 11β-hydroxylase mRNA levels. Furthermore, HMGA1 significantly increased cortisol secretion under both basal conditions and ANG II stimulation. NFIL3 decreased endogenous and reporter gene 11β-hydroxylase expression, producing a small, though significant inhibitory effect on cortisol secretion. Finally, expression of SERTAD1 and VDR potently inhibited endogenous 11β-hydroxylase mRNA expression, but neither gene decreased 11β-hydroxylase reporter gene expression or cortisol secretion.
Human H295R adrenocortical cells were used as the experimental model because this is the only well-characterized adrenal cell line that expresses all of the steroidogenic enzymes in the adrenal cortex and has a steroid secretion pattern and regulation similar to primary cultures of adrenal cells (40, 41). Although the H295R cell is a well-established human adrenocortical cell model that has greatly advanced the adrenal cell physiology field in the last 20 years, generating more than 400 publications, it is not an ideal adrenocortical cell line experimental model. One disadvantage is that H295R cells function as both adrenal gland zona glomerulosa and zona fasciculata cells. It is possible that this phenotype may have been acquired during transformation, since a new recently described human adrenocortical cell line, HAC15, also has a mixed zona glomerulosa/zona fasciculata steroid secretion pattern and regulation (38). The fact that no adrenal zona glomerulosa- or zona fasciculata-specific cell lines have been described belies the great effort to produce such cell lines. Once produced, such cells would provide better models for the study of adrenal gland zone-specific steroidogenesis and its control.
In summary, we describe the effects of a set of 24 ANG II-regulated transcription regulatory genes on the synthesis of aldosterone and cortisol in H295R human adrenocortical cells. In addition, our data raise concern about the lack of correlation between the effect of specific genes on reporter gene activity of key steroidogenic enzymes and the ultimate physiological effect, steroid secretion, for some genes. Many of the genes reported in the present report may be involved in dysregulation of either glucocorticoid or mineralocorticoid secretion and consequently involved in the pathophysiology of abnormal steroid secretion by the adrenal gland.
GRANTS
This work was supported by Medical Research funds from the Department of Veterans Affairs (to C. E. Gomez-Sanchez and E. P. Gomez-Sanchez), National Heart, Lung, and Blood Institute grants HL-27255 (to C. E. Gomez-Sanchez) and HL-75321 (to E. P. Gomez-Sanchez), and the University of Mississippi Medical Center Intramural Research Support Program (to D. G. Romero).
DISCLOSURES
The authors have nothing to disclose.
ACKNOWLEDGMENTS
We thank Dr. William E. Rainey (Medical College of Georgia, Augusta, GA) for generously providing the H295R cell line.
REFERENCES
- 1.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene Ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25: 25–29, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamforth SD, Braganca J, Eloranta JJ, Murdoch JN, Marques FI, Kranc KR, Farza H, Henderson DJ, Hurst HC, Bhattacharya S. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat Genet 29: 469–474, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Bassett MH, Suzuki T, Sasano H, De Vries CJ, Jimenez PT, Carr BR, Rainey WE. The orphan nuclear receptor NGFIB regulates transcription of 3beta-hydroxysteroid dehydrogenase. Implications for the control of adrenal functional zonation. J Biol Chem 279: 37622–37630, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol 18: 279–290, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya S, Michels CL, Leung MK, Arany ZP, Kung AL, Livingston DM. Functional role of p35srj, a novel p300/CBP binding protein, during transactivation by HIF-1. Genes Dev 13: 64–75, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE. Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology 133: 1555–1561, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Bittman EL, Doherty L, Huang L, Paroskie A. Period gene expression in mouse endocrine tissues. Am J Physiol Regul Integr Comp Physiol 285: R561–R569, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Cleynen I, Van de Ven WJ. The HMGA proteins: a myriad of functions. Int J Oncol 32: 289–305, 2008 [PubMed] [Google Scholar]
- 9.Connell JM, Davies E. The new biology of aldosterone. J Endocrinol 186: 1–20, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Coulter CL. Fetal adrenal development: insight gained from adrenal tumors. Trends Endocrinol Metab 16: 235–242, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Davis IJ, Lau LF. Endocrine and neurogenic regulation of the orphan nuclear receptors Nur77 and Nurr-1 in the adrenal glands. Mol Cell Biol 14: 3469–3483, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du B, Fu C, Kent KC, Bush H, Jr, Schulick AH, Kreiger K, Collins T, Mc Caffrey TA. Elevated Egr-1 in human atherosclerotic cells transcriptionally represses the transforming growth factor-beta type II receptor. J Biol Chem 275: 39039–39047, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Else T, Hammer GD. Genetic analysis of adrenal absence: agenesis and aplasia. Trends Endocrinol Metab 16: 458–468, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fahrenkrug J, Hannibal J, Georg B. Diurnal rhythmicity of the canonical clock genes Per1, Per2 and Bmal1 in the rat adrenal gland is unaltered after hypophysectomy. J Neuroendocrinol 20: 323–329, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Fernandez PM, Brunel F, Jimenez MA, Saez JM, Cereghini S, Zakin MM. Nuclear receptors Nor1 and NGFI-B/Nur77 play similar, albeit distinct, roles in the hypothalamo-pituitary-adrenal axis. Endocrinology 141: 2392–2400, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Ferraz-de-Souza B, Martin F, Mallet D, Hudson-Davies RE, Cogram P, Lin L, Gerrelli D, Beuschlein F, Morel Y, Huebner A, Achermann JC. CBP/p300-interacting transactivator, with Glu/Asp-rich C-terminal domain, 2, and pre-B-cell leukemia transcription factor 1 in human adrenal development and disease. J Clin Endocrinol Metab 94: 678–683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gashler AL, Swaminathan S, Sukhatme VP. A novel repression module, an extensive activation domain, and a bipartite nuclear localization signal defined in the immediate-early transcription factor Egr-1. Mol Cell Biol 13: 4556–4571, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerstein MB, Bruce C, Rozowsky JS, Zheng D, Du J, Korbel JO, Emanuelsson O, Zhang ZD, Weissman S, Snyder M. What is a gene, post-ENCODE? History and updated definition. Genome Res 17: 669–681, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Giannini G, Di Marcotullio L, Ristori E, Zani M, Crescenzi M, Scarpa S, Piaggio G, Vacca A, Peverali FA, Diana F, Screpanti I, Frati L, Gulino A. HMGI(Y) and HMGI-C genes are expressed in neuroblastoma cell lines and tumors and affect retinoic acid responsiveness. Cancer Res 59: 2484–2492, 1999 [PubMed] [Google Scholar]
- 20.Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev 20: 689–725, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Girotti M, Weinberg MS, Spencer RL. Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab 296: E888–E897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Sanchez CE, Foecking MF, Ferris MW, Chavarri MR, Uribe L, Gomez-Sanchez EP. The production of monoclonal antibodies against aldosterone. Steroids 49: 581–587, 1987 [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Origin of aldosterone in the rat heart. Endocrinology 145: 4796–4802, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Haase M, Schott M, Bornstein SR, Malendowicz LK, Scherbaum WA, Willenberg HS. CITED2 is expressed in human adrenocortical cells and regulated by basic fibroblast growth factor. J Endocrinol 192: 459–465, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Hammer GD, Parker KL, Schimmer BP. Minireview: transcriptional regulation of adrenocortical development. Endocrinology 146: 1018–1024, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2: 297–307, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kim AC, Barlaskar FM, Heaton JH, Else T, Kelly VR, Krill KT, Scheys JO, Simon DP, Trovato A, Yang WH, Hammer GD. In search of adrenocortical stem and progenitor cells. Endocr Rev 30: 241–263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krones-Herzig A, Mittal S, Yule K, Liang H, English C, Urcis R, Soni T, Adamson ED, Mercola D. Early growth response 1 acts as a tumor suppressor in vivo and in vitro via regulation of p53. Cancer Res 65: 5133–5143, 2005 [DOI] [PubMed] [Google Scholar]
- 29.LeHoux JG, Fleury A, Ducharme L. The acute and chronic effects of adrenocorticotropin on the levels of messenger ribonucleic acid and protein of steroidogenic enzymes in rat adrenal in vivo. Endocrinology 139: 3913–3922, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Lemos DR, Downs JL, Urbanski HF. Twenty-four-hour rhythmic gene expression in the rhesus macaque adrenal gland. Mol Endocrinol 20: 1164–1176, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Kelm RJ, Jr, Strauch AR. Transforming growth factor beta1-mediated activation of the smooth muscle alpha-actin gene in human pulmonary myofibroblasts is inhibited by tumor necrosis factor-alpha via mitogen-activated protein kinase kinase 1-dependent induction of the Egr-1 transcriptional repressor. Mol Biol Cell 20: 2174–2185, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin LJ, Tremblay JJ. The human 3beta-hydroxysteroid dehydrogenase/Delta5-Delta4 isomerase type 2 promoter is a novel target for the immediate early orphan nuclear receptor Nur77 in steroidogenic cells. Endocrinology 146: 861–869, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Gonzalez J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res 65: 609–618, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Mukai K, Mitani F, Agake R, Ishimura Y. Adrenocorticotropic hormone stimulates CYP11B1 gene transcription through a mechanism involving AP-1 factors. Eur J Biochem 256: 190–200, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Mukai K, Mitani F, Shimada H, Ishimura Y. Involvement of an AP-1 complex in zone-specific expression of the CYP11B1 gene in the rat adrenal cortex. Mol Cell Biol 15: 6003–6012, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nogueira EF, Vargas CA, Otis M, Gallo-Payet N, Bollag WB, Rainey WE. Angiotensin-II acute regulation of rapid response genes in human, bovine, and rat adrenocortical cells. J Mol Endocrinol 39: 365–374, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Nogueira EF, Xing Y, Morris CA, Rainey WE. Role of angiotensin II-induced rapid response genes in the regulation of enzymes needed for aldosterone synthesis. J Mol Endocrinol 42: 319–330, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parmar J, Key RE, Rainey WE. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J Clin Endocrinol Metab 93: 4542–4546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rainey WE, Bird IM, Mason JI. The NCI-H295 cell line: a pluripotent model for human adrenocortical studies. Mol Cell Endocrinol 100: 45–50, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Rainey WE, Saner K, Schimmer BP. Adrenocortical cell lines. Mol Cell Endocrinol 228: 23–38, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Rincon Garriz JM, Suarez C, Capponi AM. c-Fos mediates angiotensin II-induced aldosterone production and protein synthesis in bovine adrenal glomerulosa cells. Endocrinology 150: 1294–1302, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Romero DG, Plonczynski M, Vergara GR, Gomez-Sanchez EP, Gomez-Sanchez CE. Angiotensin II early regulated genes in H295R human adrenocortical cells. Physiol Genomics 19: 106–116, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Romero DG, Plonczynski MW, Gomez-Sanchez EP, Yanes LL, Gomez-Sanchez CE. RGS2 is regulated by angiotensin II and functions as a negative feedback of aldosterone production in H295R human adrenocortical cells. Endocrinology 147: 3889–3897, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Romero DG, Plonczynski MW, Welsh BL, Gomez-Sanchez CE, Zhou MY, Gomez-Sanchez EP. Gene expression profile in rat adrenal zona glomerulosa cells stimulated with aldosterone secretagogues. Physiol Genomics 32: 117–127, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Romero DG, Rilli S, Plonczynski MW, Yanes LL, Zhou MY, Gomez-Sanchez EP, Gomez-Sanchez CE. Adrenal transcription regulatory genes modulated by angiotensin II and their role in steroidogenesis. Physiol Genomics 30: 26–34, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Romero DG, Vergara GR, Zhu Z, Covington GS, Plonczynski MW, Yanes LL, Gomez-Sanchez EP, Gomez-Sanchez CE. Interleukin-8 synthesis, regulation, and steroidogenic role in H295R human adrenocortical cells. Endocrinology 147: 891–898, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Romero DG, Yanes LL, de Rodriguez AF, Plonczynski MW, Welsh BL, Reckelhoff JF, Gomez-Sanchez EP, Gomez-Sanchez CE. Disabled-2 is expressed in adrenal zona glomerulosa and is involved in aldosterone secretion. Endocrinology 148: 2644–2652, 2007 [DOI] [PubMed] [Google Scholar]
- 49.Romero DG, Zhou MY, Yanes LL, Plonczynski MW, Washington TR, Gomez-Sanchez CE, Gomez-Sanchez EP. Regulators of G-protein signaling 4 in adrenal gland: localization, regulation, and role in aldosterone secretion. J Endocrinol 194: 429–440, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev 84: 489–539, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Szekeres M, Turu G, Orient A, Szalai B, Supeki K, Cserzo M, Varnai P, Hunyady L. Mechanisms of angiotensin II-mediated regulation of aldosterone synthase expression in H295R human adrenocortical and rat adrenal glomerulosa cells. Mol Cell Endocrinol 302: 244–253, 2009 [DOI] [PubMed] [Google Scholar]
- 52.Tan L, Peng H, Osaki M, Choy BK, Auron PE, Sandell LJ, Goldring MB. Egr-1 mediates transcriptional repression of COL2A1 promoter activity by interleukin-1beta. J Biol Chem 278: 17688–17700, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage. Development 134: 2349–2358, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Viard I, Hall SH, Jaillard C, Berthelon MC, Saez JM. Regulation of c-fos, c-jun and jun-B messenger ribonucleic acids by angiotensin-II and corticotropin in ovine and bovine adrenocortical cells. Endocrinology 130: 1193–1200, 1992 [DOI] [PubMed] [Google Scholar]
- 55.Watanabe G, Lee RJ, Albanese C, Rainey WE, Batlle D, Pestell RG. Angiotensin II activation of cyclin D1-dependent kinase activity. J Biol Chem 271: 22570–22577, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Wilson TE, Mouw AR, Weaver CA, Milbrandt J, Parker KL. The orphan nuclear receptor NGFI-B regulates expression of the gene encoding steroid 21-hydroxylase. Mol Cell Biol 13: 861–868, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang P, Mellon SH. Multiple orphan nuclear receptors converge to regulate rat P450c17 gene transcription: novel mechanisms for orphan nuclear receptor action. Mol Endocrinol 11: 891–904, 1997 [DOI] [PubMed] [Google Scholar]