Synopsis
Infant feeding policies for HIV-infected women in developing countries differ from policies in developed countries. Here we summarize the epidemiologic data on the risks and benefits of various infant feeding practices for HIV-infected women living in different contexts. Artificial feeding can prevent a large proportion of mother-to-child HIV transmission but also is associated with increases in morbidity and mortality among both exposed-uninfected and HIV-infected children. Antiretroviral drugs can be used during lactation and reduce risks of transmission. For most of the developing world, the health and survival benefits of breastfeeding exceed the risks of HIV transmission, especially when antiretroviral interventions are provided.
Introduction
Artificial feeding has been recommended for HIV-infected women in the developed world since 1985 after an occurrence of HIV transmission through breastfeeding was first described.1 When the World Health Organization (WHO) initially recommended that HIV-infected women in the developing world continue to breastfeed (1992)2 the guidance was criticized by some as upholding a “double standard.” Twenty-five years later, with an HIV epidemic that has established itself with a vengeance in some of the poorest and most vulnerable communities of the developing world, the international community still grapples with this complex issue. However, there are now considerably more empirical data to inform this dilemma, as well as the possibility of antiretroviral and behavioral interventions that change the terms of this debate. In this review, we summarize the data describing the survival and health benefits of breastfeeding versus artificial feeding for infants and young children born to HIV-infected women. We conclude that context matters. For most of the developing world, the health and survival benefits of breastfeeding exceed the risks of HIV transmission, especially when antiretroviral interventions are provided.
HIV transmission through breastfeeding
It is now well established that HIV is transmitted throughout the duration of breastfeeding.3–5 Thus the major health benefit of artificial feeding, in both developed and developing countries, is that postnatal HIV transmission is avoided. Pregnancy and delivery transmission cannot be so easily avoided and in the absence of antiretroviral drugs approximately 20% of HIV-infected women transmit the virus via these two routes.6 Breastfeeding adds further infections with the cumulative rate of breastfeeding-associated infection determined by the nature of breastfeeding practices and the duration of all breast milk exposure. Unqualified statements that breastfeeding adds an additional transmission rate of 14%3,7 neglect the variability of normative infant feeding practices across communities and across women within communities. It is logical that the post-natal transmission rate increases with breastfeeding duration, as infections accumulate with each month of additional exposure.8 It is more difficult, however, to quantify the instantaneous hazard or force of infection during early or later periods of breastfeeding. A combined analysis of selected studies concluded that hazards were constant over time.8 But several cohort studies with tighter intervals for determining the timing of transmission have reported declining hazards as the child becomes older.4,5,9 Estimates of whether most transmission occurs early or late depend on the instantaneous hazards and the duration of breastfeeding.
A further complexity in quantifying the magnitude of postnatal HIV transmission is that, although breastfeeding is a biological process, it is also a cultural practice.10 What is healthiest and what is normative do not necessarily coincide. For example, colostrum is a fluid rich in immunologically-active components capable of protecting the newborn over the most vulnerable period immediately after delivery. Yet in some societies, colostrum is considered “dirty” and is discarded.11 Cultural practices that displace breastfeeding are detrimental to both mother and infant. Non-nutritive herbal supplements deprive the infant of essential nutrition as well as the immunologic protection afforded by milk. Inconsistent breastfeeding predisposes women to mastitis and hastens the return to menses, increasing the risk of post-partum anemia as well as pregnancy.12–14 Yet in some societies, introduction of non-nutritive herbal supplements that displace breast milk is considered essential to infant health.15
Quantifying rates of postnatal transmission have to take these cultural variations into account. One parameter that has emerged as a strong influence on the extent of postnatal transmission during the first few months of life is the quality of breastfeeding ascertained by the extent of exclusive breastfeeding.9,16–18 When breastfeeding occurs without the addition of formula, other non-human milks, non-nutritive liquids, and solids and semi-solid foods, transmission is lower 5 than when breastfeeding is inclusive of these unnecessary supplements.9,16–18 Estimates of postnatal transmission gathered from settings where support of exclusive breastfeeding is lacking or in communities with poor uptake of recommendations to breastfeed exclusively are likely to differ from settings more favorable to exclusive breastfeeding. Almost all of these data carefully clarifying risks of transmission under these different circumstances come from studies conducted in developing countries.
Survival and health benefits of breastfeeding
The harms of artificial feeding were brought to the attention of the international community most strongly following the deaths that resulted when Nestle and other formula companies began marketing their products in developing countries in the 1970s.19 Thereafter, strict controls on marketing of formula in developing countries and public health programs supporting breastfeeding were largely successful in re-establishing breastfeeding as the almost universal mode of infant feeding in most developing countries in the pre-HIV era. For example in the1980s, uptake of breastfeeding in Zambia was close to 100% with a median duration of 24 months.20 Meantime in developed countries, as infant mortality rates continued to decline through health service interventions and rising standards of living, breastfeeding practices deteriorated. In 1990 in the United States (U.S.) uptake of breastfeeding was a mere 51%.21 Moreover among the poorest sectors of wealthier countries, breastfeeding uptake was even worse.21 As the HIV epidemic among women in the U.S. has differentially affected impoverished minority communities, artificial feeding was already the norm among many of the communities most affected by HIV, independent of any recommendations. In the U.S. where racial disparities in infant health are of grave concern, lower uptake and duration of breastfeeding among socioeconomically-disadvantaged populations is one of the factors which account for poorer perinatal health outcomes among African-American women.22–24
As the defining characteristic of mammalian reproduction, it is challenging to approach study of the benefits of breastfeeding with evidence-based medicine’s reliance on the randomized clinical trial. There are very few circumstances in which randomization of an intimate personal behavior, widely considered to be healthiest for mothers and infants, can be considered ethical. There are also practical constraints. Even the most persuasive investigator faces limitations in enticing mothers and infants to obey their assigned practice. So for obvious reasons, data demonstrating survival and health advantages of breastfeeding largely come from epidemiological studies.
The results of epidemiological studies are remarkably consistent. Breastfeeding is a significant protector against diarrheal disease, respiratory disease and other infections.25–28 Breastfeeding tends to result in better nutritional outcomes, including protecting against obesity in over-fed and against wasting in under-fed populations.29–32 It has beneficial effects on cognitive functioning and psychosocial development.33–35 This is a large body of literature. There are reviews,25,26,36–38 and reviews of reviews,39,40 and even a rather long report from the Agency for Healthcare Research and Quality of studies only in developing countries.41 What is particularly striking is that the conclusions are consistent regardless of whether the studies are from the developing or the developed world. Breastfeeding protects infants not just in Bangladesh42 but also in Boston,43 not only in historical times44 but also in the new millennium.45
In order to understand why artificial feeding can be recommended for HIV-infected women in developed countries despite the known risks associated with this practice, it is important to make the distinction between an absolute risk and a relative risk. An absolute risk is the frequency with which an event occurs in the population e.g. an infant mortality rate might be 10 deaths per 1000 live-births. A relative risk requires a comparison. For example, we might say an infant mortality rate is 10/1000 live-births if women breastfeed, but 20/1000 live-births if women avoid all breastfeeding i.e. a 2-fold increased risk. The ratio of rates in the two groups is referred to as the relative risk. Studies show that the relative risk associated with artificial feeding is elevated in all populations, but what makes developed countries different is that the absolute rates of morbidity and mortality are generally low. Moreover, breastfeeding may protect against morbidity, but since most morbidity in these settings is not fatal, arguably the benefits can be ignored.
Strengths and weaknesses of using HIV-free survival as an outcome
In the spectrum of possible benefits of breastfeeding that could be weighed against the risks of HIV transmission, the field has tended to focus only on one, viz. the benefits of breastfeeding for infant survival. The concept of HIV-free survival, which refers to the absence of a combined outcome of either (1) HIV infection or (2) death prior to HIV infection, has emerged as a consensus outcome to evaluate strategies. The advantage of this approach is that it reminds us that some of the strategies we might propose to prevent HIV transmission, such as abstinence from breastfeeding or early weaning, also carry a cost in terms of lives of uninfected infants. The disadvantage of the approach is that it counts an HIV infection as equivalent to a death, a pessimistic approach and one which is out of date now that pediatric HIV infection can be successfully treated. It also stacks the deck in favor of interventions that prevent HIV transmission and neglects the range of other non-fatal, but potentially serious, adverse outcomes associated with limiting breastfeeding.
Mechanisms by which breastfeeding protects
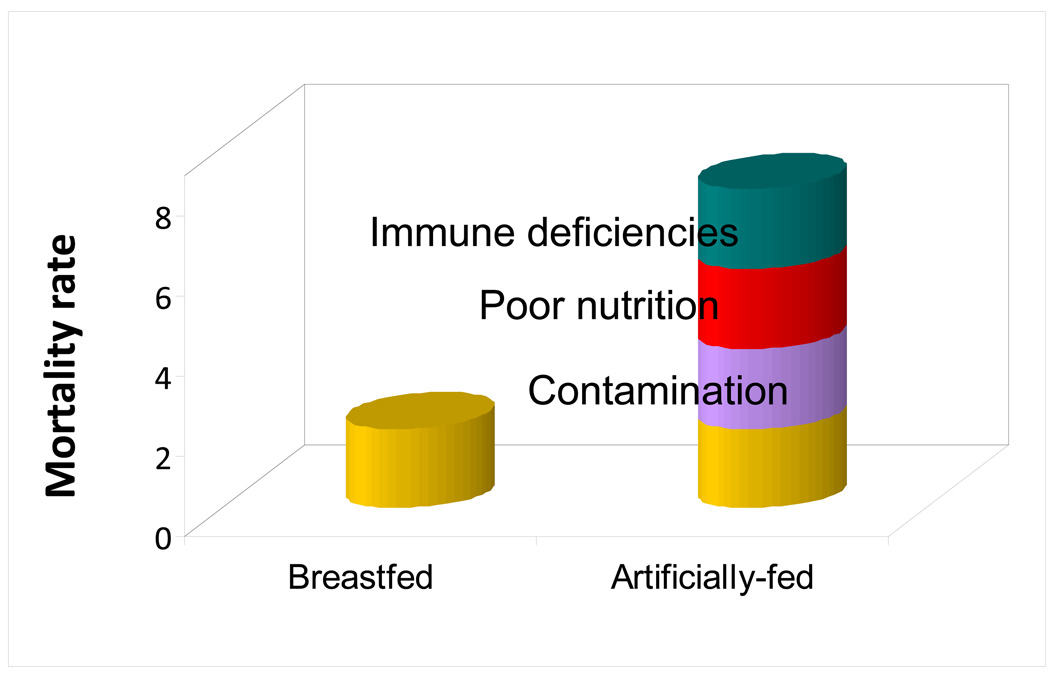
To appreciate the reasons for differential infant feeding recommendations in different circumstances, it is helpful to consider the potential mechanisms whereby breastfeeding protects infants’ health. For heuristic purposes, we separate the biological basis for the benefits of breastfeeding into three overarching mechanisms (Figure 1). (1) Contamination i.e. artificial feeding places the infant at risk through introducing environmental contaminants and creating a less hygienic feeding method. (2) Poor nutrition i.e. abstinence from breastfeeding could compromise an infant’s nutritional status if formula is not mixed correctly or not given in appropriate quantities. (3) Absence of immune protection.
Figure 1.
Theoretical mechanisms by which artificial feeding increases the risk of infant and young child death.
Contamination
It is perhaps no surprise that studies have shown that it is extremely difficult for women to prepare formula hygienically in resource-poor settings.46,47 In a study conducted in KwaZulu-Natal, South Africa, about 80% of formula mothers prepared at home after instructions from the counselors were contaminated with fecal bacteria.48 It is interesting that about 20% of the samples that the counselors prepared at the clinic while showing the mothers how to do everything correctly were also contaminated.48 The dramatic epidemic of diarrhea-related deaths that occurred in Botswana among formula-fed infants after severe flooding affected urban areas is another clear example of the dangers of contaminated water.49–51 Contaminated water is clearly a major threat to child health and the provision of a sustained supply of adequate clean water at the point of use in the household is clearly a major priority for public health.52 But is it enough?
Infant formula is not the only source of exposure to pathogens, especially in contaminated environments. Despite their parent’s best efforts, children do not live in aseptic environments. They explore their world with their hands and mouths. Breast milk has evolved to protect children from these pathogens. Diarrhea morbidity and mortality are significantly reduced even when breastfeeding is not exclusive53 and even in young children when consuming relatively small quantity of breast milk.54 It is also interesting that breastfeeding reduces the risk of respiratory illness, an outcome where contaminated water plays little to no role.42,43,55,56 Breastfeeding also protects against severe infectious disease in settings with a predominantly safe water supply.28,43,45
An exemplary demonstration of the multi-factorial source of breastfeeding’s benefits came from an interesting confluence of circumstances in a clinical study in rural Kenya. As part of an evaluation of the effects of extending antiretroviral therapy during lactation on reduction in postnatal HIV transmission, HIV-infected women were encouraged to stop all breastfeeding by 6 months (the duration of the antiretroviral therapy). Elevated diarrhea morbidity was noted coincident with weaning and the study was temporarily suspended while the investigators considered what to do.57 The investigators elected to introduce a state-of-the-art home water quality improvement program that had been found to be effective in other settings. Interestingly, when they introduced this intervention, it reduced diarrhea among breastfed infants but had no effect on weaning-related diarrhea.58 Contamination plays a role in exaggerating the risks of artificial feeding59 but clean water is insufficient to mitigate artificial feeding’s risks.
Poor nutrition
Infant formula is specifically developed to mirror the nutritional composition of breast milk as closely as possible but falls short in several respects. Breast milk is exquisitely regulated such that the content varies from the beginning to the end of the feed so that a child can be most quickly satiated even with a short feed but can continue to feed for comfort and not become overfed on longer feeds.60 The composition of human milk also varies based on the amount the child consumes and over time being regulated to adapt to the unique needs of a specific child.61 This individualization cannot be achieved with formula but if given in correct volumes and frequencies should be nutritionally adequate. The primary concern related to poor nutrition of formula-fed infants is incorrect mixing and over-dilution. A related, health systems concern is sustained supply and the problem of stock-outs.
In situations of scarcity, infant formula is usually perceived as “valuable” or “precious” by members of the community. In most developing countries, the costs of formula make it prohibitive for all but a small minority unless it subsidized or provided free by the health service. Provision of free or subsidized formula poses complex ethical challenges.62,63 Qualitative research has highlighted the coercive dynamics of free formula and there are several examples of confusion and misinformation that may result in sub-optimal practices.47,64–67 For example, in a study of three sites in South Africa, a surprising pattern was noted of inadequate use of the formula that was being provided by the health service. Mothers were not giving their infants formula in sufficient quantities. Rather they were avoiding breastfeeding, were giving some formula and were also providing a substantial proportion of the infants’ diet with nutritionally inadequate foods and liquids.68 Audits of the South African national formula program have noted serious gaps in supply to both urban and rural clinics.69 Population mobility introduces further complications for sustained access.
Absence of immune protection
During pregnancy, maternal antibodies are transported across the placenta to protect the infant whose immune system is not fully mature at the time of birth. We refer to this process as “passive immunity” as the child is reliant on the mothers’ antibodies. This process continues during breastfeeding.70,71 Other than antibodies, breast milk contains many immunomodulatory components that bolster the child’s immune system and protect against disease.38,70,71 The recent introduction of long chain fatty acids and probiotics into infant formula demonstrates the awareness that formula companies have of the deficiencies of their product.72–74 We might have expected because HIV has so many immunologic effects that the quality of breast milk might be compromised. However, a study from Botswana showed that HIV-infected and uninfected women had similar quantities of the immunologic components that they measured.75
Dangers of extrapolating from clinical studies
Several factors combine to create the beneficial effects of breastfeeding. Theoretically, a strong public health program may be able to minimize risks of environmental contamination and poor nutrition associated with artificial feeding but can do nothing to mitigate the risks conferred by the absence of the immunologically-active components of breast milk. The fact that breastfeeding continues to confer benefits to infant health even in developed countries like U.S. and the United Kingdom43,45 suggests that there is a biological threshold below which it is not possible to go even with the strongest programs.
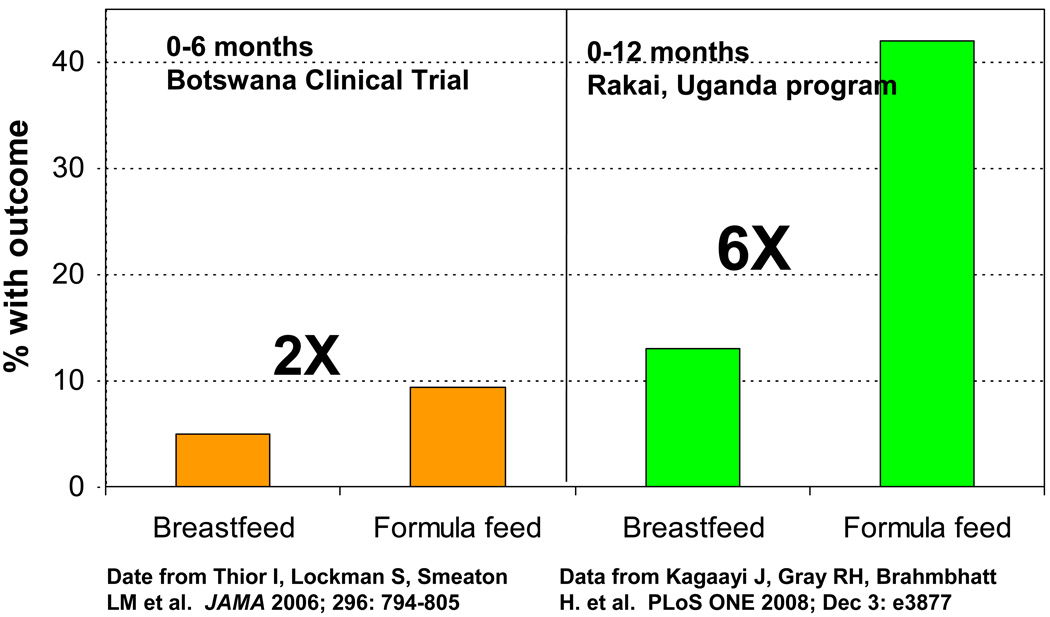
Caution is required in extrapolating results on the risks of artificial feeding from clinical studies (Figure 2). In most clinical studies, participants are highly motivated, receive the best possible educational interventions and are provided with close monitoring and a health service safety set. Yet even in this protected environment, only one study in Nairobi, Kenya has been able to demonstrate a net benefit for HIV-free survival of artificial feeding.3 The study’s strict inclusion criteria limit its relevance to the majority of HIV-infected. In a somewhat more generalizable, but otherwise, as-thoroughly supervised and monitored population in Botswana, HIV-exposed, uninfected infants randomized to infant formula from birth had a 2-fold increase in mortality compared to those randomized to breastfeeding.76 These results are to be contrasted with reports from programs in Uganda where infant mortality was increased more than 6-fold among women who chose formula feeding.77,78 Thus, under the best-case scenario, when infant formula is provided under carefully-monitored conditions, with adequate access to medical care and sufficient education and support and with optimal selection of women considered to have adequate personal resources to safely formula feed, the mortality risks of artificial feeding are about 2-fold. In programmatic settings, the risks of death are much greater.
Figure 2.
The magnitude of adverse effects of artificial feeding differs across settings. In this example, effects were stronger in a program in rural Uganda than in a clinical trial in urban Botswana.
Learning from history
Current international guidelines for the general population recommend that breast milk alone be given for the first 6 months of the child’s life (exclusive breastfeeding) and that complementary foods be introduced around 6 months of age with continued breastfeeding to 24 months or longer.79 The word “complementary” is preferred since it refers to foods given to complement the nutrients in and immunologic and other components of breast milk. The word “replacement” food is usually used to refer to foods given to replace breast milk in a non-breastfed child.80 Between 6 and 24 months, the proportion of nutrients a young child receives from breast milk gradually declines, while the proportion the child receives from breast milk substitutes and complementary foods gradually increases. Thus “weaning” extends over a period of a year or more. We refer to a child as being fully weaned once no breast milk is given at all and the child is fully supported on non-breast milk foods and liquids. This pattern of breastfeeding extending into the second year of life is well-established to be the healthiest for infants in low resource settings79 and is recommended for the general population in developed countries as well.
Over the past 10 years, complex advice given to HIV-infected women in developing countries has led to shifts away from breastfeeding and to generally shorter durations of breastfeeding than usual in these communities. A dubious positive upshot of these changes is that they introduce greater heterogeneity into infant feeding practices than usually observed allowing for epidemiologic analysis of the effects of these behavioral shifts.81 One group who initially theorized that shifts away from breastfeeding simply to avoid HIV would not result in adverse health outcomes,82 observed in their own program, substantial elevations in mortality among women who elected not to breastfeed.77 This is consistent with what has been observed in other programs which even after the benefits of HIV prevention are taken into account observed either worse or, at best, no benefit of artificial feeding.77,78,83–85
Four separate cohorts (two in Malawi, one in Kenya and one in Uganda), all recommending to women enrolled in their trials to wean early, reported elevated morbidity and mortality associated with diarrheal disease around the time of weaning (Table 1).57,86–88 All of these studies included close monitoring and follow-up, and education/counseling which was expected by the investigators to make early weaning safe. Two of the studies were interrupted by their Data Safety and Monitoring Boards who noticed the elevations in morbidity after weaning. Subsequent comparisons with historical cohorts at the same sites revealed worse outcomes in the more recent eras89,90 which is surprising as access to antiretroviral therapy and prophylaxis as well as other child-related services had mostly improved over time. Epidemiologic analyses of mortality among breastfed and non-breastfed infants and young children between birth and 24 months in two trials in Malawi revealed that breastfeeding was associated with a 2.9-fold lower risk of mortality among exposed-uninfected infants after adjustment for confounders.91
Table 1.
Studies on the effects of artificial feeding on HIV transmission and mortality among infants born to HIV-infected mothers in developing countries.
| Study design | Comparisons | Uninfected or all cause child mortality/morbidity | HIV-free survival | |
|---|---|---|---|---|
| Randomized Trials | ||||
| Nairobi, Kenya3 | Randomized trial (n=401) | BF vs. FF from birth | Trend towards higher 2-year mortality 24%) in FF than in BF (20%) group | Net benefit in FF group |
| Botswana (MASHI)76 | Randomized trial (n=1200) | BF for 6 months vs. FF | Significantly higher mortality at 7 months in FF (9.3%) vs. BF (4.9%) | No net benefit of FF |
| Lusaka, Zambia (ZEBS)92–94 | Randomized trial (n=958) | 16 months of BF vs. early weaning at 4 months | 2 to 4-fold increase in uninfected child mortality due to weaning | No net benefit of early weaning |
| Historical controls | ||||
| Kampala, Uganda88,90 | Observations during a trial vs. previous study (n=1307) | BF then weaning at median 4 months vs. median 9 months | Peak of diarrhea post-weaning. Trend towards higher diarrhea-related and all cause mortality in cohort encouraged to wean earlier | Not reported |
| Malawi86,89 | Comparison to prior trial with longer BF (n=3845) | BF > 24 months vs. weaning ~6 months | Significantly higher rate of diarrhea-related morbidity and mortality and all cause mortality in cohort encouraged to wean at 6 months | Not reported |
| Kisumu, Kenya57,58 | Comparison to prior study with longer BF (n=491) | Wean at 6 months vs. BF ad lib | Significantly higher rate of diarrhea hospitalizations post-weaning Water safety intervention ineffective | Not reported |
| Epidemiologic studies | ||||
| South Africa99 | Program evaluation | BF vs. FF adjusted for socioeconomic factors | Both BF and FF had higher rates of adverse outcomes if poor socioeconomic status relative to FF and good socioeconomic status | No net benefit of FF if poor socioeconomic status |
| Cote D’Ivoire98 | Self-selected feeding choice (n=557) | Exclusive BF plus early weaning at 4 months vs. FF | No increase in mortality or morbidity in either group | No net benefit of FF |
| Malawi91 | Combined studies (n=2000) | Multivariate analysis of actual feeding practices | Significant reduction (hazard ratio=0.44) in mortality if breastfed both infected & uninfected children) | Not reported |
| Rakai, Uganda77 | Program evaluation (n=182) | BF vs. FF | 6-fold increase in mortality if FF | Non-significant trend to worse outcomes if FF |
| Rwanda83 | Self-selected feeding choice (n=532) | BF then early weaning at 6 months or FF | Non-significant trend towards higher mortality in FF (5.6%) than BF (3.3%) | No net benefit of FF |
| Pune, India85 | Program evaluation (n=148) | BF vs. FF | Significantly elevated risks of hospitalization if FF | Not reported. |
| Rural Uganda78 | Self-selected feeding practices (n=109) | Wean before 6 months vs. wean after 6 months | 6-fold increase in death if wean before 6 months | Not reported |
| Western Kenya84 | Self-selected feeding practices (n=2477 but high drop-out) | BF with weaning at 3–4 months vs. FF | Not reported | No net benefit of FF |
| Botswana49,51 | Public Health outbreak investigation in emergency rooms after severe floods | BF vs. not BF | 25-fold increase in diarrhea deaths if not breastfed | Not reported |
Any duration breastfeeding (BF), formula feeding from birth (FF)
The observational data are consistent with the findings from our trial in Zambia in which women were randomized to either stop breastfeeding at 4 months or to continue breastfeeding for their own preferred duration. Women in the intervention group were provided with infant formula and a specially-developed, fortified weaning cereal for their infants. Since the cereal required cooking, contamination of the water source would, theoretically, be less of a concern. Infants from both groups were weighed regularly and were provided with food supplements if there was any evidence of failure to thrive. Children in both groups also received cotrimoxazole as well as routine childhood interventions (vaccines, vitamin A etc.). Counseling and education, including about safe water and hygiene, was intensive and monitoring and follow-up was close.92 Since early weaning was not well accepted by the study population, we have analyzed the effects of non-compliance. Infants born to women who adhered to their assignment to the early weaning group and weaned early as instructed, had worse outcomes than those whose mothers ignored their random assignment and continued breastfeeding; as did infants born to women who refused to adhere to their assignment to the control group and weaned early.93 Benefits of breastfeeding on infant and young child survival persisted into the second year of life to around 18 months.94 Benefits of continued breastfeeding were also observed for child growth95 and for diarrheal morbidity and mortality.96
Special needs of HIV-infected children
HIV-infected children who are formula-fed or who are weaned off breast milk early are at high risk of dying prematurely.76,91,92 The decision to formula feed is usually made during pregnancy or soon after delivery. At that time the infant’s HIV status, even in the most well-organized programs, is unknown and remains unknown for weeks. HIV testing is rarely done at birth and, when done at 6 weeks, results are usually not available for 2 weeks. Early infant testing programs have been difficult to establish for multiple reasons and even when the laboratory capacity is in place, tend to identify only a small proportion of HIV-infected children. Once identified as HIV-infected, there are also major logistic challenges and delays in entering pediatric HIV care and treatment programs. Rapid progression of HIV infection in infants means that delays in identifying children and delays in starting therapy can lead to death.97 Thus any means of slowing the progression of HIV infection is particularly important for HIV-infected infants if they are to benefit from antiretroviral therapy.
Can the risks of artificial feeding be justified?
The increased risks of mortality among HIV-exposed uninfected infants due to artificial feeding might be justifiable if a net benefit in terms of HIV-free survival could be accomplished. However, other than in the original study in Nairobi, Kenya,3 in which no antiretroviral prophylaxis or treatment was available, this is not what has been found (Table 1). In the clinical trial in Botswana, mentioned above, there was no net benefit of artificial feeding on HIV-free survival. The reduced risk of HIV transmission as a result of formula feeding was outweighed by the increased risk of mortality among uninfected children.76 Nor was there a net benefit for HIV-free survival of artificial feeding from birth vs. short breastfeeding in a study in Cote d’Ivoire.98 At best, artificial feeding results in no improvements in health status. When implemented under real-world conditions, HIV-free survival has generally been worse, as shown in programs from South Africa and Uganda.77,99 For example, in an evaluation of the South African national program, women’s infant feeding choices bore little relation to their living circumstances. For women who lived in two of the poorer urban and rural sites, both HIV infection and death was increased among women who opted for formula feeding.99
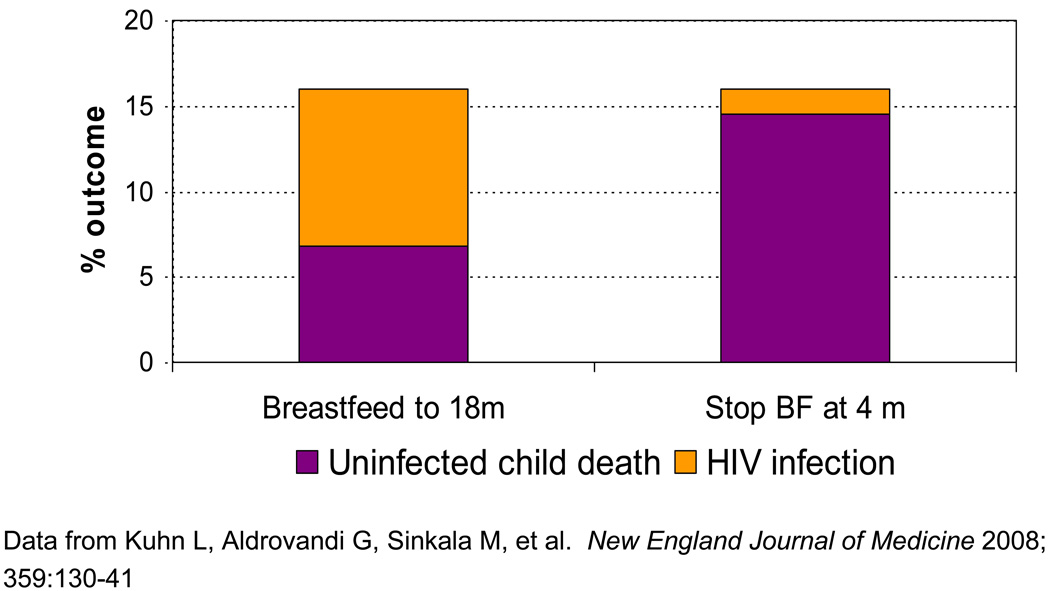
For early weaning, the net benefit for HIV prevention is less. The older the child when all breastfeeding ends, the less there is to gain. In essence, the horse is already out of the barn. Because there is less to gain by early weaning, the risks take on greater weight. Even a small increase in mortality can offset a small benefit of HIV prevented. In the primary intent-to-treat analysis of our trial in Lusaka, Zambia, we reported no benefit of cessation of breastfeeding at 4 months for the combined outcome of HIV infection or death (HIV-free survival) compared to standard practice of breastfeeding ad lib.92 Further analysis of the actual practices of the study population revealed that the magnitude of benefit (i.e. the amount of HIV prevented) was almost the same as the magnitude of the harm (i.e. the numbers of deaths caused in the population overall).93 Overall in the study population, a woman who stopped breastfeeding by 5 months added a HIV transmission rate of 1.1% after 4 months and a mortality rate among the uninfected children of 17.4%. A woman who continued breastfeeding for 18 months added a transmission rate of 11.2% of late postnatal transmission and a mortality rate of 9.7%93 (Figure 3).
Figure 3.
Benefits of early weaning for HIV prevention are counter-balanced by risks of uninfected mortality in resource-poor countries. Hence there is no benefit for HIV-free survival of early weaning in such settings [93]
These data are in the absence of either maternal antiretroviral treatment or extended antiretroviral regimens that continue during breastfeeding. As these interventions reduce postnatal transmission considerably, we can extrapolate from these results that the magnitude of mortality caused by artificial feeding will be larger than the magnitude of HIV transmission prevented. In our trial, among women who were not yet at an advanced enough disease stage to require antiretroviral therapy for their own health, stopping breastfeeding at 4 months led to a 3-fold increase in the combined outcome of HIV infection or death occurring between 4–24 months.93
Better ways to prevent HIV transmission
There have been important scientific breakthroughs in recent years informing us how best to use antiretroviral drugs during the breastfeeding period.100 It is helpful to make the distinction between using antiretroviral drugs primarily to treat maternal HIV infection and improve the health of the mother (therapeutic regimens), with prevention of transmission as a beneficial side effect vs. using antiretroviral drugs primarily for preventing transmission (prophylaxis regimens to either mother or child). The new WHO guidelines that expand treatment criteria to include all pregnant women with CD4 counts <350 cells/mm3 101 go a long way to also reduce postnatal transmission. Programs that have proactively initiated treatment among pregnant women with low CD4 counts have also consistently reported low rates of postnatal transmission as well.102 This is even in the absence of providing additional interventions for women and/or infants born to women with higher CD4 counts. This is because, like morbidity in the mother, transmission is strongly concentrated in women with low CD4 counts.103 Studies in Mozambique, Tanzania, Kenya, Botswana and Cote D’Ivoire have all observed low rates (<5%) of HIV transmission (via all routes combined i.e. intrauterine, intrapartum and postpartum) among breastfeeding women receiving therapeutic regimens initiated during pregnancy and then continued thereafter.83,102,104–109 For women who meet clinical criteria, treatment has to continue indefinitely and thus can protect the infant throughout the course of breastfeeding.
For women who do not require therapy for their own health, some of the short-course regimens currently recommended for prophylaxis, including single-dose nevirapine and nevirapine combined with short-course zidovudine, appear to reduce the risk of early postnatal transmission during the first weeks of life.110 In addition, three clinical trials, one multi-country study in Ethiopia, Uganda and India111, and two in Malawi112,113 have demonstrated that extended infant prophylaxis with nevirapine can significantly reduce postnatal HIV transmission. Lamivudine given to the infant during breastfeeding also appears to reduce transmission114 but zidovudine does not.76 In the first nevirapine study, prophylaxis was continued for only 6 weeks, in the second for only 14 weeks, and in the third for 24 weeks. Benefits were observed while prophylaxis was given and stopped once the drug was withdrawn.115 With the wisdom of hindsight we can see that the decisions to evaluate only short periods of extended prophylaxis rather than periods extending over a normal duration of breastfeeding were unfortunate. It has thus been necessary to extrapolate the results of these clinical trials to longer durations of use given the now well-appreciated dangers of early weaning. Fortunately, toxicity does not appear to be cumulative but the adherence and programmatic challenges of long term prophylaxis will need to be investigated.
Individualizing community risks
For the time being, advice given to HIV-infected women in developing countries regarding infant feeding differs from that given to HIV-infected women in developed countries. The prime reason for this difference is to protect the health of exposed-uninfected infants in developing countries who are at high risk of morbidity and mortality if not breastfed. Less attention has been given to discussion of the infant feeding policies for HIV-infected women in developed countries, in part, because background rates of mortality are low and in part because the numbers of women and infants affected is so much lower. Nevertheless, it is interesting, that debates around this issue in the developing world may be beginning to spillover to developed countries. With growing appreciation of the effectiveness of antiretrovirals, some have argued for less zealous promotion of artificial feeding for HIV-infected women in developed countries.116 A more complex issue is the heterogeneity of socioeconomic status between the many countries which fall within the general category of “developing” and the heterogeneity within developing countries. Previous WHO guidelines attempted to individualize choice, introducing the concept that artificial feeding should be recommended when women met certain personal criteria making artificial feeding affordable, feasible, acceptable, sustainable and safe (AFASS).117,118 These attempts have largely failed in practice66,67,69 and programs continue to struggle with how to support individual freedoms without compromising the health of infants. New WHO guidelines attempt to bring greater attention to community-level parameters, including background rates of infectious diseases and the adequacy of child health services in making recommendations for infant feeding practices.119
Conclusions
The gross economic inequalities between the developed and the developing world create global inequities in health status that are ethically unacceptable and should be tackled at every level. However, the unreflective desire to simply enforce the same programs in vastly different circumstances does nothing to address these inequalities and has been shown in the field of infant feeding and HIV to do considerable harm. Antiretroviral drugs markedly reduce all forms of mother-to-child HIV transmission, and culturally-appropriate counseling programs can improve the quality of breastfeeding and thereby reduce HIV transmission and improve child survival. Therefore, the time has come to implement these programs in the developing countries where they are most needed. Effective use of antiretroviral drugs can now reduce transmission to such low levels that there are few circumstances in developing countries where artificial feeding can be justified.
Acknowledgements
We would like to acknowledge support from the National Institutes of Child Health and Human Development (HD 57161, HD 39611, and HD 40777).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
Reference List
- 1.Centers for Disease Control and Prevention. Recommendations for assisting in the prevention of perinatal transmission of human T-lymphotropic virus type III/lymphadenopathy-associated virus and acquired immunodeficiency syndrome. MMWR. 1985;34:721-6–731-2. [PubMed] [Google Scholar]
- 2.World Health Organization Global Programme on AIDS. Consensus statement from the WHO/UNICEF consultation on HIV transmission and breastfeeding. Geneva-Switzerland: WHO; Report No WHO/GAPA/INF/92 1. 1992
- 3.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: A randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 4.Miotti PG, Taha TE, Kumwenda NI, et al. HIV transmission through breast feeding: a study in Malawi. JAMA. 1999;282:744–749. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 5.Fawzi W, Msamanga G, Spiegelman D, et al. Transmission of HIV-1 through breastfeeding among women in Dar es Salaam, Tanzania. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2002;31:331–338. doi: 10.1097/00126334-200211010-00010. [DOI] [PubMed] [Google Scholar]
- 6.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994;331:1173–1180. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 7.Dunn DT, Newell ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet. 1992;340:585–588. doi: 10.1016/0140-6736(92)92115-v. [DOI] [PubMed] [Google Scholar]
- 8.Breastfeeding and HIV International Transmission Study Group. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn L, Sinkala M, Kankasa C, et al. High Uptake of Exclusive Breastfeeding and Reduced Early Post-natal HIV Transmission. PLOS ONE. 2007;2(12):e1363. doi: 10.1371/journal.pone.0001363. doi:10.1371/journal.pone.0001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pak-Gorstein S, Haq A, Graham EA. Cultural influences on infant feeding practices. Pediatr Rev. 2009;30:e11–e21. doi: 10.1542/pir.30-3-e11. [DOI] [PubMed] [Google Scholar]
- 11.Kumar D, Goel NK, Mittal PC, Misra P. Influence of infant-feeding practices on nutritional status of under-five children. Indian J Pediatr. 2006;73:417–421. doi: 10.1007/BF02758565. [DOI] [PubMed] [Google Scholar]
- 12.Fewtrell MS, Morgan JB, Duggan C, et al. Optimal duration of exclusive breastfeeding: what is the evidence to support current recommendations? Am J Clin Nutr. 2007;85 suppl:635S–638S. doi: 10.1093/ajcn/85.2.635S. [DOI] [PubMed] [Google Scholar]
- 13.Flores M, Filteau S. Effect of lactation counselling on subclinical mastitis among Bangladeshi women. Ann Trop Paediatr. 2002;22:85–88. doi: 10.1179/027249302125000210. [DOI] [PubMed] [Google Scholar]
- 14.Kramer MS, Kakuma R. The optimal duration of exclusive breastfeeding: a systematic review. Adv Exp Med Biol. 2004;554:63–77. doi: 10.1007/978-1-4757-4242-8_7. 63–77. [DOI] [PubMed] [Google Scholar]
- 15.Fjeld E, Siziya S, Katepa-Bwalya M, Kankasa C, Moland KM, Tylleskar T. 'No sister, the breast alone is not enough for my baby' a qualitative assessment of potentials and barriers in the promotion of exclusive breastfeeding in southern Zambia. Int Breastfeed J. 2008;3:26. doi: 10.1186/1746-4358-3-26. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 17.Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV-1 infection during exclusive breastfeeding in the first 6 months of life: an intervention cohort study. Lancet. 2007;369:1107–1116. doi: 10.1016/S0140-6736(07)60283-9. [DOI] [PubMed] [Google Scholar]
- 18.Iliff P, Piwoz E, Tavengwa N, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 19.Jelliffe DB, Jelliffe EF. Human milk in the modern world. New York: Oxford University Press; 1978. [Google Scholar]
- 20.Ng'andu NH, Watts TE. Child growth and duration of breast feeding in urban Zambia. J Epidemiol Community Health. 1990;44:281–285. doi: 10.1136/jech.44.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grummer-Strawn LM, Shealy KR. Progress in protecting, promoting, and supporting breastfeeding: 1984–2009. Breastfeed Med. 2009;4 Suppl 1:S31–S39. doi: 10.1089/bfm.2009.0049. S31–S39. [DOI] [PubMed] [Google Scholar]
- 22.Chen A, Rogan WJ. Breastfeeding and the risk of postneonatal death in the United States. Pediatrics. 2004;113:e435. doi: 10.1542/peds.113.5.e435. [DOI] [PubMed] [Google Scholar]
- 23.Forste R, Weiss J, Lippincott E. The decision to breastfeed in the United States: does race matter? Pediatrics. 2001;108:291–296. doi: 10.1542/peds.108.2.291. [DOI] [PubMed] [Google Scholar]
- 24.Bartick M, Reinhold A. The burden of suboptimal breastfeeding in the United States: A pediatric cost analysis. Pediatrics. 2010;125:e1048–e1056. doi: 10.1542/peds.2009-1616. [DOI] [PubMed] [Google Scholar]
- 25.Feachem RG, Koblinsky MA. Interventions for the control of diarrhoeal diseases among young children: promotion of breast-feeding. Bull WHO. 1984;62:271–291. [PMC free article] [PubMed] [Google Scholar]
- 26.Habicht JP, DaVanzo J, Butz WP. Does breastfeeding really save lives, or are apparent benefits due to biases? Am J Epidemiol. 1986;123:279–290. doi: 10.1093/oxfordjournals.aje.a114236. [DOI] [PubMed] [Google Scholar]
- 27.WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–455. [PubMed]
- 28.Kramer MS, Chalmers B, Hodnett E, et al. Promotion of Breastfeeding Intervention Trial (PROBIT) A randomized Trial in the Republic of Belarus. JAMA. 2001;285:413–420. doi: 10.1001/jama.285.4.413. [DOI] [PubMed] [Google Scholar]
- 29.Hummel S, Pfluger M, Kreichauf S, Hummel M, Ziegler AG. Predictors of overweight during childhood in offspring of parents with type 1 diabetes. Diabetes Care. 2009;32:921–925. doi: 10.2337/dc08-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koletzko B, von Kries R, Monasterolo RC, et al. Can infant feeding choices modulate later obesity risk? Am J Clin Nutr. 2009;89:1502S–1508S. doi: 10.3945/ajcn.2009.27113D. [DOI] [PubMed] [Google Scholar]
- 31.Owen CG, Martin RM, Whincup PH, Davey SG, Gillman MW, Cook DG. The effect of breastfeeding on mean body mass index throughout life: a quantitative review of published and unpublished observational evidence. Am J Clin Nutr. 2005;82:1298–1307. doi: 10.1093/ajcn/82.6.1298. [DOI] [PubMed] [Google Scholar]
- 32.Owen CG, Whincup PH, Kaye SJ, et al. Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. Am J Clin Nutr. 2008;88:305–314. doi: 10.1093/ajcn/88.2.305. [DOI] [PubMed] [Google Scholar]
- 33.Mortensen EL, Michaelsen KF, Sanders SA, Reinisch JM. The association between duration of breastfeeding and adult intelligence. JAMA. 2007;18:2365–2371. doi: 10.1001/jama.287.18.2365. [DOI] [PubMed] [Google Scholar]
- 34.Kramer MS, Aboud F, Mironova E, et al. Breastfeeding and child cognitive development: New evidence from a large randomized trial. Archives of General Psychiatry. 2008;65:578–584. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
- 35.Caspi A, Williams B, Kim-Cohen J, et al. Moderation of breastfeeding effects on the IQ by genetic variation in fatty acid metabolism. Proc Natl Acad Sci USA. 2007;104:18860–18865. doi: 10.1073/pnas.0704292104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cunningham AS, Jelliffe DB, Jelliffe EF. Breast-feeding and health in the 1980s: a global epidemiologic review. J Pediatr. 1991;118:659–666. doi: 10.1016/s0022-3476(05)80023-x. [DOI] [PubMed] [Google Scholar]
- 37.Jason JM, Nieburg P, Marks JS. Mortality and infectious disease associated with infant feeding practices in developing countries. Pediatrics. 1984;74:702–727. [PubMed] [Google Scholar]
- 38.Morrow AL, Rangel JM. Human milk protection against infectious diarrhea: implications for prevention and clinical care. Semin Pediatr Infect Dis. 2004;15:221–228. doi: 10.1053/j.spid.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 39.American Academy of Pediatrics. Breast feeding and the use of human milk. Pediatrics. 1997;100:1035–1039. doi: 10.1542/peds.100.6.1035. [DOI] [PubMed] [Google Scholar]
- 40.American Academy of Pediatrics. Policy Statement. Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. [Google Scholar]
- 41.Ip S, Chung M, Raman G, Trikalinos TA, Lau J. A summary of the Agency for Healthcare Research and Quality's evidence report on breastfeeding in developed countries. Breastfeed Med. 2009;4 Suppl 1:S17–S30. doi: 10.1089/bfm.2009.0050. S17–S30. [DOI] [PubMed] [Google Scholar]
- 42.Arifeen S, Black RE, Antelman G, Baqui A, Caulfield L, Becker S. Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics. 2001;108:E67. doi: 10.1542/peds.108.4.e67. [DOI] [PubMed] [Google Scholar]
- 43.Chantry CJ, Howard CR, Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117:425–432. doi: 10.1542/peds.2004-2283. [DOI] [PubMed] [Google Scholar]
- 44.Dunn PM. Sir Hans Sloane (1660–1753) and the value of breast milk. Arch Dis Child Fetal Neonatal Ed. 2001;85:F73–F74. doi: 10.1136/fn.85.1.F73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quigley MA, Kelly YJ, Sacker A. Breastfeeding and hospitalization for diarrheal and respiratory infection in the United Kingdom Millennium Cohort Study. Pediatrics. 2007;119:e837–e842. doi: 10.1542/peds.2006-2256. [DOI] [PubMed] [Google Scholar]
- 46.Dunne EF, Angoran-Benie H, Kamelan-Tano A, et al. Is drinking water in Abidjan, Cote d'Ivoire, safe for infant formula? Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2001;28:393–398. doi: 10.1097/00126334-200112010-00014. [DOI] [PubMed] [Google Scholar]
- 47.Doherty T, Chopra M, Nkonki L, Jackson D, Greiner T. Effect of the HIV epidemic on infant feeding in South Africa: "When they see me coming with the tins they laugh at me". Bull WHO. 2006;84:90–96. doi: 10.2471/blt.04.019448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andresen E, Rollins NC, Sturm AW, Conana N, Griener T. Bacterial contamination and over-dilution of commercial infant formula prepared by HIV-infected mothers in a prevention of mother-to-child transmission (PMTCT) programme in South Africa. Journal of Tropical Pediatrics. 2007;53:410–414. doi: 10.1093/tropej/fmm059. [DOI] [PubMed] [Google Scholar]
- 49.Creek T, Arvelo W, Kim A, et al. Role of infant feeding and HIV in a severe outbreak of diarrhea and malnutrition among young children, Botswana, 2006. 14th Conference on Retroviruses and Opportunistic Infections; 25–28 February; 2007; Los Angeles, CA. (Abstract # 770) ed. [Google Scholar]
- 50.Mach O, Lu L, Creek T, et al. Population-based study of a widespread outbreak of diarrhea associated with increased mortality and malnutrition in Botswana, January–March, 2006. Am J Trop Med Hyg. 2009;80:812–818. [PubMed] [Google Scholar]
- 51.Creek TL, Kim A, Lu L, et al. Hospitalization and mortality among primarily nonbreastfed children during a large outbreak of diarrhea and malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr. 2010;53:14–19. doi: 10.1097/QAI.0b013e3181bdf676. [DOI] [PubMed] [Google Scholar]
- 52.Marino DD. Water and food safety in the developing world: global implications for health and nutrition of infants and young children. J Am Diet Assoc. 2007;107:1930–1934. doi: 10.1016/j.jada.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Victora CG, Smith PG, Vaughan JP, et al. Evidence for protection by breast-feeding against infant deaths from infectious diseases in Brazil. Lancet. 1987;2:319–322. doi: 10.1016/s0140-6736(87)90902-0. [DOI] [PubMed] [Google Scholar]
- 54.Brown KH, Black RE, Lopez de Romana G, Creed de Kanashiro H. Infant-feeding practices and their relationship with diarrheal and other diseases in Huascar (Lima) Peru. Pediatrics. 1989;83:31–40. [PubMed] [Google Scholar]
- 55.Cesar JA, Victora CG, Barros FC, Santos IS, Flores JA. Impact of breast feeding on admission for pneumonia during the postnatal period in Brazil: nested case-control study. BMJ. 1999;318:1316–1320. doi: 10.1136/bmj.318.7194.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bahl R, Frost C, Kirkwood BR, et al. Infant feeding patterns and risks of death and hospitalization in the first half of infancy: multicentre cohort study. Bull WHO. 2005;83:418–426. [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas T, Masaba R, van Eijk A, et al. Rates of diarrhea associated with early weaning among infants in Kisumu, Kenya. 14th Conference on Retroviruses and Opportunistic Infections; 25–28 February; 2007; Los Angeles, CA. (Abstract # 774) ed. [Google Scholar]
- 58.Harris JR, Greene SK, Thomas TK, et al. Effect of a point-of-use water treatment and safe water storage intervention on diarrhea in infants of HIV-infected mothers. J Infect Dis. 2009;200:1186–1193. doi: 10.1086/605841. [DOI] [PubMed] [Google Scholar]
- 59.Habicht JP, DaVanzo J, Butz WP. Mother's milk and sewage: their interactive effects on infant mortality. Pediatrics. 1988;81:456–461. [PubMed] [Google Scholar]
- 60.Neville MC. Determinants of milk volume and composition. In: Jensen RG, editor. Handbook of milk composition. San Diego: Academic Press; 1995. pp. 87–114. [Google Scholar]
- 61.Neville MC, Keller RP, Seacat J, Casey CE, Allen JC, Archer P. Studies on human lactation. I. Within-feed and between-breast variation in selected components of human milk. Am J Clin Nutr. 1984;40:635–646. doi: 10.1093/ajcn/40.3.635. [DOI] [PubMed] [Google Scholar]
- 62.Coutsoudis A, Goga AE, Rollins N, Coovadia HM. Free formula milk for infants of HIV-infected women: blessing or curse? Health Policy and Planning. 2002;17:154–160. doi: 10.1093/heapol/17.2.154. [DOI] [PubMed] [Google Scholar]
- 63.Coutsoudis A, Coovadia HM, Wilfert CM. HIV, infant feeding and more perils for poor people: new WHO guidelines encourage review of formula milk policies. Bull WHO. 2008;86:210–214. doi: 10.2471/BLT.07.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sibeko L, Coutsoudis A, Nzuza S, Gray-Donald K. Mothers' infant feeding experiences: constraints and supports for optimal feeding in an HIV-impacted urban community in South Africa. Public Health Nutrition. 2009;10:1–8. doi: 10.1017/S1368980009005199. [DOI] [PubMed] [Google Scholar]
- 65.Bland RM, Rollins NC, Coovadia HM, Coutsoudis A, Newell ML. Infant feeding counselling for HIV-infected and unifnected women: appropriateness of choice and practice. Bull WHO. 2007;85:289–296. doi: 10.2471/BLT.06.032441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doherty T, Chopra M, Nkonki L, Jackson D, Persson LA. A longitudinal qualitative study of infant-feeding decision making and practices among HIV-positive women in South Africa. J Nutr. 2006;136:2421–2426. doi: 10.1093/jn/136.9.2421. [DOI] [PubMed] [Google Scholar]
- 67.Desclaux A, Alfieri C. Counseling and choosing between infant-feeding options: overall limits and local interpretations by health care providers and women living with HIV in resource-poor countries (Burkina Faso, Cambodia, Cameroon) Soc Sci Med. 2009;69:821–829. doi: 10.1016/j.socscimed.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 68.Goga A, Colvin M, Doherty T, et al. How do routine PMTCT programmes influence infant feeding practices and infant outcome? Results from an observational cohort study, South Africa. 2010 submitted. [Google Scholar]
- 69.Chopra M, Rollins N. Infant feeding in the time of HIV: rapid assessment of infant feeding policy and programmes in four African countries scaling up prevention of mother to child transmission programmes. Arch Dis Child. 2008;93:288–291. doi: 10.1136/adc.2006.096321. [DOI] [PubMed] [Google Scholar]
- 70.Goldman AS. The immune system of human milk: antimicrobial, antiinflammatory and immunomodulating properties. Pediatr Infect Dis J. 1993;12:664–671. doi: 10.1097/00006454-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nature Reviews. 2004;4:565–572. doi: 10.1038/nri1393. Immunology. [DOI] [PubMed] [Google Scholar]
- 72.Lonnerdal B. Personalizing nutrient intakes of formula-fed infants: breast milk as a model. Nestle Nutr Workshop Ser Pediatr Program. 2008;62:189–198. doi: 10.1159/000146272. discussion 198–203. [DOI] [PubMed] [Google Scholar]
- 73.Heird WC. Progress in promoting breast-feeding, combating malnutrition, and composition and use of infant formula, 1981–2006. J Nutr. 2007;137:499S–502S. doi: 10.1093/jn/137.2.499S. [DOI] [PubMed] [Google Scholar]
- 74.Koletzko B, Baker S, Cleghorn G, et al. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr. 2005;41:584–599. doi: 10.1097/01.mpg.0000187817.38836.42. [DOI] [PubMed] [Google Scholar]
- 75.Shapiro RL, Lockman S, Kim S, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis. 2007;196:562–565. doi: 10.1086/519847. [DOI] [PubMed] [Google Scholar]
- 76.Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 77.Kagaayi J, Gray RH, Brahmbhatt H, et al. Survival of infants born to HIV-positive mothers by feeding modality in Rakai, Uganda. PLOS ONE. 2008;3:e3877. doi: 10.1371/journal.pone.0003877. doi:10.1371/journal.pone.0003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Homsy J, Moore D, Barasa A, et al. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-Infected women on highly active antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr. 2010;53:28–35. doi: 10.1097/QAI.0b013e3181bdf65a. [DOI] [PubMed] [Google Scholar]
- 79.World Health Organization. Planning guide for national implementation of the global strategy for infant and young child feeding. 2006 http://www who int/nutrition/publications/Planning_guide pdf.
- 80.Greiner T. The concept of weaning: definitions and their implications. Commentary. Journal of Human Lactation. 1996;12:123–128. doi: 10.1177/089033449601200216. [DOI] [PubMed] [Google Scholar]
- 81.Simondon KB. Early breast-feeding cessation and infant mortality in low-income countries: workshop summary. Adv Exp Med Biol. 2009;639:319–329. doi: 10.1007/978-1-4020-8749-3_23. 319–329. [DOI] [PubMed] [Google Scholar]
- 82.Brahmbhatt H, Gray RH. Child mortality associated with reasons for non-breastfeeding and weaning: is breastfeeding best for HIV-positive mothers? AIDS. 2003;17:879–885. doi: 10.1097/00002030-200304110-00013. [DOI] [PubMed] [Google Scholar]
- 83.Peltier CA, Ndayisaba GF, Lepage P, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS. 2009;23:2415–2423. doi: 10.1097/QAD.0b013e32832ec20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nyandiko WM, Otieno-Nyunya B, Musick B, et al. Outcomes of HIV-exposed children in western Kenya: efficacy of prevention of mother to child transmission in a resource-constrained setting. J Acquir Immune Defic Syndr. 2010;54:42–50. doi: 10.1097/QAI.0b013e3181d8ad51. [DOI] [PubMed] [Google Scholar]
- 85.Phadke MA, Gadgil B, Bharucha KE, et al. Replacement-fed infants born to HIV-infected mothers in India have a high early postpartum rate of hospitalization. J Nutr. 2003;133:3153–3157. doi: 10.1093/jn/133.10.3153. [DOI] [PubMed] [Google Scholar]
- 86.Kafulafula G, Thigpen M, Hoover DR, et al. Post-weaning gastroenteritis and mortality in HIV-uninfected African infants receiving antiretroviral prophylaxis to prevent MTCT of HIV-1. 14th Conference on Retroviruses and Opportunistic Infections; 25–28 February; 2007; Los Angeles, CA. (Abstract # 773) ed. [Google Scholar]
- 87.Kourtis AP, Fitzgerald D, Hyde L, et al. Diarrhea in uninfected infants of HIV-infected mothers who stop breastfeeding at 6 months: the BAN study experience. 14th Conference on Retroviruses and Opportunistic Infections; 25–28 February; 2007; Los Angeles, CA. (Abstract # 772) ed. [Google Scholar]
- 88.Onyango C, Mmiro F, Bagenda D, et al. Early breastfeeding cessation among HIV-exposed negative infants and risk of serious gastroenteritis: findings from a perinatal prevention trial in Kampala, Uganda. 14th Conference on Retorviruses and Opportunistic Infections; 25–28 February; 2007; Los Angeles, CA. (Abstract #775) ed. [Google Scholar]
- 89.Kafulafula G, Hoover DR, Taha TE, et al. Frequency of Gastroenteritis and Gastroenteritis-Associated Mortality with Early Weaning in HIV-1-Uninfected Children Born to HIV-Infected Women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13. doi: 10.1097/QAI.0b013e3181bd5a47. [DOI] [PubMed] [Google Scholar]
- 90.Onyango-Makumbi C, Bagenda D, Mwatha A, et al. Early Weaning of HIV-Exposed Uninfected Infants and Risk of Serious Gastroenteritis: Findings from Two Perinatal HIV Prevention Trials in Kampala, Uganda. J Acquir Immune Defic Syndr. 2010;53:20–27. doi: 10.1097/QAI.0b013e3181bdf68e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taha TE, Kumwenda NI, Hoover DR, et al. The impact of breastfeeding on the health of HIV-positive mothers and their children in sub-Saharan Africa. Bull WHO. 2006;84:546–554. doi: 10.2471/blt.05.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuhn L, Aldrovandi GM, Sinkala M, et al. Effects of early, abrupt cessation of breastfeeding on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–141. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuhn L, Aldrovandi GM, Sinkala M, et al. Differential effects of early weaning for HIV-free survival of children born to HIV-infected mothers by severity of maternal disease. PLOS ONE. 2009;4:e6059. doi: 10.1371/journal.pone.0006059. doi:10.1371/journal.pone.0006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuhn L, Sinkala M, Semrau K, et al. Elevations in mortality due to weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis. 2010;54:437–444. doi: 10.1086/649886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arpadi SM, Fawzy A, Aldrovandi GM, et al. Growth faltering due to breastfeeding cessation among uninfected children born to HIV-infected mothers in Zambia. Am J Clin Nutr. 2009;90:344–350. doi: 10.3945/ajcn.2009.27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fawzy A, Arpadi S, Aldrovandi GM, et al. Diarrhea morbidity and mortality increases with weaning prior to 6 months among uninfected infants born to HIV-infected mothers in Zambia; International AIDS Society Conference in Cape Town July; 2009. TUAC104. [Google Scholar]
- 97.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Becquet R, Bequet L, Ekouevi DK, et al. Two-year morbidity-mortality and alternatives to prolonged breastfeeding among children born to HIV-infected mothers in Cote d'Ivoire. PLOS Medicine. 2007;4:e17. doi: 10.1371/journal.pmed.0040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doherty T, Chopra M, Jackson D, Goga A, Colvin M, Persson LA. Effectiveness of the WHO/UNICEF guidelines on infant feeding for HIV-positive women: results from a prospective cohort study in South Africa. AIDS. 2007;21:1791–1797. doi: 10.1097/QAD.0b013e32827b1462. [DOI] [PubMed] [Google Scholar]
- 100.Bulterys M, Wilfert C. HAART during pregnancy and during breastfeeding among HIV-infected women in the developing world: has the time come? AIDS. 2009;23:2473–2477. doi: 10.1097/QAD.0b013e328333866c. [DOI] [PubMed] [Google Scholar]
- 101.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Geneva: 2009 http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf. [PubMed]
- 102.Tonwe-Gold B, Ekouevi DK, Viho I, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLOS Medicine. 2007;4:e257. doi: 10.1371/journal.pmed.0040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Mwiya M, Thea DM. Potential impact of new World Health Organization criteria for antiretroviral treatment for prevention of mother-to-child HIV transmission. AIDS. 2010;24:1374–1371. [PMC free article] [PubMed] [Google Scholar]
- 104.Thomas T, Masaba R, Ndivo R, et al. Prevention of Mother-to-Child Transmission of HIV-1 among Breastfeeding Mothers Using HAART: The Kisumu Breastfeeding Study, Kisumu, Kenya, 2003–2007. 15th Conference of Retrovirus and Opportunistic Infections; Feb. 2008; Boston, USA. 2008. Abstract 45aLB. [Google Scholar]
- 105.Palombi L, Marazzi MC, Voetberg A, Magid MA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21 Suppl 4:S 65–S 71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 106.de Vincenzi I The Kesho Bora Study Group. HIV-free survival at 12 months among children born to HIV-infected women receiving antiretrovirals from 34 to 36 weeks of pregnancy. 15th Conference of Retrovirus and Opportunistic Infections; Feb. 2008; Boston, USA. 2008. Abstract 638. [Google Scholar]
- 107.Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 108.Shapiro RL, Hughes M, Ogwu A. A randomized trial comparing highly active antiretroviral therapy regimens for virologic effiacy and the prevention of mother-to-child transmission among breastfeeding women in Botswana (The Mma Bana Study); 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2009. WELBB101: Cape Town 19–22 July. [Google Scholar]
- 109.Marazzi MC, Nielsen-Saines K, Buonomo E, et al. Increased infant human immunodeficiency virus-type one free survival at one year of age in sub-saharan Africa with maternal use of highly active antiretroviral therapy during breast-feeding. Pediatr Infect Dis J. 2009;28:483–487. doi: 10.1097/INF.0b013e3181950c56. [DOI] [PubMed] [Google Scholar]
- 110.Chung MH, Kiarie JN, Richardson BA, Lehman DA, Overbaugh J, John-Stewart GC. Breast milk HIV-1 suppression and decreased transmission: a randomized trial comparing HIVNET 012 nevirapine versus short-course zidovudine. AIDS. 2005;19:1415–1422. doi: 10.1097/01.aids.0000181013.70008.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–313. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 112.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 113.Chasela C, Hudgens M, Jamieson DJ. Both maternal HAART and daily infant nevirapine (NVP) are effective in reducing HIV-1 transmission during breastfeeding in a randomized trial in Malawi: 28 week results of the Breastfeeding, Antiretroviral and Nutrition (BAN) Study; 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2009. WELBC103:Cape Town-19–22 July. [Google Scholar]
- 114.Kilewo C, Karlsson K, Massawe A, et al. Prevention of mother-to-child transmission of HIV-1 through breast-feeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: the Mitra Study. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2008;48:315–323. doi: 10.1097/QAI.0b013e31816e395c. [DOI] [PubMed] [Google Scholar]
- 115.Taha TE, Kumwenda J, Cole SR, et al. Postnatal HIV-1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. J Infect Dis. 2009;200:1490–1497. doi: 10.1086/644598. [DOI] [PubMed] [Google Scholar]
- 116.Tudor-Williams G. Changing UK practice: influence from resource-poor setting, including new infant feeding guidance. Second Joint Conference of the British HIV Association (BHIVA) and the British Association for Sexual Health and HIV (BASHI) Plenary Session 3; 20–23 April 2010; Imperial College London-Manchester Central Convention Complex. 2010. [Google Scholar]
- 117.World Health Organization. New data on the prevention of mother-to-child transmission of HIV and their policy implications. Conclusions and recommendations. WHO technical consultation on behalf of the UNFPA/UNICEF/WHO/UNAIDS Inter-agency Task Team on Mother-to-child Transmission of HIV. [11–13 October 2000];2000 Available at: www.who.int/reproductive-health/publications/new_data_prevention_mtct_hiv/index.html.
- 118.World Health Organization. HIV and Infant Feeding. Guidelines for decision-makers. Geneva: 2003 http://www.who.int/child-adolescent-health/New_Publications/NUTRITION/HIV_IF_DM.pdf.
- 119.World Health Organization. HIV and Infant Feeding: Revised principles and recommendations: Rapid Advice November 2009. [accessed April 1, 2010];2009 http://whqlibdoc who int/publications/2009/9789241598873_eng pdf.