Abstract
Objective:
We sought to evaluate the clinical, economic, and humanistic burden of illness in patients with psoriatic arthritis (PsA).
Study Design:
We performed a literature review.
Methods:
Our literature search, conducted between 1998 and 2009, included published studies that (1) considered the direct and indirect costs of PsA; reported measures of clinical burden, including mortality, physical function, quality of life, and productivity; and (3) reported comorbid conditions in patients with PsA.
Results:
We retrieved and reviewed a total of 49 studies. Compared with the general population, patients with PsA had lower health-related quality of life and an increased risk of co-morbid conditions, especially cardiovascular disease. In the U.S., the direct annual health care costs for PsA are estimated to be as high as $1.9 billion. Total indirect costs associated with PsA account for 52% to 72% of total costs. Both direct and indirect costs of PsA increase with worsening physical function and disease activity.
Conclusion:
PsA imposes a considerable economic and quality-of-life burden to patients and society. Clinical features of PsA, including comorbid conditions and disease activity, contribute to reduced physical and psychosocial health-related quality of life. The clinical burden of PsA contributes to direct medical costs attributable to the utilization of health care resources. As a result of the physical functioning limitations imposed by PsA, indirect costs such as disability and lost productivity are substantial drivers of the total costs of care.
Keywords: psoriatic arthritis, burden, costs, quality of life, psoriasis
INTRODUCTION
Psoriatic arthritis (PsA) is a chronic, progressive, inflammatory arthropathy associated with psoriasis. The prevalence of PsA in patients with psoriasis ranges from 6% to 39% and is equally likely to occur in males and females.1,2 The overall prevalence of PsA in the U.S. ranges from 101 to 250 per 100,000 people, with an annual incidence reported at 6.6 out of every 100,000 people.3 The prevalence of PsA has been historically difficult to determine, partly because of the lack of a widely accepted classification or diagnostic criteria and partly because experts often misdiagnose the condition. The original diagnostic criteria of Moll and Wright (1973) are the simplest and the most frequently used.
Patients with PsA are usually affected with psoriasis before signs of joint disease have developed; the mean time to onset of PsA is 10 years after the first signs of psoriasis appear.4 PsA can be distinguished from other inflammatory arthritic diseases by its clinical and radiographic features. In general, PsA affects fewer joints than rheumatoid arthritis (RA), and it often has an asymmetrical distribution of the affected joints rather than the symmetrical pattern seen in RA.4 By definition, all patients with PsA have psoriasis, which may be present for many years.5 Skin involvement can occur anywhere on the body, but most often the scalp, nails, trunk, elbows, and knees are affected.6 Other common clinical features of PsA that are not seen in RA or ankylosing spondylitis (AS) include dactylitis and nail disease.7
Although the burden of psoriasis has been described extensively in the literature, the burden of illness associated with PsA has not been as well quantified. For example, patients with psoriasis alone require more than twice the health care resources (e.g., medical and drug-related services) over 12 months compared with the general population, and psoriasis may account for up to 25% of the total cost of skin disorders in the U.S.8,9 It might be expected that PsA patients incur even greater costs, because they have the added burden of joint involvement.
Similarly, quality of life (QOL) in patients with psoriasis is substantially lower than that in patients with other chronic conditions. Because of the dual skin and joint involvement, QOL and functioning may be further impaired in patients with PsA. Indeed, the clinical burden of PsA is comparable to that of patients with RA.10 Manifestations of PsA contribute to disease burden in terms of negative effects on patients’ psychological and psychosocial functioning, dissatisfaction with the management of their disease, and a negative impact on daily living activities.4,11
The availability of biologic agents such as infliximab (Remicade, Centocor Ortho Biotech Inc.), etanercept (Enbrel, Amgen), adalimumab (Humira, Abbott), and golimumab (Simponi, Centocor Ortho Biotech Inc.) over the last 20 years has provided additional improvements in efficacy and QOL in patients with PsA.12–15 However, the cost of these agents and the expanding pipeline may have a significant impact on limited health care resources, requiring decision makers and payers to increase their scrutiny and management. As such, we conducted a literature search to evaluate the clinical, economic, and humanistic burden of illness in patients with PsA. Our results may help the managed care community understand the current burden of illness experienced by these patients, relative to other chronic inflammatory diseases.
METHODS
We conducted a literature search on documents and articles published in the English language between 1998 and 2009. We used the following databases to identify relevant studies: Medline (via PubMed), the Cochrane Library, the Health Economics Evaluation Database, Psycinfo, and citation lists of published systematic reviews and health technology assessments. We also searched abstracts presented from 2004 to 2009 at major rheumatology conferences, including the European League Against Rheumatism, the American College of Rheumatology, and the American Academy of Dermatology. Because the objective of our review was to evaluate the clinical and economic burden of illness of PsA, we included studies that:
considered the direct and indirect costs of PsA from the perspective of patients, caregivers, health systems, or society.
reported measures of clinical burden (e.g., mortality, physical function, productivity, and quality of life).
reported comorbid conditions in patients with PsA.
The included studies did not have to be empirical in design. We excluded publications that were not in the English language, those that evaluated only non-PsA disorders or psoriasis alone, and those that were economic evaluations of drugs, treatments, or therapy. Personal papers, editorials, commentaries, and case studies were also excluded.
Searches used index and text words encompassing the following terms (synonyms and combinations): psoriatic arthritis, arthritis, psoriasis, economics, costs, cost analysis, cost of illness, burden of illness, work, employment, disability, productivity, patient-reported outcomes, quality of life, the Short-Form Health Survey (SF-36), physical function, functioning, fatigue, Health Assessment Questionnaire (HAQ), modified HAQ, utility, mortality, life expectancy, caregiver, EQ-5D (European Quality of Life–5 Dimensions; EurQoL-5D), health states, time trade off, standard gamble, willingness to pay, co-morbid condition, comorbidities, cardiovascular risk, cardiovascular event, and others.
Search terms were chosen to cast the broadest net of publications considering the burden of PsA. Consequently, disease-specific measures and QOL instruments were not included as search terms, because we decided that more general terms such as “quality of life” would encompass relevant studies.
Search filters limited the studies to the date ranges of 1998 to 2009, the English language, and humans. The last search was undertaken on September 3, 2009. A reviewer manually inspected the titles and abstracts identified in the initial literature search in order to select potentially relevant publications. The full texts of these publications were retrieved and evaluated. Because the objective of the review was qualitative in nature, retrieved publications were not scored on the basis of predefined criteria.
RESULTS OF THE LITERATURE REVIEW
We identified 49 studies that evaluated the clinical and/or economic burden of PsA. Of these, eight were economic cost-of-illness studies, consisting of seven longitudinal studies and one patient survey. Twelve studies were conducted in Canada, five in the U.S., and four each in the United Kingdom, Germany, Spain, and Italy. Table 1 summarizes the patient characteristics of studies reviewed.
Table 1.
Characteristics of Patients with Psoriatic Arthritis (PsA) in Selected Studies
| Study | Country | No. Patients | Mean Age (Years) | Female (%) | Mean Disease Duration (Years) |
|---|---|---|---|---|---|
| Agarwal, 200731 | U.K. | 53 | 50 | 43 | NR |
| Ali, 200755 | Canada | 680 | 43.7 | 43.4 | 7.6 |
| Borman, 200738 | Turkey | 40 | 41.5 | 70 (assumed) | 9.1 |
| Borman, 200856 | Turkey | 47 (18 with arthritis) | Arthritis +: 40.5 Arthritis –: 38.7 |
Arthritis +: 55.5 Arthritis –: 48.3 |
2.35 |
| Brodszky, 200948 | Hungary | 183 | 50.1 | 57 | 9.2 |
| Callis, 200942 | U.S. | 320 (psoriasis patients) | 50.1 | 52.9 | NR |
| Chandran, 200757 | Canada | 135 | 52 | 40.7 | 17 |
| Frediani, 200158 | Italy | 186 | PsA: 63.4 Controls: 63.1 |
PsA: 67.2 Controls: 68 |
3.5 |
| Gladman, 199859 | Canada | 428 | 43 (median) | 45.3 | 4.5 (arthritis) (median) |
| Gladman, 200930 | Canada | 648 | 43.5 at first visit (51.8 at last visit) | 43.8 | 7.4 |
| Gonzalez-Juanatey, 200634 | Spain | 50 | 49.7 | 46 | 7.1 |
| Gonzalez-Juanatey, 200735 | Spain | 59 (healthy controls) | 48.8 | 47.4 | 7.8 |
| Gonzalez-Juanatey, 200736 | Spain | 50 (healthy controls) | 49.7 | 46 | 7.1 |
| Han, 200629 | U.S. | 3,066 | 49.7 | 50.9 | NR |
| Hu, 200845 | U.S. | 60 | 70% ≥ 45 years | 44 | NR |
| Huscher, 20066 | Germany | 908 | 49 | 55.5% | <5 years (n = 274) 5–10 years (n = 263) >10 years (n = 345) |
| Husted, 200140 | Canada | 107 | 50.2 | 38.3 | 14.2 (arthritis) |
| Husted, 200520 | Canada | 341 | 45.9 | 41.1 | 10.6 |
| Husted, 200719 | Canada | 382 | 46.1 | 40.1 | 10.6 |
| Husted, 200843 | Canada | 499 | 48.5 | 43.0 | 12.7 |
| Khraishi, 200944 | Canada | 148 | 53 (PsA > 2 years) 48 (PsA < 2 years) |
42.6% (PsA > 2 years) 60.5% (PsA < 2 years) |
8.0 (PsA > 2 years) 12.6 months (PsA < 2 years) |
| Kimhi, 200737 | Israel | 47 (100 controls) | 50 | 52 | 12.1 |
| Lindqvist, 200760 | Sweden | 135 | 47.3 | 58 | 0.95 |
| Long, 200061 | Canada | 169 | 48.6 | 36 | 14 |
| Mau, 200551 | Germany | 6,041 | 45 | 50 | ≤5 years: 47%, 6–20 years: 25%, >10 years: 28% |
| Mease, 200462 | NR | 205 (101 assigned to etanercept) | 47.6 | NR | 9.0 |
| Nannini, 200928 | Italy | 98/16 (case series patients/controls with PsA) | 51.8/52.0 (case series/controls) | 50.5/56.3 (case series/controls) | 6.7/7.2 (case series/controls) |
| Radtke, 200963 | Germany | 375 | 53.6 | 45.3 | 24.0 (psoriasis; PsA NR) |
| Rohekar, 200826 | Canada | 665 | 62.4 | Full cohort: NR; patients with malignancies: 55.9 | NR |
| Salaffi, 200922 | Italy | 101 (peripheral PsA) | 60.7 | 61.4 | 7.5 |
| Salaffi, 200922 | Italy | 65 (axial PsA) | 58.2 | 50.7 | 8.4 |
| Sokoll, 200141 | U.K. | 47 | 45.2 | 48.9 | 9.6 |
| Taccari, 199864 | Italy | 72 | 55 | 30.1 | 11.1 |
| Tam, 200833 | China | 102 | 48.7 | 52.9 | 9.0 |
| Tam, 200832 | China | 82 | 49 | 40 | 9.4 |
| Wallenius, 200954 | Norway | 102/169 (females/males) | 35.5/36.5 (females/males) | 37.6 | 6.9/5.6 (females/males) |
| Williamson, 200421 | U.K. | 69 (57 [83%] with nail psoriasis) | NR | 46 | 34 (for arthritis) 32 (evident nail disease) 28 (skin disease) |
| Zink, 200646 | Germany | 1,863 | 53.0 | 56.8 | 10.6 |
NR = not reported.
Clinical Features
The course of PsA can be variable and unpredictable, ranging from mild and nondestructive disease to erosive and deforming arthritis, seen in 40% to 60% of PsA patients.16 Untreated patients may have persistent inflammation, progressive joint damage, severe physical limitations, disability, and increased mortality.16 In a prospective cohort of 100 patients with PsA who were observed for approximately five years (mean duration, 11 years), joint damage progressed at a median of 0.42 peripheral joints per year.17 Flares and remissions frequently occur; more than half of patients with PsA have had at least one flare over two years.10
The burden of physical disability is substantial in patients with PsA. The HAQ Disability Index (HAQ–DI) is commonly used to assess physical function in PsA. A score of 0 to 1 represents mild to moderate disability, 1 to 2 represents moderate-to-severe disability, and 2 to 3 represents severe to very severe disability.18 Physical function generally worsens as the number of inflamed joints and disease activity increases.19
Progression of disability may follow three patterns: stable state of disability, steady improvement or worsening in HAQ scores, or fluctuating periods of disability. Predictors of change in the HAQ include age, sex, disease duration, and the number of actively inflamed and deformed joints.19,20 As shown in Table 2, although mean HAQ scores are generally lower for patients with PsA than those with RA, pain scores are generally comparable.
Table 2.
Physical Function and Pain in Patients with Psoriatic Arthritis (PsA) and Rheumatoid Arthritis
| Psoriatic Arthritis | Rheumatoid Arthritis | P Value | |
|---|---|---|---|
| HAQ–DI | |||
| Gladman, 200930 | 0.58 | 1.11 | NR |
| Brodszky, 200948 | 1.0 | 1.4 | <0.05 |
| Husted, 200140 | 0.58 | 1.11 | 0.0001 |
| Lindqvist, 2008 (at baseline)60 | 0.66–0.90 | 1.04 | <0.0001 |
| Lindqvist, 2008 (at 2-year follow-up exam)60 | 0.55–0.74 | 0.62 | NS |
| Gibbs, 200665 | 0.99 | 1.36 | <0.05 |
| Zink, 2006 (% patients with HAQ > 1.0)46 | 43.4% | 53.6% | NR |
| Sokoll, 2001 (median)41 | 1.25 | 1.63 | 0.09 |
| Pain | |||
| Brodszky, 2009, VAS 0–10048 | 52.0 | 48.7 | 0.177 |
| Borman, 2007, VAS38 | 2.78 | 5.9 | NR |
| Lindqvist, 2008, VAS (at baseline)60 | 44–51 | 49 | 0.0311 vs PsA; NS vs. PsA with polyarthritis |
| Lindqvist, 2008, VAS (at 2-year follow-up exam)60 | 34–36 | 29 | NS |
| Sokoll, 2001, VAS range 0–17 (median)41 | 9 | 9 | 0.75 |
| Husted 2001 (HAQ-pain, 0–3)40 | 0.96 | 1.0 | NS |
HAQ–DI = Health Assessment Questionnaire Disability Index; NR = not reported; NS = not significant; VAS = Visual Analogue Scale.
Nail psoriasis is a frequent and cosmetically disfiguring presentation of PsA, occurring in as many as 83% of patients, and often causing functional impairment, pain, and emotional distress. The severity of nail psoriasis is associated with enthesitis, polyarticular disease, and progressive arthritis in PsA.21 Severe nail disease is also associated with functional impairment (higher HAQ scores), higher depression scores, and higher anxiety scores.21
Comorbid Conditions
In addition to skin and joint involvement, PsA may be associated with other inflammatory conditions, including auto-immune disorders (such as iritis and uveitis), and increased risk factors for cardiovascular disease (CVD). In a study by Salaffi et al. that included 166 patients with PsA and 1,579 healthy controls, more than half of all patients reported at least one comorbidity, with hypertension, heart disease, gastrointestinal (GI) conditions, and chronic respiratory diseases the most commonly documented. When assessed by the self-administered Comorbidity Questionnaire, which measures the presence, severity, and functional impact of 12 medical conditions, patients with PsA had higher comorbidity scores (3.34–3.75 on a 36-point score) compared with healthy controls (1.95) and patients with AS (2.48). Patients with RA had the highest scores, indicating the greatest degree of comorbidity (4.35). Comorbidity scores were significantly higher for patients with inflammatory rheumatic disease (PsA, RA, and AS) than for the general population (P < 0.001).22
Patients with PsA are also at greater risk for bone de mineralization and osteoporosis than healthy people. In a prospective observational study, PsA patients had lower bone mineral density in the total body, total body subregions, lumbar spine, and femur (P < 0.05 for all comparisons vs. healthy controls).58 Based on multivariate analysis, age (P = 0.01), years of menopause, HAQ scores (P = 0.009 corrected for age and years of menopause), and body mass index (BMI) (P = 0.01 corrected for age and years of menopause) were significant predictors of osteoporosis.58
Autoimmune gut disorders are more common in patients with PsA. The bowel mucosa of patients with PsA without bowel symptoms shows microscopic lesions even when the mucosa appears macroscopically normal. Along with ileocolonoscopic studies showing inflammatory gut lesions in PsA patients, this may support a pathogenic link between the skin, joints, and gut in psoriatic patients with arthritis, even in the absence of bowel symptoms.23,24 The prevalence of inflammatory bowel disease is also higher in PsA patients (3.9%) than in the general population (0.4%) (P < 0.001), according to a sample of 103 patients with PsA recruited from rheumatology out-patient clinics in the United Kingdom (U.K.), compared with the general U.K. population.25
Although RA and other inflammatory rheumatic disorders have been associated with an elevated risk of malignancy26 and even though psoriasis might be linked to an increased prevalence of lymphomas and other non-melanoma cancers,7 it is not clear whether PsA increases cancer risk. Nannini et al. evaluated the occurrence of malignancies in patients receiving anti-tumor necrosis factor (TNF) agents for the treatment of PsA, RA, or AS.28 Approximately 10% of the patients receiving therapy (n = 363) had at least one abnormal finding upon cancer screening, compared with no such findings in the historical control group. The overall occurrence of occult cancer did not differ significantly.28 In a prospective study by Rohekar et al. (n = 682), malignancy developed in 10.2% of PsA patients over the 14-year study period. Compared with the general Ontario population, however, there was no difference in the incidence of cancer in the PsA cohort.26
The association of psoriasis with increased CVD has been known for decades, but its relationship with PsA is just beginning to come to light. The prevalence of traditional cardiovascular risk factors and comorbid cardiometabolic disease is as high in PsA as it is in RA and AS—and substantially higher than in the general population.29–31 The severity of psoriasis has been shown to be an independent predictor of time to first CVD event, even after adjusting for sex and age (P = 0.05).31 Initially, this finding was attributed to behavioral risk factors that were linked to the psychosocial burden of psoriasis, such as obesity and smoking. However, more recent studies suggest that psoriasis itself might be associated with the chronic inflammatory process in PsA.30
Several small studies have indicated that patients with PsA have significantly elevated levels of inflammatory cardiovascular markers and subclinical ultrasonographic findings. Tam and colleagues compared risk factors for CVD among 82 consecutive PsA patients and 82 controls.32 Although there was no overt CVD, patients with PsA had a higher prevalence of subclinical atherosclerosis, as determined by increased carotid artery intima–media thickness (CIMT), a marker of macrovascular atherosclerotic disease (37% vs. 5%; P < 0.001). In a cross-sectional study of 102 PsA patients by the same authors, patients with PsA demonstrated low-grade inflammation, as indicated by significantly higher levels of high-sensitivity C-reactive protein (hsCRP) compared with healthy controls (P < 0.001).33 After adjusting for BMI, the prevalence of traditional cardiovascular risk factors, including hypertension, low high-density lipoprotein-cholesterol (HDL-C) levels, and diabetes, was significantly higher in PsA patients than in the general population (P < 0.05).33
Gonzalez-Juanatey and colleagues conducted a series of CVD biomarker studies in patients with PsA.34–36 In the first of the series, no significant echocardiographic abnormalities were observed in PsA patients compared with healthy controls without CVD (each n = 50).34
In a subsequent study, the authors observed impaired endothelial dysfunction in PsA patients, an early feature of atherosclerosis, compared with matched controls (P = 0.008).36 The authors then assessed whether PsA was associated with macrovascular atherosclerosis in the same patient population.35 Compared with matched controls, PsA patients had greater CIMT (P = 0.031). CIMT was positively correlated with age (P < 0.001), disease duration (P = 0.04), and cholesterol levels (P = 0.01) but not with the Disease Activity Score (DAS28), an indicator of RA disease activity and response to treatment.
In a cohort study, Kimhi et al. observed a similar correlation between CIMT, the patient’s age and sex, diabetes, duration of arthritis, and severity and duration of skin disease.37
Health-Related Quality of Life
PsA places a substantial burden on patients, diminishing their capacity to carry out daily activities and reducing their quality of life. Measures of physical function and health-related quality of life (HRQoL) are lower in patients with PsA than in healthy people and in patients with other inflammatory arthritidies.38 Because of the visibility of skin involvement, patients with PsA may also experience poor psychosocial function, resulting in embarrassment, self-consciousness, and, in some cases, depression.39
As shown in Table 3, patients with PsA have lower HRQoL scores than the general population, as measured on the SF-36, and they show similar reductions in HRQoL scores when compared with those of patients with RA and AS on scales of bodily pain, general health perceptions, social functioning, and mental health.
Table 3.
Short-Form Health Survey (SF-36) Scores in Patients with Inflammatory Arthritic Diseases and in the General Population
| Psoriatic Arthritis | Rheumatoid Arthritis | Ankylosing Spondylitis | General or Healthy Population | |
|---|---|---|---|---|
| SF-36 subscales | ||||
| Physical functioning | ||||
| Husted, 200140 | 67 | 45.3* | NR | NR |
| Gibbs, 200665 | 54.8*† | 41.9† | 50† | 83.2 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 43.5/50.6† | 41.8† | 52.6† | 82.5 |
| Role limitations—physical | ||||
| Husted, 200140 | 62.6 | 33.7* | NR | NR |
| Gibbs, 200665 | 28.6† | 15.5† | 20.3† | 80.5 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 34.3/38.4† | 29.8† | 38.2† | 73.1 |
| Bodily pain | ||||
| Husted, 200140 | 60.5 | 57.1 | NR | NR |
| Husted, 200843 | 48.6 | NR | NR | NR |
| Gibbs, 200665 | 36.8† | 33.5† | 31.6† | 77.6 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 36.3/45.9† | 30.1† | 45.0† | 78.5 |
| General health perception | ||||
| Husted, 200140 | 58.8 | 54.2 | NR | NR |
| Gibbs, 200665 | 47.1† | 40.1† | 41.0† | 73.8 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 45.1/43.8† | 44.0† | 47.2† | 60.1 |
| Vitality | ||||
| Husted, 200140 | 56.2 | 42.7* | NR | NR |
| Gibbs, 200665 | 41.2† | 33.3† | 32.6† | 64.8 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 45.1/41.8† | 41.9† | 48.5† | 56.8 |
| Social functioning | ||||
| Husted, 200140 | 80.7 | 75.6 | NR | NR |
| Gibbs, 200665 | 57.9† | 50.9† | 52.3† | 84.1 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 43.1/44.7† | 46.9† | 54.7† | 71.6 |
| Role limitations—emotional | ||||
| Husted, 200140 | 68.5 | 52.8 | NR | NR |
| Gibbs, 200665 | 55.4† | 43.9† | 45.7† | 83.2 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 28.0/37.6† | 38.2† | 42.0† | 72.1 |
| Mental health | ||||
| Husted, 200140 | 72.7 | 76 | NR | NR |
| Husted, 200843 | 68.7 | NR | NR | NR |
| Gibbs, 200665 | 67.4 | 65.4 | 62.3 | 77.8 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 44.7/47.6† | 50.3† | 54.3† | 63.6 |
| Physical Health Summary (PCS) | ||||
| Husted, 200140 | 42.1 | 34.3* | NR | NR |
| Gibbs, 200665 | 32.9*† | 28.6† | 31.1† | 51.1 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 34.1/37.5† | 32.5† | 37.1† | 49.6 |
| Mental Component Summary | ||||
| Husted, 200140 | 50 | 50.4 | NR | NR |
| Gibbs, 200665 | 45.9 | 44.4† | 42.1 | 55.2 |
| Salaffi, 2009 (peripheral/axial PsA)22 | 36.9/36.5† | 39.4† | 40.7† | 45.6 |
P = significant versus PsA.
P = significant versus general and healthy population.
NR = not reported.
In a study by Husted et al. (2001), PsA patients had higher vitality scores than RA patients; after adjustments were made for vitality, PsA patients reported greater role limitations, caused by emotional problems, as well as more bodily pain.40 Similarly, both RA and PsA patients had significantly worse HRQoL on all dimensions (pain, physical mobility, energy, sleep, social isolation, and emotional reaction) compared with a healthy population, as indicated by the Nottingham Health Profile (NHP). Although pain and physical mobility scores were worse in patients with RA, both groups reported comparable psychosocial distress and reduction in life satisfaction.38
Overall, SF-36 mental dimensions typically affected by PsA are mental health, limitations resulting from emotional health, and social functioning. Depression is also often present.41,44 In an observational Canadian study reported by Khraishi et al., patients who had PsA for longer than two years had rates of depression that were two to five times higher than those of age-matched controls who had no history of PsA or psoriasis.44
Disease activity (joint and skin) is associated with worsened quality of life; psychological domains of HRQoL are also related to disease activity and pain scores.38 The extent of disability and the impact on physical and mental HRQoL is possibly related to the fact that these patients have the dual burden of psoriatic skin lesions and peripheral and/or axial joint disease.22,41 Although a high DAS28 score and chronic co-morbid conditions are associated with the SF-36 physical component in RA, PsA, and AS, disease activity and psoriatic skin lesions are associated strongly with poor mental health in patients with PsA. When skin disease is severe, for example, median scores on the anxiety/depression domain of the EuroQoL-5D, a generic instrument used to measure health status, functioning, and QoL, are comparable to those of patients with RA.41
Patients with PsA commonly complain of fatigue and sleep disturbances, which can contribute to poor HRQoL. Almost 50% of patients with psoriasis report some level of sleep disturbance; the presence of PsA is a strong predictor of sleep disturbance (odds ratio 3.27; P < 0.001).42 The degree of fatigue observed in PsA patients is significantly worse than that of the general population and comparable to that of patients with systemic lupus erythematous; approximately 50% of patients complain of moderate-to-severe fatigue, and 29% complain of severe fatigue.43
A pilot test of a willingness to pay for a cure found similar results. On an instrument measuring HRQoL, the top four domains affected by PsA were physical comfort (88%), emotional health (63%), sleep (60%), and work (57%). Most of the participants were willing to pay for a cure in these domains—a median of $10,000 for physical comfort, sleep, and work and $5,000 for emotional health. More patients with higher incomes ($65,000 or more per year) stated that PsA affected their work and self-care. Patients with higher incomes were willing to pay higher amounts for improvement in the work, sleep, concentration, and emotional domains.45
Economic Burden
The costs associated with PsA can be considerable. In our review, 10 studies were identified that assessed the direct and indirect costs of the disease (nine cost-of-illness studies and one study evaluating the level of health care resource utilization). Of these studies, seven were conducted to evaluate the impact of lost productivity. Given the clinical burden imposed by the disease, it is not surprising that PsA patients are significant users of health care resources. In a study conducted in German collaborative arthritis centers in 2002, patients made 20.3 visits to a general practitioner each year and 3.9 visits to a rheumatologist, and 12.7% were hospitalized in the previous year.46 These rates of health care resource utilization were similar to those for RA patients and were slightly higher than those for AS patients.46
Because of the variety of cost-collection methods, patient populations, and differences among countries, it is difficult to compare costs in all studies; however, a substantial economic burden is definitely associated with PsA. In the U.S., direct annual health care costs for PsA are estimated to be as high as $1.9 billion, based on a mean cost per patient of $3,638 (from 1999 to 2000), multiplied by the estimated prevalence of 520,000 PsA patients in the U.S. in 2000.47 These costs were based on a sex- and age-adjusted, patient-reported prevalence of 0.25% from the National Psoriasis Foundation and applied to 2000 U.S. Census Bureau figures.1,47 In a study by Brodszky et al., however, direct costs were probably underestimated because patients receiving biologic therapy were excluded from the analysis.48
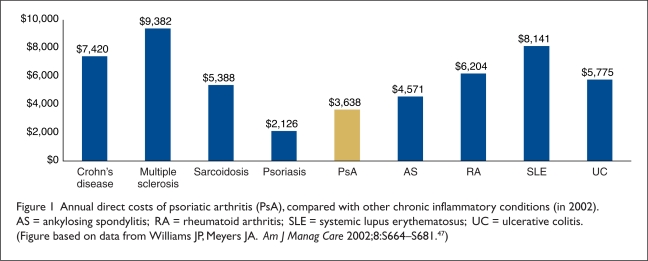
Average direct costs ranged from $4,008 in Hungary to $5,646 in the U.S. (Table 4). Figure 1 compares the average annual direct costs of PsA with those of other chronic inflammatory conditions. The primary driver of direct costs is hospitalizations (about 60% of direct costs).6,49 Total indirect costs associated with PsA account for 52% to 72% of total costs. As expected, both direct and indirect costs of PsA increase with worsening physical function and disease activity. For example, total direct and indirect costs rise (in euros, converted to 2008 U.S. dollars) from €2,331 (∼$3,800) and €5,599 (∼$9,155) in patients with low HAQ scores (below 1.2) to €5,721 (∼$9,350) and €37,440 (∼$61,220) in patients with HAQ scores (1.7 or higher), according to 2002 values.6
Table 4.
Average Annual Direct and Indirect Costs Associated with Psoriatic Arthritis (PsA)
| Study | Data Year | Direct Costs | Indirect Costs | ||
|---|---|---|---|---|---|
| As Published | 2008 (Dollars)* | As Published | 2008 (Dollars)* | ||
| Williams, 200247 | 1999 | $3,638 (attributable to PsA) | $5,646 | NR | NR |
| Huscher, 200610 | 2002 | €3,156 | $5,161 | €7,919 | $12,949 |
| Brodszky, 200948 | 2007 | €2,670 | $4,008 | €2,904 | $4,359 |
Adjusted to 2008 U.S. dollar values. NR = not reported.
Figure 1.
Annual direct costs of psoriatic arthritis (PsA), compared with other chronic inflammatory conditions (in 2002). AS = ankylosing spondylitis; RA = rheumatoid arthritis; SLE = systemic lupus erythematosus; UC = ulcerative colitis. (Figure based on data from Williams JP, Meyers JA. Am J Manag Care 2002;8:S664–S681.47)
As with RA, disability and lost productivity are substantial components of the economic burden of PsA. Reported employment rates of patients with PsA range from 54% to 63%.10,46,50,51 Compared with the general population, patients with PsA have significantly lower employment rates,51 although the rate is higher in PsA patients than in RA patients but is similar to patients with AS.10,51 In a cohort study of PsA patients in Spain, factors significantly associated with employment were age (P < 0.001) and HAQ (P = 0.018).52
Short-term disability claims also impart a substantial burden to employers whose workers have PsA. Almost one-third of patients with PsA claim either short-term or permanent disability.6,46,52,53 As reported by Huscher et al., the annual costs of permanent work disability (in 2008 dollars) increase sharply with the duration of disease—from €2,526 (∼$4,130) for less than five years’ duration to €5,692 (∼$9,307) for five to 10 years, up to €10,255 (∼$1,678) for more than 10 years’ duration.6
Similar levels of work disability were observed in the Norwegian Disease Modifying Antirheumatic Drug study. For PsA patients 18 to 45 years of age (n = 268), 23% were unable to work and were receiving a disability pension.54 Factors independently associated with the need to take disability leave were low educational level, increasing disability scores in the modified HAQ, presence of erosive disease, female sex, and disease duration.54
An overall assessment of the literature on the burden of illness of PsA suggests that there are gaps in current knowledge. In the economic literature, for example, there is a paucity of data on the indirect costs of lost productivity and absenteeism attributable to PsA in the U.S. There were also few to no studies that measured the financial burden and impact of HRQoL on caregivers for patients with PsA in the U.S. and in the rest of the world. Inclusion of these costs is likely to increase the burden of PsA to society.
Implications for Formulary and Drug Policy Decision-Making
The clinical and economic burden of illness of PsA has implications for managed care decision-making and policy formulation, especially in view of the growing drug therapy pipeline. One of the challenges facing health care decision-makers is determining medical and pharmacy policies regarding coverage of these frequently high-cost therapies. In order to assess the clinical, cost-effectiveness, and budgetary impact of a therapy, decision-makers require a thorough understanding of the epidemiology, societal and economic burden of illness, and current approaches to treatment of the disease.66 Indeed, organizations have developed standard formats to aid in coverage determinations and medical and pharmacy policies that include an assessment of the burden of illness (Format for Formulary Submission)66 and the potential impact of new therapy on reducing the burden of illness from the perspective of patients, society, or managed care organizations (MCOs), for example, WellPoint’s Health Technology Assessment Guidelines,67 the National Institute for Health and Clinical Excellence (NICE),68 and the Scottish Medicines Consortium.69
Knowledge of the burden of illness of a disease also aids decision-makers in valuing aspects of the disease that might be mitigated with drug therapy. With PsA, for example, consideration may be given not only to disease activity (joint involvement, psoriatic skin lesions) but also to physical functioning and disability, pain, and patient-reported HRQoL. Outcomes associated with these measures (including increased health care resource utilization by PsA patients and their caregivers, lost employment, and productivity) may have direct consequences to MCOs. A holistic view of the impact of PsA may reveal potential reductions in direct and indirect costs to organizations and society and improvements in patients’ HRQoL, with disease-modifying therapy beyond clinical trial efficacy and safety.
CONCLUSION
For many patients, psoriatic arthritis encompasses not only joint disease but also psoriasis. Our literature review reveals that the emotional toll of the disease can be higher than that of other arthritic conditions. Similar to the other inflammatory rheumatic conditions, PsA is progressive, erosive, and destructive, resulting in diminished functional capacity and poor quality of life. Patients with PsA may also have an increased risk of comorbid conditions, especially cardiovascular disease, compared with the general population.
PsA imposes a substantial economic burden to patients and society. The clinical burden of PsA contributes to direct medical costs. Indirect costs, including lost productivity and disability caused by limitations in functioning and activities of daily living also contribute to the total costs of PsA.
Footnotes
Disclosure. The research and development of the manuscript were supported by funding from Johnson & Johnson Pharmaceutical Services. All authors contributed to the conceptualization and critical review of the manuscript. Dr. Lee and Dr. Mendelsohn are employees of Johnson & Johnson. Dr. Sarnes is an employee of Xcenda, which received funding for this research.
REFERENCES
- 1.Gelfand JM, Gladman DD, Mease PJ, et al. Epidemiology of psoriatic arthritis in the population of the United States. J Am Acad Dermatol. 2005;53(4):573–577. doi: 10.1016/j.jaad.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 2.Taylor WJ. Epidemiology of psoriatic arthritis. Curr Opin Rheumatol. 2002;14:98–103. doi: 10.1097/00002281-200203000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of psoriatic arthritis: A systematic review. J Rheumatol. 2008;35:1354–1358. [PubMed] [Google Scholar]
- 4.Yu AP, Tang J, Xie J, et al. Economic burden of psoriasis compared to the general population and stratified by disease severity. Curr Med Res Opin. 2009;25(10):2429–2438. doi: 10.1185/03007990903185557. [DOI] [PubMed] [Google Scholar]
- 5.Steele T, Pawaskar M, Balkrishnan R, et al. Does cost-effectiveness play a role in clinical trials? Dermatol Ther. 2007;20(2):110–119. doi: 10.1111/j.1529-8019.2007.00119.x. [DOI] [PubMed] [Google Scholar]
- 6.Huscher D, Merkesdal S, Thiele K, et al. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and systemic lupus erythematosus in Germany. Ann Rheum Dis. 2006;65(9):1175–1183. doi: 10.1136/ard.2005.046367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Korte J, Sprangers MA, Mombers FM, Bos JD. Quality of life in patients with psoriasis: A systematic literature review. J Investig Dermatol Symp Proc. 2004;9(2):140–147. doi: 10.1046/j.1087-0024.2003.09110.x. [DOI] [PubMed] [Google Scholar]
- 8.Donahue KE, Gartlehner G, Jonas DE, et al. Comparative effectiveness of drug therapy for rheumatoid arthritis and psoriatic arthritis in adults. Rockville, Md: Agency for Healthcare Research and Quality; Nov, 2007. Comparative Effectiveness Review No 11 Prepared by Research Triangle Institute–University of North Carolina Evidence-Based Practice Center, under Contract No 290-02-0016. Available at: www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 9.Enbrel (etanercept), package insert. Thousand Oaks, Calif: Amgen; 2008. [Google Scholar]
- 10.Remicade (infliximab), package insert. Malvern, Pa: Centocor OrthoBiotech, Inc; 2008. [Google Scholar]
- 11.Humira (adalimumab), package insert. North Chicago, Ill: Abbott; 2008. [Google Scholar]
- 12.Krueger GG. Clinical features of psoriatic arthritis. Am J Manag Care. 2002;8:S160–S170. [PubMed] [Google Scholar]
- 13.Gladman DD, Antoni C, Mease P, et al. Psoriatic arthritis: Epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher VS. Clinical monograph for drug formulary review: Systemic agents for psoriasis/psoriatic arthritis. J Manag Care Pharm. 2005;11(1):33–55. doi: 10.18553/jmcp.2005.11.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gladman DD. Clinical, radiological, and functional assessment in psoriatic arthritis: Is it different from other inflammatory joint diseases? Ann Rheum Dis. 2006;65(Suppl III):iii22–iii24. doi: 10.1136/ard.2006.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis, Section 2. J Am Acad Dermatol. 2008;58:851–864. doi: 10.1016/j.jaad.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 17.McHugh N, Balachrishnan C, Jones SM. Progression of peripheral joint disease in psoriatic arthritis: A 5-year prospective study. Rheumatology. 2003;42:778–783. doi: 10.1093/rheumatology/keg217. [DOI] [PubMed] [Google Scholar]
- 18.Kavanaugh A, Fransen J. Defining remission in psoriatic arthritis. Clin Exp Rheumatol. 2006;24(Suppl 43):S83–S87. [PubMed] [Google Scholar]
- 19.Husted JA, Tom BD, Farewell VT, et al. A longitudinal study of the effect of disease activity and clinical damage on physical function over the course of psoriatic arthritis: Does the effect change over time? Arthritis Rheum. 2007;56(3):840–849. doi: 10.1002/art.22443. [DOI] [PubMed] [Google Scholar]
- 20.Husted JA, Tom BD, Farewell VT, et al. Description and prediction of physical functional disability in psoriatic arthritis: A longitudinal analysis using a Markov model approach. Arthritis Rheum. 2005;53(3):404–409. doi: 10.1002/art.21177. [DOI] [PubMed] [Google Scholar]
- 21.Williamson L, Dalbeth N, Dockerty JL, et al. Extended report: Nail disease in psoriatic arthritis—clinically important, potentially treatable, and often overlooked. Rheumatology (Oxford) 2004;43(6):790–794. doi: 10.1093/rheumatology/keh198. [DOI] [PubMed] [Google Scholar]
- 22.Salaffi F, Carotti M, Gasparini S, et al. The health-related quality of life in rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis: A comparison with a selected sample of healthy people. Health Qual Life Outcomes. 2009;7:25. doi: 10.1186/1477-7525-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritchlin C. Psoriatic disease—from skin to bone. Natl Clin Pract Rheumatol. 2007;3(12):698–706. doi: 10.1038/ncprheum0670. [DOI] [PubMed] [Google Scholar]
- 24.Schatteman L, Mielants H, Veys EM. Gut inflammation in psoriatic arthritis: A prospective ileocolonoscopic study. J Rheumatol. 1995;22:680–683. [PubMed] [Google Scholar]
- 25.Williamson L, Dockerty JL, Dalbeth N, Wordsworth P. Gastrointestinal disease and psoriatic arthritis. J Rheum. 2004;31(7):1469. [PubMed] [Google Scholar]
- 26.Rohekar S, Tom BD, Hassa A, et al. Prevalence of malignancy in psoriatic arthritis. Arthritis Rheum. 2008;58(1):82–87. doi: 10.1002/art.23185. [DOI] [PubMed] [Google Scholar]
- 27.Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis, Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 28.Nannini C, Cantini F, Niccoli L, et al. Single-center series and systematic review of randomized controlled trials of malignancies in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis receiving anti-tumor necrosis factor-alpha therapy: Is there a need for more comprehensive screening procedures? Arthritis Rheum. 2009;61(6):801–812. doi: 10.1002/art.24506. [DOI] [PubMed] [Google Scholar]
- 29.Han C, Robinson DW, Jr, Hackett MV, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–2172. [PubMed] [Google Scholar]
- 30.Gladman DD, Ang M, Su L, et al. Cardiovascular morbidity in psoriatic arthritis. Ann Rheum Dis. 2009;68(7):1131–1135. doi: 10.1136/ard.2008.094839. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal S, Gullick N, Macedo A, et al. 10-year cardiovascular risk in patients with psoriatic arthritis (Abstract 611). Presented at the 71st annual scientific meeting of the American College of Rheumatology; Boston. November 11–16, 2007. [Google Scholar]
- 32.Tam LS, Shang Q, Li EK, et al. Subclinical carotid atherosclerosis in patients with psoriatic arthritis. Arthritis Rheum. 2008;59(9):1322–1331. doi: 10.1002/art.24014. [DOI] [PubMed] [Google Scholar]
- 33.Tam LS, Tomlinson B, Chu TT-W, et al. Cardiovascular risk profile of patients with psoriatic arthritis compared to controls: The role of inflammation. Rheumatology (Oxford) 2008;47(5):718–723. doi: 10.1093/rheumatology/ken090. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Juanatey C, Amigo-Diaz E, Miranda-Filloy JA, et al. Lack of echocardiographic and Doppler abnormalities in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Semin Arthritis Rheum. 2006;35(5):333–339. doi: 10.1016/j.semarthrit.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Juanatey C, Llorca J, Amigo-Diaz E, et al. High prevalence of subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57(6):1074–1080. doi: 10.1002/art.22884. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Juanatey C, Llorca J, Miranda-Filloy JA, et al. Endothelial dysfunction in psoriatic arthritis patients without clinically evident cardiovascular disease or classic atherosclerosis risk factors. Arthritis Rheum. 2007;57(2):287–293. doi: 10.1002/art.22530. [DOI] [PubMed] [Google Scholar]
- 37.Kimhi O, Caspi D, Bornstein NM, et al. Prevalence and risk factors of atherosclerosis in patients with psoriatic arthritis. Semin Arthritis Rheum. 2007;36(4):203–209. doi: 10.1016/j.semarthrit.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Borman P, Toy GG, Babaoglu S, et al. A comparative evaluation of quality of life and life satisfaction in patients with psoriatic and rheumatoid arthritis. Clin Rheumatol. 2007;26(3):330–334. doi: 10.1007/s10067-006-0298-y. [DOI] [PubMed] [Google Scholar]
- 39.Mease PJ. Assessing the impact of psoriatic arthritis on patient function and quality of life: Lessons learned from other rheumatologic conditions. Semin Arthritis Rheum. 2009;38(4):320–335. doi: 10.1016/j.semarthrit.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Husted JA, Gladman DD, Farewell VT, Cook RJ. Health-related quality of life of patients with psoriatic arthritis: A comparison with patients with rheumatoid arthritis. Arthritis Rheum. 2001;45(2):151–158. doi: 10.1002/1529-0131(200104)45:2<151::AID-ANR168>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 41.Sokoll KB, Helliwell PS. Comparison of disability and quality of life in rheumatoid and psoriatic arthritis. J Rheumatol. 2001;28(8):1842–1846. [PubMed] [Google Scholar]
- 42.Callis DK, Wong B, Horn EJ, Krueger GG. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. J Am Acad Dermatol. 2009;60(4):604–608. doi: 10.1016/j.jaad.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 43.Husted JA, Tom BD, Schentag CT, et al. Occurrence and correlates of fatigue in psoriatic arthritis (PsA) Ann Rheum Dis. 2009;68(10):1553–1558. doi: 10.1136/ard.2008.098202. [DOI] [PubMed] [Google Scholar]
- 44.Khraishi MM, Longo N, Pellegrino A, Sampalis JS. The prevalence of comorbidities in a psoriatic arthritis cohort (Abstract 514). Presented at the 73rd annual scientific meeting of the American College of Rheumatology; Philadelphia. October 17–21, 2009. [Google Scholar]
- 45.Hu SW, Holt EW, Husni ME, Qureshi AA. Willingness-to-pay stated preferences for 8 health-related quality-of-life domains in psoriatic arthritis: A pilot study. Semin Arthritis Rheum. 2010;39(5):384–397. doi: 10.1016/j.semarthrit.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Zink A, Thiele K, Huscher D, et al. Healthcare and burden of disease in psoriatic arthritis: A comparison with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33(1):86–90. [PubMed] [Google Scholar]
- 47.Williams JP, Meyers JA. Immune-mediated inflammatory disorders (I.M.I.D.s): The economic and clinical costs. Am J Manag Care. 2002;8:S664–S681. [PubMed] [Google Scholar]
- 48.Brodszky V, Balint P, Geher P, et al. Disease burden of psoriatic arthritis compared to rheumatoid arthritis, Hungarian experiment. Rheumatol Int. 2009;30(2):199–205. doi: 10.1007/s00296-009-0936-1. [DOI] [PubMed] [Google Scholar]
- 49.Javitz HS, Ward MM, Farber E, et al. The direct cost of care for psoriasis and psoriatic arthritis in the United States. J Am Acad Dermatol. 2002;46(6):850–860. doi: 10.1067/mjd.2002.119669. [DOI] [PubMed] [Google Scholar]
- 50.Verstappen SM, Watson KD, McGrother K, et al. Working status in the BSR Biologics Register (Abstract 798). Rheumatol (Oxford); Presented at the 72nd annual scientific meeting of the American College of Rheumatology; San Francisco. October 24–28, 2008; 2010. pp. 1570–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mau W, Listing J, Huscher D, et al. Employment across chronic inflammatory rheumatic diseases and comparison with the general population. J Rheumatol. 2005;32(4):721–728. [PubMed] [Google Scholar]
- 52.Fernandez-Sueiro JL, Willisch A, Pertega S, et al. Factors associated to employment in ankylosing spondylitis and psoriatic arthritis (Abstract 596). Presented at the 71st annual scientific meeting of the American College of Rheumatology; Boston. November 11–16, 2007. [Google Scholar]
- 53.Fernandez-Sueiro JL, Willisch A, Pertega S, et al. Burden of ankylosing spondylitis and psoriatic arthritis on work capability, physical function, and quality of life (Abstract 1187). Presented at the 71st annual scientific meeting of the American College of Rheumatology; Boston. November 11–16, 2007. [Google Scholar]
- 54.Wallenius M, Skomsvoll JF, Koldingsnes W, et al. Work disability and health-related quality of life in males and females with psoriatic arthritis. Ann Rheum Dis. 2009;68(5):685–689. doi: 10.1136/ard.2008.092049. [DOI] [PubMed] [Google Scholar]
- 55.Ali Y, Tom BD, Schentag CT, et al. Improved survival in psoriatic arthritis with calendar time. Arthritis Rheum. 2007;56(8):2708–2714. doi: 10.1002/art.22800. [DOI] [PubMed] [Google Scholar]
- 56.Borman P, Babaoglu S, Gur G, et al. Bone mineral density and bone turnover in patients with psoriatic arthritis. Clin Rheumatol. 2008;27(4):443–447. doi: 10.1007/s10067-007-0725-8. [DOI] [PubMed] [Google Scholar]
- 57.Chandran V, Bhella S, Schentag C, Gladman DD. Functional assessment of chronic illness therapy–fatigue scale is valid in patients with psoriatic arthritis. Ann Rheum Dis. 2007;66(7):936–939. doi: 10.1136/ard.2006.065763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frediani B, Allegri A, Falsetti P, et al. Bone mineral density in patients with psoriatic arthritis. J Rheumatol. 2001;28(1):138–143. [PubMed] [Google Scholar]
- 59.Gladman DD, Farewell VT, Wong K, Husted J. Mortality studies in psoriatic arthritis: Results from a single outpatient center: II. Prognostic indicators for death. Arthritis Rheum. 1998;41(6):1103–1110. doi: 10.1002/1529-0131(199806)41:6<1103::AID-ART18>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 60.Lindqvist UR, Alenius GM, Husmark T, et al. The Swedish early psoriatic arthritis register—2-year follow-up: A comparison with early rheumatoid arthritis. J Rheumatol. 2008;35(4):668–673. [PubMed] [Google Scholar]
- 61.Long JA, Husted JA, Gladman DD, Farewell VT. The relationship between patient satisfaction with health and clinical measures of function and disease status in patients with psoriatic arthritis. J Rheumatol. 2000;27(4):958–966. [PubMed] [Google Scholar]
- 62.Mease P, Ganguly R, Wanke L, et al. How much improvement in functional status is considered important by patients with active psoriatic arthritis: Applying the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) Group guidelines (Abstract SAT0015). Ann Rheum Dis; Presented at 5th annual European League Against Rheumatism (EULAR) Congress; Berlin, Germany. June 9–12, 2004; 2004. p. 39. [Google Scholar]
- 63.Radtke MA, Reich K, Blome C, et al. Prevalence and clinical features of psoriatic arthritis and joint complaints in 2009 patients with psoriasis: Results of a German national survey. J Eur Acad Dermatol Venereol. 2009;23(6):683–691. doi: 10.1111/j.1468-3083.2009.03159.x. [DOI] [PubMed] [Google Scholar]
- 64.Taccari E, Spadaro A, Rinaldi T, et al. Comparison of the Health Assessment Questionnaire and Arthritis Impact Measurement Scale in patients with psoriatic arthritis. Rev Rhum Engl Ed. 1998;65(12):751–758. [PubMed] [Google Scholar]
- 65.Gibbs A, Veale D, Fitzgerald O, Bresnihan B. Health-related quality of life in patients with psoriatic arthritis and ankylosing spondylitis: A comparison with rheumatoid arthritis (Abstract THU0543) Ann Rheum Dis. 2006;65(Suppl II):280. [Google Scholar]
- 66.Foundation for Managed Care Pharmacy/Academy of Managed Care Pharmacy (FMCP) A Format for Submission of Clinical and Economic Data in Support of Formulary Consideration by Health Care Systems in the United States. Alexandria, Va: Academy of Managed Care Pharmacy; Oct, 2009. The AMCP Format for Formulary Submissions (Version 3.0) Available at: www.fmcpnet.org. Accessed April 22, 2010. [Google Scholar]
- 67.WellPoint Health Technology Assessment Guidelines: Drug Submission Guidelines for Reevaluation of Products, Indications, and Formulations. Updated September 2008. Available at: https://www.wellpointnextrx.com/shared/noapplication/f1/s0/t0/pw_ad080614.pdf. Accessed April 22, 2010.
- 68.National Institute for Clinical Excellence (NICE) Guide to the Methods of Technology Appraisal. (reference N0515). National Health Services. Available at: www.nice.org.uk/aboutnice/howwework/devnicetech/technologyappraisalprocessguides/guide_to_the_methods_of_technology_appraisal_reference_n0515.jsp. Accessed April 22, 2010. [PubMed] [Google Scholar]
- 69.Scottish Medicines Consortium New Product Assessment Form. Available at: www.scottishmedicines.org.uk/smc/22.html. Accessed April 22, 2010.