Abstract
Regulatory T cells (Tregs) play a critical role in the maintenance of immunological self-tolerance. Naïve human or murine T cell treatment with the inhibitory cytokine IL-35 induces a regulatory population, termed iTR35, that mediates suppression via IL-35, but not IL-10 or TGFβ, neither express nor require Foxp3, are strongly suppressive in five in vivo models, and exhibit in vivo stability. Treg-mediated suppression induces iTR35 generation in an IL-35- and IL-10-dependent manner in vitro, and in inflammatory conditions in vivo in Trichuris-infected intestines and within the tumor microenvironment, where they appear to contribute to the regulatory milieu. iTR35 may constitute a key mediator of infectious tolerance, may contribute to Treg-mediated tumor progression, and ex vivo generated iTR35 may possess therapeutic utility.
Regulatory T cells (Tregs) are a unique subset of CD4+ T cells that are essential for maintaining peripheral tolerance, preventing autoimmunity, and limiting chronic inflammatory diseases. However, they also prevent beneficial anti-tumor responses and sterilizing immunity against certain chronic infections. Consequently, the modulation of Treg activity or generation of Tregs ex vivo are important goals of immunotherapy. Naturally-occurring, thymus-derived CD4+ Tregs (nTregs) express the lineage specific transcription factor Foxp3 (forkhead box P3), which is required for their development, homeostasis and function1–4. Despite their limited numbers (5–10% of CD4+ T cells), Tregs play a pivotal role in immune homeostasis. Indeed, it has been suggested that the suppressive milieu is potentiated by in vivo conversion of non-Tregs into suppressive cells, a process termed ‘infectious tolerance’. This contagious spread of suppression is thought to be a primary mechanism underlying transplantation tolerance5 and modulating autoimmune and inflammatory diseases, such as experimental allergic encephalomyelitis (EAE)6 and asthma7. While the mechanisms that mediate infectious tolerance remain obscure, both TGFβ and IL-10 have been implicated.
Induced regulatory T cell populations (iTR) can be generated in the periphery, or in vitro, from conventional CD4+Foxp3− T cells (Tconv)8–10. There is substantial interest in the therapeutic potential of iTR as it has been shown that antigen-specific regulatory populations can be generated that are potently inhibitory in vivo11, 12. Two types of iTR have been described based on the cytokines that induce them; TGFβ- and IL-10-iTR. TGFβ-iTR are generated following T cell activation in the presence of TGFβ with or without retinoic acid and IL-2. Both types of iTR are potently suppressive both in vitro and in vivo11, 13, 14, but possess distinct molecular signatures. While TGFβ iTR express Foxp3 and primarily secrete TGFβ, IL-10 iTR cells remain Foxp3− following conversion and are defined by high IL-10 secretion.
Treg-based approaches to treating inflammatory conditions such as allergy, autoimmune diseases, and graft-versus-host responses have great potential, but also have limitations [reviewed in 12]. The therapeutic potential of human Tregs is limited by their polyclonal specificity, poorly defined markers for enrichment, and reduced proliferative capacity which limits ex vivo expansion. Antigen-specific iTR (IL-10 iTR or TGFβ iTR) can be generated ex vivo but their utility is restricted by technical complexities in their generation, limited potency and/or ambiguity regarding stability and longevity in vivo. Thus, the identification of a well-defined population of Tregs which can be readily generated ex vivo, and are stable and potently inhibitory in vivo is a critical goal for effective cell-based immunotherapy.
We have recently described a novel Treg-specific cytokine, IL-35, that is required for maximal regulatory activity of murine Tregs in vitro and in vivo15. In this study, we show that IL-35, like IL-10 and TGFβ, can generate human and murine iTR and address four questions: (1) what is their in vivo efficacy and stability, (2) can they be generated by nTregs, (3) are they generated at inflammation sites, and (4) what is their physiological contribution to the regulatory milieu established by nTregs?
RESULTS
Human IL-35 treated Tconv acquire a regulatory phenotype
Human IL-35 can suppress the proliferation of umbilical cord-derived human CD4+ Tconv cells to a degree similar to that seen by activated Tregs (see Supplementary Information and Supplementary Figs. 1 and 2). Tconv cells activated with anti-CD3- + anti-CD28-coated latex beads (αCD3/CD28) in the presence of IL-35 dramatically upregulated EBI3 and IL12A mRNA, the two constituents of IL-35 (Ebi3 and p35, respectively) (Fig. 1a), but not IL-10 or TGFβ (Supplementary Fig. 3). Single cell analysis by both intracellular cytokine staining (Fig. 1b) and confocal microscopy (Fig. 1c) suggests that IL-35, but not control protein, treatment induces homogeneous expression of IL-35 in human CD4+ Tconv cells. Similarly, CD4+CD45RA+CD25− Tconv cells from adult peripheral blood expressed EBI3 and IL12A, but not TGFB or IL10 mRNA following activation in the presence of IL-35 (Supplementary Fig. 3i,j and data not shown).
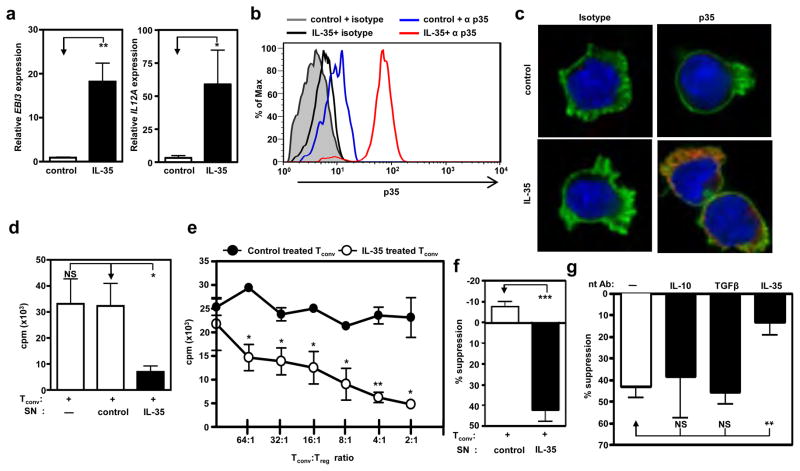
Figure 1. Human IL-35 treatment of Tconv induces autocrine IL-35 expression and confers a regulatory phenotype.
Tconv purified by FACS from cord blood were treated with IL-35 or control at 25% of total culture volume for 9 days during activation (αCD3/CD28, and IL-2). (a) RNA was extracted, cDNA generated and qPCR performed. Relative Ebi3 (left panel) and Il12a (right panel) mRNA expression. (b) Cytokine treated cells were re-purified and stained with an isotype or a p35-specific antibody following 4h activation with PMA and ionomycin. Intracellular staining of IL-35 was determined by FACS. (c) Microscopic analysis of p35 expression was similarly determined following 4h activation with PMA and ionomycin. Anti- p35 or isotype control antibodies (shown in red), phalloidin (shown in green) and DAPI (shown in blue). (d) Proliferation of cytokine treated cells was determined by [3H]-thymidine incorporation (e) Tconv cells were mixed at indicated ratios (Tconv: suppressor) with control or IL-35 treated Tconv, hIL-2 and anti-CD3- + anti-CD28-coated latex beads for 9 days. Proliferation was determined by [3H]-thymidine incorporation. The mean of 4 representative experiments with similar cpm is shown. (f) Control or IL-35 treated Tconv were cultured in the top chambers of a Transwell™ culture plate as indicated. Freshly purified responder Tconv were cultured in the bottom chamber of the 96-well flat bottom plates in medium containing hIL-2 and anti-CD3- + anti-CD28-coated latex beads. Top chambers were removed and [3H]-thymidine was added directly to the responder Tconv cells in the bottom chambers of the original Transwell™ plate for the final 8 h of the 9 day assay. (g) Tconv cells were activated in the presence of IL-35 at 25% of total culture volume. Following conversion with cytokines, suppression assays were supplemented with neutralizing IL-10, TGFβ, or IL-35 to assess their requirement for indicated cytokines to mediate suppression. Counts per minute of Tconv cells activated alone, in the absence of control or IL-35, were 20,000–125,000 (f) and 25,000 – 570,000 (g). Data represent the mean ± SEM of (a)10 (b) 4, (c-g) independent experiments [* p < 0.05, ** p < 0.005, *** p < 0.001, NS = not significant].
We next assessed if IL-35-treated cells assumed the functional phenotype of iTR. Tconv cells activated in the presence of IL-35 but not control were hyporesponsive to secondary restimulation (Fig. 1d). To determine whether IL-35-pretreated Tconv cells had acquired regulatory capacity, they were co-cultured as potential suppressors with freshly purified responder Tconv. While control-treated cells lacked any suppressive capacity, IL-35 treated cells were strongly suppressive (Fig. 1e). Human IL-35, but not control-treated, Tconv cells also suppressed responder Tconv cell proliferation across a permeable membrane, in the absence of direct cell contact, supporting a role for cytokine-mediated suppression (Fig. 1f). Moreover, neutralizing mAbs to IL-35, but not IL-10 or TGFβ, blocked their suppressive capacity (Fig. 1g, Supplementary Information and Supplementary Fig. 3). Taken together, these data suggest that IL-35 can convert human Tconv into a homogeneous population of iTR cells that suppress via IL-35.
IL-35 treated murine Tconv acquire a regulatory phenotype in vitro
Given that human IL-35 can mediate iTR generation, we then asked if murine IL-35 possessed a similar capacity (see Supplementary Information and Supplementary Fig. 4). Analysis of Tconv cells activated in the presence of murine IL-35 upregulated both Ebi3 and Il12a, but not Il10 or Tgfb mRNA (Fig. 2a, Supplementary Information and Supplementary Fig. 5). Immunoprecipitation and western blot analysis demonstrated that only IL-35 treated cells secrete IL-35, which was equivalent to the amount of IL-35 produced by natural Tregs. Both control-treated Tconv cells and IL-35-treated Ebi3−/− Tconv cells did not secrete IL-35 (Fig. 2b). We next assessed if IL-35-treated murine cells, like their human counterparts, assumed an iTR phenotype. Consistent with earlier reports16, previously activated Tconv cells proliferated well in response to secondary re-stimulation (Fig. 2c). IL-10 and IL-27 pre-treated Tconv also proliferated strongly in response to re-stimulation (note that short-term IL-10 treatment alone, in the absence of DCs, is insufficient to mediate IL-10 iTR conversion14) (Supplementary Fig. 6). However, both IL-35 and TGFβ pretreated Tconv cells were hyporesponsive to re-stimulation, albeit to a lesser degree than freshly purified nTregs. To determine whether these cytokine-pretreated Tconv cells had acquired regulatory capacity, they were co-cultured as potential suppressors with freshly purified responder Tconv cells (Fig. 2d and Supplementary Fig. 6). Whereas the control, IL-10- and IL-27-treated Tconv cells had no effect on responder proliferation, TGFβ-treated Tconv cells suppressed responder T cell proliferation13. As seen with human T cells, murine Tconv cells pretreated with IL-35 were also capable of suppressing responder T cell proliferation. Furthermore, IL-35- but not control-treated Tconv could suppress T cell proliferation in a contact-independent manner, across a permeable membrane, implicating soluble suppressive mediators (Fig. 2e). Using an IL-35 sandwich ELISA we also showed that approximately 500–700pg/ml IL-35 is required to mediate the induction of Ebi3 and Il12a expression and of the suppressive phenotype (Supplementary Fig. 4).
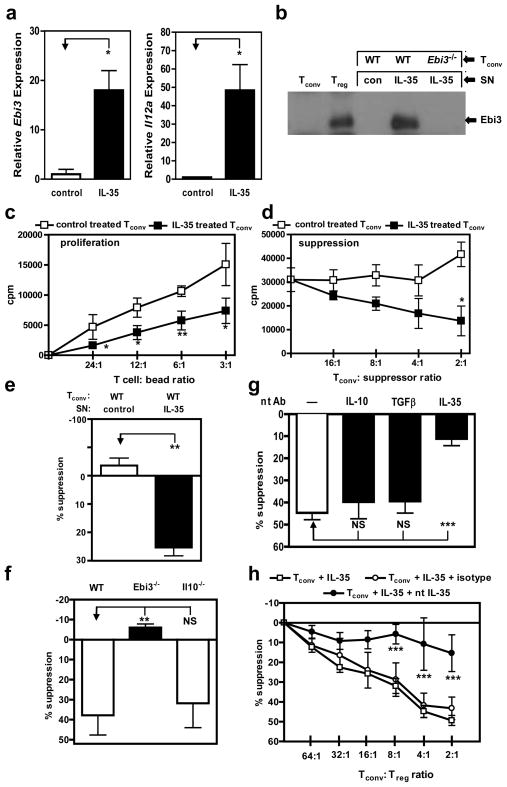
Figure 2. Murine IL-35 treatment of Tconv converts cells to an IL-35 producing suppressive population.
Tconv purified by FACS from C57BL/6, Ebi3−/− or Il10−/− mice were treated with indicated cytokines for 72 h during activation (αCD3/CD28). (a) RNA was extracted and cDNA generated from Tconv following control or IL-35 treatment. Relative Ebi3 (left panel) and Il12a (right panel) mRNA expression. (b) Tconv cells were cultured with control protein or IL-35 for 72 h. Cells were re-purified and cultured for an additional 24 h to facilitate IL-35 secretion. Culture supernatants from indicated cultures, or Tconv and Treg as control, were immunoprecipitated with anti-p35 mAb, resolved by SDS-PAGE and probed with anti-Ebi3 mAb to identify IL-35 secretion. (c) Proliferative capacity, determined by [3H]-thymidine incorporation, of Tconv treated with indicated cytokines for 72 h. (d) Tconv cells were mixed at indicated ratios (Tconv: suppressor) with control or IL-35 treated Tconv and anti-CD3- + anti-CD28-coated latex beads for 72 h. Proliferation was determined by [3H]-thymidine incorporation (e) Control or IL-35 treated Tconv were cultured in the top chambers of a Transwell™ culture plate as indicated. Freshly purified wild-type responder Tconv were cultured in the bottom chamber of the 96-well flat bottom plates in medium containing anti-CD3- + anti-CD28-coated latex beads. After 60 h in culture, top chambers were removed and [3H]-thymidine was added directly to the responder Tconv cells in the bottom chambers of the original Transwell™ plate for the final 8 h of the 72 h assay. (f) Tconv from C57BL/6, Ebi3−/− or Il10−/− mice were activated in the presence of IL-35 at 25% of total culture volume, for 72 h to generate suppressive cells. Cells were re-purified and mixed at 4:1 ratio (Tconv: suppressor) and proliferation was determined. (g) Wild-type Tconv cells were activated in the presence of IL-35 at 25% of total culture volume. Following conversion with cytokines, suppression assays were supplemented with neutralizing IL-10, TGFβ, or IL-35 to assess their requirement for indicated cytokines to mediate suppression. Cells were cultured at a 4:1 ratio in suppression assays as described in e. (h) Suppressive capacity of IL-35 treated Tconv cells supplemented with titrations of isotype control or neutralizing IL-35 mAbs. Counts per minute of Tconv cells activated alone were 21,000–64,000 (e-h). Data represent the mean ± SEM of 4–8 independent experiments [* p < 0.05, ** p < 0.005, *** p < 0.001, NS = not significant].
To determine the mechanism of suppression, we first showed that IL-35-pretreated Il10−/− (which cannot make IL-10), but not Ebi3−/− (which cannot make IL-35), Tconv could suppress responder T cells (Fig. 2f). In addition, TGFβR.DN Tconv that are unable to respond to TGFβ were fully suppressed by IL-35-treated Tconv (see Supplementary Information and Supplementary Fig. 7). Using cytokine neutralizing mAbs, we then showed that IL-35-pretreated Tconv mediated suppression via IL-35 but not IL-10 or TGFβ (Fig. 2g,h [note that the anti-Ebi3 mAb used neutralizes IL-35 but not IL-27; Supplementary Fig. 8]). Collectively, these data suggest that murine IL-35 converts Tconv into an iTR population that appears to mediate suppression exclusively via IL-35.
IL-35-treated murine Tconv exhibit a highly restricted genetic signature
Given that IL-35 can convert proliferative, Foxp3− Tconv cells into hypo-responsive, strongly suppressive iTR, we next sought to define their genetic signature. Interestingly, Foxp3 is neither induced nor required for the generation of IL-35-iTR. While natural Tregs and TGFβ-iTR express Foxp3, neither control nor IL-35 treated Tconv cells express Foxp3 (Supplementary Fig. 9). Moreover, Foxp3−/− Tconv cells could be converted to IL-35 iTR which expressed IL-35 and mediated suppression in a manner indistinguishable from their wild type counterparts (Supplementary Fig. 9c). In addition, iTR35 cells do not express Foxp3 following in vivo inoculation as demonstrated by utilizing iTR35 generated from Foxp3gfp mice in an in vivo model of homeostatic expansion. Seven days post-transfer into Rag1−/− mice, iTRcontrol, wild-type iTR35 and Ebi3−/− iTR35 were purified based on congenic Thy1 markers and Foxp3 expression assessed by flow cytometric analysis of GFP expression. No induction of Foxp3 was seen in any of the transferred iTR (Supplementary Fig. 9d).
We next compared the global gene expression of IL-35- and control-treated Tconv cells using Affymetrix GeneChip microarrays. Prior to analyses, we verified that the IL-35-iTR used for analysis expressed Ebi3 and p35 mRNA, secreted IL-35 and suppressed responder Tconv cells (Supplementary Fig. 10). While clear differences were observed between nTreg and Tconv, no genes appear to be significantly up- or down-regulated between IL-35- and control-treated Tconv (>3-fold; Supplementary Information and Supplementary Fig. 11). Nevertheless, the IL-35-iTR cells generated from five independent experiments expressed Ebi3 and Il12a mRNA, secreted IL-35 and mediated potent in vitro suppression. The minimalistic genetic alteration observed in IL-35- compared to control-treated Tconv was further supported by analysis of T cell activation and co-stimulatory molecule expression and cytokine production. While the expression of most of the proteins/cytokines examined was indistinguishable between IL-35- and control-treated Tconv, reductions in the secretion of GM-CSF, IFNy, and IL-4 were observed, albeit not statistically significant (Supplementary Fig. 12). In addition, surface molecules such as CLTA-4 and CD25, which have been previously described as mediators of nTreg suppression, were similarly upregulated in both control and IL-35-treated Tconv, cells, arguing against an exclusive role in the latter. Furthermore, the percentage of CTLA4+ IL-35-iTR was relatively small (<15%). Taken together, these results suggest that IL-35 treatment mediates surgical rather than global gene expression changes.
Given that IL-35 is central to both the generation and suppressive function of IL-35-iTR, we refer to this novel iTR population as iTR35 (for induced T regulatory population making IL-35) [Control-treated Tconv, which do not acquire a suppressive phenotype, are referred to as iTRcontrol]. While our data suggest that iTR35 have a highly restricted Cd4+/Foxp3−/Ebi3+/Il12a+/Il10−/Tgfb− genetic signature, we cannot rule out the possibility that there are some molecular changes that might distinguish IL-35- from control-treated Tconv that were not revealed by this analysis, or that this signature may apply to an as yet undefined non-regulatory population (See Supplementary Information).
iTR35 are potently suppressive in vivo
The regulatory capacity of iTR35 was tested in five different in vivo models. We first assessed whether iTR35 could restore immune homeostasis and prevent lethal autoimmunity in Foxp3−/− mice17, 18. iTR35 and various control populations were transferred into newborn (2–3 day old) Foxp3−/− mice. Approximately 25 days later, an external clinical score, splenic and lymph node CD4+ T cell numbers, and a histological score (lungs, liver and skin) were determined (see Supplementary Information)19. As expected, nTregs and TGFβ-iTR, but not control-treated Tconv (iTRcontrol) were able to restore immune homeostasis and prevent autoimmunity (Fig. 3a-d and Supplementary Fig. 13). Interestingly, iTR35 were as effective as nTregs at restoring immune homeostasis and preventing autoimmunity in Foxp3−/− mice. Importantly, neither Ebi3−/− nor p35−/− iTR35 could restore immune homeostasis demonstrating the necessity for IL-35 production in vivo by iTR35.
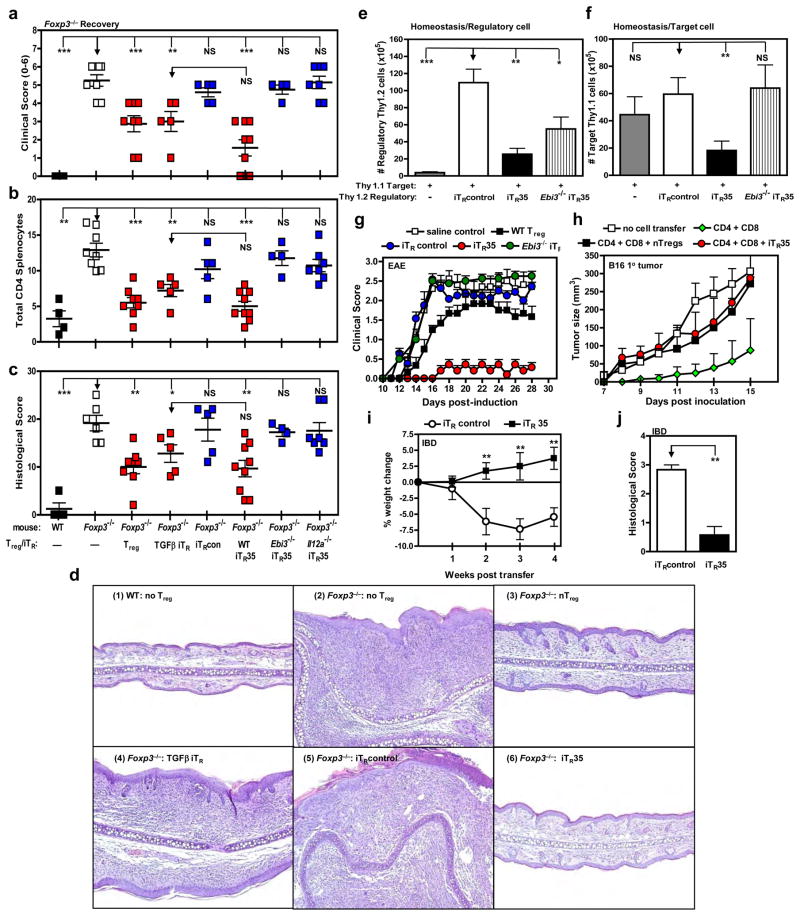
Figure 3. iTR35 are suppressive in vivo.
Control treated (iTRcontrol), IL-35 treated (iTR35) or TGFβ treated (TGFβ iTR) cells were generated from FACS purified Tconv from wild type C57BL/6 or Ebi3−/− mice. (a-c) Cells were injected i.p. into 2–3 day old Foxp3−/− mice. Recovery from disease was monitored and reported by reduction in (a) clinical score (b) splenic CD4+ T cell number as determined by flow cytometric analysis and (c) combined histological score from lungs, liver and skin (d) Representative histopathology images of ear pinna following T cell transfer into Foxp3−/− mice. Panel 1: Wild type mouse receiving no Tregs is normal and has no inflammatory cells in the dermis. Panel 2: Foxp3−/− mouse receiving no Tregs is thickened with a dense inflammatory cell infiltrate causing distortion of the cartilage. Panel 3: Foxp3−/− mouse receiving nTregs is mildly thickened with mild edema and a sparse inflammatory cell infiltrate. Panel 4: Foxp3−/− mouse receiving TGFβ iTR cells is moderately thickened with inflammatory cells separated by edema fluid. Panel 5: Foxp3−/− mouse receiving iTRcontrol cells is markedly thickened with edema dispersed inflammatory cells causing distortion of the cartilage. Panel 6: Foxp3−/− mouse receiving iTR35 cells is mildly thickened with edema and a mild inflammatory cell infiltrate. (e-j) iTRcontrol or iTR35 were generated from FACS purified Tconv from C57BL/6 or Ebi3−/− (Thy1.2) or B6.PL (Thy1.1) mice. (e) Homeostatic expansion was monitored by i.v. injection of Thy1.1+ Tconv cells alone or with Thy1.2+ iTRcontrol or iTR35 cells (as regulatory cells) into Rag1−/− mice. Seven days after transfer, splenic T cell numbers were determined by flow cytometry. Thy1.2+ regulatory T cell numbers (e). Thy1.1+ target Tconv cell numbers (f). (g) EAE was induced by immunizing mice with MOG35–55 peptide in complete Freund’s adjuvant followed by pertussis toxin administration. iTRcontrol, iTR35 or nTreg (106) were transferred i.v. into C57BL/6 mice 12–18 hours prior to disease induction. Clinical disease was monitored daily. (h) Rag1−/− mice received indicated cells via the tail vein on day –1 of experiment. On day 0, all were injected with 120,000 B16 cells i.d. in the right flank. Tumor diameter was measured daily for 15 days and is reported as mm3. (i-j) IBD was induced by injecting Rag1−/− mice with Tconv cells via the tail vein. After 3–4 weeks, mice developed clinical symptoms of IBD and were given iTRcontrol or iTR35 cells. Percentage weight change after iTRcontrol or iTR35 cell transfer (i). (j) Colonic histology scores of experimental mice. Data represent the mean ± SEM of 8–12 mice per group from at least 2 independent experiments [* p < 0.05, ** p < 0.005, *** p < 0.001, NS = not significant].
Second, Tregs can control the homeostatic expansion of Tconv cells in lymphopenic recombination activating gene 1 (Rag1)−/− mice15, 20, 21. Purified wild-type Thy1.1+ Tconv cells, either alone or in the presence of control- or IL-35-treated Thy1.2+ T cells were adoptively transferred into Rag1−/− mice, and splenic responder (Thy1.1+) and suppressor (Thy1.2+) T cell numbers determined 7 days later19. Thy1.2+ iTRcontrol expanded significantly and failed to block the expansion of Thy 1.1+ responder Tconv cells (Fig. 3e, f). In contrast, Thy1.2+ Tconv iTR35, but not Ebi3−/− iTR35, had low proliferative capacity and significantly limited proliferation of Thy 1.1+ responder Tconv cells.
Third, experimental autoimmune encephalomyelitis (EAE) is a model for the human autoimmune disease multiple sclerosis and can be induced in C57BL/6 mice following immunization with MOG35–55 peptide. Adoptively transferred nTregs have been shown to reduce EAE disease severity6, 23, 24. To determine whether iTR35 could slow or prevent EAE, 106 nTregs, iTRcontrol or iTR35 cells were transferred into mice prior to EAE induction19. Consistent with previous reports, clinical scores were reduced in nTregs recipients, while disease course in mice receiving the iTRcontrol cells or saline alone was unaffected (Fig. 3g). Strikingly, the iTR35-treated mice were completely protected from EAE, while mice receiving Ebi3−/− iTR35 were indistinguishable from saline control mice, suggesting that IL-35 production by iTR35 in vivo is required for protection.
Fourth, Tregs can prevent anti-tumor CD8+ T cell responses against the poorly-immunogenic B16 melanoma25, 26. Wild type naïve CD4+CD25− and CD8+ T cells alone or in combination with nTregs or iTR35 cells were adoptively transferred into Rag1−/− mice followed by i.d. injection of B16 melanoma cells and tumor size monitored daily19. As expected, tumor size was reduced in CD4+/CD8+ T cell recipients lacking Tregs compared with the untreated Rag1−/− mice (Fig. 3h). In contrast, transfer of either nTregs or iTR35 cells completely blocked the anti-tumor response resulting in more aggressive tumor growth that was comparable to untreated Rag1−/− mice. Surgical excision of the primary tumor and subsequent secondary tumor challenge at a distal site demonstrated that concomitant tumor immunity was also prevented by both nTregs and iTR35 (Supplementary Fig. 13).
Fifth, inflammatory bowel disease (IBD) is initiated by the adoptive transfer of naïve CD4+CD45RBhiCD25− T cells into Rag1−/− recipient mice and disease assessed by weight loss and colonic histopathology22. After mice developed clinical symptoms of IBD (~4 weeks post T cell transfer), they received iTRcontrol or iTR35 and were monitored daily19. Recovery from disease, marked by weight gain (Fig. 3i) and decreased histopathology (Fig. 3j and Supplementary Fig. 13), was observed in mice that received iTR35 but not the iTRcontrol cells. We also used this model to further demonstrate that TGFβ is not required for the in vivo suppressive capacity of iTR35 (see Supplementary Information and Supplementary Fig. 14).
Finally, we further assessed the importance of IL-35 production by iTR35 in vivo using a unique Ebi3-specific mAb that neutralized IL-35 but not IL-27 (Supplementary Fig. 8). Administration of this mAb, but not an isotype control, blocked the suppressive capacity of iTR35 in an in vivo model of homeostatic expansion (Supplementary Fig. 13g), consistent with our observations using Ebi3−/− or p35−/− iTR35 in three in vivo models (Fig. 3). Taken together, these data clearly demonstrate that iTR35 have potent suppressive capacity in a wide variety of in vivo models and that this activity is dependent on IL-35 production in vivo.
iTR35 are stable in vivo
Some have suggested that ex vivo generated iTR cells are unstable in vivo. Although our five in vivo transfer experiments suggest that iTR35 have some degree of stability, we used two approaches to assess this question directly. First, CD45.2+ iTR35 or TGFβ-iTR were generated in vitro and adoptively transferred into CD45.1+ C57BL/6 mice to monitor cell recovery and function over time (see Supplementary Information). Both iTR35 and TGFβ-iTR can be recovered from the spleen post-transfer, and retain expression of their signature genes, Ebi3/Il12a and Foxp3/Tgfb, respectively (Supplementary Fig. 15d). As in vitro, iTR35 inoculated in vivo fail to induce Foxp3 expression suggesting that this critical nTreg transcription factor is not required for iTR35 maintenance and function. While 33% of the initial iTR35 inoculum was recovered 3 weeks post-transfer, only 12% of TGFβ iTR cells were recovered (Fig. 4a). In addition, purified iTR35 cells still retained strong suppressive capacity, whereas the function of TGFβ-iTR cells was reduced by approximately 50% (Fig. 4b). While this suggests that iTR35 may be more stable in vivo, it does not exclude the possibility that iTR35 and TGFβ-iTR may home to different anatomical locations in the mouse, which could affect their recovery from the spleen. Second, we transferred nTregs, iTR35 or TGFβ-iTR into 2–3 day old Foxp3−/− mice and determined how long they could prevent the onset of a moribund state (clinical score ≥4). By 5 weeks post-transfer, all the TGFβ-iTR recipients were moribund compared with 40% and 33% of nTregs and iTR35 recipients, respectively (Fig. 4c). Furthermore, survival of the remaining nTreg and iTR35 recipients was longer, with 100% moribund not being reached until 6.5 and 8 weeks, respectively. Although additional experiments will be required to fully evaluate the long-term stability of iTR35 in homeostatic and inflammatory environments, these data suggest that they may be functionally stable in vivo.
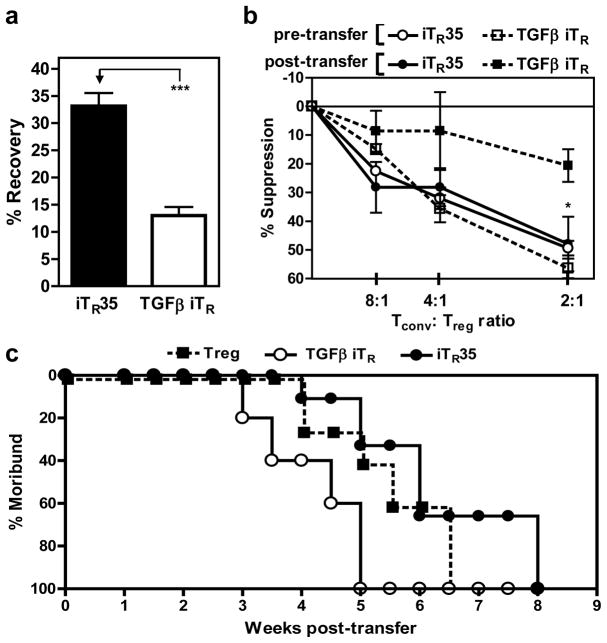
Figure 4. Comparative stability of iTR35 and TGFβ iTR in vivo.
iTR35 or TGFβ iTR were generated in vitro with CD45.2+ Tconv cells and adoptively transferred into CD45.1+ C57BL/6 mice to assess stability. (a) Splenic iTR cell number was determined by flow cytometric analysis of CD45.2+ cells. Percentage of total cells injected that were recovered 25 days post-transfer. (b) Tconv cells were mixed at indicated ratios (Tconv: suppressor) with either freshly generated iTR cells (pre-transfer) or iTR cells recovered after in vivo resting (post-transfer) and anti-CD3- + anti-CD28-coated latex beads for 72 h. Proliferation was determined by [3H]-thymidine incorporation. (c) Natural Tregs or iTR were injected into 2–3 day old Foxp3−/− mice. Mice were monitored for clinical signs of sickness and scored accordingly. Mice obtaining a clinical score of 4 were considered moribund. Survival, based on longevity of mice, was significantly less in recipients of TGFβ iTR when compared to nTregs. Conversely, mice that received iTR35 survived significantly longer than those that received nTregs (p < 0.005). Counts per minute of Tconv cells activated alone were 18,000–44,000 (b). Data represent the mean ± SEM of 5–12 mice per group from at least 2 independent experiments [* p < 0.05, *** p < 0.005].
Treg-mediated suppression generates iTR35
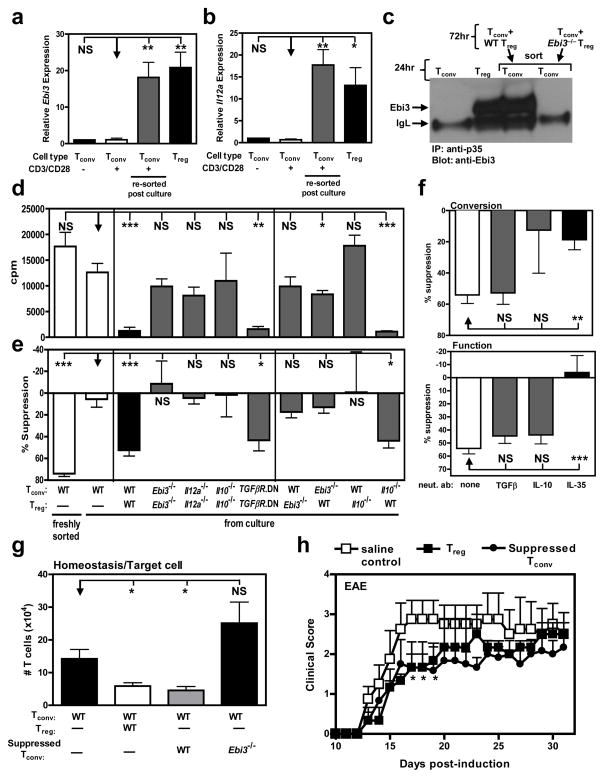
It has been suggested that Tregs can amplify their suppressive capacity by converting non-regulatory populations into suppressive cells, consistent with the concept of infectious tolerance, and that this process might be cytokine-mediated27–29. We have previously shown that nTregs are a natural source of IL-35, which increases 5–10-fold upon contact with the target Tconv cells15, 30. Thus, we asked whether nTreg-derived IL-35 could mediate iTR35 conversion. We first purified Tconv cells that had been cultured with, and suppressed by, nTregs for 3 days (which we refer to as suppressed Tconv) and found that expression of both Ebi3 and Il12a (p35) mRNA was significantly up-regulated following co-culture, to a level comparable with nTregs (Fig. 5a,b). Furthermore, suppressed Tconv generated by co-culture with wild type, but not Ebi3−/− nTregs, secrete a significant amount of IL-35 (Fig. 5c). This demonstrates that IL-35 secretion by nTregs is required to induce IL-35 secretion by co-cultured, suppressed Tconv cells. To determine whether suppressed Tconv express Foxp3, a prerequisite for mediating the regulatory activity of nTregs and TGFβ-iTR, we activated Thy1.2+ Foxp3gfp Tconv cells alone or in combination with Thy1.1+ nTregs. Unlike TGFβ-iTR, but similar to iTR35, suppressed Tconv remain Foxp3− following activation in the presence of Tregs suggesting that TGFβ may not mediate this conversion (Supplementary Fig. 16). These data raise the possibility that iTR35 are generated within the suppressed Tconv population.
Figure 5. Tregs generate iTR35 in an IL-35- and IL-10-dependent manner.
Tconv were activated in the presence of Treg at a 4:1 ratio (responder: suppressor) for 72 h. (a) RNA was extracted and cDNA generated from resting or activated Tconv cells or from Tconv:Treg co-cultures (resorted based on differential Thy1 markers). Ebi3 (a) and Il12a (b) expression of the populations indicated. (c) Following co-culture, suppressed Tconv were re-purified and cultured for an additional 24 h. Secretion of IL-35 was determined by IP/WB of culture supernatants and compared to that of freshly cultured Tconv and Treg. (d) Following co-culture, suppressed Tconv were re-purified and activated (αCD3/CD28). Proliferative capacity was assayed by [3H]-thymidine incorporation. (e) The suppressive capacity of suppressed Tconv upon fresh responder Tconv cells was determined by [3H]-thymidine incorporation. (f) Anti-IL-10, anti-TGFβ, or anti-IL-35 neutralizing antibodies were added to co-cultures to inhibit cytokine driven “conversion” into suppressed Tconv (top panel) or added in secondary proliferation assays to inhibit cytokine driven suppression of “function” (bottom panel). (g) Tconv cells alone or with C57BL/6, Ebi3−/− suppressed Tconv (as regulatory cells) were injected into Rag1−/− mice. Seven days after transfer, splenic T cell numbers were determined by flow cytometry. (h) EAE was induced by immunizing mice with MOG35–55 peptide in complete Freund’s adjuvant followed by pertussis toxin administration. 1 × 106 suppressed Tconv or natural Treg were transferred i.v. into C57BL/6 mice 12–18 hours prior to disease induction. Clinical disease was monitored daily. Clinical score was statistically significant between mice receiving saline control and Treg or suppressed Tconv at days 16–18. Counts per minute of Tconv cells activated alone were 14,000–34,000 (e, f). Data represent the mean ± SEM of 8–12 mice per group from at least 2 independent experiments [* p < 0.05, ** p < 0.005, *** p < 0.001, NS = not significant].
We next assessed whether suppressed Tconv gained the phenotypic characteristics of a regulatory population. Interestingly, suppressed Tconv were profoundly unresponsive to anti-CD3 stimulation and were potently suppressive in vitro (Fig. 5d, e). Tregs can secrete IL-10, TGFβ and IL -35 which may influence their ability to convert Tconv into suppressed Tconv. Likewise, the same cytokines could be secreted by suppressed Tconv and contribute in an autocrine fashion to their conversion and/or their suppressive activity. To address these questions we first co-cultured Tconv and Tregs that were wild type or lacked the capacity to produce IL-35 (Ebi3−/− or Il12a−/−) or IL-10 (Il10−/−), or were unable to respond to TGFβ (TGFβR.DN). While the generation of suppressed Tconv that were hyporesponsive and possessed regulatory capacity did not require TGFβ-mediated signaling, the absence of both IL-35 and IL-10 in the nTreg:Tconv co-culture blocked their development and/or function (Fig. 5d, e). Further analysis using nTreg:Tconv co-cultures in which only one population was mutant revealed that both nTreg-derived IL-10 and IL-35 was required for the generation of the regulatory suppressed Tconv population. Interestingly, suppressed Tconv-derived IL-35 was required for conversion as suggested by qPCR analysis of signature genes (see Supplementary Information and Fig. 15b).
Neutralizing antibodies were included during the conversion process or in the secondary suppression assay to further assess the role of IL-35, IL-10 and TGFβ (Fig. 5f). While anti-TGFβ had no effect at either stage, IL-10 neutralization partially blocked conversion but not the regulatory capacity of suppressed Tconv, suggesting that IL-10 is required for optimal conversion of suppressed Tconv into a regulatory population. In contrast, IL-35 neutralization prevented both the conversion and regulatory function of suppressed Tconv. Collectively, these data suggest that some or all of the suppressed Tconv are iTR35. The precise contribution of IL-10 to Treg–mediated conversion of iTR35 remains to be fully elucidated, as IL-10 alone does not induce Ebi3 and Il12a mRNA expression (Supplementary Fig. 5). However, the addition of low dose IL-10 appears to augment IL-35-mediated conversion, which may help offset the delayed production of IL-35 by nTregs (see Supplementary Information and Supplementary Fig. 16c). Taken together, these data suggest that IL-35, either from a natural source (nTregs) or supplemented exogenously, mediates iTR35 conversion.
Next, the regulatory capacity of nTregs-suppressed Tconv (nTregs-induced iTR35) was assessed in vivo. First, they were able to significantly suppress the homeostatic expansion of co-transferred naïve Tconv in Rag1−/− mice in a manner comparable to nTregs and iTR35 (Fig. 5g). However, suppressed Tconv generated from Ebi3−/− Tconv cultured with wild-type nTreg, failed to suppress the expansion of co-transferred Tconv. Second, in the EAE model, peak clinical disease scores were decreased by suppressed to a level Tconv comparable with nTregs (Fig. 5h). However, suppressed Tconv could not ameliorate EAE as effectively as iTR35 suggesting either that only a proportion of this suppressed Tconv population are iTR35 or that conversion in vitro is suboptimal due the time required for potentiation of IL-35 production by nTregs15, 30. Nevertheless, these data support the notion that iTR35 are generated from Tconv, to some degree, by nTregs during suppression. In contrast, there is no evidence for the generation of IL-10-iTR or TGFβ-iTR in this setting.
Treg-mediated induction of iTR35 in vivo
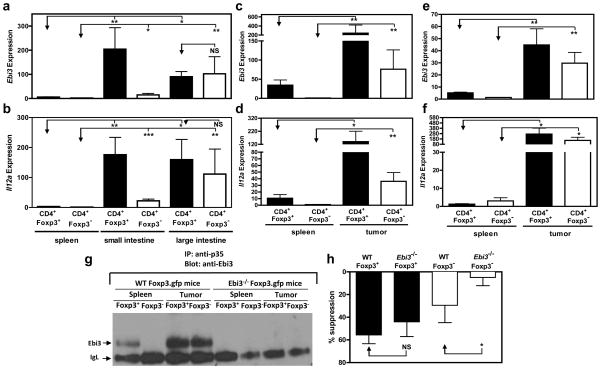
We reasoned that iTR35 generation in vivo would occur predominantly in inflammatory or disease environments where optimally stimulated nTregs might be secreting high amounts of IL-35. Infection with Trichuris muris, an intestinal nematode, is known to promote Tregs responses at the site of infection, the large intestine31. Thus, we assessed whether iTR35 could be detected following Trichuris muris infection, using the CD4+/Foxp3−/Ebi3+/Il12a+ iTR35 signature. Foxpgfp mice were infected with Trichuris muris and Foxp3+ and Foxp3− T cells were purified from spleens, small intestines and large intestines 14 days post-infection. Both Ebi3 and Il12a (p35) expression were dramatically increased in Foxp3+ Tregs, in both the small and large intestines, compared with splenic Tregs, consistent with our previous observations that nTregs increase IL-35 expression ~10-fold in the presence of Tconv cells15 (Fig. 6a,b). While essentially no Ebi3/Il12a expression was observed in splenic Foxp3−CD4+ T cells, expression was substantial in comparable isolates from the small and, especially, the large intestines (the primary site of infection). Indeed, Ebi3/Il12a expression in Foxp3+ Tregs and Foxp3−CD4+ T cells from the large intestines was statistically indistinguishable. It should be emphasized that we have never observed IL-35 expression by naïve, activated or memory CD4+ T cells15, and Foxp3−CD4+ T cells from the intestines or MLN of uninfected mice do not express Ebi3/Il12a (data not shown) raising the possibility that iTR35 are being generated by Tregs within this inflammatory microenvironment.
Figure 6. IL-35-producing Foxp3− iTR35 develop in vivo.
Foxp3gfp mice were infected with Trichuris muris. CD4+Foxp3− and CD4+Foxp3+ cells were purified from spleen, small intestine IEL and LPL, or large intestine IEL and LPL by FACS, RNA extracted and cDNA generated. Ebi3 (a) and Il12a (b) expression of the populations indicated. (c, d, g, h) Foxp3gfp mice or Ebi3−/− Foxp3gfp were injected with 120,000 B16 cells i.d. on the right flank. Tumors and spleens were excised after 15–17 days, CD4+Foxp3− and CD4+Foxp3+ cells, purified by FACS, RNA extracted and cDNA generated. Ebi3 (c) and Il12a (d) expression of the populations indicated. (e, f) Foxp3gfp mice or Ebi3−/− Foxp3gfp were injected with 2×106 MC38 cells subcutaneously on the right flank. Tumors and spleens were excised after 12 days, CD4+Foxp3− and CD4+Foxp3+ cells purified by FACS, RNA extracted and cDNA generated. Ebi3 (e) and Il12a (f) expression of the populations indicated. (g) Following B16 cell inoculation, purified T cells from the spleen or tumor were cultured for 24 h to allow secretion of IL-35. Culture supernatants from indicated cultures were immunoprecipitated with anti-p35 mAb, resolved by SDS-PAGE and probed with anti-Ebi3 mAb to identify IL-35 secretion. No IL-35 secretion was seen in either the splenic or tumor-infiltrating lymphocytes from Ebi3−/− mice (g). Purified cells were assayed for regulatory capacity by mixing populations indicated at a 4:1 ratio with fresh responder Tconv cells for 72 h. Proliferation was determined by [3H]-thymidine incorporation. Counts per minute of Tconv cells activated alone were 16,000–33,000 (h). Data represent the mean ± SEM of 8–10 mice per group from 2–3 independent experiments (B16) and 1 experiment (MC38) [* p < 0.05, ** p < 0.005, *** p < 0.001, NS = not significant].
The inflammation induced by solid tumors is known to attract Tregs32–37. Using B16 melanoma and MC38 colorectal adenocarcinoma as model systems25, 38, 39, tumor cells were inoculated into Foxpgfp mice, solid tumors resected 15–17 days (for B16) or 12 days (for MC38) post-transfer and Foxp3+ and Foxp3− T cells purified from spleens and tumors. Interestingly, tumor-infiltrating Foxp3+ Tregs had substantially increased expression of both Ebi3 and Il12a (Fig. 6c-f). Surprisingly, tumor-infiltrating Foxp3− T cells also dramatically upregulated Ebi3 and Il12a expression. It is noteworthy that comparable observations were made in two distinct tumor types. We further analyzed IL-35 secretion and its physiological relevance using the B16 melanoma system. While a moderate amount of IL-35 secretion by splenic Foxp3+ Tregs was observed ex vivo, a substantial and comparable amount of IL-35 was secreted by both Foxp3+ Tregs and Foxp3−CD4+ tumor-infiltrating T cells (Fig. 6e). Lastly, we assessed whether tumor-infiltrating CD4+/Foxp3−/Ebi3+/Il12a+ T cells were able to suppress the proliferation of fresh responder Tconv in vitro. Although their suppressive capacity was not as potent as that of tumor-infiltrating Foxp3+ T cells, our results clearly demonstrate that tumor-derived Foxp3−CD4+ T cells can mediate effective suppression in vitro in an IL-35-dependent manner (Fig. 6f).
iTR35 contribute to the regulatory milieu in vivo
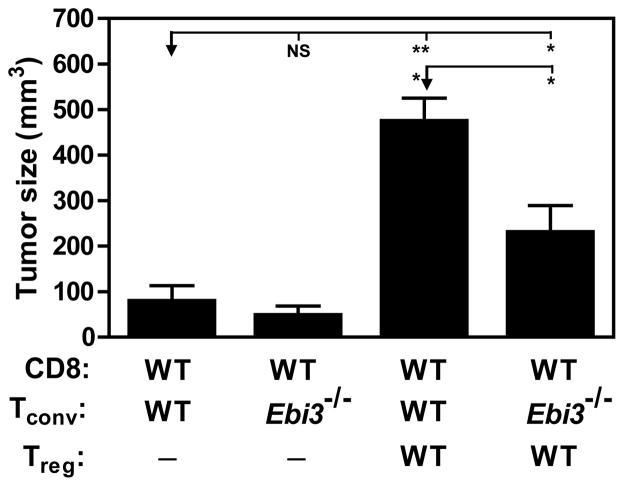
Finally, we assessed the physiological contribution of iTR35 to the Treg-induced regulatory milieu. We reasoned that if iTR35 development within the tumor played a significant role in blocking anti-tumor immunity, then mice reconstituted with Tconv cells that lacked the ability to be converted into iTR35 would develop smaller tumors. Therefore, Rag1−/− mice were reconstituted with wild type CD8 cells, with or without wild type Tregs plus either wild type or Ebi3−/− CD4+ Tconv cells. As expected, tumor size was reduced in CD4+/CD8+ T cell recipients lacking Tregs (50–90mm3) regardless of whether wild type or Ebi3−/− CD4 Tconv cells were transferred. Co-transfer of nTregs with wild type CD4+/CD8+ T cells blocked anti-tumor immunity resulting in aggressive tumor growth (470mm3) (Fig. 7). Analysis of congenically-marked tumor-infiltrating CD4+ T cells confirmed high Ebi3/Il12a expression that was comparable to the Tregs (see Supplementary Information and Supplementary Fig. 17). Thus it should be noted that in this instance both IL-35-producing nTregs and iTR35 contribute to the suppressive milieu (Fig. 6, 7 and Supplementary Fig. 17). Strikingly, co-transfer of nTregs and CD8+ T cells with Ebi3−/− CD4+ Tconv (which are unable to be converted to IL-35-producing iTR35) only partially blocked anti-tumor immunity resulting in intermediate tumor growth (220mm3). These results suggest that Treg-mediated induction of iTR35 development has a significant impact on tumor burden and is responsible for approximately half the regulatory milieu within the tumor microenvironment (as the tumor burden is reduced from 470mm3 to 220mm3). Furthermore, these data suggest that nTregs mediate their suppressive activity, in part, by inducing iTR35.
Figure 7. The suppressive T cell milieu in the tumor microenvironment is largely due to iTR35.
Rag1−/− mice were reconstituted with wild type C57BL/6 CD8+ T cells and wild type or Ebi3−/− Tconv cells with or without wild type Tregs. The following day, all mice were injected with 120,000 B16 cells i.d. on the right flank. Tumors were excised on day 15 post-inoculation to facilitate analysis of tumor infiltrating lymphocytes and tumor diameter reported as mm3. Data represent the mean ± SEM of 6–12 mice per group from at least 2 independent experiments [* p < 0.05, *** p < 0.005, NS = not significant].
DISCUSSION
iTR35 cells represent a new and unique member of the regulatory T cell family that are generated by, and mediate their suppression via, IL-35. They do not express Foxp3 and are quite distinct from the currently known induced regulatory populations, TGFβ iTR and IL-10 iTR (see Supplementary Information and Supplementary Fig. 18). The concept of infectious tolerance, whereby Treg confer a suppressive phenotype upon Tconv cells, has been previously described in both murine and human systems27, 29. It has been suggested that TGFβ may play a critical role in mediating infectious tolerance40. Since IL-35-secreting Tregs can convert Tconv cells into iTR35, a suppressed Tconv population with regulatory potential, this raises the possibility that IL-35 and iTR35 may represent additional, important mediators of infectious tolerance. Indeed, our data suggest that up to half the regulatory microenvironment within the tumor is mediated by Treg-induced iTR35. This also suggests that iTR35 may contribute to tumor progression. While iTR35 may play a significant physiological contribution to the regulatory milieu established by Treg, further studies will be required to fully elucidate their contribution in diverse disease settings.
Whether a similar system operates in humans remains to be determined, but may be controversial given recent studies suggesting that human Tregs do not make IL-3541, 42, findings that await further confirmation. Nevertheless, human iTR35 can be generated and can suppress primary human T cell proliferation in an IL-35-dependent manner. The potential therapeutic application of ex vivo generated IL-10 iTR and TGFβ iTR is complicated by complexities in their generation, their short half-life and reversal of their suppressive capacity in time or by IL-2 (see Supplementary Information)13, 43, 44. Although additional experiments are needed to fully assess the clinical potential of iTR35, our data suggest that they represent a new, stable iTR population that may have significant therapeutic utility.
METHODS
Methods and any associated references are available in the online version of the paper at http://www.nature.com/natureimmunology/.
Supplementary Material
Acknowledgments
The authors wish to thank Doug Green, Terrence Geiger and Hongbo Chi for advice and critical discussion, and Samir Burns for technical guidance, regarding EAE experiments. We also thank Terrence Geiger, Jim Ihle, Richard Blumberg, Vijay Kuchroo and Alexander Rudensky for mice, Dario Campana for the 8E permeabilization buffer, Alan Korman and Mark Selby for MC38 cells and advice, Brandon Triplett, Michelle Howard, and Melissa McKenna at St. Louis Cord Blood Bank for cord blood samples, and staff of the St. Jude Blood Donor Center for aphaeresis rings. We are also grateful to Karen Forbes, Tara Moore and Amy Krause for maintenance and breeding of mouse colonies, Andrea Szymczak-Workman for IBD histological analysis, Lindsay Jones for Ab testing for IP/WB and ELISA, Richard Cross, Greig Lennon and Stephanie Morgan for FACS, the staff of the Shared Animal Resource Center at St Jude for the animal husbandry, and the Hartwell Center for Biotechnology and Bioinformatics at St Jude for real-time PCR primer/probe synthesis and MOG synthesis and purification. This work was supported by the National Institutes of Health (R01 AI39480, to D.A.A.V.) and (R01 AI61570 / R01 AI74878, D.A.), an Individual NRSA (F32 AI072816, L.W.C.), the Australian National Health and Medical Research Council (NHMRC) Overseas Biomedical Fellowship program (P.G.), NCI Comprehensive Cancer Center Support CORE grant (CA21765, D.A.A.V.), and the American Lebanese Syrian Associated Charities (ALSAC, D.A.A.V.).
Footnotes
AUTHOR CONTRIBUTIONS
L.W.C. designed (with help from D.A.A.V) and executed all mouse experiments, analyzed data and wrote the manuscript; V.C. performed human experiments; A.L.H. (with L.W.C) performed B16 tumor experiments; J.B. performed MC38 tumor experiments; P.R.G. carried out Trichuris muris infections; C.G. did confocal microscopy analyses; D.F. performed Affymetrix analyses; K.F. and S.A.B (with C.J.W) generated and screened anti-IL35 mAbs; C.J.W. coordinated anti-IL35 mAb development, testing and purification, and aided in figure preparation; M.L.J. generated and purified mEbi3 protein for immunizations and mAb development; J.N. provided key reagents and information; J.E.R. created and performed histological analyses of Foxp3−/− mice; D.A. designed Trichuris muris experiments and provided input on interpretation; M.J.T provided training in the B16 tumor model, and provided input to the research design and interpretation; and D.A.A.V. conceptualized the research, directed the study, and edited the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests.
References
- 1.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Gavin MA, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM, et al. The lifestyle of naturally occurring CD4+ CD25+ Foxp3+ regulatory T cells. Immunol Rev. 2006;212:60–73. doi: 10.1111/j.0105-2896.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- 5.Waldmann H, Adams E, Fairchild P, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev. 2006;212:301–313. doi: 10.1111/j.0105-2896.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 6.Selvaraj RK, Geiger TL. Mitigation of experimental allergic encephalomyelitis by TGF-beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J Immunol. 2008;180:2830–2838. doi: 10.4049/jimmunol.180.5.2830. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen KD, Vanichsarn C, Nadeau KC. Impaired IL-10-dependent induction of tolerogenic dendritic cells by CD4+CD25hiCD127lo/- natural regulatory T cells in human allergic asthma. Am J Respir Crit Care Med. 2009;180:823–833. doi: 10.1164/rccm.200905-0761OC. [DOI] [PubMed] [Google Scholar]
- 8.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 9.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;25:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Workman CJ, Szymczak-Workman AL, Collison LW, Pillai MR, Vignali DA. The development and function of regulatory T cells. Cell Mol Life Sci. 2009 doi: 10.1007/s00018-009-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roncarolo MG, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 12.Verbsky JW. Therapeutic use of T regulatory cells. Curr Opin Rheumatol. 2007;19:252–258. doi: 10.1097/BOR.0b013e3280ad46bb. [DOI] [PubMed] [Google Scholar]
- 13.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groux H, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 15.Collison LW, et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566–569. doi: 10.1038/nature06306. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 17.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 18.Huter EN, et al. TGF-beta-induced Foxp3+ regulatory T cells rescue scurfy mice. Eur J Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Workman CJ, et al. In vivo Treg Suppression Assays. Regulatory T cells: Methods and Protocols. 2010 In Press. [Google Scholar]
- 20.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Bandeira A. On the ontogeny and physiology of regulatory T cells. Immunol Rev. 2001;182:5–17. doi: 10.1034/j.1600-065x.2001.1820101.x. [DOI] [PubMed] [Google Scholar]
- 21.Workman CJ, et al. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 22.Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256–271. doi: 10.1111/j.0105-2896.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 23.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 24.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 25.Turk MJ, et al. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang P, Cote AL, de Vries VC, Usherwood EJ, Turk MJ. Induction of postsurgical tumor immunity and T-cell memory by a poorly immunogenic tumor. Cancer Res. 2007;67:6468–6476. doi: 10.1158/0008-5472.CAN-07-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jonuleit H, et al. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milojevic D, Nguyen KD, Wara D, Mellins ED. Regulatory T cells and their role in rheumatic diseases: a potential target for novel therapeutic development. Pediatr Rheumatol Online J. 2008;6:20. doi: 10.1186/1546-0096-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waldmann H. Tolerance can be infectious. Nat Immunol. 2008;9:1001–1003. doi: 10.1038/ni0908-1001. [DOI] [PubMed] [Google Scholar]
- 30.Collison LW, Pillai MR, Chaturvedi V, Vignali DA. Regulatory T cell suppression is potentiated by target T cells in a cell contact, IL-35- and IL-10-dependent manner. J Immunol. 2009;182:6121–6128. doi: 10.4049/jimmunol.0803646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Elia R, Behnke JM, Bradley JE, Else KJ. Regulatory T cells: a role in the control of helminth-driven intestinal pathology and worm survival. J Immunol. 2009;182:2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Stasi A, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113:6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liyanage UK, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 34.North RJ, Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J Exp Med. 1984;159:1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olkhanud PB, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 37.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 38.Kocak E, et al. Combination therapy with anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. Cancer Res. 2006;66:7276–7284. doi: 10.1158/0008-5472.CAN-05-2128. [DOI] [PubMed] [Google Scholar]
- 39.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersson J, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–1981. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allan SE, Song-Zhao GX, Abraham T, McMurchy AN, Levings MK. Inducible reprogramming of human T cells into Treg cells by a conditionally active form of FOXP3. Eur J Immunol. 2008;38:3282–3289. doi: 10.1002/eji.200838373. [DOI] [PubMed] [Google Scholar]
- 42.Bardel E, Larousserie F, Charlot-Rabiega P, Coulomb-L’Hermine A, Devergne O. Human CD4+ CD25+ Foxp3+ regulatory T cells do not constitutively express IL-35. J Immunol. 2008;181:6898–6905. doi: 10.4049/jimmunol.181.10.6898. [DOI] [PubMed] [Google Scholar]
- 43.Horwitz DA, et al. Regulatory T cells generated ex vivo as an approach for the therapy of autoimmune disease. Semin Immunol. 2004;16:135–143. doi: 10.1016/j.smim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.