Abstract
Macrophage scavenger receptors have been implicated as key players in the pathogenesis of atherosclerosis. To assess the role of the class B scavenger receptor CD36 in atherogenesis, we crossed a CD36-null strain with the atherogenic apo E–null strain and quantified lesion development. There was a 76.5% decrease in aortic tree lesion area (Western diet) and a 45% decrease in aortic sinus lesion area (normal chow) in the CD36-apo E double-null mice when compared with controls, despite alterations in lipoprotein profiles that often correlate with increased atherogenicity. Macrophages derived from CD36-apo E double-null mice bound and internalized more than 60% less copper-oxidized LDL and LDL modified by monocyte-generated reactive nitrogen species. A similar inhibition of in vitro lipid accumulation and foam cell formation after exposure to these ligands was seen. These results support a major role for CD36 in atherosclerotic lesion development in vivo and suggest that blockade of CD36 can be protective even in more extreme proatherogenic circumstances.
Introduction
In vitro and in vivo studies support the hypothesis that macrophages are key early mediators of atherogenesis (1–3) and their impaired recruitment and activation protects against lesion development (4–6). A significant amount of research also supports the hypothesis that subendothelial modified LDLs provide the initiating ligands for the macrophage (7, 8), and these are recognized by scavenger receptors (9–13).
Oxidized LDL is a ligand for the class A scavenger receptors type I and II (SRA-I/II), MARCO, the class B scavenger receptor, CD36, and the class D receptor, CD68. This modified lipoprotein has been considered the most important atherogenic LDL (14–16). In vitro studies have provided evidence that SRA-I/II and CD36 are the major oxidized LDL receptors mediating lipid accumulation and foam cell formation, whereas MARCO and CD68 play a more minor role (17–21). Absence of SRA-I/II in atherogenic murine models has had a variable impact on atherosclerosis (22–24). Thus, an essential contribution of scavenger receptors to the pathogenesis of atherosclerosis in vivo remains unresolved.
CD36 has been shown to be highly regulated in monocytes/macrophages during differentiation (25, 26) and to be present in lipid-laden macrophages in atherosclerotic lesions (27, 28). This scavenger receptor is upregulated by IL-4 (25), macrophage colony stimulating factor (26), modified LDL (17, 18), cellular cholesterol content (29), and peroxisome proliferator–activated receptor-γ (PPAR-γ) ligands (30, 31). Unlike SRA I/II, CD36 is more broadly expressed (32–35) and has been shown to play a strategic role in lipoprotein and lipid metabolism (36, 37). The phenotype of CD36-null mice generated in our laboratory included increased plasma levels of cholesterol, triacylglycerol, and fatty acids and supported a major role for CD36 in fatty acid uptake and lipid metabolism in vivo (36). In transgenic mice, overexpression of CD36 in muscle enhanced fatty acid oxidation during stimulation/contraction and also had significant influence on plasma lipoprotein and fatty acid levels (37). Absence of CD36 was implicated recently in insulin resistance in the spontaneous hypertensive rat by using genetic analysis (37, 38). These studies point to an essential role for CD36 not only in uptake of lipid but in determination of cellular lipid stores.
To determine if CD36 is a major macrophage scavenger receptor responsible for early lipid accumulation and foam cell formation, which can predispose animals to the development of fatty streaks and ultimately more advanced atherosclerotic lesions, we generated CD36-apo E double-null mice and evaluated aortic lesions on normal chow and Western diets.
Methods
Reagents.
Cell culture reagents were from Life Technologies, Inc. (Grand Island, New York, USA) and chemicals were from Sigma Chemical Co. (St. Louis, Missouri, USA).
Animals and diets.
Apo E-null mice were obtained from The Jackson Laboratory (Bar Harbor, Maine, USA). Animals were maintained ad libitum on standard rodent chow (Rodent Diet 5001; Lab Diet, PMI Nutrition International, Brentwood, Missouri, USA). For studies using the Western diet, the animals were maintained ad libitum on a diet composed of 21% (wt/wt) adjusted calories from fat and 1.5% (wt/wt) cholesterol (TD 88137; Harlan Teklad Laboratory, Madison, Wisconsin, USA). CD36-apo E double-null mice developed more subcutaneous xanthomas then apo E–null mice, but were otherwise indistinguishable.
Morphometry.
Mice were sacrificed by pentobarbital overdose and hearts were perfused with 20 mL saline and 10 mL 10% buffered formalin. For aortic sinus analysis, serial cryosections of 10-μm thickness were taken from the region of the proximal aorta through the aortic sinuses and stained with oil red-O and fast green. Morphometric evaluations of lesion size were made using NIH Image software (National Institutes of Health, Bethesda, Maryland, USA) (39). For aortic tree analysis, the entire aorta from the heart, extending 5–10 mm after bifurcation of the iliac arteries and including the subclavian right and left common carotid arteries, was removed, dissected, and evaluated for lesion development by en face oil red-O staining and morphometry of scanned images using the software Scion Image (Scion Corp., Fredrick, Maryland, USA).
Plasma lipid and lipoprotein analysis.
One week before sacrifice, mice were fasted overnight and blood was collected for lipid analysis. Total serum cholesterol and triacylglycerol were determined by enzymatic kits (Sigma Chemical). Appropriate standards and controls were included in each assay.
For fast protein liquid chromatography (FPLC) analysis, blood was pooled from 2–3 females after an overnight fast. Lipoproteins were separated on 2 serial Superose 6 columns (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA), at the Rogosin Institute Clinical Research Laboratory for Comprehensive Lipid Analysis (New York, New York, USA). Fractions were analyzed for cholesterol and triacylglycerol using an automated Cobas Fara system (Roche Diagnostic, Indianapolis, Indiana, USA) with appropriate standards and controls.
Binding and cell association assays.
Human LDL was isolated from pooled plasma by density gradient ultracentrifugation (d = 1.019 to 1.063 g/mL), dialyzed against 0.15 M NaCl containing 0.3 mM Na2 EDTA and 100 μM diethylenetriamine pentaacetic acid (DPTA), pH 7.4, sterilized by filtration, and stored under nitrogen gas at 4°C. Protein concentration was measured by the Markwell-modified Lowry assay (40). LDL was labeled as described previously (41, 42). For oxidation, LDL or 125I-LDL was dialyzed against PBS, adjusted to a concentration of 500 μg/mL, and incubated with 5 μM CuSO4 for 24 hours at 37°C. Oxidation was terminated by addition of 40 μM butylated hydroxytoluene (BHT) and dialysis against PBS containing 100 μM DPTA. The purity and charge of the lipoproteins were evaluated by agarose gel electrophoresis. The extent of oxidation was assessed by measuring the thiobarbituric acid reactive substances, by fluorescence analysis (43), and by HPLC quantification of lipid hydroperoxides (21). The specific activity of the 125I-oxidized LDL was 100–250 dpm/ng protein. LDL was acetylated by repeated additions of acetic acid anhydride, and the modification was confirmed by assessing electrophoretic mobility. NO2-LDL was prepared by incubating LDL (0.2 mg/mL) at 37°C in 50 mM sodium phosphate (pH 7.0) and 100 μM DPTA in the presence of 30 nM myeloperoxidase, 100 μg/mL glucose, 20 ng/mL glucose oxidase (grade II; Roche Molecular Biochemicals, Indianapolis, Indiana, USA), and 0.5 mM NaNO2 for 8 or 24 hours, as indicated (44). Myeloperoxidase was purified from detergent extracts of human leukocytes by sequential lectin affinity and gel filtration chromatography (45).
Mouse peritoneal macrophages were harvested 3–4 days after intraperitoneal injection of 4% sterile thioglycolate by peritoneal lavage. Similar numbers of cells were obtained from both genotypes. Cells were serum starved for 2 hours, and assays were performed in Macrophage Serum Free Media (Life Technologies, Gaithersburg, Maryland, USA) containing 20 μM BHT, 300 nM catalase, and 100 μM DPTA. Binding was carried out at 4°C for 4 hours at a ligand concentration of 10 μg/mL. Determination of LDL uptake was carried out at 37°C for 5 hours (44). Nonspecific binding, assessed in the presence of 50-fold unlabeled reagent, was less than 10% of total binding and was subtracted from values in the figures presented here. The mean values of triplicates plus or minus SD from representative experiments are shown, but experiments were repeated at least 3 times and gave similar results.
In vitro foam cell assay.
Thioglycolate-elicited macrophages were cultured on sterile coverslips. After 2-hour serum starvation, 25 μg/mL Cu-oxLDL or 75 μg/mL NO2-LDL (for 8 hours or 24 hours) was added. After PBS wash, the cells were fixed in paraformaldehyde and stained with oil red-O.
Results
Absence of CD36 protects against atherosclerotic lesion development in mice.
CD36-null mice were mated to apo E-null mice to generate CD36-apo E double-null animals. As a result of this cross, the mice were backcrossed 4 times to a C57Bl/6 background. Genotype was confirmed by Southern blot hybridization and plasma cholesterol levels (data not shown). The parental apo E–null strain was propagated to generate control animals.
At 4 weeks of age, mice were placed on a Western diet for 12 weeks. As shown in Figure 1a, en face analyses of total aorta surfaces in CD36-apo E double-null mice revealed greater than a 76% reduction in lesion area compared with apo E–null mice. Apo E–null male mice had extensive lesion development, covering a mean area of 30.32 ± 3.6% of the total aortic tree, as compared with only 5.42 ± 1.46% for CD36-apo E double-null males. Similar results were seen with female mice, with apo E–null animals having lesions covering a mean area of 32.24 ± 3.38% of the total aortic surface, as compared with 9.88 ± 2.56% in CD36-apo E double-null females.
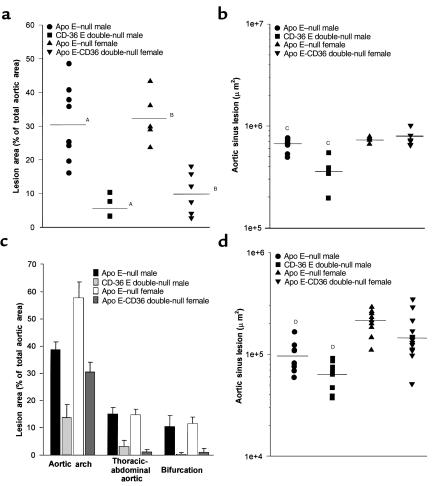
Figure 1.
Disruption of CD36 decreases atherosclerotic lesion development in apo E–null mice. Circles, apo E-null male (n = 9); squares, CD36-apo E double-null male (n = 5); triangle, apo E–null female (n = 5); reversed triangle, CD36-apo E double-null female (n = 6). (a) Lesion surface area in the aortic tree was assessed by en face oil red-O positive staining. The aortic tree from the heart to below the bifurcation of the iliac arteries was removed and dissected for staining and lesion analysis from mice fed a Western diet for 12 weeks. Each sample was scanned, and total oil red-O positive area was determined using Scion Image software. AP < 0.001, BP < 0.001, Mann-Whitney test. (b) Aortic sinus cross-sectional lesion area was measured in mice fed the atherogenic Western diet for 12 weeks. Atherosclerotic plaque area was determined by oil red-O positivity and morphology and measured using NIH Image software. CP < 0.0005, Mann Whitney test. (c) Prevalence of lesions at different sites in the aortic tree. En face oil red-O positive lesions from a were assessed in 3 regions of the aorta, aortic arch, thoracic-abdominal aorta, and at the iliac bifurcation, and lesion area was expressed as percent of total area of that site. (d) Aortic sinus lesion area measured as in b in mice fed a normal chow diet. Mice were 16 weeks old at sacrifice. Apo E–null male (n = 11); CD36-apo E double-null male (n = 9); apo E–null female (n = 9); CD36-apo E double-null female (n = 13). DP < 0.01, Mann Whitney test.
Cross-sectional analyses of aortic sinuses, shown in Figure 1b, revealed that CD36-apo E double-null male mice developed significantly smaller lesions than apo E–null males (3.65 ± 0.557 × 105 μm2 vs. 6.57 ± 0.308 × 105 μm2). This represents a 45% decrease in mean lesion size. CD36-apo E double-null female mice developed larger lesions than male mice, but these were similar in size to those found in apo E–null females (apo E–null females: 7.39 ± 0.211 × 105 μm2 vs. 7.66 ± 0.559 × 105 μm2 for CD36-apo E double-null females).
During evaluation of the aortic tree, it was noted that lesion development by site differed between the 2 genotypes. As shown in Figures 1c and 2a, apo E–null mice developed lesions throughout the aortic tree (although with a predilection for the aortic arch). CD36-apo E double-null animals showed lesions almost exclusively in the aortic arch, with only an occasional lesion in the thoracic-abdominal aorta (Figure 2b).
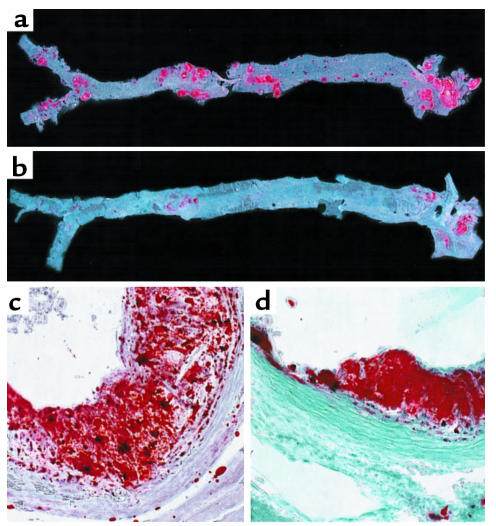
Figure 2.
Atherosclerotic lesion distribution and morphology is altered in CD36-deficient mice. The entire aorta from apo E–null (a) and CD36-apo E double-null (b) mice were dissected and opened longitudinally. Oil red-O–stained lesions occur in all regions of the aorta from the apo E–null mouse, whereas in the aorta from the CD36-apo E double-null mouse lesions are seen primarily in the aortic arch. ×20. Hearts were dissected from apo E–null (c) and CD36-apo E double-null (d) mice fed a normal chow diet, cryosectioned at the level of the valve leaflets, and stained with oil red-O and fast green. Lesions in apo E–null mice contained lipid-laden, intensely oil red-O–stained foam cells, cellular areas of less oil red-O positivity, empty spaces, and cholesterol clefts. Those in CD36-apo E double-null mice contained only rare areas lacking cells or containing cholesterol clefts. ×250.
In a separate study, to evaluate early lesion development, age- and sex-matched apo E–null and CD36-apo E double-null animals were maintained on normal chow and evaluated for aortic sinus lesions at 16 weeks of age. As shown in Figure 1d, CD36-apo E double-null animals developed smaller lesions than apo E-null controls. The mean lesion size in CD36-apo E double-null males was 6.56 ± 0.537 × 104 μm2 as compared with 9.78 ± 1.12 × 104 μm2 for apo E–null males, representing a 33% decrease. Female CD36-apo E double-null mice also developed smaller lesions (1.64 ± 0.231 × 105 μm2 for CD36-apo E double-null mice vs. 2.15 ± 0.196 × 105 μm2 for apo E–null mice). The difference did not reach statistical significance.
Morphologically, aortic sinus lesions in CD36-apo E double-null animals differed from apo E–null animals. As shown in Figure 2, c and d, large lesions in the apo E–null animals often contained areas of less intense oil red-O staining, spaces between cells, and cholesterol clefts, even on the chow diet. These were much less frequently observed in lesions from CD36-apo E double-null animals, which consisted almost exclusively of densely packed lipid-laden foam cells.
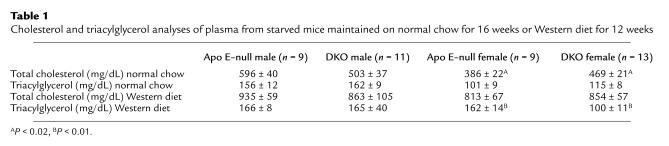
Plasma cholesterol, triacylglycerol, and weight gain differed in CD36-apo E double-null mice compared with apo E–null mice.
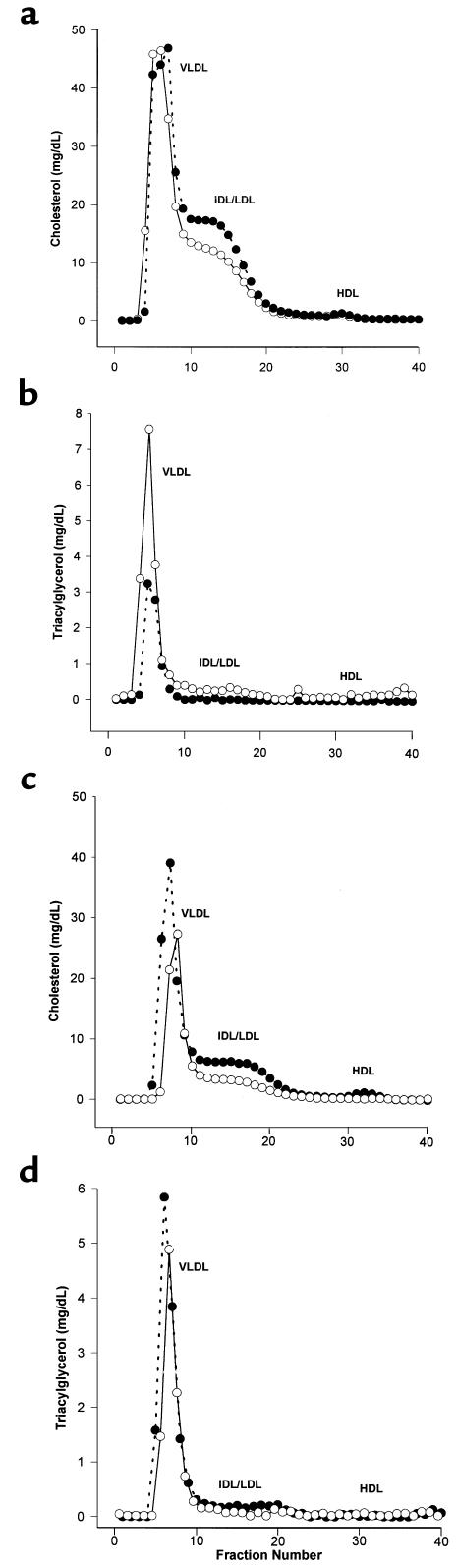
Because CD36 plays an important role in lipid metabolism (36), we measured plasma cholesterol, triacylglycerol, and body weight. As shown in Table 1, on the Western diet total cholesterol levels were similar in the 2 genotypes. Triacylglycerol levels were significantly lower in CD36-apo E double-null females. FPLC analysis (Figure 3) revealed that CD36-apo E double-null mice had increased IDL and LDL cholesterol (Figure 3a), as compared with apo E–null mice. There was no difference in HDL cholesterol. The decrease in triacylglycerol was within the VLDL fraction in CD36-apo E double-null female mice (Figure 3b).
Table 1.
Cholesterol and triacylglycerol analyses of plasma from starved mice maintained on normal chow for 16 weeks or Western diet for 12 weeks
Figure 3.
Comparative analysis of the distribution of cholesterol and triacylglycerol in plasma lipoproteins from apo E–null and CD36-apo E double-null female mice. Pooled plasma samples from mice fed a Western diet for 12 weeks (a, b) or normal chow for 16 weeks (c, d) were separated by FPLC. Fractions were assayed for cholesterol (a, c) and triacylglycerol (b, d). Open circles, apo E–null mice; filled circles, CD36-apo E double-null mice.
On normal chow total cholesterol was increased in female CD36-apo E double-null mice (Table 1). FPLC analysis showed that the increased cholesterol migrated with VLDL, IDL, and LDL in the CD36-apo E double-null mice (Figure 3c). HDL cholesterol and triacylglycerol (Figure 3d) were unchanged.
CD36-apo E double-null mice maintained on the Western diet gained significantly more weight than apo E–null mice (mean weight ± SEM of CD36-apo E double-null males at sacrifice: 37.54 ± 1.8 g vs. 31.33 ± 1.3 g for apo E–null males; CD36-apo E double-null females: 25.14 ± 0.96 g vs. 21.56 ± 0.71 g for apo E–null females; P < 0.02, Student’s unpaired t test). On normal chow CD36-apo E double-null male mice were again significantly heavier than apo E–null male mice (30.14 ± 0.74 g vs. 27.04 ± 0.88 g; P < 0.02). Female mice in this study were similar (CD36-apo E double-null mice: 21.38 ± 0.41 g vs. 20.7 ± 0.56 g for apo E–null mice).
Binding and uptake of modified LDL are decreased in elicited peritoneal macrophages from CD36-apo E double-null mice.
Binding and uptake of oxidatively modified LDL by free copper (Cu-oxLDL) or the myeloperoxidase-hydrogen peroxide-nitrite system of monocytes (NO2-LDL) (44), were evaluated in vitro using thioglycolate-elicited peritoneal macrophages. As shown in Figure 4a, CD36-apo E double-null macrophages bound significantly less Cu-oxLDL (87%) and NO2-LDL (89% decrease for 8-hour NO2-LDL and 88% for 24-hour NO2-LDL) when compared with apo E–null macrophages. Binding of acetylated LDL, which is a ligand for CD36 as well as SRA-I/II, was reduced by 64%. Binding of native LDL, however, was similar in both cell types.
Figure 4.
Binding (a) and uptake (b) of native and modified LDLs by elicited peritoneal macrophages from apo E–null and CD36-apo E double-null mice. Modified LDLs were prepared and assays performed as described in Methods. Nonspecific binding was subtracted, and mean values of triplicates ± SD are shown. –NO2-LDL refers to LDL modified by the myeloperoxidase system in the absence of NO2–.
Cellular uptake of these ligands was also significantly decreased in CD36-apo E double-null macrophages as compared with apo E–null macrophages (Figure 4b). Specific uptake of Cu-oxLDL was reduced by 60%; the decrease for 8- and 24-hour myeloperoxidase-modified LDL was 77 and 65%, respectively. Cellular uptake of acetylated LDL was reduced (52%) and that of native LDL was unchanged, as expected. In controls where a component of the myeloperoxidase system was absent, there was no greater binding or cell association than that seen with unmodified LDL in cells from either genotype.
Development of lipid-laden foam cells is decreased in elicited peritoneal macrophages from CD36-apo E double-null mice.
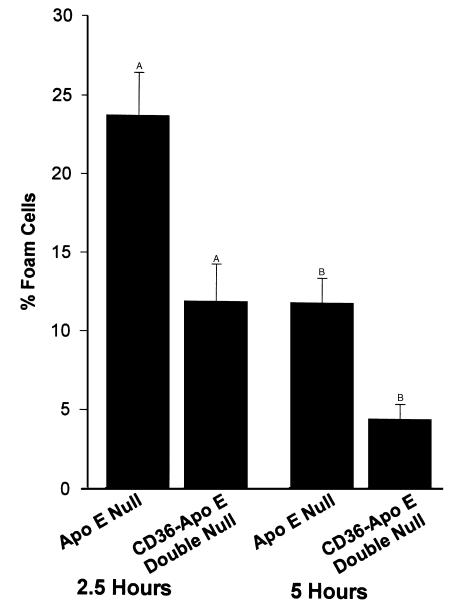
We next assessed lipid accumulation and foam cell formation in vitro in elicited macrophages incubated for 2.5 and 5 hours with Cu-oxLDL. As shown in Figure 5, there were 50% fewer oil red-O stained CD36-apo E double-null macrophages as compared with apo E-null macrophages (23.75 ± 2.75% vs. 11.97 ± 2.37%) at 2.5 hours and 61% fewer at 5 hours (11.98 ± 1.56% vs. 4.67 ± 0.92%; P < 0.05 for both groups, Student’s t test). Dramatically, as shown in Figure 6, after 72 hours of incubation with 8- or 24-hour myeloperoxidase-treated LDL, there was a significant decrease in oil red-O stained lipid in CD36-apo E double-null macrophages.
Figure 5.
Comparison of in vitro foam cell development in elicited peritoneal macrophages from apo E–null and CD36-apo E double-null mice in response to oxidized LDL. Macrophages were incubated with 25 μg/mL Cu-oxLDL, and fixed and stained with oil red-O after 2.5 or 5 hours. The mean number of lipid containing cells ± SE is shown. AP < 0.05, BP < 0.05, Student’s t test.
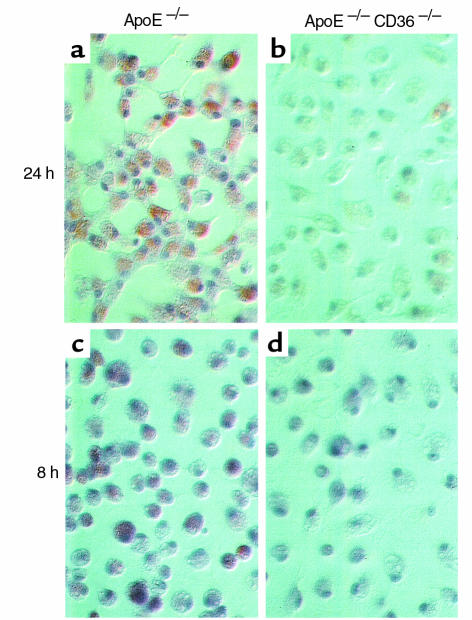
Figure 6.
Comparison of in vitro foam cell development in elicited peritoneal macrophages from apo E–null (a, c) and CD36-apo E double-null (b, d) mice in response to exposure to LDL modified by the myeloperoxidase-hydrogen peroxide-nitrite system (NO2-LDL) for 24 hours (a, b) or 8 hours (c, d). Macrophages were incubated for 72 hours with 75 μg/mL NO2-LDL, fixed and stained with oil red-O. Nearly all apo E–null macrophages contained neutral lipid, whereas the number of lipid-containing cells and the degree of accumulation of lipid in CD36-apo E double-null macrophages were dramatically less.
Discussion
These studies address the in vivo role of CD36 in the etiology of atherosclerosis in the apo E–null mouse model and provide compelling data for the importance of this macrophage scavenger receptor in the pathophysiology of the disease. On an atherogenic diet, apo E–null animals had advanced lesion development, whereas CD36-apo E double-null animals had 77% less lesion area as assessed by en face analysis of whole aortas. CD36-apo E double-null male animals also developed significantly smaller aortic sinus lesions on normal chow and Western diets. Morphologically, on both diets, lesions in apo E–null mice had characteristics of more advanced atherosclerosis, but, on normal chow, lesions from CD36-apo E double-null mice had few areas containing cholesterol clefts or lacking cells.
When assessed by site, CD36-apo E double-null animals were markedly resistant to lesion formation in all but the most highly prone sinus and arch, and here lesions were smaller, in general. In apo E–null animals, the aortic sinus and arch are among the most prominent early sites for lesion development, and lesions there are usually the most advanced (46–48). It is possible that retention time and accumulation and aggregation of proatherogenic modified LDL in these areas is greater, resulting in the formation of more heavily oxidized LDL that can be recognized by receptors other than CD36, such as SRA-I/II and CD68 (12, 20, 49, 50). It is also possible that proatherogenic stimuli in these regions may upregulate the expression of these scavenger receptors, and, in fact, this has been demonstrated in murine studies similar to ours (23). Our data suggest that absence of CD36 diminishes lesion size in these areas enough to slow progression even on a high-fat diet, and in less proatherogenic areas of the aorta, absence of CD36 virtually eliminates lesion formation.
The protective effect seen in our studies is perhaps more compelling because apo E–null animals that lack CD36 had lipoprotein profiles that, if anything, could be more atherogenic, that is, an increase in IDL and LDL levels. The moderate increase in body weight of CD36-apo E double-null mice could also be proatherogenic. The gender difference in lesion development in animals on normal chow could be related to the significantly elevated levels of total cholesterol in atherogenic lipoproteins seen in the females. Interestingly, whereas apo E-null male lesion development catches up with female lesion development on the Western diet, this was not the case for the CD36-apo E double-null males. Thus, delayed lesion progression in males was apparent on both diets.
The differences observed in weight gain and lipoprotein profiles may reflect other functions of CD36, for example its role in fatty acid transport, or may reflect differences in degree of inbreeding or genetic background between the apo E–null and CD36-apo E double-null mice. We observed previously significant differences in fatty acid and lipoprotein profiles in littermate-matched CD36-null and wild-type mice (36). The interaction of the 2 null phenotypes and the different diets make it difficult to outline a mechanism for the resulting phenotype without further studies.
Our studies support the prevailing model of fatty streak formation in the vessel wall, emphasizing the importance of macrophage recruitment to the subendothelial space and specific uptake of modified lipoproteins by scavenger receptors. Oxidative modification of LDL is considered to be an essential step in the conversion of LDL into an atherogenic ligand for macrophage scavenger receptors. There have been many reports documenting its potential lesion-promoting effects and properties, and there is evidence that oxidation of LDL occurs in vivo (51–53). In previous in vitro work, antibody to CD36 was shown to inhibit up to 60% of binding of Cu-oxLDL by human monocyte-derived macrophages (54), and monocytes obtained from human subjects with CD36 deficiency were shown to bind and internalize 40% less Cu-ox-LDL (55). In this report we show an 85% decrease in binding of Cu-oxLDL to peritoneal macrophages derived from CD36-apo E double-null animals compared with those from apo E–null mice. Lipid accumulation and in vitro foam cell formation were also decreased by at least 50%. Thus, our data are consistent with the hypothesis that decreased uptake of oxidized forms of LDL and decreased in vitro foam cell formation correlate with decreased lesion development in vivo.
Recently, LDL modified by myeloperoxidase-generated oxidants has been shown to be present in atherosclerotic lesions (56). A specific marker of myeloperoxidase catalyzed oxidation, 3-chlorotyrosine, was found to be enriched 6-fold in atherosclerotic tissue obtained during vascular surgery and was present in 30-fold greater levels in LDL isolated from atherosclerotic intima compared with circulating plasma LDL from healthy donors (56). Modification of LDL by myeloperoxidase-generated reactive nitrogen species generates a specific ligand for CD36 and promotes macrophage foam cell formation (21, 44). Data in this report further support these observations: only after extensive exposure (24 hours) to this system, when the modified LDL also becomes a ligand for SRA-I/II (44), was there any significant binding or cell association by peritoneal macrophages from CD36-apo E double-null mice. CD36-apo E double-null macrophages failed to accumulate any neutral lipid after prolonged exposure to NO2-LDL, whereas more than 75% of apo E–null macrophages stained with oil red-O. Thus, this report demonstrates that the in vitro atherogenic potential of 2 model ligands, Cu-ox-LDL and NO2-LDL, was substantially reduced in the absence of CD36, and this is the most likely mechanism for the decrease in lesion development observed in vivo.
The role of SRA-I/II in atherosclerosis has also been studied in murine models (22–24). This receptor recognizes only extensively modified LDL and, in vitro, can account for 30–50% of Cu-oxLDL binding (12, 20, 22, 54). Results in vivo have ranged from 50% protection to enhancement of advanced lesion development (22–24). A possible reason for this result may be increased expression of other scavenger receptors, including CD36 (23). This may relate to the recent discovery that CD36 gene expression is regulated by PPAR-γ and that oxidized LDL and other modified lipoproteins may deliver PPAR-γ ligands to macrophages (30, 31). In support of this hypothesis is the recent observation that disruption of 12/15-lipoxygenase in the apo E–null model of atherosclerosis resulted in a profound decrease in aortic tree lesions by en face analysis and decreased sinus lesion size in early stages of lesion development (57). Whereas these macrophage cytosolic enzymes may be important mediators of LDL modification in vivo, their more important role may be to provide ligands for PPAR-γ, which in turn activates transcription of CD36.
The data in this report suggest that CD36 is a major macrophage receptor for proatherogenic modified LDLs. Although the absence of CD36 resulted in an alteration of lipid profiles in apo E–null mice that may be more atherogenic, there was substantial decrease in lesion size and progression, even in areas of enhanced predisposition. Our data support the hypothesis that macrophages are indeed key early mediators of atherogenesis and that the scavenger receptor CD36 plays an essential role in the atherogenic process. Furthermore, these studies suggest that specific targeting of the type B scavenger receptor CD36 could have a profound impact on lesion development.
Acknowledgments
We would like to acknowledge Thomas Parker and Daniel Levine of the Rogosin Institute Comprehensive Lipid Laboratory. This work was supported by National Institutes fo Health grants HL-56987 (to D.P. Hajjar), HL-42540 (to R.L. Silverstein), and HL-62526 (to S.L. Hazen). M. Febbraio is the recipient of a Silberman Foundation Fellowship.
References
- 1.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 2.Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPAR-gamma: differentiation-dependent peroxisomal proliferator-activated receptor gamma (PPAR-gamma) expression and reduction of MMP-9 activity through PPAR-gamma activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yla-Herttuala S, et al. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci USA. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2–/– mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 5.de Villiers WJ, et al. Macrophage phenotype in mice deficient in both macrophage-colony-stimulating factor (op) and apolipoprotein E. Arterioscler Thromb Vasc Biol. 1998;18:631–640. doi: 10.1161/01.atv.18.4.631. [DOI] [PubMed] [Google Scholar]
- 6.Geng YJ, Hansson GK. Interferon-gamma inhibits scavenger receptor expression and foam cell formation in human monocyte-derived macrophages. J Clin Invest. 1992;89:1322–1330. doi: 10.1172/JCI115718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berliner JA, et al. Induction of chemotactic cytokines by minimally oxidized LDL. Adv Exp Med Biol. 1993;351:13–18. doi: 10.1007/978-1-4615-2952-1_2. [DOI] [PubMed] [Google Scholar]
- 8.Frostegard J, et al. Oxidized low density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell line U937. Proc Natl Acad Sci USA. 1990;87:904–908. doi: 10.1073/pnas.87.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramprasad MP, Terpstra V, Kondratenko N, Quehenberger O, Steinberg D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1996;93:14833–14838. doi: 10.1073/pnas.93.25.14833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parthasarathy S, Printz DJ, Boyd D, Joy L, Steinberg D. Macrophage oxidation of low density lipoprotein generates a modified form recognized by the scavenger receptor. Arteriosclerosis. 1986;6:505–510. doi: 10.1161/01.atv.6.5.505. [DOI] [PubMed] [Google Scholar]
- 11.Krieger M. Molecular flypaper and atherosclerosis: structure of the macrophage scavenger receptor. Trends Biochem Sci. 1992;17:141–146. doi: 10.1016/0968-0004(92)90322-z. [DOI] [PubMed] [Google Scholar]
- 12.Endemann G, et al. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 13.Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol. 1998;8:365–372. doi: 10.1016/s0962-8924(98)01329-4. [DOI] [PubMed] [Google Scholar]
- 14.Parthasarathy S, Steinberg D, Witztum JL. The role of oxidized low-density lipoproteins in the pathogenesis of atherosclerosis. Annu Rev Med. 1992;43:219–225. doi: 10.1146/annurev.me.43.020192.001251. [DOI] [PubMed] [Google Scholar]
- 15.Penn MS, Chisolm GM. Oxidized lipoproteins, altered cell function and atherosclerosis. Atherosclerosis. 1994;108(Suppl.):21–29. doi: 10.1016/0021-9150(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 16.Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida H, Quehenberger O, Kondratenko N, Green S, Steinberg D. Minimally oxidized low-density lipoprotein increases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arterioscler Thromb Vasc Biol. 1998;18:794–802. doi: 10.1161/01.atv.18.5.794. [DOI] [PubMed] [Google Scholar]
- 18.Han J, Hajjar DP, Febbraio M, Nicholson AC. Native and modified low density lipoproteins increase the functional expression of the macrophage class B scavenger receptor, CD36. J Biol Chem. 1997;272:21654–21659. doi: 10.1074/jbc.272.34.21654. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Nicholson AC. Lipoproteins modulate expression of the macrophage scavenger receptor. Am J Pathol. 1998;152:1647–1654. [PMC free article] [PubMed] [Google Scholar]
- 20.Lougheed M, et al. High affinity saturable uptake of oxidized low density lipoprotein by macrophages from mice lacking the scavenger receptor class A type I/II. J Biol Chem. 1997;272:12938–12944. doi: 10.1074/jbc.272.20.12938. [DOI] [PubMed] [Google Scholar]
- 21.Podrez EA, et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki H, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–296. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 23.Sakaguchi H, et al. Role of macrophage scavenger receptors in diet-induced atherosclerosis in mice. Lab Invest. 1998;78:423–434. [PubMed] [Google Scholar]
- 24.de Winther MP, et al. Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APO-E3 Leiden transgenic mice. Atherosclerosis. 1999;144:315–321. doi: 10.1016/s0021-9150(98)00332-3. [DOI] [PubMed] [Google Scholar]
- 25.Yesner LM, Huh HY, Pearce SF, Silverstein RL. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler Thromb Vasc Biol. 1996;16:1019–1025. doi: 10.1161/01.atv.16.8.1019. [DOI] [PubMed] [Google Scholar]
- 26.Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood. 1996;87:2020–2028. [PubMed] [Google Scholar]
- 27.Wal AC, Das PK, Tigges AJ, Becker AE. Macrophage differentiation in atherosclerosis: an in situ immunohistochemical analysis in humans. Am J Pathol. 1992;141:161–168. [PMC free article] [PubMed] [Google Scholar]
- 28.Nakata A, et al. CD36, a novel receptor for oxidized low-density lipoproteins, is highly expressed on lipid-laden macrophages in human atherosclerotic aorta. Arterioscler Thromb Vasc Biol. 1999;19:1333–1339. doi: 10.1161/01.atv.19.5.1333. [DOI] [PubMed] [Google Scholar]
- 29.Han J, Hajjar DP, Tauras JM, Nicholson AC. Cellular cholesterol regulates expression of the macrophage type B scavenger receptor, CD36. J Lipid Res. 1999;40:830–838. [PubMed] [Google Scholar]
- 30.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPAR-gamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 31.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPAR-gamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 32.Van Nieuwenhoven FA, et al. Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem Biophys Res Commun. 1995;207:747–752. doi: 10.1006/bbrc.1995.1250. [DOI] [PubMed] [Google Scholar]
- 33.Ryeom SW, Sparrow JR, Silverstein RL. CD36 participates in the phagocytosis of rod outer segments by retinal pigment epithelium. J Cell Sci. 1996;109:387–395. doi: 10.1242/jcs.109.2.387. [DOI] [PubMed] [Google Scholar]
- 34.Greenwalt DE, Watt KW, So OY, Jiwani N. PAS IV, an integral membrane protein of mammary epithelial cells, is related to platelet and endothelial cell CD36 (GP IV) Biochemistry. 1990;29:7054–7059. doi: 10.1021/bi00482a015. [DOI] [PubMed] [Google Scholar]
- 35.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 36.Febbraio M, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahimi A, et al. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem. 1999;274:26761–26765. doi: 10.1074/jbc.274.38.26761. [DOI] [PubMed] [Google Scholar]
- 38.Aitman TJ, et al. Identification of Cd36 (Fat) as an insulin-resistance gene causing defective fatty acid and glucose metabolism in hypertensive rats. Nat Genet. 1999;21:76–83. doi: 10.1038/5013. [DOI] [PubMed] [Google Scholar]
- 39.Plump AS, Scott CJ, Breslow JL. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA. 1994;91:9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 41.Hoppe G, O’Neil J, Hoff HF. Inactivation of lysosomal proteases by oxidized low density lipoprotein is partially responsible for its poor degradation by mouse peritoneal macrophages. J Clin Invest. 1994;94:1506–1512. doi: 10.1172/JCI117490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci USA. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 44.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rakita RM, Michel BR, Rosen H. Differential inactivation of Escherichia coli membrane dehydrogenases by a myeloperoxidase-mediated antimicrobial system. Biochemistry. 1990;29:1075–1080. doi: 10.1021/bi00456a033. [DOI] [PubMed] [Google Scholar]
- 46.Plump AS, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 47.Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 48.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. Apo E-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 49.Ottnad E, et al. A macrophage receptor for oxidized low density lipoprotein distinct from the receptor for acetyl low density lipoprotein: partial purification and role in recognition of oxidatively damaged cells. Proc Natl Acad Sci USA. 1995;92:1391–1395. doi: 10.1073/pnas.92.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramprasad MP, et al. The 94- to 97-kDa mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proc Natl Acad Sci USA. 1995;92:9580–9584. doi: 10.1073/pnas.92.21.9580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palinski W, et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palinski W, et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Invest. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yla-Herttuala S, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicholson AC, Frieda S, Pearce A, Silverstein RL. Oxidized LDL binds to CD36 on human monocyte-derived macrophages and transfected cell lines. Evidence implicating the lipid moiety of the lipoprotein as the binding site. Arterioscler Thromb Vasc Biol. 1995;15:269–275. doi: 10.1161/01.atv.15.2.269. [DOI] [PubMed] [Google Scholar]
- 55.Nozaki S, et al. Reduced uptake of oxidized low density lipoproteins in monocyte-derived macrophages from CD36-deficient subjects. J Clin Invest. 1995;96:1859–1865. doi: 10.1172/JCI118231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hazen SL, Heinecke JW. 3-Chlorotyrosine, a specific marker of myeloperoxidase-catalyzed oxidation, is markedly elevated in low density lipoprotein isolated from human atherosclerotic intima. J Clin Invest. 1997;99:2075–2081. doi: 10.1172/JCI119379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cyrus T, et al. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999;103:1597–1604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]