Abstract
The subgenual anterior cingulate cortex (sgACC) presents altered functional connections with other regions of the brain in individuals with depression. However, the developmental nature of this phenomenon remains largely unexplored. Functional connections of the sgACC were examined in 36 school age children, 17 with a history of preschool depression (PO-MDD). The sgACC exhibited increased connections with cognitive control regions in healthy children and increased connections with thalamic and parietal regions in the PO-MDD group. A significant correlation between dysregulated emotional behavior and connectivity of the sgACC and dorsal medial prefrontal cortex was also found. These findings demonstrate that atypical sgACC functional connections are evident as early as school age in children with a history of PO-MDD and suggest an association with a very early episode of depression.
Keywords: Functional Connectivity, Depression, Cingulate, fMRI, Pediatric, Preschool, Psychopathology
Introduction
Multiple neuroimaging studies have pointed to the importance of the subgenual anterior cingulate cortex (sgACC) in the onset and course of depression [1]. In general, these studies have suggested that the sgACC exhibits reduced gray matter volume as well as increases in metabolism (after controlling for volume) that decline toward normative levels with successful treatment and symptom remission [2]. More recent research has used the relatively new approach of resting state functional connectivity (rs-fcMRI), a method based on the discovery that spontaneous low-frequency (<~0.1Hz) blood-oxygen level-dependent (BOLD) activity is correlated between functionally related grey matter regions [3]. These studies have noted the presence of altered correlations between activity in the sgACC and other brain regions in people with depression [4]. Atypical correlations between activity in the sgACC and other regions suggest that it may be beneficial to conceptualize functional brain abnormalities in clinical depression as reflecting a disordered neural system rather than impairments in isolated regions [1].’
Given the recognized need to study the relationships between psychopathology and brain development at younger ages [5], we explored the functional relationships of the sgACC in a unique sample of school age children with a history of Preschool Onset Major Depressive Disorder (PO-MDD). As part of our Preschool Depression Study (see [6]) we have followed these children and their families for the past 9 years. Results from the Preschool Depression Study have suggested that clinical depression can arise in preschool children as young as age 3, that children experiencing PO-MDD exhibit differences from their same age healthy peers both behaviorally and biologically, and that there is continuity between PO-MDD and the well-known school age form of the disorder [6–8]. Building on this work and that of others, we suggest that the emergence of this putative clinical depressive syndrome very early in life may leave a ‘developmental’ fingerprint that persists following the remittance of symptoms and leaves one vulnerable to future episodes and/or continued emotionally dysregulated behavior [9]. Using our sample of children with a well-characterized history of PO-MDD, we began addressing this question by examining the functional relationships of the sgACC in rs-fcMRI data collected from them. While the sgACC is not the only brain region implicated in Major Depression, we choose to focus on this region given the large body of literature supporting it as a key region in multiple neurobiological models of this disorder [1,2,10].
Following neurobiological models of depression and early brain development [9], we hypothesized that the sgACC would demonstrate reduced functional connections with earlier maturing cognitive control regions [11], as well as increased functional relationships with areas processing visceromotor/sensory information, when comparing children with PO-MDD and their same age healthy peers. Due to previous rs-fcMRI studies failing to find differences in sgACC-amygdala connectivity when comparing depressed and healthy subjects, we did not anticipate their presence in the current study [12]. To further build upon previous studies of older subjects with depression, which have treated the sgACC as a single region [4,12], we examined the functional relationships of this area separately in each hemisphere. Additional post hoc examinations were carried out to explore the relationship between brain findings and clinically relevant behavior.
Methods and Materials
Participants
The Preschool Depression Study is an ongoing longitudinal investigation of 306 preschoolers and their families funded by the National Institute of Mental Health. Details regarding recruitment and assessment of this sample have been published in detail previously [6]. The current study reports on a subsample of 36 children from this study that have participated in the initial phase of a longitudinal neuroimaging study. Prior to enrollment in the neuroimaging study, each family had participated in at least 4 annual comprehensive age appropriate mental health assessments (spanning at least 3 years) at the Early Emotional Development Program at the Washington University School of Medicine in St. Louis. Using this information, children were placed into groups based upon the presence/absence of PO-MDD (see Diagnostic Assessment below). Children with a history of head trauma, neurological disease, developmental delay, or medication were excluded. This resulted in a group of 17 PO-MDD children and 19 healthy controls. Prior to their scan, children completed the Children’s Depression Inventory [13] and the Children’s Sadness Management Scale [14]. Parental written consent was obtained and the Institutional Review Board at Washington University approved all experimental procedures.
Diagnostic Assessment
Diagnostic assessments were conducted by trained research assistants using the Preschool Age Psychiatric Assessment [15], a valid and reliable diagnostic interview for identifying psychiatric disorders during the preschool period. PO-MDD was defined in the current study as meeting developmentally adjusted DSM-IV MDD criteria prior to 6 years of age (see [16,17] for further detail). Similarly, comorbid disorders (e.g., Attention Deficit Hyperactivity Disorder) were identified using this assessment instrument. The healthy control group was defined by an absence of any psychiatric diagnosis at all previous assessments. However, diagnostic status at time of scan was not available for either group. To measure current depressive symptom status and avoid mis-classifying a child as healthy who had a new onset of Major Depression, the Child Depression Inventory was used as a measure of current depression symptoms (see below).
Child Depression Inventory
The Child Depression Inventory-Child and Parent versions were administered at the time of scan [13]. Child Depression Inventory scores were unavailable for 4 children (3 control/1 PO-MDD). Any child in the control group with a T-score of 65 or greater (indicating risk for clinically significant mood difficulties) on either the child or parent measures were excluded. This lead to a total of 15 controls used in all subsequent analyses as 4 control children were excluded based on these criteria.
Children’s Sadness Management Scale
The Children’s Sadness Management Scale is a reliable and valid measure of sadness management which provides 3 scales measuring a child’s ability to manage (Inhibition), regulate (Dysregulated Expression), and cope with (Coping) the experience of sadness [14].
Data Acquisition
Imaging data were collected using a 3T TIM TRIO Siemens whole body system and included a T1 [sagittal acquisition, TE=2.9ms, TR=6.6ms, flip angle=8°, 1 acquisition, 128 slices, 1×1×1 mm voxels] image and functional images collected with a 12-channel head coil using an asymmetric spin-echo echo-planar sequence sensitive to BOLD contrast (T2*) (TR=2500ms, TE=27ms, FOV=256mm, flip=90°). Each functional run consisted of 36 contiguous axial images with isotropic voxels (4mm3) acquired parallel to the anterior-posterior commissure plane. Two functional runs of 128 TRs (~11 minutes total) were collected while children rested with their eyes closed. For 2 PO-MDD and 3 controls only one run was available.
Preprocessing
Preprocessing and resting state functional connectivity (rs-fcMRI) analyses were carried out using in-house software and were based on previously published techniques [18,19]. Prior to preprocessing, the first 5 frames of each run were discarded to allow for signal stabilization. Data were reconstructed into images and normalized across runs by scaling whole-brain signal intensity to a fixed value and removing the linear slope to counteract effects of drift. MR data was then corrected for head motion using rigid-body rotation and translation correction algorithms. Following motion correction, the functional images were registered to Talairach [20] space using a 12 parameter linear (affine) transformation and smoothed with a 6mm FWHM Gaussian filter. Several additional preprocessing steps including temporal filtering with a high pass filter (cutoff frequency of 0.009Hz) and removal by regression of several sources of spurious variance including 6 rigid body motion correction parameters, ventricle signal, deep white matter signal and whole brain signal. Average SNR values did not differ between the groups (p>.05)
Subgenual Anterior Cingulate Region of Interest Identification
Seed regions for the left and right subgenual anterior cingulate were derived from a previous study of sgACC connectivity in healthy school age children [21]. Specifically, the coordinates x=±4 y=22 z=−5 were used to create 6 mm spheres centered in the sgACC for each hemisphere.
Seed-Based Whole-Brain Correlational Analysis
Correlation maps for each child were produced for each seed by extracting their BOLD time course and computing the correlation coefficient between these time courses and those from all other brain voxels. Fisher’s r to z transform was applied to the individual correlation maps, and group comparisons were conducted with this transformed data using a random effects analysis. Monte Carlo alpha simulations were used to determine the necessary cluster size/uncorrected p-value combination for a corrected false positive rate of .05 (uncorrected p-value [<.0001]+cluster size thresholding [13 voxels]) [22]. Given the exploratory nature of this study and the potential for missing meaningful functional relationships, we choose to use a whole brain analysis approach rather than a region of interest one.
Brain Behavior Relationships
In order to identify potential individual differences in emotion regulation and expression, associations between sgACC connectivity and Children’s Sadness Management Scale scores were evaluated with regression analyses in SPSS. Age, gender, history of comorbid disorder(s), Child Depression Inventory total score, and sgACC connectivity with regions identified as showing group differences (see Table 2) were used as independent variables. A Bonferroni correction was applied to account for the number of regions in each hemisphere (L: p < .01; R: p < .002) and only associations surviving this are reported.
Table 2.
Regions of significant difference between groups
| Healthy > PO-MDD | ||||||
|---|---|---|---|---|---|---|
| Region | Hemisphere | BA | X | Y | Z | Cluster(Voxels) |
| Medial Frontal Gyrus | R | 10 | −23 | 38 | 0 | 45 |
| Medial Frontal Gyrus | R | 9 | 1 | 33 | 32 | 81 |
| Medial Frontal Gyrus* | R | 8 | 22 | 26 | 40 | 22 |
| Precentral Gyrus | R | 9 | 39 | 25 | 34 | 23 |
| Anterior Cingulate | R | 32 | −14 | 32 | 11 | 13 |
| Claustrum | R | 34 | 0 | 3 | 182 | |
| Caudate | R | 14 | 14 | 14 | 43 | |
| Medial Frontal Gyrus | L | 10 | −29 | 46 | 1 | 20 |
| Putamen | L | 26 | 1 | 4 | 21 | |
| PO-MDD > Healthy | ||||||
| Inferior Frontal Gyrus | R | 47 | −19 | 28 | −12 | 13 |
| Thalamus | R | −1 | −20 | 16 | 17 | |
| Precuneus | L | 7 | 0 | −57 | 52 | 50 |
| Paracentral Lobule | L | 5 | 11 | −38 | 53 | 13 |
| Cerebellum | L | −5 | −40 | −25 | 14 | |
Note. BA = Brodmann Area. PO-MDD = History of Preschool-Onset Major Depressive Disorder.
Region identified in brain-behavior analysis.
Results
Demographics
Groups were did not differ in age, gender, handedness, Child Depression Inventory total score, or any of the Children’s Sadness Management Scale scales. (all p > .05; see Table 1).
Table 1.
Group Characteristics
| Characteristic | PO-MDD (N = 17) | Healthy (N = 15) |
|---|---|---|
| Age (years) | 9.6 (±.93) | 9.5 (±1) |
| Gender | 11F/6M | 8F/7M |
| Handedness | 17R | 13R/2L |
| Comorbidity | ||
| Internalizing | 8 | NA |
| Externalizing | 9 | NA |
| Child Depression Inventory-Child | ||
| Total T-score | 45.4(10.6) | 42.3(4.2) |
| Children’s Sadness Management Scale | ||
| Inhibition | 7.5(1.5) | 7.3(2) |
| Coping | 10.5(1.7) | 10.7(1.4) |
| Dysregulation | 5.3(1.8) | 4.6(1.4) |
Note. PO-MDD = History of Preschool-Onset Major Depressive Disorder. Data presented as mean(standard deviation)
Whole Brain fcMRI Analysis
Healthy Controls
Similar to previous research [21], the healthy control group exhibited a strong positive functional relationship between both sgACC seeds and a large cluster extending bilaterally from dorsomedial frontal gyrus to ventromedial prefrontal cortex (sgACC seed/center of mass coordinates x,y,z/cluster size (voxels); R/0 38 14/2516; L/−2 34 9/3100). Additional clusters within subcortical and temporal lobe regions were noted for both seeds as well (see Figures 1A and 2A). As can be seen in Figure 2A, a large region of bilateral posterior parietal cortex was negatively related to the left sgACC seed region in the healthy control group (−6 −48 45/3526 mm3). We did not find a functional relationship between the sgACC and amygdala in either hemisphere.
Figure 1.
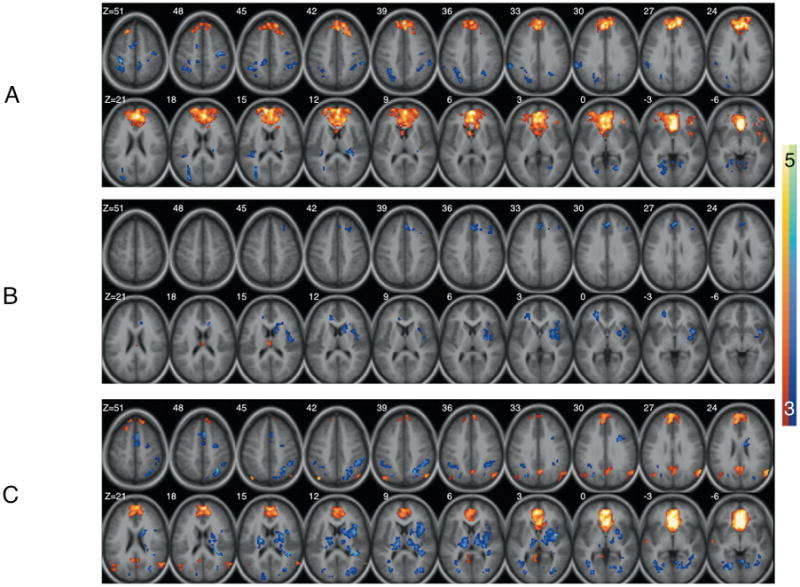
Functional connectivity maps for the right subgenual anterior cingulate seed in (A) Healthy (C) PO-MDD and (B) group differences. Images show regions significantly correlated with the subgenual anterior cingulate seed volume as well as group differences after correction for multiple comparisons (see text). In A and B warm and cool coolers indicate positive and negative connectivity respectively. In C cool coolers indicate increased connectivity in the Healthy group while warm colors indicate increased connectivity in the PO-MDD group.
Figure 2.
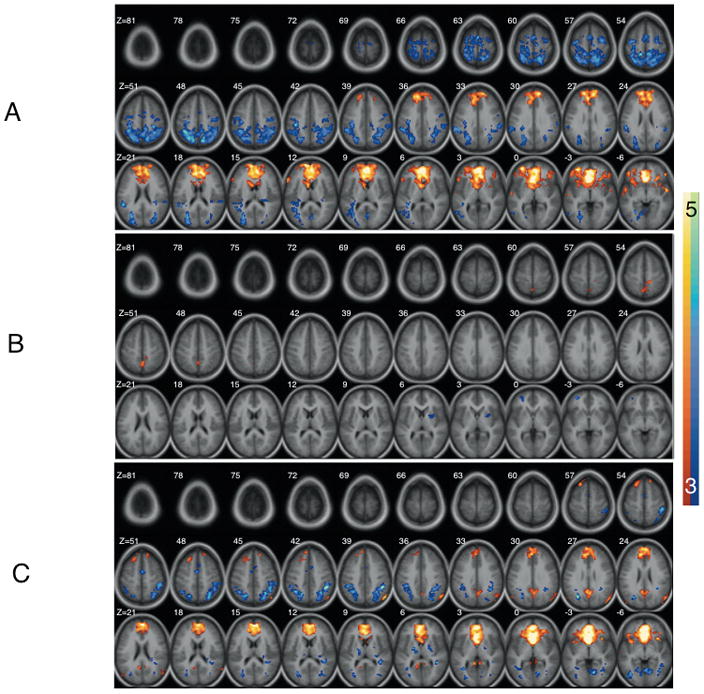
Functional connectivity maps for the left subgenual anterior cingulate seed in (A) Healthy (C) PO-MDD and (B) group differences. Images show regions significantly correlated with the subgenual anterior cingulate seed volume as well as group differences after correction for multiple comparisons (see text). In A and B warm and cool coolers indicate positive and negative connectivity respectively. In C cool coolers indicate increased connectivity in the Healthy group while warm colors indicate increased connectivity in the PO-MDD group.
Preschool-Onset Major Depressive Disorder
When the right and left sgACC seed regions were examined in the PO-MDD group, a strong positive functional relationship between the sgACC and a large bilateral cluster extending between the dorsomedial frontal gyrus and ventromedial prefrontal cortex (sgACC seed/center of mass coordinates x,y,z/cluster size (voxels); R/0 35 3/1392; L/−2 33 5/2086) was again observed. However, the extent of this cluster was notably smaller and less extensive than that seen for controls (see Figures 1C and 2C). Additionally, strong negative relationships between the right sgACC and midline structures such as the striatum were noted (see Figure 1C) as well. Positive relationships between both sgACC seeds and bilateral posterior cingulate cortex were also observed in the PO-MDD group. As in the healthy controls, a functional relationship between the sgACC and amygdala was absent.
Group Differences
Group differences were evident in both hemispheres (see Table 2). Specifically, the right sgACC in the healthy comparison group was found to have a stronger functional relationship with the dorsal anterior cingulate (BA32), medial frontal gyrus (BA 10), caudate, claustrum, and dorsal medial prefrontal regions (BAs 8,9). Conversely, children with a history of PO-MDD demonstrated a stronger functional relationship between the right sgACC and the inferior frontal gyrus (BA 47) and thalamus (figure 1B). When the left sgACC was examined, the healthy comparison group demonstrated increased functional connections between this region and the medial prefrontal gyrus (BA 10) and putamen while children with a history of PO-MDD exhibited increased functional connections with the periaqueductal gray, precuneus (BA 7), and paracentral lobule (BA 5; see figure 2B).
Brain Behavior Relationships
A negative relationship between the right sgACC-right dorsal medial prefrontal cortex (see Table 2) functional connection and current level of emotional dysregulation reported on the Children’s Sadness Management Scale (i.e, decreased connectivity/increased dysregulation; pearson r = −0.523, p = .002 [2-tailed]) was found. Further, right sgACC-right dorsal medial prefrontal cortex scores were found to account for a significant proportion of variance in emotional dysregulation scores even after current age, gender, history of internalizing/externalizing disorder, and negative affect at time of scan were included in the model (B = −6.4, SE = 2.7, p = .028); R2 = .167, F(1,21) = 5.54, p = .028). Further examination at the individual group level revealed that this relationship was primarily driven by the PO-MDD group (r = −0.624, p = .007 [2-tailed]), however, the expected trend was still apparent in the healthy group (r = −0.311, p = .259 [2-tailed]).
Discussion
Using an a priori identified seed region in the sgACC, significant differences in the functional connections of this area were found between the PO-MDD and healthy groups in both hemispheres. As hypothesized, greater functional connections were found between sgACC and dorsomedial prefrontal regions for the healthy control group and with subcortical and posteriomedial parietal regions for the PO-MDD group. Additional connections between the sgACC and striatum were found to be stronger in the healthy group as well. When considered as a whole, the results suggest a pattern of atypical functional relationships between the sgACC and cortical and subcortical regions generally associated with cognitive control/behavioral flexibility (decreased) and visceromotor/self-focused (increased) operations in children with a history of PO-MDD. As such, these findings are consistent with neurobiological models pointing to the importance of the sgACC in depression onset and course [1]. They also provide initial support for neurodevelopmental theories suggesting the importance of ‘self-organizing’ processes in depressive disorders. Put simply, frequently occurring interactions between brain regions (e.g., corticolimbic) are those that become most stable over development [9]. We suggest that children with a history of PO-MDD demonstrate atypical patterns of sgACC functional connectivity that have been ‘sculpted’ by frequent, earlier occurring dysregulated mood states that have left a lasting and potentially indelible impression on the functional architecture of the developing brain.
The results of the current study are also consistent with previous research suggesting decreased connectivity between the sgACC and dorsomedial prefrontal regions in depressed adolescents. However, unlike Cullen et al. [12], we also found regions with increased connectivity to the sgACC in our PO-MDD group. This may have resulted from examining sgACC connectivity separately in each hemisphere rather than using a single seed placed between them. As such, the observed pattern of sgACC connectivity differences suggests that the right and left sgACC may individually contribute to dysregulated cognitive control and increased visceromotor/sensory processing, respectively, in children with a history of PO-MDD. It is also worth noting that, in contrast to Cullen et al.’s study of sgACC connectivity in depressed adolescents, our PO-MDD group had no history of psychotropic medication use. Given the previously reported effects of antidepressant treatment on brain function in depression [23], it is possible that the increased sgACC connectivity with thalamic and parietal regions found in our PO-MDD group were attenuated in the Cullen et al. [12] study as a result of medication exposure.
We failed to find a functional connection between the sgACC and amygdala for either group. Additionally, as reported in previous studies of sgACC connectivity in depressed and healthy subjects [12], we failed to find a significant difference in the functional relationship between the sgACC and amygdala when the groups were compared. While sgACC seed location and/or our whole brain approach to significance may have contributed to this in both the within group analyses and between group comparisons, it is also possible that identification of any potential differences is dependent upon emotionally evocative events (e.g., induced sadness) that were absent in the current study.
A statistically significant ‘brain-behavior’ relationship between atypical functional connectivity of the right sgACC-right dorsal medial prefrontal cortex (BA 8) and disruptions in emotional behavior was also identified, suggesting a link between dysregulated functional relationships of the sgACC and clinically relevant behavior. It has previously been suggested that regions such as the sgACC may act to recruit prefrontal regions for cognitive control of emotional responses [24]. Given the prominent role the dorsal medial prefrontal cortex plays in behavioral monitoring and decision making in childhood and adolescence [11,25] this finding suggests that the normative relationship between these regions is not only disrupted in MDD but may also be related to the experience of depression very early in development.
Several limitations of the current study should be noted. While our sample represents the first functional imaging study of school age children with a history of PO-MDD, it is still relatively modest in size. Additionally, diagnostic status at time of scan was not available. However, given our focus on the history of PO-MDD and given the relatively low Child Depression Inventory scores (suggesting that a current diagnosis of depression was unlikely), this concern is somewhat minimized. Future analyses using a seed region not implicated in clinical depression will be of importance for testing the specificity of the current findings as will additional study of functional connections between other brain regions believed to be important in clinical depression.
Conclusion
The current study reports findings of abnormal functional relationships between the sgACC and multiple cortical and subcortical regions in children with a very early history of a putative clinical depressive syndrome. They also suggest that the early experience of a clinical depressive syndrome may have a lasting impact on brain function and future emotional behavior.
Acknowledgments
Funding: Grant numbers MH64769 (JL), MH090786 (JL, DB, KB) from the National Institute of Mental Health funded the current study. Dr. Belden’s work was supported by the National Institutes of Mental Health (1K01MH090515-01).
We wish to acknowledge our child participants and their parents whose participation and cooperation made this research possible.
References
- 1.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 4.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry. 2007;62:429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pine DS, Alegria M, Cook EH, Jr, Costello EJ, Dahl RE, Merikangas KR, et al. Advances in developmental science and DSM-V. In: Kupfer VD, First MR, Reiger DA, editors. A Research Agenda for DSM. Washington D.C: American Psychiatric Publisher, Inc; 2002. pp. 85–122. [Google Scholar]
- 6.Luby JL, Si X, Belden AC, Tandon M, Spitznagel E. Preschool depression: homotypic continuity and course over 24 months. Arch Gen Psychiatry. 2009;66:897–905. doi: 10.1001/archgenpsychiatry.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch Gen Psychiatry. 2003;60:1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- 8.Luby JL. Early childhood depresson. Am J Psychiatry. 2009;166:974–979. doi: 10.1176/appi.ajp.2009.08111709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis MD. Self-organizing individual differences in brain development. Dev Rev. 2005;25:252–277. [Google Scholar]
- 10.Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry. 2002;51:342–344. doi: 10.1016/s0006-3223(01)01280-x. [DOI] [PubMed] [Google Scholar]
- 11.Rueda MR, Rothbart MK, McCandliss BD, Saccomanno L, Posner MI. Training, maturation, and genetic influences on the development of executive attention. Proc Natl Acad Sci U S A. 2005;102:14931–14936. doi: 10.1073/pnas.0506897102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, et al. A preliminary study of functional connectivity in comorbid adolescent depression. Neurosci Lett. 2009;460:227–231. doi: 10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovacs M. The Children’s Depression, Inventory (CDI) Psychopharmacol Bull. 1985;21:995–998. [PubMed] [Google Scholar]
- 14.Zeman J, Shipman K, Penza-Clyve S. Development and Initial validation of the Children’s Sadness Management Scale. Journal of Nonverbal Behavior. 2001;25:187–205. [Google Scholar]
- 15.Egger HL, Ascher B, Angold A. Center for Developmental Epidemiology, Department of Psychiatry and Behavioral Sciences. Durham, NC: Duke University Medical Center; 1999, 2003. The Preschool Age Psychiatric Assessment: Version 1.4. [Google Scholar]
- 16.Luby J, Heffelfinger A, Mrakeotsky C, Hessler M, Brown K, Hildebrand T. Preschool major depressive disorder: preliminary validation for developmentally modified DSM-IV criteria. J Am Acad Child Adolesc Psychiatry. 2002;41:928–937. doi: 10.1097/00004583-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Stalets MM, Luby JL. Preschool depression. Child Adolesc Psychiatr Clin N Am. 2006;15:899–917. viii–ix. doi: 10.1016/j.chc.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. Stuttgart: Thieme Medical Publishers; 1988. [Google Scholar]
- 21.Kelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, et al. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- 22.McAvoy MP, Ollinger JM, Buckner RL. Cluster size thresholds for assessment fo significant activation in fMRI. Neuroimage. 2001;13:S198. [Google Scholar]
- 23.Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 24.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 25.Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


