Abstract
Zinc transporter 2 (ZnT2) plays a major role in zinc (Zn) export from the mammary gland. Recently, we determined that ZnT2 is associated with secretory vesicles reflecting its role in Zn secretion during lactation. Herein, we identified two distinct single nucleotide polymorphisms (SNPs) in SLC30A2, which encodes ZnT2. SNP1 (rs35235055) results in a leucine-to-proline substitution (Leu23Pro), while SNP2 (rs35623192) results in an arginine-to-cysteine substitution (Arg340Cys). We examined the localization and function of each SNP in cells generated to express these polymorphic variants. SNP1 was mislocalized to lysosomes, while SNP2 was mislocalized to the Golgi apparatus. FluoZin-3 fluorescence illustrated increased lysosomal accumulation of Zn in cells expressing SNP1 concomitant with the abrogation of Zn secretion. In contrast, ectopic expression of SNP2 was associated with the expansion of cytoplasmic Zn pools, elevated reactive oxygen species, and increased Zn efflux. Taken together, our data indicate that polymorphic variants in ZnT2 distinctly alter mammary cell Zn metabolism. We speculate that these SNPs may compromise mammary cell function, which may have important implications in human health and breast disease.
Keywords: zinc transporters, lactation, mammary cells, reactive oxygen species
the lactating mammary gland has an extraordinary zinc (Zn) requirement due to the need to secrete abundant Zn (1–3 mg Zn/day) into milk. While the Zn concentration of breast milk declines during lactation, breast milk usually contains adequate Zn to meet the requirement for term infants up to 4–6 mo of age (18). However, milk Zn concentration varies greatly between mothers (13). Neither nutritional factors (e.g., Zn intake, nutrient interactions, undernutrition), environmental factors (e.g., cigarette smoking, oral contraceptives), nor physiological variables (premature delivery, parity, maternal age, infection, diabetes) consistently affect milk Zn concentration or predict its rate of decline during lactation (12). The physiological relevance of this variability reflects the fact that low milk Zn concentration can quickly result in severe Zn deficiency in nursing infants (7, 19, 33). Neonatal Zn deficiency compromises neuronal and behavioral development, impairs immune function, and increases infant morbidity and/or mortality (1). This may be of particular importance to infants born preterm who may have increased Zn requirements during postnatal development. Thus understanding how the mammary gland regulates Zn secretion is critical to optimizing infant growth and development.
Recent identification of genetic mutations and polymorphisms in Zn transporters has drawn attention to the consequences of dysregulated cellular and physiological Zn homeostasis in human health. In humans, acrodermatitis enteropathica (AE) is a rare, autosomal recessive disorder that results in severe Zn deficiency as a consequence of impaired intestinal Zn absorption (2) due to mutations in SLC39A4, which encodes the Zn importer Zip4 (9, 41). It has been recently shown that single nucleotide polymorphisms (SNPs) in the gene encoding ZnT8 (SLC30A8) are associated with diabetes. We previously identified a missense mutation in the gene that encodes ZnT2 (SLC30A2) that substitutes an arginine for a highly conserved histidine residue (H54R) in the NH2-terminal domain results in aggresomal accumulation of the mutated form of ZnT2. Importantly, women who are heterozygotes for this mutation secreted ∼75% less Zn into their milk relative to women of similar lactational stage (7), indicating that ZnT2 plays a major role in Zn secretion into milk. Our recent studies suggest that ZnT2 facilitates Zn accumulation into exocytotic vesicles in secreting mammary epithelial cells (MECs) (23) thereby participating in Zn transfer into milk. Therefore, we hypothesized that polymorphic variants in ZnT2 would modulate Zn export from MECs and thus may play a determinant role in the variability observed in the Zn concentration of milk.
Herein we identified two distinct SNPs in ZnT2 (PubMed reference rs35235055, rs35623192): SNP1 corresponds to an amino acid substitution from leucine to proline (Leu23Pro) in the NH2-terminal region, while SNP2 corresponds to an amino acid substitution from arginine to cysteine (Arg340Cys) in the COOH terminus (Table 1). Topology prediction of ZnT2 using the proteomic server TopPred (8) predicts that both amino acid 23 and amino acid 340 reside intracytoplasmically. This study aimed to determine how these specific polymorphisms in the NH2 or COOH terminus of ZnT2 affect MEC Zn metabolism and explored the functional consequences of polymorphic expression of ZnT2 proteins. We determined that SNP1 was mislocalized to the lysosomal compartment, whereas SNP2 was mislocalized to the Golgi apparatus. Ectopic expression of SNP1 resulted in increased lysosomal Zn accumulation concomitant with decreased Zn secretion. In contrast, SNP2 did not appear to be functional, and ectopic expression of SNP2 led to increased cellular oxidative stress.
Table 1.
Identification of two nonsynonymous ZnT2 gene polymorphisms in the dbSNP database
| dbSNP Cluster ID, rs# | mRNA Position | Allele Change | Amino Acid Position | Protein Residue | |
|---|---|---|---|---|---|
| SNP1 | rs35235055 | 285 | thymidine (T) | 23 | leucine (L) |
| Leu23Pro | cytidine (C) | proline (P) | |||
| SNP2 | rs35623192 | 1235 | cytidine (C) | 340 | arginine (R) |
| Arg340Cys | thymidine (T) | cysteine (C) |
dbSNP database available at http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=7780.
MATERIALS AND METHODS
Generation of plasmid DNA constructs.
A COOH-terminal tandem hemagglutinin (HA) tagged ZnT2 plasmid was generated as previously described (7). Two missense SNPs were identified in the dbSNP database (PubMed references rs35235055 and rs35623192) corresponding to a T/C transition at the +285 nucleotide position and to a C/T transition at the +1235 nucleotide position in the coding region of the human SLC30A2 gene, respectively. Mutagenesis was performed using the Phusion site-directed mutagenesis kit (New England Biolab, Beverly, MA). The two polymorphic variants were generated using mutagenic oligonucleotides: mutated nucleotides are indicated in parentheses. Sense, 5′-phos-(C)GTGGCAGGAAGGGGCTG-3′ and anti-sense, 5′-phos-GAGATCCCGTGTATGACCGG-3′ for rs35235055; sense, 5′-phos-(T)GCCTCCAAGGGAAGTTCCAC-3′ and anti-sense, 5′-phos-GCTGCTGGCTGTCTTCAGC-3′ for rs35623192. The entire coding region of the mutated forms of human SLC30A2 was amplified and fused to two tandem HA epitopes at the COOH terminus and inserted into the pcDNA3.1/V5-His TOPO vector (Invitrogen). The site-directed mutation, orientation, and fidelity of the insert and incorporation of the epitope tag were confirmed by directed sequencing (The Pennsylvania State University, Nucleic Acid Facility).
Cell culture and expression of polymorphic variants.
HC11 cells were a gift from Dr. Jeffery Rosen (Houston, TX) and used with permission of Dr. Bernd Groner (Institute for Biomedical Research, Frankfurt, Germany). HC11 cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, gentamicin (50 mg/l, Sigma), insulin (5 mg/l, Sigma), and epidermal growth factor (10 μg/l, Sigma) at 37°C and 5% CO2. Cells were plated in antibiotic-free growth medium in six-well plates (2.5 × 106 cells/well) for expression of SNP-HA fusion proteins or in 24-well plates (6 × 105 cells/well) for Zn secretion and subcellular localization (on glass coverslips) experiments and cultured overnight until ∼95% confluent. Cells were transiently transfected with 0.8 μg (24-well plates) or 4 μg (6-well plates) of pcDNA3.1, pcDNA3.1-ZnT2, pcDNA3.1-SNP1, or pcDNA3.1-SNP2 in antibiotic-free Opti-MEM medium using Lipofectamine 2000 (Invitrogen) at a transfection reagent:DNA ratio of 1:2.5 according to manufacturer's specifications for up to 24 h prior to experiments.
Subcellular localization of HA-tagged SNPs.
To determine the subcellular distribution of SNP1-HA or SNP2-HA proteins, transfected cells plated onto glass coverslips were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min and permeabilized with 0.2% Triton X-100 in PBS for 5 min. Nonspecific binding was blocked with 4% bovine serum albumin in PBS for 30 min, and transfected ZnT2-HA, SNP1-HA, or SNP2-HA protein was detected following incubation with Alexa 488-conjugated anti-mouse HA (1 μg/ml, Invitrogen) for 1 h. Cells were extensively washed, and nuclei were stained with TOPRO Nuclear Stain 647 (1 μM, Invitrogen) for 30 min. Cells were washed extensively in PBS; coverslips were drained, mounted in ProLong Gold (Invitrogen), and sealed with nail polish. To determine the subcellular localization of HA-tagged SNPs, transfected cells were fixed and permeabilized as described above. Nonspecific binding was blocked with 5% goat serum/1% bovine serum albumin in PBS for 1 h. Lysosomes, the Golgi apparatus, and late endosomes were visualized as follows. Rabbit anti-Lamp1 (7 μg/ml, Abcam) was used as a lysosomal marker; rabbit anti-p58 (1 μg/ml, Sigma) was used as a Golgi apparatus marker; rabbit anti-mannose-6-phosphate receptor (M6PR; 5 μg/ml, Abcam) was used as a late endosomal marker. Detection of Lamp1, p58, and M6PR was performed using an anti-rabbit IgG antibody conjugated to Alexa 568 for 20 min. After extensive washing with PBS, nonspecific binding was blocked with 5% goat serum/1% bovine serum albumin in PBS for 20 min followed by detection with Alexa 488-conjugated anti-mouse HA (1 μg/ml, Invitrogen) for 1 h, shielded from light. Immunofluorescent imaging was performed using an Olympus FV1000 with PlanApo 60X oil lens N.A. 1.42, and digital images were captured sequentially (FV10-ASW, version 4.5; Olympus) to eliminate potential interference between fluorochromes and images were saved as .tif files to maintain image quality. Colocalization analysis was performed through the use of the “color composite” and “colocalization” functions in Image Pro Plus (version 4.5; Olympus), and colocalized pixels were pseudocolored yellow.
Immunoblotting.
Cells were washed in PBS, scraped into lysis buffer containing protease inhibitors as previously described (7), and sonicated for 20 s on ice. Cellular debris and nuclei were pelleted by centrifugation at 500 g for 5 min, and protein concentration of the postnuclear supernatant was determined by the Bradford assay. To isolate the crude membrane fraction, the postnuclear supernatant was centrifuged at 150,000 g for 20 min at 4°C. Protein (50–100 μg) was diluted in Laemmli sample buffer containing DTT (100 mM) and incubated at 95°C for 5 min. Proteins were separated by electrophoresis, transferred to nitrocellulose for 60 min at 350 mA, then immunoblotted with anti-mouse HA (0.4 μg/ml; Roche), and detected with horseradish peroxidase-conjugated IgG as previously described (7). Proteins were visualized by chemiluminescence after exposure to autoradiography film, and relative band density and molecular mass relative to standard molecular mass markers (Amersham Pharmacia) was assessed using the Gel Quantification System (Carestream Health, Rochester, NY).
Cytoplasmic Zn pool assay (4×-metal response element luciferase reporter assay).
The 4×-MRE (metal-responsive element) pGL3-luciferase reporter was kindly provided by Dr. Colin Duckett (University of Michigan Medical School, Ann Arbor, MI) and used as previously described (25). Briefly, cells were cotransfected with either the empty pGL3 vector (0.8 μg) or the 4×-MRE pGL3 luciferase reporter (0.8 μg) and pRL-TK Renilla vector (0.05 μg) in addition to pcDNA3.1-ZnT2, pcDNA3.1-SNP1, or pcDNA3.1-SNP2 (0.8 μg). After 24 h, cells were treated with ZnSO4 (1 μM) for 24 h to activate the promoter and then analyzed for luciferase activity as previously described (39). After incubation, cells were rinsed with 1× PBS and harvested in 1× passive lysis buffer (Promega, Madison, WI) following the manufacturer's instructions, and luminometry was assessed (Turner Biosystems, Sunnyvale, CA) using Dual-Luciferase reporter assay system (Promega) for firefly and Renilla luciferase activity. Relative light units were determined by the ratio of firefly:Renilla luciferase activity.
Vesicular Zn accumulation assay (FluoZin-3 fluorometry).
To determine whether SNPs in ZnT2 affect the accumulation of labile Zn, cells were plated in antibiotic-free growth medium in 96-well optical bottom plates and cultured until 90–95% confluent. Cells were transfected with plasmids containing ZnT2, SNP1-HA, or SNP2-HA for 24 h. Cells were rinsed with 1× PBS, pH 7.4, and loaded with FluoZin-3 AM (2 μM, Invitrogen) in Opti-MEM containing 0.2% pluoronic acid 127 for 1 h as recommended by the manufacturer. Cells were rinsed twice with PBS, pH 7.4, and incubated for 30 min at 25°C with constant shaking. Fluorescence of FluoZin-3 (emission 485 nm/excitation 520 nm) was measured at 25°C using a FLUOstar OPTIMA spectrofluorimeter with FLUOstar OPTIMA software version 1.32R2 (BMG Labtech). Cellular protein concentration was determined by the Bradford assay, and fluorescence measurements were normalized to total protein concentration. To visualize compartment-specific alterations in Zn pools (e.g., cytoplasmic or vesicular), cells were cultured on glass coverslips and transfected with plasmids containing SNP1-HA or SNP2-HA for 24 h. Cells were rinsed with 1× PBS, pH 7.4, and loaded with FluoZin-3 AM (1 μM, Invitrogen) in Opti-MEM containing 0.2% pluoronic acid 127 for 1 h as recommended by the manufacturer. Cells were rinsed twice with PBS, pH 7.4, and incubated in PBS for 30 min at 25°C with constant shaking. Immunofluorescent imaging was performed using an Olympus FV1000, with PlanApo 60X oil lens N.A. 1.42, and digital images were captured sequentially.
LysoTracker and FluoZin-3 staining for live cell imaging.
To identify the vesicular compartments that accumulate Zn, cells were cultured on glass coverslips and transfected with plasmids containing SNP1-HA for 24 h. Cells were loaded with LysoTracker Red DND-99 (75 nM, Invitrogen) in Opti-MEM for 1 h and rinsed twice with PBS, pH 7.4, and secondarily treated with FluoZin-3 AM (1 μM, Invitrogen) as described above. As a positive control, cells expressing SNP1 were preincubated with chloroquine disphosphate (Sigma, 100 μM) for 3 h to disrupt lysosomes as previously described (16) and compared with untreated cells. Imaging of “vesicular” Zn pools was performed using an Olympus FV1000, with PlanApo 60X oil lens N.A. 1.42, and digital images were captured sequentially.
65Zn secretion assay.
To determine if cells expressing SNP1 and SNP2 were able to secrete Zn, HC11 cells were generated to express either SNP1 or SNP2 as described above. Cells were loaded serum-free medium containing 0.1 μM ZnSO4 and 65Zn (0.1 μCi; Oakridge National Laboratory, Oakridge, TN) for 3 h at 37°C and then washed briefly with cold PBS containing 1 mM EDTA to remove exofacial-bound Zn. Cells were cultured in serum-free medium for up to 120 min, and the amount of 65Zn exported into the culture medium was quantified in a gamma counter.
Reactive oxygen species assay (2, 7-dichlorodihydrofluoroscein diacetate).
2, 7-Dichlorodihydrofluoroscein diacetate (DCFH-DA, Invitrogen) were used to measure reactive oxygen species (ROS) production. DCFH-DA is converted to DCFH by intracellular esterases, and then nonfluorescent DCFH is oxidized to DCF (fluorescent dichlorofluorescein) by reaction with ROS. Cells were transfected with plasmid containing ZnT2-HA, SNP1-HA, or SNP2-HA for 24 h. Cells were rinsed with 1× PBS, pH 7.4, and loaded with 10 μM DCFH-DA for 1 h. Fluorescence of DCF (emission 495 nm, excitation 520 nm) was measured at 25°C using FLUOstar OPTIMA plate reader. Cellular protein concentration was determined by the Bradford assay, and fluorescence measurements were normalized to total protein concentration. Cells were treated with hydrogen peroxide (H2O2, 100 μM) for 1 h as a positive control.
Statistical analysis.
Results are presented as means ± standard deviation from triplicate samples from three independent experiments. Statistical comparisons were performed using Student's t-test (Prism Graph Pad, Berkeley, CA), and a significant difference was demonstrated at P < 0.05. To quantify the subcellular localization of each SNP with specific subcellular compartments, statistical analysis of the correlation of the intensity values of green and red pixels in a dual-channel image was performed using correlation coefficient [Pearson's coefficient (PC)]. The value can range from +1 to −1, with +1 illustrating a positive correlation, −1 a negative correlation, and zero illustrating no correlation (4).
RESULTS
Expression of ZnT2 polymorphic variants in mammary cells.
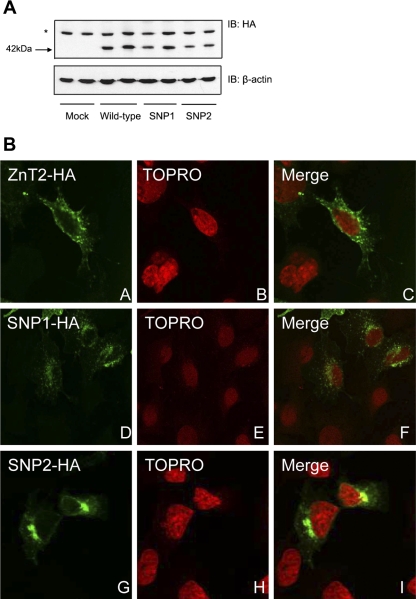
To explore the function of polymorphic variants in ZnT2 and determine effects they have on Zn metabolism in MECs, we first generated MECs expressing wild-type ZnT2-HA, SNP1-HA, or SNP2-HA. Immunoblotting with HA antibody verified that both variants could be successfully translated (Fig. 1A). Data indicated that cells transfected with wild-type ZnT2, SNP1, or SNP2 resulted in similar translation efficiency of a ZnT2 protein with the predicted molecular mass (∼42 kDa).
Fig. 1.
Polymorphic variants in zinc transporter 2 (ZnT2) result in mislocalization of the protein. A: representative immunoblot (IB) of crude membrane proteins (50 μg protein/lane) from cells transfected with pcDNA3.1 (Mock), or cells expressing wild-type ZnT2-hemagglutinin (HA) fusion protein (ZnT2), single nucleotide polymorphism (SNP)1-HA fusion protein (SNP1), or SNP2-HA fusion protein (SNP2) detected with HA antibody. *Nonspecific protein recognized by the anti-HA antibody. B: localization of wild-type ZnT2-HA (A–C), SNP1-HA (D–F), or SNP2-HA (G–I) in transfected HC11 cells examined using confocal microscopy. ZnT2 was detected with Alexa 488-conjugated anti-HA antibody (green; A, D, and G), and the nucleus was stained with TOPRO nucleic acid dye (red; B, E, and H).
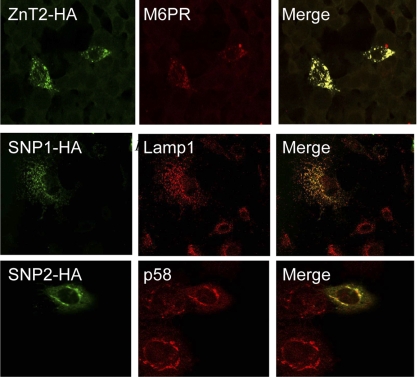
We next aimed to determine the subcellular localization of polymorphic variants of ZnT2 in mammary cells. MECs were generated to ectopically express SNP1-HA or SNP2-HA, or wild-type ZnT2-HA, and localization was visualized by confocal microscopy. Our data indicated that in contrast to the punctate vesicular staining pattern observed for wild-type ZnT2 in endosomal compartments (23), SNP1 and SNP2 were detected in different cellular compartments (Fig. 1B). These data suggested that polymorphic variants in ZnT2 are mislocalized to cellular compartments distinct from wild-type ZnT2. We next examined the subcellular localization of these two ZnT2 variants using confocal microscopy. Consistent with our previous reports (23), wild-type ZnT2 was detected in late endosomal/secretory vesicles that specifically colocalized with M6PR (Fig. 2). Unlike wild-type ZnT2, confocal microscopy revealed that SNP1 was almost exclusively colocalized with Lamp1, a lysosomal marker (Pearson's coefficient = 0.9). In contrast, SNP2 was almost exclusively colocalized with p58, a Golgi apparatus marker (Pearson's coefficient = 0.8) (Fig. 2). The localization of SNP1 and SNP2 in compartments discrete from wild-type ZnT2 suggested that expression of polymorphic variants of ZnT2 may affect the functional role of ZnT2 in cellular Zn distribution in MECs.
Fig. 2.
Confocal microscopy identified lysosomes and the Golgi apparatus as the specific subcellular compartments associated with SNP1 and SNP2, respectively. Cells transfected with pcDNA3.1-ZnT2, -SNP1, and -SNP2 to express HA-tagged ZnT2 (ZnT2-HA), SNP1 (SNP1-HA), and SNP2 (SNP2-HA) were detected with HA antibody and visualized with Alexa 488 conjugated anti-mouse IgG. Confocal micrographs illustrate that the wild-type ZnT2 (green) colocalizes (merge, yellow) with mannose-6-phosphate receptor (M6PR, red, endosome marker). SNP1 (green) colocalizes (merge, yellow) with Lamp1 (red, lysosomal marker) [Pearson's coefficient (PC) = 0.9]. SNP2 colocalizes (merge, yellow) with p58 (red, Golgi marker) (PC = 0.8). The value of PC can range from 1 to −1, with 1 standing for complete positive correlation and −1 for a negative correlation, with zero standing for no correlation.
Effect of ZnT2 polymorphisms on cellular Zn distribution.
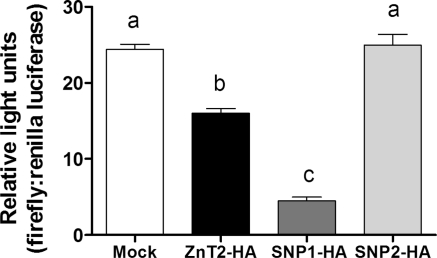
To investigate effects of ZnT2 mislocalization to lysosomes and the Golgi apparatus, we first aimed to determine if polymorphic variants in ZnT2 affected changes in cytoplasmic Zn pools. We assessed cytoplasmic Zn pools using a luciferase reporter assay that is a sensitive method of quantifying relative changes in cytoplasmic Zn pools (24). Cells were cotransfected with a luciferase reporter construct containing a 4×-MRE in the presence of SNP1-HA, SNP2-HA, or wild-type ZnT2-HA. This novel transcription-based Zn biosensor is based on the endogenous capacity of Zn to activate metal responsive transcription factor-1 and bind to MREs in the promoter region of specific genes. Ectopic expression of SNP1 significantly decreased cytoplasmic Zn pools (−72%, P < 0.05) relative to cells overexpressing wild-type ZnT2 (Fig. 3). In contrast, cells expressing SNP2 had significantly higher (P < 0.05) luciferase activity compared with cells overexpressing wild-type ZnT2. In addition, there was no effect of ectopic expression of SNP2 on cytoplasmic Zn pools relative to mock-transfected cells, suggesting that SNP2 was not able to transport Zn into the Golgi apparatus.
Fig. 3.
Cytoplasmic zinc (Zn) pools were significantly reduced in cells expressing SNP1 but not affected by ectopic expression of SNP2. Cytoplasmic Zn level was measured using a 4X-metal response element (MRE) luciferase reporter construct in cells co-transfected with each SNP and compared with cells transfected to overexpress wild-type ZnT2. Data represent mean luminescence (arbitrary units/μg protein) (n = 3) from 2 independent experiments. Means with different letters are significantly different as analyzed by 1-way analysis of variance followed by a Tukey posttest (P < 0.05).
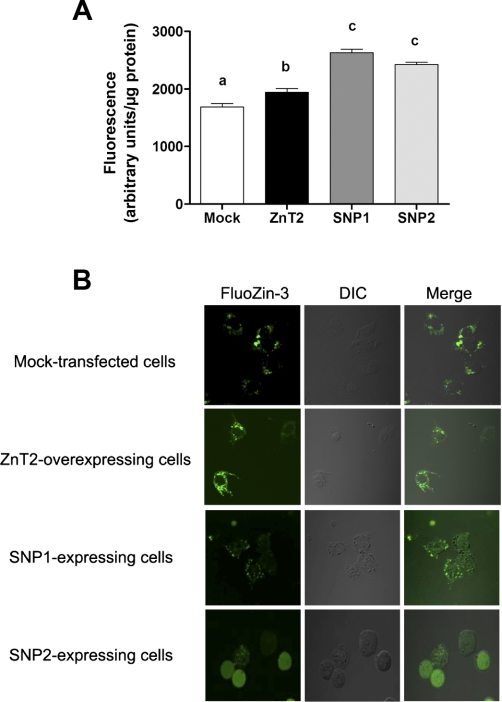
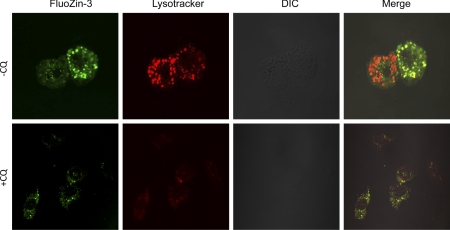
To determine if polymorphic variants in ZnT2 affect the accumulation of labile Zn into vesicles we used the Zn-specific fluorophore, FluoZin-3. Upon binding to labile Zn, FluoZin-3 fluoresces, permitting quantification of relative changes in labile Zn pools using fluorimetry. Our results clearly revealed that cells expressing SNP1-HA or SNP2-HA had significantly greater fluorescence (+35 and +25%, respectively, P < 0.05) compared with cells overexpressing wild-type ZnT2 (Fig. 4A). To determine if expression of SNP1 resulted in vesicular accumulation of Zn, labile Zn pools were detected with FluoZin-3 and visualized by confocal microscopy. As illustrated in Fig. 4B, cells expressing SNP1-HA displayed the classic punctuate staining pattern of vesicularized Zn pools. To verify that lysosomes accumulated Zn pools in SNP1-expressing cells, we treated cells expressing SNP1-HA with chloroquine (CQ), a weak base known to disrupt lysosomal function by reducing the acidification of the lysosomal compartments (28). As illustrated in Fig. 5, in the absence of CQ FluoZin-3 exclusively colocalized with Lysotracker (Pearson's coefficient = 0.9), indicating that lysosomes are a site of Zn accumulation in SNP1-expressing cells. It is important to note that the different intensity in FluoZin-3 fluorescence between cells (Fig. 5, top) may reflect the transfection efficiency of the SNP1-HA construct. In addition, CQ pretreatment disrupted lysosomes and importantly, disrupted the colocalization of FluoZin-3 and Lysotracker, thus directly confirming a role for SNP1 in the expansion of lysosomal Zn pools. Together our data suggest that expression of SNP1 enhanced Zn accumulation into lysosomes at the expense of cytoplasmic Zn pools. In contrast, cells expressing SNP2 displayed a hazy, diffuse FluoZin-3 staining pattern reflecting the detection of nonvescularized, cytoplasmic Zn pools. This is consistent with our determination that labile Zn pools in the cytoplasm were significantly affected by ectopic expression of SNP2 (Fig. 4).
Fig. 4.
Expression of SNP1 leads to increased Zn accumulation into vesicles. Zn accumulation was assessed using FluoZin-3 fluorescence in cells transfected to express each SNP and compared with cells transfected to overexpress wild-type ZnT2. Fluorescence was quantified by fluorimetry (A) and visualized by confocal microscopy (B). Data represent mean fluorescence ± SD (n = 8 samples/group). Means with different letters are significantly different as analyzed by 1-way analysis of variance followed by a Tukey posttest (p < 0.05). DIC, differential interference contrast.
Fig. 5.
Ectopic expression of SNP1 accumulates Zn into lysosomes. Cells were transfected with pcDNA3.1-SNP1 to express HA-tagged SNP1. Confocal micrographs illustrate that colocalization (yellow) of FluoZin-3 (green) with the lysosomal marker Lysotracker (red) in the absence of chloroquine disphosphate (−CQ). Colocalization is eliminated in chloroquine disphosphate (+CQ)-treated SNP1-expressing cells, indicating lysosomes are a site of Zn accumulation in SNP1-expressing cells.
Role of ZnT2 polymorphisms on Zn secretion.
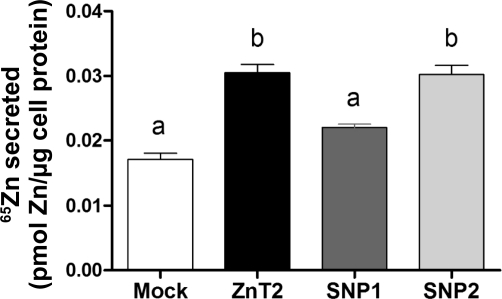
We next aimed to determine effects of polymorphic ZnT2 variants on Zn secretion from MECs. To do so, we measured the ability of SNP1- or SNP2-expressing cells to efflux Zn into the culture medium after being preloaded with 65Zn. Consistent with our previous reports (23) the overexpression of wild-type ZnT2 increased Zn secretion ∼2-fold. Our data clearly demonstrated that cells expressing SNP1 completely eliminated the increase in Zn secretion observed in ZnT2-overexpressing cells (Fig. 6). In contrast, our data suggest that ectopic expression of SNP2 was associated with enhanced Zn export.
Fig. 6.
Zn secretion was significantly reduced in cells expressing SNP1 but increased by ectopic expression of SNP2. Cells were preincubated in serum-free medium containing 0.1 μM ZnSO4 and 0.1 μCi 65Zn for 3 h. Medium was replaced, and 65Zn was measured after 120 min. Values represent mean pmol Zn/μg protein ± SD (n = 3 samples/genotype). Means with different letters are significantly different as analyzed by 1-way analysis of variance followed by a Tukey posttest (P < 0.05).
Effect of ZnT2 polymorphisms on oxidative stress.
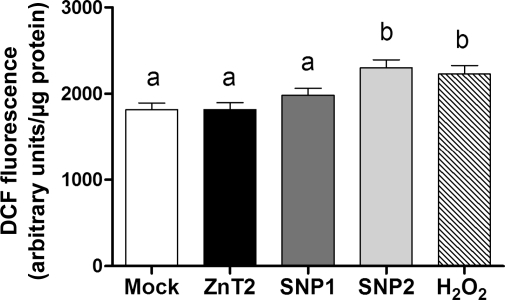
These disparate observations led us to postulate that the misdistribution of cellular Zn pools affected by polymorphic ZnT2 variants would have functional consequences in MECs. Previous studies have demonstrated that alterations in cellular Zn levels result in oxidative stress, which is associated with cancer, degenerative diseases, and other pathological conditions. Given the importance of Zn in the regulation of oxidative stress and our ability to modulate cellular Zn pools by expression of polymorphic variants in ZnT2, we explored whether the misdistribution of cellular Zn pools induced oxidative stress in our MEC model. ROS was detected in these experiments using DCFH-DA as fluorescence increases when DCFH is oxidized to DCF by O2−, H2O2, or •OH. As shown in Fig. 7, ROS levels were significantly increased in cells ectopically expressing SNP2 (+27%, P<0.05), compared with cells overexpressing wild-type ZnT2, while no effect of SNP1 expression on ROS levels were observed.
Fig. 7.
Reactive oxygen species (ROS) levels were significantly increased in cells ectopically expressing SNP2. ROS level was assessed using dichlorofluorescein (DCF)-HA fluorescence in cells transfected to express each SNP and compared with cells generated to overexpress wild-type ZnT2. To validate our assay, H2O2 (100 μM) was used as a positive control. Data represent mean fluorescence ± SD (n = 8 samples/group). Means with different letters are significantly different as analyzed by 1-way analysis of variance followed by a Tukey posttest (P < 0.05).
DISCUSSION
Increasing evidence gives credence to the postulate that genetic mutations and SNPs in Zn management proteins play significant roles in human health and disease. For example, AE is a rare, autosomal recessive disorder that results in severe Zn deficiency as a consequence of impaired intestinal Zn absorption (2). AE results from mutations in the gene SLC39A4, which encodes the Zn importer Zip4 (9, 41). The study of the murine ortholog, mZip4, further demonstrated that AE mutations disrupt the transport activity, subcellular localization and/or Zn-responsive protein trafficking of the mZip4 protein (40). Additionally, it has been recently shown that SNPs in the gene encoding ZnT8 (SLC30A8) are associated with diabetes. Several studies carried out in different European populations revealed a strong association of SNP rs13266634, a nonsynonymous (C>T) variant that results in the substitution of tryptophan for arginine at amino acid 325 (Arg325Trp) in ZnT8 with Type 2 diabetes (36, 37). We previously determined that the Zn transporter ZnT2 plays a major role in Zn secretion from the mammary gland into milk (7, 23). Importantly, lactating women with a mutation in the gene that encodes ZnT2 (SLC30A2) that results in the substitution of a highly conserved histidine residue for an arginine at amino acid 54 (H54R) secrete ∼75% less Zn into their milk (7). Thus a primary aim of this study was to determine if two specific polymorphic variants in ZnT2 affect Zn secretion from MECs. Secondly, we explored the consequences of ectopic expression of polymorphic ZnT2 variants on cellular Zn homeostasis and the functional consequences.
ZnT2 is normally localized to endosomal compartments (23) in MECs. Importantly, we have documented that ZnT2 is specifically not associated with lysosomes (23), nor does Zn normally accumulate in lysosomes in MECs (26). In contrast, the colocalization of SNP1 with Lamp1 indicated that SNP1 was mislocalized to lysosomes. An important question that arises from this observation is, does mislocalization of SNP1 to lysosomes alter Zn secretion? Our results clearly reveal that ectopic expression of SNP1 directly resulted in the accumulation of Zn into lysosomes at the expense of cytoplasmic Zn levels. However, lysosomal contents do not enter the secretory pathway, and thus the expanded lysosomal Zn pool was not destined for secretion.
The mislocalization of SNP1 to lysosomes combined with Zn hyperaccumulation into this compartment suggests that the Leu23Pro substitution may alter protein targeting motifs and/or provide “gain-of-function” over wild-type ZnT2. Topology prediction of ZnT2 using TopPred (8) predicts that similar to other members of the ZnT family (ZnT1–10, with the exception of ZnT5), ZnT2 displays the classic ZnT structure of six transmembrane domains with both NH2 and COOH termini on the cytoplasmic side of the membrane (31). Thus the Leu23Pro substitution resides in the NH2-terminal, cytoplasmic region. Sequence motifs found in the terminal cytoplasmic regions of membrane proteins generally control their subcellular localization, trafficking, and function. It is important to note that the change from a leucine to a proline is a particularly significant substitution because proline provides a unique conformational property not found with any other residues (35). Leucine, which has a hydrophobic aliphatic side chain, is known to favor formation of α-helices. In contrast, proline, which has a cyclic structure that influences protein secondary and tertiary architecture considerably, does not favor formation of α-helices but instead introduces breaks and kinks into α-helical parts of the peptide backbone (15). Bioinformatic prediction indicates that the Leu23Pro substitution indeed results in a change in the secondary protein structure from an α-helix (leucine) to a c-turn conformation (proline) consistent with our observation of mistargeting. Our previous studies have documented diffuse vesicular staining of wild-type ZnT2 (7) and further documented its localization to the endosomal compartments (23). A key question is, how does the Leu23Pro substitution target SNP1 to the lysosome? Lysosomal proteins contain sorting signals {[DE]XXXL[LI] and tyrosine-based motifs, YXXØ (where X = any residue, Ø = large hydrophobic residues)} in their cytosolic tails. Examination of the amino acid sequence of ZnT2 reveals that there is no identifiable lysosomal targeting signal within which the Leu23Pro substitution is contained. Despite the fact that there is no canonical motif for lysosomal targeting, many lysosomal proteins have noncanonical sequences including tyrosine-based (34) and dileucine-based motifs (32, 38), as well as other sequences that are not known to fit any particular motif (20). Thus it is currently unclear how SNP1 is targeted to lysosomes.
Our data suggest that the Leu23Pro substitution may also facilitate a gain-of-function resulting in Zn hyperaccumulation into lysosomes. This is evidenced by the quantitative difference in Zn accumulation into vesicles by SNP1-expressing cells. In support of this concept, a recent report indicates that ZnT5 and ZnT6 may require a proton gradient to transport Zn (27); thus mislocalization to an acidic compartment may enhance the Zn transporting rate of ZnT2 resulting in Zn hyperaccumulation into lysosomes. The importance of this observation may reflect the fact of Zn hyperaccumulation has been implicated in the development and progression of breast cancer (10) and tumor acidification (17). However, the precise mechanisms through which ZnT proteins transport Zn and the role of protein structure in Zn transporting function have not been characterized, and further studies will be required to determine the effects of alternative protein structure on the Zn transporting function of ZnT2.
In contrast to SNP1, our data indicated that the Arg340Cys substitution in SNP2 mislocalized ZnT2 to the Golgi apparatus. This mislocalization combined with abrogated function appeared to trap “labile” Zn in the cytoplasm. An interesting observation was that Zn efflux in cells expressing SNP2 was actually similar to cells overexpressing wild-type ZnT2. We speculate that this resulted from Zn export through other Zn exporting mechanisms such as ZnT1 or endogenous ZnT2 as expression of both are increased by excess Zn (21, 22).
An important question is, how is Arg340Cys mislocalized to the Golgi apparatus? Previous studies indicate that ZnT2 is colocalized with M6PR, a late endosomal vesicle marker (23). Trafficking of proteins to endosomes is dependent on sorting signals present in the COOH-terminal tails. The main signals responsible for this sorting consist of a cluster of acidic residues followed by a double leucine (i.e., DDSDEDLL and EESEERDDHLL) (6, 14), in which both aspartate and leucine residues are critical for signaling (5). Moreover, serine residues embedded within the acidic clusters are phosphorylated, and this modification causes enhanced sorting. In fact, the amino acid sequence of ZnT2 contains two aspartate residues located six residues apart upstream from a dileucine motif at Leu293 followed by a serine residue (DXXXXXDLLLS). This suggests that this sorting motif may contribute to the correct targeting of ZnT2 to the late endosomes. The Arg340Cys substitution downstream from this motif may negatively impact these sorting signals, thus retaining and accumulating ZnT2 within the Golgi apparatus. Alternatively, the Arg340Cys substitution may dramatically alter ZnT2 conformation as a result of the difference in isoelectric points between arginine (11.15) and cysteine (5.02). Ionic interactions between amino acids may affect protein stability through repulsion and attraction properties, and the net contribution of these interactions governs protein folding and stability. Thus we speculate that the Arg340Cys substitution may affect trafficking of ZnT2 protein to the late endosomes due to its conformational change (30). Our data suggest that the Arg340Cys substitution is associated with a “loss-of-function” eliminating the ability to transport Zn. We speculate that the Arg340Cys substitution in ZnT2 results in protein misfolding or enhanced reactivity and results in incorrect localization and loss-of-function, but further studies are required to explore this hypothesis.
A physiologically relevant observation from our study is that the inability to appropriately redistribute cellular Zn pools by ectopic expression of SNP2 induced oxidative stress. Several lines of evidence point to dysregulated Zn metabolism as an important factor in the onset and/or progression of multifactorial diseases (11). In addition, emerging data suggest that altered subcellular Zn pools may contribute to increased generation of ROS, which has been observed in cancer, degenerative diseases, and other pathological conditions (29). Therefore, we speculate that excessive accumulation of labile Zn in the cytoplasm by SNP2 expression sensitizes the mammary cells to oxidative stress. A growing body of evidence suggests that polymorphisms resulting perturbations in metal homeostasis supports this theory. For example, a strong association between polymorphisms in manganese superoxide dismutase (MnSOD) and cancer risk exists. A polymorphism in MnSOD (Val9Ala) disrupts protein targeting enzyme from cytosol to mitochondrial matrix where it acts on O2·− to dismutate it to H2O2. A change in the level of O2·− and of H2O2 in mitochondria modulates the molecular mechanisms of apoptosis, cellular adhesion, and cell proliferation and thus play a key role in cancer development (reviewed in Ref. 3).
In summary, our current study provides direct and functional evidence that nonsynonymous ZnT2 gene polymorphisms affect cellular Zn homeostasis in mammary epithelial cells. Our data indicate that the mislocalization of SNP1 to lysosomes results in hyperaccumulation of Zn into lysosomes and reduced Zn secretion. In contrast, mislocalization of SNP2 to the Golgi apparatus does not appear to facilitate Zn accumulation into the Golgi apparatus but is associated with oxidative stress through inability to appropriately regulate intracellular Zn pools. Due to the role of ZnT2 in Zn secretion into milk, we speculate that expression of three different ZnT2 variants in the human population may provide an explanation behind the variability in milk Zn concentration. Taken together, these results from genetic variations elegantly highlight the interplay between genetics and nutrient regulation. Further studies are needed to evaluate the incidence and penetrance of these polymorphisms in the human population and the relevance to human health and disease.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD-058614 to S. L. Kelleher.
DISCLOSURES
No conflicts of interest (financial or otherwise) are declared by the author(s).
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Linxi Qian for technical assistance, Dr. Colin Duckett (University of Michigan Medical School) for the 4×-MRE luciferase plasmid, and Kelleher lab members for excellent support. All confocal microscopy was done at the Cytometry Facility, University Park (Huck Institutes of the Life Sciences, Penn State University).
REFERENCES
- 1.Aggett PJ, Harries JT. Current status of zinc in health and disease states. Arch Dis Child 54: 909–917, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atherton DJ, Muller DP, Aggett PJ, Harries JT. A defect in zinc uptake by jejunal biopsies in acrodermatitis enteropathica. Clin Sci (Lond) 56: 505–507, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Bag A, Bag N. Target sequence polymorphism of human manganese superoxide dismutase gene and its association with cancer risk: a review. Cancer Epidemiol Biomarkers Prev 17: 3298–3305, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bolte S, Cordelieres FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224: 213–232, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Chen HJ, Yuan J, Lobel P. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J Biol Chem 272: 7003–7012, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Chen SS, Lee CN, Lee WR, McIntosh K, Lee TH. Mutational analysis of the leucine zipper-like motif of the human immunodeficiency virus type 1 envelope transmembrane glycoprotein. J Virol 67: 3615–3619, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowanadisai W, Lonnerdal B, Kelleher SL. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem 281: 39699–39707, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Claros MG, von Heijne G. TopPred II: an improved software for membrane protein structure predictions. Comput Appl Biosci 10: 685–686, 1994 [DOI] [PubMed] [Google Scholar]
- 9.Cragg RA, Christie GR, Phillips SR, Russi RM, Kury S, Mathers JC, Taylor PM, Ford D. A novel zinc-regulated human zinc transporter, hZTL1, is localized to the enterocyte apical membrane. J Biol Chem 277: 22789–22797, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Cui Y, Vogt S, Olson N, Glass AG, Rohan TE. Levels of zinc, selenium, calcium, and iron in benign breast tissue and risk of subsequent breast cancer. Cancer Epidemiol Biomarkers Prev 16: 1682–1685, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Devirgiliis C, Zalewski PD, Perozzi G, Murgia C. Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res 622: 84–93, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Dorea JG. Zinc deficiency in nursing infants. J Am Coll Nutr 21: 84–87, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Dorea JG. Zinc in human milk. Nutr Res 20: 1645–1688, 2000 [Google Scholar]
- 14.Johnson KF, Kornfeld S. A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function. J Biol Chem 267: 17110–17115, 1992 [PubMed] [Google Scholar]
- 15.Karvonen MK, Pesonen U, Koulu M, Niskanen L, Laakso M, Rissanen A, Dekker JM, Hart LM, Valve R, Uusitupa MI. Association of a leucine(7)-to-proline(7) polymorphism in the signal peptide of neuropeptide Y with high serum cholesterol and LDL cholesterol levels. Nat Med 4: 1434–1437, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Koh YH, von Arnim CA, Hyman BT, Tanzi RE, Tesco G. BACE is degraded via the lysosomal pathway. J Biol Chem 280: 32499–32504, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kokkonen N, Rivinoja A, Kauppila A, Suokas M, Kellokumpu I, Kellokumpu S. Defective acidification of intracellular organelles results in aberrant secretion of cathepsin D in cancer cells. J Biol Chem 279: 39982–39988, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Krebs NF. Dietary zinc and iron sources, physical growth and cognitive development of breastfed infants. J Nutr 130: 358S–360S, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Krieger I, Alpern BE, Cunnane SC. Transient neonatal zinc deficiency. Am J Clin Nutr 43: 955–958, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Kyttala A, Ihrke G, Vesa J, Schell MJ, Luzio JP. Two motifs target Batten disease protein CLN3 to lysosomes in transfected nonneuronal and neuronal cells. Mol Biol Cell 15: 1313–1323, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem 275: 34803–34809, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Liuzzi JP, Blanchard RK, Cousins RJ. Differential regulation of zinc transporter 1, 2, and 4 mRNA expression by dietary zinc in rats. J Nutr 131: 46–52, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lopez V, Kelleher SL. Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem J 422: 43–52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez V, Kelleher SL. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp Cell Res 316: 366–375 [DOI] [PubMed] [Google Scholar]
- 25.Lopez V, Kelleher SL. Zip6-attenuation promotes epithelial-to-mesenchymal transition in ductal breast tumor (T47D) cells. Exp Cell Res 2009 [DOI] [PubMed] [Google Scholar]
- 26.McCormick N, Velasquez V, Finney L, Vogt S, Kelleher SL. X-ray fluorescence microscopy reveals accumulation and secretion of discrete intracellular zinc pools in the lactating mouse mammary gland. PLoS One 5: e11078, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J Biol Chem 284: 17677–17686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci USA 75: 3327–3331, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oteiza PI, Mackenzie GG. Zinc, oxidant-triggered cell signaling, and human health. Mol Aspects Med 26: 245–255, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Otten C, Wagener R, Paulsson M, Zaucke F. Matrilin-3 mutations that cause chondrodysplasias interfere with protein trafficking while a mutation associated with hand osteoarthritis does not. J Med Genet 42: 774–779, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflügers Arch 447: 744–751, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Piccirillo R, Palmisano I, Innamorati G, Bagnato P, Altimare D, Schiaffino MV. An unconventional dileucine-based motif and a novel cytosolic motif are required for the lysosomal and melanosomal targeting of OA1. J Cell Sci 119: 2003–2014, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piela Z, Szuber M, Mach B, Janniger CK. Zinc deficiency in exclusively breast-fed infants. Cutis 61: 197–200, 1998 [PubMed] [Google Scholar]
- 34.Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J Cell Sci 120: 3838–3849, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Reiersen H, Rees AR. The hunchback and its neighbours: proline as an environmental modulator. Trends Biochem Sci 26: 679–684, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316: 1341–1345, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445: 881–885, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Storch S, Pohl S, Braulke T. A dileucine motif and a cluster of acidic amino acids in the second cytoplasmic domain of the batten disease-related CLN3 protein are required for efficient lysosomal targeting. J Biol Chem 279: 53625–53634, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Van den Berghe PV, Folmer DE, Malingre HE, van Beurden E, Klomp AE, van de Sluis B, Merkx M, Berger R, Klomp LW. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J 407: 49–59, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang F, Kim BE, Dufner-Beattie J, Petris MJ, Andrews G, Eide DJ. Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum Mol Genet 13: 563–571, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J. A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet 71: 66–73, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]