Abstract
Recently, the IL-17 family member cytokines have become prominent subjects of investigation. IL-17 (IL-17A) is the best-described member of this family where its production has been mainly attributed to a specialized T helper subset of the adaptive immune response termed Th17. However, recent research on this and other Th17 cytokines has revealed new sources and functions of IL-17 family members in the innate immune response. This review will highlight recent advances in the field of IL-17 family member cytokines and will predominately focus on the innate regulation and function of IL-17, IL-17F, and IL-25.
Keywords: IL-17, IL-17F, IL-25, innate immunity
1. Introduction
The IL-17 family of cytokines consists of six family members of varying homology and function: IL-17 (also called IL-17A), IL-17B, IL-17C, IL-17D, IL-17E (also called IL-25), and IL-17F. In general, these cytokines range from 20–30 kDA and incorporate four conserved C-terminal cysteine residues (1, 2). The cytokines IL-17 and IL-17F share the strongest homology, which is likely the reason why these mediators are thought to use similar signaling mechanisms in exerting their pro-inflammatory functions(1, 3, 4). Little is known about the regulation and function of IL-17B and IL-17C; however, there is some evidence that these cytokines are also regulators of the inflammatory response as well(5–7). IL-25 has the least homology with IL-17A, which may translate to its unique abilities in regulating allergy and T helper (Th) 2 responses (8–10). A summary of the known sources and functions of the IL-17 family of cytokines is presented in Table 1.
Table 1.
The IL-17 family member cytokines
| Cytokine | Receptor | Source | Known functions | Role in disease | References |
|---|---|---|---|---|---|
| IL-17A (IL-17) | IL-17RA, IL-17RC | CD4+ and CD8+ T cells, γδ T cells, NKT cells, other innate cells | Induce pro-inflammatory cytokines and chemokines in various target cells which promote inflammation and neutrophil recruitment | Pro-inflammatory functions in host defense against extracellular bacteria, fungi, and possibly some viral infections and cancers. Promotes inflammation associated with autoimmunity | (11, 15, 17, 59, 146–148) |
| IL-17B | IL-17RB | Pancreas, gut, cartilage, neurons, embryonic limb buds | Stimulates TNFα and IL-1β from monocytes; stimulates chondrocyte proliferation | Associated with arthritis; bone fracture healing | (5, 7, 149–151) |
| IL-17C | ? | Prostate, kidney, psoriatic skin, pneumonia-infected lung | Stimulates TNFα and IL-1β in THP-1 cells | Expression correlates with arthritis and psoriasis; upregulated following pneumonia infection | (5–7, 26, 152) |
| IL-17D | ? | Nervous system, muscle, heart, adipose | Stimulates IL-6, IL-8, and GM-CSF from endothelial cells | Reduced in psoriasis | (152, 153) |
| IL-17E (IL-25) | IL-17RB, IL-17RA | Epithelial ells, myeloid cells, eosinophil, basophil, mast cells | Induces Th2 cytokines in NBNT, monocytes and NKT cells; promotes Th2 differentiation, enhances Th9 differentiation | Promotion of type 2 helper responses in vivo, promotes allergic lung disease, important in host defense against parasites; possible roles in autoimmune inflammatory disorders | (8, 9, 114, 118, 120, 121, 130) |
| IL-17F | IL-17RA, IL-17RC | CD4+ and CD8+ T cells, γδ T cells, epithelial cells and other innate cells | Induces pro-inflammatory mediator production in hematopoietic and non-hematopoietic cells (less potent than IL-17A), involved in neutrophil recruitment, induces IP-10 from bronchial epithelial cells | Similar pro-inflammatory functions to IL-17; not as important as IL-17A in EAE; inhibitory in asthma and colitis; potent in psoriasis | (1, 11, 17, 30, 50, 154, 155) |
The cytokines IL-17 and IL-17F have been associated with a distinct lineage of CD4+ T helper lymphocytes (Th) known as Th17 cells (11, 12). However, recent reports have strongly suggested that IL-17 and IL-17F expression is not strictly limited to Th17 cells or for that matter T cells at all. Additionally, the sources of IL-25 include both innate immune cells as well as cells from non-hematopoietic origins. Thus, this review will highlight recent developments in IL-17A, IL-17F, and IL-25 biology, with a special emphasis on the innate sources and functions of these cytokines.
2. IL-17 and IL-17F
IL-17 has been extensively studied since its discovery in human peripheral blood (13, 14). The identification of IL-17 led to the unearthing of the specialized Th17 subset that was found to produce the cytokines IL-17 as well IL-17F (15–17). Upon recognition of cognate ligands, naïve CD4+ helper T (Th) cells differentiate into various effector lineages that can be characterized by the induction of specialized transcription factors and their subsequent patterns of cytokine expression. Naïve Th cells, however, do not simply autonomously decide their fate; environmental signals present during the initial activation events instead determine their effector properties (18, 19). These environmental factors, produced by innate cells following host insult, are crucial for Th17 lineage commitment and maintenance and include transforming growth factor beta (TGFβ), IL-6, IL-1β, and IL-23 (17, 20). However, the hallmark of inflammation is the rapid production of pro-inflammatory factors that are vital in the recruitment and activation of infiltrating leukocytes with the overall goal of combating host insult (21). Therefore, due to the known importance of IL-17 and IL-17F in the promotion of inflammation, there are likely many other sources of these cytokines, including the innate arm of immunity, which would provide a rapid means for IL-17 effector function.
2.1. The pro-inflammatory function of IL-17A and IL-17F
IL-17F was also discovered through homologous IL-17 sequence comparison in humans following the identification of IL-17 (4, 22). Both IL-17 and IL-17F readily form homodimers, however, there is evidence that suggests that an IL-17/IL-17F heterodimer forms as well, where IL-17, IL-17F, or IL-17/IL-17F bind to ubiquitously expressed receptors, IL-17RA, IL-17RC, or IL-17RA/IL-17RC, respectively (1, 23–26). The cytokines IL-17 and IL-17F employ the adaptors TRAF6 and Act1 in their signaling transduction pathways as well(27, 28). Both IL-17 and IL-17F readily activate innate and tissue resident cells, such as fibroblasts and epithelial cells, to induce the production of pro-inflammatory cytokines and chemokines (reviewed in (29)). Although somewhat redundant in function, research has shown that in some cases IL-17F is less potent compared to IL-17 in activating target cells to produce pro-inflammatory products (30). Moreover, other pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNFα), have been shown to cooperate with IL-17 to synergistically enhance the inflammatory capacity of innate cells (31, 32). Overall, one of the major outcomes attributed to considerable IL-17 and IL-17F production is the recruitment and subsequent activation of neutrophils during inflammation (3).
In vivo, IL-17 and IL-17F perform a wide variety of immunoregulatory roles during infection. For example, IL-17 production is readily induced following infection with the extracellular bacterium, Klebsiella pneumoniae (33–35). In humans, Pseudomonas aeruginosa infection stimulates IL-17 and IL-17F production (36). Blocking IL-17 during fungal infection, such as that observed in a model of Pneumocystis carinii, leads to exacerbated disease (37). Taken together, these examples along with many other studies (extensively reviewed in (29)) demonstrate the importance of IL-17 and IL-17F in combating microbial infections, through the activation of innate cells as well as the recruitment of inflammatory leukocytes.
Besides infection, one of the most prominent roles associated with IL-17 and IL-17F production is in the regulation of autoimmunity, where dysregulation of Th17 and other IL-17-producing cells may lead to severe disease. During multiple sclerosis (MS), Th17 cells and their associated cytokines have been shown to be critical for lesion development and central nervous system (CNS) inflammation (15, 38, 39). In contrast, the mouse model of human MS, experimental autoimmune encephalomyelitis (EAE) still develops, albeit partially, in mice lacking IL-17 expression (reviewed in (29)). This evidence suggests that although IL-17 is important during EAE pathogenesis, it is not the sole pro-inflammatory mediator contributing to CNS inflammation. Moreover, in models of rheumatoid arthritis, both IL-17 and IL-23 have been implicated in joint destruction (40–42). Blocking IL-17 following arthritis induction results in a protective effect (43). Furthermore, IL-17 deficiency results in a similar protection in other autoimmune inflammatory disease models, including colitis (44), and IL-17 and IL-23-producing cells have known roles in promoting psoriasis (45–48).
2.2. Differential regulation and function of IL-17A and IL-17F
Research into the differential regulation requirements for IL-17 and IL-17F to date has been minimal. However, there is one such report that suggests that inducible T cell kinase (Itk) expression, an important mediator for T cell receptor (TCR) signaling, is required for IL-17 induction (49). Ikt-deficient T cells had impaired ability to produce IL-17 but not IL-17F, even though Ikt levels did not influence RORγ activity. Further analysis revealed that the promoter for IL-17 contained an NFATc1 binding site but the IL-17F promoter did not, providing mechanistic insight into this differential regulation. Thus, IL-17 and IL-17F are not always necessarily induced in the same manner, where further examination is needed to determine what other factors can regulate IL-17 or IL-17F-specific responses.
Although IL-17 and IL-17F are generally accepted to have redundant functions in promoting inflammation, there are a few prominent examples of differential functions of these highly related cytokines. In a seminal paper by our group, we examined many different aspects of IL-17 and IL-17F function (30). For example, IL-17F, like IL-17, was able to induce inflammatory molecules from MEF cells in vitro, however IL-17F was not nearly as effective when MEF cells were stimulated at similar concentrations of IL-17 and IL-17F. In vivo, IL-17 was shown to be critical in the early priming phase of EAE, possibly through the regulation of IFNγ-producing cells, whereas IL-17F was almost dispensable. In an allergic airway inflammation model, IL-17F-deficient animals, but not IL-17−/−, exhibited substantially decreased CXCL5 expression, which corresponded to reduced neutrophila. Conversely, in a mouse model of asthma IL-17-deficent animals exhibited a reduction of cellular infiltrates into the airways, most notably eosinophils. However, IL-17F-deficient mice exhibited the opposite phenotype; high levels of IL-5 and IL-13 in the airways were observed and subsequent eosinophil infiltration was greatly increased compared to wild-type and IL-17-deficient animals. Using the DSS-induced colitis model, our group also observed that IL-17-deficient mice had exacerbated disease compared to a milder phenotype in IL-17F−/− animals. Finally, human epidermal keratinocytes induced IL-8, a cytokine important for the development of psoriatic lesions, upon stimulation with IL-17F but not IL-17, suggesting a more prominent role for IL-17F in psoriasis (50, 51). Taken together, these studies demonstrate that although these cytokines are somewhat redundant in promoting inflammation, these individual factors do in fact have unique effector functions depending on the environment and the nature of host insult.
3. IL-17 and IL-17F in innate immunity
As previously mentioned, the production of IL-17 and IL-17F has largely been attributed to specialized Th17 cells residing in the adaptive arm of immunity (17). However, a vast array of exciting research performed in the past few years has concretely demonstrated that Th17 cells are just one of many sources of both IL-17 and IL-17F (Table 2). Not only do certain components of the innate immune response readily produce Th17 cytokines (IL-17, IL-17F, IL-22), they can be the predominate source of theses cytokines in an assortment of disease settings. The most famous example of IL-17-producing innate cells to date is the innate-like γδ T cell, however, additional known IL-17 and IL-17F innate producers will be discussed as well.
Table 2.
Sources of Th17-related cytokines in innate immunity
| Source | Secreted cytokine(s) | Induction signals | Transcription factors | Role in disease | References |
|---|---|---|---|---|---|
| γδ T cell | IL-17, IL-17F, IL-22 | IL-1, IL-23, TLRs, TGFβ | RORγ, AhR | Prominent during bacterial infection (i.e. Staphylococcus and Mycobacterium); potential roles in autoimmunity | (63–66, 68, 78, 79, 83) |
| LTi and LTi-like cells | IL-17, IL-17F, IL-22 | IL-23, TLR2 | RORγ, AhR | Rapid IL-17 responses; specific pathogens? | (89–92) |
| NK cells | IL-17, IL-22 | IL-6 | RORγ, AhR | Toxoplasmosis | (93–95) |
| NKT cells | IL-17, IL17F? | IL-23 | RORγ | Rapid IL-17 responses, specific pathogens? | (97, 156) |
| Macrophages | IL-17 | Chitin | ? | Breast cancer, allergic inflammation | (101–103) |
| Paneth cells | IL-17 | TNF α + pattern recognition receptor | ? | Gut immunity | (106) |
| Neutrophils | IL-17 | Pathogen product? | ? | Ischemia-reperfusion injury; Bordetella pertussis infections | (108, 109) |
| Epithelial cells | IL-17F | Pathogen product? | ? | Lung and colon immunity | (104, 110) |
| Mast cells | IL-17 | TLR | RORγ | Arthritis | (111) |
3.1. γδ T cells
The γδ T cells are a relatively specialized population, comprising 1–5% of the total lymphocyte fraction in humans and mice (52). γδ T cells represent the first wave of T lymphocytes generated in ontogeny, where the presence of IL-7 in the thymus is crucial to their survival and expansion. Indeed, thymic precursors expressing high levels of IL-7 receptor are more likely to develop into γδ T cells. As fetal maturation proceeds, the number of γδ TCR-expressing cells remains constant as γδ TCR-expressing cells dramatically increase (52–54). Traditionally, γδ T cells were thought to function in the innate immune response as a “first line of defense” against invading pathogens mainly due to their strategic localization in pockets of vulnerable tissues such as the gut, skin, and lungs. Additionally, γδ T cells express common γδ T cell scavenger receptors, Toll-like Receptors (TLRs), and apoptosis-promoting molecules such as FasL and NKG2D (55). However, γδ T cells do undergo VDJ gene rearrangements, a major facet of adaptive immunity. Thus, it is now widely believed that γδ T cells may function in the so-called “transitional response” at the crossroads of innate and adaptive immunity (52, 56–58).
3.1.1. IL-17-producing γδ T cells: development and cytokine regulation
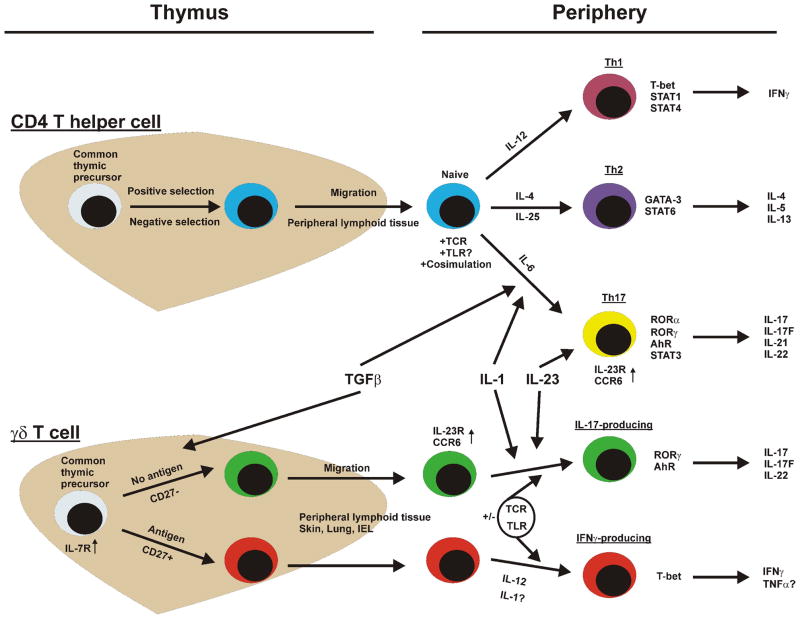
Recent studies have indeed shown that γδ T cells have the ability to produce both IL-17 and IL-17F (59). However, for a while it was unclear if γδ T cells exhibited functional plasticity in cytokine production or were generally restricted to either being IL-17 or IFNγ producers. An important study recently published by Jensen and colleagues has helped clarified this issue (60). The authors first demonstrated that the commitment towards IL-17 or IFNγ production was determined during the thymic selection process for γδ T cells. Cells that had previously interacted with antigen, the so-called “experienced cells”, demonstrated an IFNγ-producing phenotype. Conversely, naïve γδ T cells leaving the thymus were primarily IL-17 producers. Furthermore, IL-17-and IFNγ-producing γδ T cells also express the Th17 and Th1 transcriptional factors RORγ and T-bet, respectively (61, 62). Another recent report demonstrated that during thymic selection, CD27+ γδ T cells exhibit high levels of T-bet and subsequently become IFNγ producers. In contrast, γδ T cells lacking CD27 expression had constitutive levels of RORγ and became IL-17-producing γδ T cells (62). Finally, IL-17-prodcucing γδ T cells exhibit high expression of CCR6, a common marker present on Th17 cells (63, 64). Thus, γδ T cells are programmed to produce certain cytokines during developments and can be easily identified based on certain IL-17-producing or IFNγ-producing markers.
Th17 cell differentiation and maintenance involves various cytokines, with known roles of IL-6, TGFβ, IL-1, IL-21, and IL-23 in this process (17, 20). Thus, many researchers have questioned whether IL-17-producing γδ T cells are governed by a similar set of factors. Interestingly, IL-6 does not appear to be a critical factor for the development of IL-17- and IL-17F-producing γδ T cells (reviewed in (65)). In contrast, TGFβ was recently demonstrated to be a pivotal factor for the development of γδ T cells in the thymus (66). The role of IL-23 in promoting IL-17 responses by γδ T cells is well established (59). Indeed, IL-23 receptor expression is a hallmark of IL-17-producing γδ T cells (67). Moreover, γδ T cells express IL-1 receptor, where treatment of this subset with a combination of IL-1 and IL-23 readily induces IL17 and IL-17F cytokine production in a TCR-independent manner (68). IL-21 is an autocrine factor necessary for the maintenance of Th17 populations (69). In the case of γδ T cells, IL-21 has been discovered to expand human γδ T cell populations (70, 71), and is also important for γδ T cell for the development of IL-17-producing γδ T cells (69). Finally, although IL-23 is sufficient to induce IL-17 production from γδ T cells alone, a synergistic effect of inducing IL-17 production is observed when γδ T cells are treated with a combination of IL-23 plus TLR2 ligand (64). Moreover, our group has shown that treament of IL-23 enhances the expression of TLR2 on γδ T cells; whereas TLR2 but not IL-23 promotes proliferation of γδ T cells. Finally, both TLR2 and IL-23-deficient γδ T cells exhibit substantial defects in their ability to produce IL-17 in the periphery; however, both of these genes were not required for thymic development of IL-17-producing γδ T cell populations (72). Therefore, IL-17-producing γδ T cells do in fact incorporate many of the Th17-polarizing/maintenance factors to regulate cytokine production, however, it appears that these cells are utilizing these cytokines in a different context (i.e. the direct induction of IL-17 and IL-17F).
Th17 cells have well-defined transcription factors that are utilized for lineage commitment and cytokine production, including RORγ, RORα, IRF-4, and AhR (73–75). Consequently, research is now being directed to determine if IL-17-producing γδ T cells utilize some if not all of the same transcription factors. As previously mentioned, IL-17-producing γδ T cells do in fact utilize RORγ (61, 68). Furthermore, AhR is also enhanced in IL-17-producing γδ T cells when stimulated with IL-23 and TLR2 ligand (64). Thus, there is some overlap in transcription factors utilized by Th17 and γδ T cells, however the relative contributions of other factors such as RORα and IRF-4 have yet to be determined. A comparison of Th and γδ T cell cytokine commitment is presented in Figure 1.
Figure 1.
Differentiation pathways of CD4+ T helper cells compared to IL-17- and IFNγ-producing γδ T cells. γδ T cells utilize many of the same cytokines and transcription factors as Th cells for the regulation of cytokine production. The primary difference between the development of cytokine-committed αβ and γδ T cells seems to be that this process occurs in the thymus in the case of γδ T cells compared to the periphery in CD4+ t helper cells.
3.1.2 IL-17-producing γδ T cells: importance in disease
γδ T cells are localized in mucosal tissues throughout the host to allow for the innate-like ability to rapidly produce immunoregulatory mediators, which is especially true of IL-17-producing γδ T cells (59). In fact, it has been shown that γδ T cells are crucial regulators of early inflammation and the dominant source of IL-17 and IL-17F in many disease models. For example, two recent publications eloquently demonstrated that IL-17-producing γδ T cells were critical in the early stages of defense against Listeria monocytogenes infection (76, 77). γδ T cell-deficient mice were also found to have more severe skin lesions as a result of Staphylococcus aureus infection, and that the lack of IL-17 production normally associated with this subset was the primary cause for this phenotype (78). Moreover, mouse models of Mycobacterium infection have demonstrated that not only are IL-17-producing γδ T cells crucial in host defense; they are also the primary source of IL-17 during these infections (79, 80). Finally, Shibata and colleagues published research that showed that in Escheria coli-induced peritonitis, resident γδ T cells were responsible for rapid IL-17 induction and neutrophil recruitment in a TLR4-dependent manner (81). Thus, this specialized subset of γδ T cells clearly plays a key role in the control of bacterial infection.
In addition to critical protective roles in the early response to infection, IL-17-producing γδ T cells may be critical for the development of autoimmune inflammatory disorders as well. Depletion of γδ T cells prior to EAE induction led to drastically reduced EAE development and subsequent CNS inflammation (82). Moreover, recent reports have demonstrated that IL-17-producing γδ T cells are found in the CNS tissues of EAE mice (68, 83). Conversely, one must also consider the importance of the hallmark Th1 cytokine, IFNγ, in EAE pathogenesis. Wohler and colleagues have shown that IFNγ- and TNFα-producing γδ T cells are critical as well for full EAE development (84). Although the function of IL-17-producing γδ T cells in inducing EAE is somewhat undetermined, this specialized subset has additionally been linked to other CNS-related inflammatory disorders. For example, during ischemic brain injury, γδ T cells are the predominant source of IL-17 following activation in an IL-23-dependent manner (85). Finally, a recent study has shown the importance of IL-17 in experimental autoimmune uveitis, where IL-17-producing γδ T cells were key mediators in the activation of autoreactive IL-17-producing αβ T cells (86). Overall, recent evidence has suggested a function of these innate IL-17 generators in autoimmunity, however further experimental validation is needed to fully understand the regulation and function of this subset.
3.2. Lymphoid tissue inducer cells
Fetal lymphoid tissue inducer cells (LTi) are critical mediators for the development of lymphoid tissues (reviewed in (87)). Furthermore, LTi cells are related to certain types of natural killer (NK) cells in terms of development and surface molecule expression (88). LTi-like cells are found in adult spleen and share many of the same characteristics as LTi cells. LTi-like cells express high levels of OX40 ligand and have previously been shown to be major producers of innate IL-22 (89). LTi cells express RORγ as well and most likely rely on this transcription factor to induce innate IL-17 expression (90). Additionally, LTi-like cells expressing IL-23R, AhR, and CCR6 will rapidly produce IL-17, IL-17F, and IL-22 in an innate capacity when stimulated with IL-23 along with the TLR2 ligand zymosan (91). Therefore, as recently described for γδ T cells, a combination of innate-derived IL-23 with an innate-activation signal (TLR2) can lead to rapid IL-17 production in non-αβ T cells. However, other factors such as potential contributions of additional Th17-polarizing cytokines and transcription factors as well as the physiological relevance of IL-17 and IL-17F production in LTi and LTi-like cells needs to be further addressed.
3.3. Natural Killer and Natural Killer T cells
NK cells are innate cells that function in two distinct capacities: 1) cytotoxic ability through granzyme and death receptor expression and 2) cytokine production to activate other immune cells. NK cells utilize both activating and inhibitory receptors to regulate their potent functions, where activating receptors recognize danger signals and inhibitory receptors recognize normal healthy conditions (reviewed in (92)). A role for NK cells in exhibiting certain Th17-like factors has previously been described. In certain situations NKp44+ NK cells produce high levels of innate IL-22 (93). Additionally, NK cells can express high levels of the transcription factor RORγ (94). Thus, it seems plausible that NK cells would be another innate source of IL-17 and IL-17F, especially considering their developmental similarity to LTi cells. However, only one report has demonstrated that NK cells have this potential. In a model of toxoplasmosis, NK cells were able to generate IL-17 through a mechanism that was dependent on IL-6 (95). Thus, further assessments of NK cells as IL-17 producers are warranted.
Natural killer T cells (NKT) are class of T lymphocytes that express NK cells markers such as NK.1.1 and that recognize lipid antigens presented by antigen presenting cells on the CD1d molecule. NKT cells can be divided into different categories based on TCR expression and reactivity to the ligand α-galactosylceramide (αGalCer) (reviewed in (96)). Of particular importance are the invariant NKT cells (iNKT) that express a single restricted TCR receptor and are activated by αGalCer. These cells were also shown to produce IL-17 in an innate capacity following TCR ligation and IL-23 stimulation (97). iNKT cells did not, however, require IL-6 to induce IL-17. Furthermore, CD4-/NK1.1+ NKT T cells were recently described as being the most potent IL-17 producers compared to the CD4+ and NK1.1+ NKT subsets (98). As in the case of other recently identified sources of innate IL-17, the factors regulating the production of IL-17 needs to be further examined. For example, IL-1 can induce NKT expansion (99) and NKT cells highly express the IL-21 receptor (100). Thus, it would be interesting to examine if these cytokines can influence IL-17 production in NKT cells.
3.4. Macrophages
Macrophages have been shown in a few reports to produce IL-17 under special circumstances as well. However, the signaling pathways utilized by macrophages for the innate production of IL-17 remain largely unknown. One such example came from testing for the presence of IL-17 in human breast cancer tissues. The authors found that CD68-stained macrophages also stained positive for IL-17 by histology (101). Moreover, in mice IL-17 production was demonstrated following chitin stimulation in a TLR2-dependent manner (102). Likewise, Song and colleagues provided evidence suggesting that IL-17 production can be dominated by alveolar macrophages in a model of allergic inflammation (103). However, a recent report has suggested that while IL-17F may be present in macrophages and other innate immune cells, IL-17 is mostly limited to T cells (104).
3.5. Additional innate sources of IL-17A and IL-17F
Paneth cells are granular epithelial cells that function in an innate capacity to produce antimicrobial products along with pro-inflammatory cytokines such as TNFα (reviewed in (105)). Recently, Paneth cells were found to contain large intracellular stores of IL-17 that could be rapidly released following TNFα stimulation (106). TLR5, an innate receptor recognizing bacterial flagella, was found to induce IL-17 from CD3-/CD127+ cells residing in the spleen and mucosa in a dendritic cell-dependent manner (107). These cells may represent an LTi-like subset, as they are similar to LTi and NK cells but lack the expression of RORγ. Neutrophils, being a major recruitment target of IL-17 and IL-17F activation in innate cells, seem to be an unlikely source of IL-17. However, a recent paper that focused on a mouse model of ischemia-reperfusion injury suggests otherwise. IL-17-producing neutrophils are present within three hours following injury and likely depend on IL-23 (108). Additionally, Bordetella pertussis infection was shown to induce IL-17 production from infiltrating neutrophils, macrophages, and T lymphocytes (109). Epithelial cells have been shown to be an innate source of IL-17F but not IL-17 in the lung and colon (104, 110). Finally, mast cells were recently show to be a dominant source of IL-17 in human arthritis; mast cells relied on RORγ and produced IL-17 following activation with pro-inflammatory cytokines or TLR signal (111). Additional analysis of these cell types will further our understanding of the innate immune system and IL-17 production.
3.6. Innate mechanisms regulating Th17 cells
Although strictly members of the adaptive arm of immunity, CD4+ and CD8+ T lymphocytes as well as B cells also express various members of the innate, evolutionary-conserved TLR family (reviewed in (112) and (113)). Therefore, our group analyzed the direct role of TLR signaling in Th17 maintenance and function (72). We found that TLR2 was enhanced in the Th17 lineage compared to the Th1 and Th2 subsets. Ligation of TLR2 led to a costimulatory effect in polarizing Th17 cells as well promoting their expansion. In vivo, the lack of TLR2 expression directly on CD4+ T cells led to reduced Th17 generation and an almost complete protection from the development of EAE. Furthermore, we demonstrated that CD4+ T cells are likely activated by endogenous TLR2 ligands generated during the inflammatory process, which as of now are still undefined. Future studies should focus on other innate pathways that CD4+ T lymphocytes may utilize for IL-17 production, expanding our knowledge of direct innate regulation by molecules expressed by adaptive immune cells.
4. IL-17E (IL-25)
IL-25, a distinct cytokine in the IL-17 family was originally identified on the basis of searching for sequence homology to the other IL-17 family members (10, 114). Seminal studies in renal carcinoma cell lines showed an effect of IL-25 in inducing the expression of the pro-inflammatory cytokine, IL-8 through NF-κB activation (114, 115). However, additional studies in vivo indicated that IL-25 plays vital roles in regulating type-2 immune response (115). While IL-17 and IL-17F induce neutrophila, participate in immunity against certain bacterial and fungal infections, and are involved in the pathogenesis of multiple autoimmune diseases; IL-25 promotes eosinophila and appears to play important roles in Th2-mediated host defense against helminthic parasite infection as well as in exacerbating allergic airway diseases.
4.1 The expression and regulation of IL-25
IL-25 was initially reported to be derived from highly polarized Th2 cells (10), but later on it was found to be expressed by several cell types both in the hematopoietic and non-hematopoietic compartment. IL-25 mRNA was expressed by IgE-activated mast cells (116), alveolar macrophages (117), microglia (118), eosinophils (119) (120), basophils (120), epithelial cells (121, 122), and endothelial cells (123). In the brain, IL-25 mRNA is constitutively expressed by mouse microglia and by brain capillary endothelial cells and its expression is involved in protection from inflammatory brain diseases such as MS (118, 123). In the gut, IL-25 can be found in the large intestine, in particular intestinal epithelial cells, and is involved in maintenance of intestinal homeostasis (122). In the lung, IL-25 was induced in mouse models of allergic lung disease (124). Our group has demonstrated that several allergens such as ragweed and fungal protease can induce IL-25 mRNA expression in lung epithelial cells (121). Therefore, IL-25 plays important roles in the pathogenesis of allergic lung disease and the tissue expression of IL-25 contributes to immune responses against pathogens and controls local inflammation.
Although many reports provide insight into which cells produce IL-25, the mechanisms governing the expression of this cytokine remain unclear. IL-25 can be detected in intestinal epithelial cells from conventionally reared mice, however, IL-25 expression is absent in the germ-free mice (122), suggesting that signals from gut pathogens can induce IL-25 expression. In the airway, IL-25 in lung epithelial cells can be induced by protease allergens (121, 125). The induction of IL-25 by allergens was shown to be suppressed by Erk, JNK, and p38 inhibitors (125), suggesting that allergens can regulate the expression of IL-25 through the MAPK pathway. Furthermore, some airway epithelial cell-derived factors, such as matrix metalloproteinase 7 (MMP7), have been shown to modulate IL-25 function in the airway during allergic asthma (126). IL-25 can be cleaved by MMP7 at multiple sites and this processed IL-25 is better at inducing Th2 cytokine production compared to native IL-25 (126). Finally, viral infection was also found to induce the expression of IL-25 in lung tissue and that this IL-25 expression may contribute to virus-induced exacerbation of allergic asthma (127). Thus, signals from pathogens themselves may be capable of regulating IL-25 expression.
4.2 IL-25 receptor and target cells
The receptor for IL-25 was identified based on homology searches for sequences related to the IL-17 receptor. The IL-25 receptor was found to be the same receptor for IL-17B (termed IL-17RB or EV127); however, IL-25 was found to have a higher binding affinity to IL-17RB compared to IL-17B (114, 128, 129). Further studies by Rickel et al. found that the functional IL-25 receptor indeed requires not only IL-17RB but also IL-17RA, where mice deficient in either IL-17RA or IL-17RB did not respond to IL-25 (130). Similar to the IL-17 receptor that requires IL-17RA and IL-17RC complex, the interaction of both IL-17RA and IL-17RB is important for IL-25 activity. While IL-17 mainly functions on structural cells such as epithelial cells and fibroblasts to induce pro-inflammatory cytokines and chemokines, IL-25 mostly targets hematopoietic cells and induces the production of Th2 cytokines. Both innate and adaptive immune cells are currently known to be potential targets for IL-25.
Several IL-25 innate immune responder cells, including monocytes (119, 131) and NKT cells (132, 133) were reported to express functional IL-17RB and IL-25 treatment of these cells induced Th2 cytokine production. In vivo, IL-25 treatment in Rag knockout mice induced Th2 cytokine production from undefined populations of non-T/non-B (NBNT) cells (10). Recently, three groups independently identified a novel population of innate effector cells that are indeed innate IL-25 responders. These cells are responsible for the production of Th2 cytokines, in particular IL-13, during helminthic parasite infection and serve to mediate worm expulsion (134–136). These innate IL-25 responder cells were named accordingly to investigator preference: nuocyte by Neill et al. (134), MPPtype2 cells by Saenz et al. (135), and ih2 cells by Price et al (136). Whether these cells are the same population and function in other Th2-associated diseases remains unclear.
Not only can IL-25 target innate immune cells to produce Th2 cytokines, it also can direct naïve T helper cells towards Th2 commitment as well as augment the cytokine production of effector/memory Th2 cells (120, 121). IL-17RB mRNA was highly expressed in Th2 cells but not in Th1 and Th17 cells (121, 137). Treatment of naïve T cells with IL-25 promoted the differentiation of Th2 cells in an IL-4 and STAT-6 dependent manner, but had no effect on the polarization of Th1 and Th17 cells. Furthermore, our group demonstrated that IL-25 could augment c1 and JunB transcription factors during early T cell differentiation, possibly resulting in upregulation of intital IL-4 levels(121). Moreover, the expression of IL-17RB can be detected in circulating human Th2 memory cells and further up-regulated in CD3/CD28-activated cells (120, 138). Thymic stromal lymphopoietin (TSLP) is known to activate dendritic cells produce express Th2-polarizing signals and TSLP-activated dendritic cells can direct the differentiation of Th2 cells (139). In the presence of TSLP-activated dendritic cells, IL-17RB on Th2 memory cells was strongly induced and further IL-25 treatment enhanced their polarization and proliferation (120). Moreover, Th9 cells, a recently identified T helper lineage that produces mainly IL-9, was also found to express IL-17RB (137). Unlike Th2 cells, the differentiation of Th9 from naïve T cells is induced by TGF-β and IL-4 (140, 141). Th9 cells demonstrated the highest expression of IL-17RB compared to other Th subsets and treatment of IL-25 on differentiating Th9 cells enhanced their IL-9 and IL-10 production (137). Enforced expression of IL-17RB by CD4+ T cells induced substantial production of IL-4, IL-5, IL-13, and IL-9, implicating the involvement of IL-25 function in not only Th2 but also Th9 differentiation (137). These data indicate that IL-25 may act on both innate responder cells and adaptive T helper cells to induce type-2 immunity.
4.3 Biological function of IL-25
IL-25 has been implicated in the regulation of type-2 immune responses. In vivo studies by IL-25 protein injection and in IL-25 transgenic mice showed the induction of eosinophila and Th2 cytokine expression in several tissues (10, 142, 143). There have been many reports on the pathogenic roles of IL-25 in Th2-based diseases, especially those pertaining to allergic lung. IL-25 is not only linked to allergic disease, but also plays a protective role in helminthic parasite infection. Additionally, IL-25 functions in the regulation of some autoimmune diseases, such as EAE. The known roles of IL-25 in disease are summarized in Table 3.
Table 3.
The role of IL-25 in disease
| Disease | Evidence in human/mouse | Source of IL-25 | IL-25 target cells | Role in disease | References |
|---|---|---|---|---|---|
| Allergic rhinitis/asthma | Human, mouse | Epithelial cells, Eosinophils, Basophils, Mast cells | Th2 cells, Th9 cells, NKT cells, Monocytes | Induce Th2 cytokines, lung inflammation Induce lung remodeling and AHR Exacerbate diseases | (121, 125, 137, 144, 157) |
| Autoimmune disease (EAE) | Mouse | Microglia, Brain capillary endothelial cells | ? | Induce Th2 cytokines, Suppress Th17 cytokines, Inhibit EAE development Maintain blood brain barrier integrity | (118, 123) |
| Colitis | Human, mouse | ? | CD14+ cells | Inhibit pro-inflammatory cytokines, Protective role in colitis | (158, 159) |
| Helminth parasite infection | Mouse | ? | NBNT cells | Induce Th2 cytokines Induce worm clearance limit chronic inflammation | (8, 9, 134–136) |
4.3.1 Pathogenic roles of IL-25 in allergic lung diseases
Studies in both mice and humans have demonstrated the crucial roles of IL-25 in the pathogenesis of allergic lung diseases. Accordingly, the expression of IL-25 and IL-17RB has been detected in asthmatic lung tissues (120, 124). In patients with allergic rhinitis, IL-25 and TSLP was found to be upregulated in nasal lavages (144). Overexpression of IL-25 in the lung epithelium of mice induced epithelial cell hyperplasia, mucus hyper-secretion, airway infiltration of eosinophils and macrophages, and the upregulation of chemokines associated with type-2 immunity (121). In contrast, IL-25 neutralization attenuated allergic inflammation with reduced eosinophils and CD4+ T cells in the airway (121). Moreover, mice treated with anti-IL-25 exhibited less Th2 cytokine production in bronchoalveolar lavage and reduced OVA-specific Th2 cytokine production in splenocytes (121). Likewise, IL-25-deficient mice showed significant attenuation of allergic inflammation, characterized by reductions in both Th2 and Th9 cytokines (137). Indeed, IL-25 appears to be important for both the sensitization and the challenge phase of airway responses (145). IL-25 blockade during the sensitization phase resulted in reduced levels of not only Th2 cytokines, but also goblet cell hyperplasia and serum IgE secretion (145). More importantly, neutralizing IL-25 either in the sensitization or the challenge phase can prevent airway hyper-responsiveness (AHR) (145). The induction of airway hyper-reactivity by IL-25 was dependent on IL-17RB-expressing NKT cells (132, 133). Similarly, inhibition of the IL-25 and IL-17RB interaction through the use of soluble IL-17RB or an anti-IL-17RB in a mouse model of asthma resulted in reduced airway inflammation, hyper-reactivity, and airway remodeling (124, 145). More recent studies have found enhanced expression of IL-25 in the lung following pneumonia virus infection, suggesting that IL-25 induced by early-life virus infection may promote further development of allergic airway responses (127). These data thus indicate the importance of IL-25 in promoting allergic lung disease and the IL-25 pathway may be a target of novel therapeutics in the future.
4.3.2 Protective roles of IL-25 in helminthic parasite infection
Helminthes are parasites known to induce strong type-2 immune responses. Recent data indicate that IL-25 strongly contributes to protective immunity against intestinal helminthic infection. The role of IL-25 in helminthic parasite infection has been examined in two different models thus far. In a mouse model of Trichuriasis (whipworm infection), exogenous IL-25 treatment induced a protective Th2 response and worm expulsion in genetically susceptible mice (9). In contrast, IL-25 knockout mice were unable to eradicate the infection. The mechanism by which IL-25 regulated worm protection in this model was shown to be lymphocyte dependent. During chronic infection with Trichuris, IL-25 was also able to ameliorate chronic inflammation and maintain mucosal integrity (9). Likewise, in hookworm Nippostrongylus braziliensis infection, IL-25 was shown to be involved in worm clearance (8). In IL-25-deficient mice, this worm could not be expelled efficiently, which correlated with a significantly reduced level of type-2 cytokines (8). Unlike whipworm infection, IL-25 seems to mediate worm expulsion independent of lymphocytes in N. braziliensis-infected mice. IL-25 treated-Rag knockout mice were still able to eradicate the worm (8). The mechanism of worm protection mediated by IL-25 in this mouse model was found to be dependent on NBNT cells (8). More recent work has clearly demonstrated that NBNT cells are indeed the novel innate type-2 effector cells responsible for the IL-25 effect in N. braziliensis infection (134–136). These studies indicate the essential function of IL-25 in protection to helminthic parasite infection. Whether IL-25 plays roles in other helminthic parasite infections or utilizes a similar pathway(s) for protection remains unclear.
5. Summary and conclusions
Although traditionally thought of as adaptive responders, Th17-related cytokines (IL-17 and IL-17F) have various innate sources and function as rapidly produced pro-inflammatory mediators. Innate IL-17-producing cells also employ many of the cytokine and transcriptional regulators utilized by Th17 cells. Clearly, γδ T cells have been shown to have important immunological consequences as a function of their IL-17 production. However, further research needs to expand on the regulation of IL-17, IL-17F, and IL-22 in this cell type. Moreover, the relative contributions of IL-17 and IL-17F expression from NK cells, LTi cells, and other hematopoietic and non-hematopoietic lineages needs to be more thoroughly addressed to determine the physiological relevance of their innate IL-17 production. The unifying factors of innate IL-17 and IL-17F production in various cell types include ROR family expression, IL-23 reactivity, and possibly TLR-mediated activation.
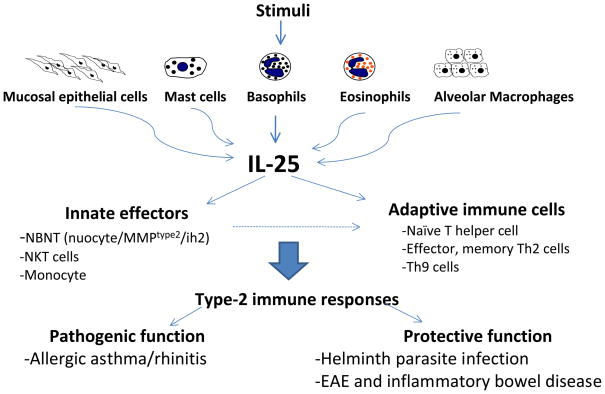
Unlike IL-17 and IL-17F, IL-25 has unique functions in promoting type-2 immunity as well as inhibiting pro-inflammatory responses. IL-25, expressed by several innate immune cell types including mucosal epithelial cells, eosinophils, basophils, and mast cells, is capable of inducing Th2 cytokines from both innate and adaptive IL-25 responders. IL-25 can target innate immune cells, including NKT cells, monocytes, and NBNT cells, which are recently identified novel innate effector cells that produce the initial Th2 cytokines during helminth parasite infection. IL-25 also can act on naïve T cells by promoting polarization toward the Th2 lineage and on memory/effector Th2 cells by enhancing their Th2 cytokine production. Additionally, a novel T helper lineage, Th9 can also respond to IL-25, which functions in augmenting IL-9 production. The effect of IL-25 on multiple cells can thus result in dysregulated Th2 responses in asthma, protective immunity to helminth infection, and pathological suppression of autoimmune diseases (Figure 2).
Figure 2.
Many stimuli such as allergens or pathogens can induce several cell types to express IL-25. IL-25 functions on both innate effectors and adaptive T cells to promote type-2 immune responses. IL-25 plays pathogenic roles in allergic lung diseases and protective roles in helminth parasite infection and some organ-specific autoimmune diseases such as experimental encephalitis autoimmune disease (EAE) and colitis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine. 2009;46:7–11. doi: 10.1016/j.cyto.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 3.Dong C. Differentiation and function of pro-inflammatory Th17 cells. Microbes Infect. 2009;11:584–588. doi: 10.1016/j.micinf.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hymowitz SG, Filvaroff EH, Yin JP, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. Embo J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Fujio K, Shoda H, et al. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007;179:7128–7136. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 6.Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW. IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumoniae infection. Microbes Infect. 2007;9:78–86. doi: 10.1016/j.micinf.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Chen J, Huang A, et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci U S A. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fallon PG, Ballantyne SJ, Mangan NE, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owyang AM, Zaph C, Wilson EH, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fort MM, Cheung J, Yen D, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 11.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6:329–334. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 12.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Yao Z, Painter SL, Fanslow WC, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483–5486. [PubMed] [Google Scholar]
- 14.Aarvak T, Chabaud M, Miossec P, Natvig JB. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J Immunol. 1999;162:1246–1251. [PubMed] [Google Scholar]
- 15.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 17.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 18.Dong C, Flavell RA. Cell fate decision: T-helper 1 and 2 subsets in immune responses. Arthritis Res. 2000;2:179–188. doi: 10.1186/ar85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glimcher LH, Murphy KM. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 2000;14:1693–1711. [PubMed] [Google Scholar]
- 20.Chung Y, Chang SH, Martinez GJ, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35:555–562. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi M, Onuchic LF, Li XD, et al. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J Immunol. 2001;167:4430–4435. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 23.Wright JF, Guo Y, Quazi A, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 24.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 25.Kuestner RE, Taft DW, Haran A, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian Y, Liu C, Hartupee J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 28.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 29.Martinez GJ, Nurieva RI, Yang XO, Dong C. Regulation and function of proinflammatory TH17 cells. Ann N Y Acad Sci. 2008;1143:188–211. doi: 10.1196/annals.1443.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XO, Chang SH, Park H, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruddy MJ, Wong GC, Liu XK, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 32.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 33.Happel KI, Zheng M, Young E, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Happel KI, Dubin PJ, Zheng M, et al. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aujla SJ, Chan YR, Zheng M, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun. 2007;75:3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 40.Murphy CA, Langrish CL, Chen Y, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 42.Nakae S, Saijo S, Horai R, Sudo K, Mori S, Iwakura Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci U S A. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 45.Piskin G, Sylva-Steenland RM, Bos JD, Teunissen MB. In vitro and in situ expression of IL-23 by keratinocytes in healthy skin and psoriasis lesions: enhanced expression in psoriatic skin. J Immunol. 2006;176:1908–1915. doi: 10.4049/jimmunol.176.3.1908. [DOI] [PubMed] [Google Scholar]
- 46.Ma HL, Liang S, Li J, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008;118:597–607. doi: 10.1172/JCI33263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma HL, Napierata L, Stedman N, et al. Tumor necrosis factor alpha blockade exacerbates murine psoriasis-like disease by enhancing Th17 function and decreasing expansion of Treg cells. Arthritis Rheum. 2010;62:430–440. doi: 10.1002/art.27203. [DOI] [PubMed] [Google Scholar]
- 48.Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128:1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Rodriguez J, Sahu N, Handon R, et al. Differential expression of interleukin-17A and -17F is coupled to T cell receptor signaling via inducible T cell kinase. Immunity. 2009;31:587–597. doi: 10.1016/j.immuni.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe H, Kawaguchi M, Fujishima S, et al. Functional characterization of IL-17F as a selective neutrophil attractant in psoriasis. J Invest Dermatol. 2009;129:650–656. doi: 10.1038/jid.2008.294. [DOI] [PubMed] [Google Scholar]
- 51.Fujishima S, Watanabe H, Kawaguchi M, et al. Involvement of IL-17F via the induction of IL-6 in psoriasis. Arch Dermatol Res. 2010 doi: 10.1007/s00403-010-1033-8. [DOI] [PubMed] [Google Scholar]
- 52.Chien YH, Bonneville M. Gamma delta T cell receptors. Cell Mol Life Sci. 2006;63:2089–2094. doi: 10.1007/s00018-006-6020-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7 −/− mice. J Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- 54.Xiong N, Raulet DH. Development and selection of gammadelta T cells. Immunol Rev. 2007;215:15–31. doi: 10.1111/j.1600-065X.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- 55.De Rosa SC, Andrus JP, Perfetto SP, Mantovani JJ, Herzenberg LA, Roederer M. Ontogeny of gamma delta T cells in humans. J Immunol. 2004;172:1637–1645. doi: 10.4049/jimmunol.172.3.1637. [DOI] [PubMed] [Google Scholar]
- 56.Girardi M. Immunosurveillance and immunoregulation by gammadelta T cells. J Invest Dermatol. 2006;126:25–31. doi: 10.1038/sj.jid.5700003. [DOI] [PubMed] [Google Scholar]
- 57.Tough DF, Sprent J. Lifespan of gamma/delta T cells. J Exp Med. 1998;187:357–365. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Born WK, Reardon CL, O’Brien RL. The function of gammadelta T cells in innate immunity. Curr Opin Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Roark CL, Simonian PL, Fontenot AP, Born WK, O’Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jensen KD, Su X, Shin S, et al. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 62.Ribot JC, deBarros A, Pang DJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haas JD, Gonzalez FH, Schmitz S, et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 64.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 65.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 66.Do JS, Fink PJ, Li L, et al. Cutting edge: spontaneous development of IL-17-producing gamma delta T cells in the thymus occurs via a TGF-beta 1-dependent mechanism. J Immunol. 2010;184:1675–1679. doi: 10.4049/jimmunol.0903539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riol-Blanco L, Lazarevic V, Awasthi A, et al. IL-23 receptor regulates unconventional IL-17-producing T cells that control bacterial infections. J Immunol. 2010;184:1710–1720. doi: 10.4049/jimmunol.0902796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Nurieva R, Yang XO, Martinez G, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 70.Eberl M, Engel R, Beck E, Jomaa H. Differentiation of human gamma-delta T cells towards distinct memory phenotypes. Cell Immunol. 2002;218:1–6. doi: 10.1016/s0008-8749(02)00519-1. [DOI] [PubMed] [Google Scholar]
- 71.Thedrez A, Harly C, Morice A, Salot S, Bonneville M, Scotet E. IL-21-mediated potentiation of antitumor cytolytic and proinflammatory responses of human V gamma 9V delta 2 T cells for adoptive immunotherapy. J Immunol. 2009;182:3423–3431. doi: 10.4049/jimmunol.0803068. [DOI] [PubMed] [Google Scholar]
- 72.Reynolds JM, Pappu BP, Peng J, et al. Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity. 2010;32:692–702. doi: 10.1016/j.immuni.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brustle A, Heink S, Huber M, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 75.Veldhoen M, Hirota K, Westendorf AM, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 76.Hamada S, Umemura M, Shiono T, et al. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meeks KD, Sieve AN, Kolls JK, Ghilardi N, Berg RE. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J Immunol. 2009;183:8026–8034. doi: 10.4049/jimmunol.0901588. [DOI] [PubMed] [Google Scholar]
- 78.Cho JS, Pietras EM, Garcia NC, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 80.Umemura M, Yahagi A, Hamada S, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 81.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 82.Rajan AJ, Gao YL, Raine CS, Brosnan CF. A pathogenic role for gamma delta T cells in relapsing-remitting experimental allergic encephalomyelitis in the SJL mouse. J Immunol. 1996;157:941–949. [PubMed] [Google Scholar]
- 83.Lees JR, Iwakura Y, Russell JH. Host T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell lines. J Immunol. 2008;180:8066–8072. doi: 10.4049/jimmunol.180.12.8066. [DOI] [PubMed] [Google Scholar]
- 84.Wohler JE, Smith SS, Zinn KR, Bullard DC, Barnum SR. Gammadelta T cells in EAE: early trafficking events and cytokine requirements. Eur J Immunol. 2009;39:1516–1526. doi: 10.1002/eji.200839176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–950. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 86.Cui Y, Shao H, Lan C, et al. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–567. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim MY, Kim KS, McConnell F, Lane P. Lymphoid tissue inducer cells: architects of CD4 immune responses in mice and men. Clin Exp Immunol. 2009;157:20–26. doi: 10.1111/j.1365-2249.2009.03932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cupedo T, Crellin NK, Papazian N, et al. Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat Immunol. 2009;10:66–74. doi: 10.1038/ni.1668. [DOI] [PubMed] [Google Scholar]
- 89.Lane P, Kim MY, Withers D, et al. Lymphoid tissue inducer cells in adaptive CD4 T cell dependent responses. Semin Immunol. 2008;20:159–163. doi: 10.1016/j.smim.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 90.Eberl G, Marmon S, Sunshine MJ, Rennert PD, Choi Y, Littman DR. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 91.Takatori H, Kanno Y, Watford WT, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 93.Cella M, Fuchs A, Vermi W, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luci C, Reynders A, Ivanov II, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol. 2009;10:75–82. doi: 10.1038/ni.1681. [DOI] [PubMed] [Google Scholar]
- 95.Passos ST, Silver JS, O’Hara AC, Sehy D, Stumhofer JS, Hunter CA. IL-6 promotes NK cell production of IL-17 during toxoplasmosis. J Immunol. 2010;184:1776–1783. doi: 10.4049/jimmunol.0901843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 97.Rachitskaya AV, Hansen AM, Horai R, et al. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coquet JM, Chakravarti S, Kyparissoudis K, et al. Diverse cytokine production by NKT cell subsets and identification of an IL-17-producing CD4-NK1.1-NKT cell population. Proc Natl Acad Sci U S A. 2008;105:11287–11292. doi: 10.1073/pnas.0801631105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brinster C, Shevach EM. Costimulatory effects of IL-1 on the expansion/differentiation of CD4+CD25+Foxp3+ and CD4+CD25+Foxp3-T cells. J Leukoc Biol. 2008;84:480–487. doi: 10.1189/jlb.0208085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Coquet JM, Kyparissoudis K, Pellicci DG, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–2834. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 101.Zhu X, Mulcahy LA, Mohammed RA, et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Song C, Luo L, Lei Z, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 104.Ishigame H, Kakuta S, Nagai T, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 105.Keshav S. Paneth cells: leukocyte-like mediators of innate immunity in the intestine. J Leukoc Biol. 2006;80:500–508. doi: 10.1189/jlb.1005556. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi N, Vanlaere I, de Rycke R, et al. IL-17 produced by Paneth cells drives TNF-induced shock. J Exp Med. 2008;205:1755–1761. doi: 10.1084/jem.20080588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Maele L, Carnoy C, Cayet D, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J Immunol. 2010;185:1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li L, Huang L, Vergis AL, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andreasen C, Powell DA, Carbonetti NH. Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS One. 2009;4:e7079. doi: 10.1371/journal.pone.0007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Suzuki S, Kokubu F, Kawaguchi M, et al. Expression of interleukin-17F in a mouse model of allergic asthma. Int Arch Allergy Immunol. 2007;143(Suppl 1):89–94. doi: 10.1159/000101413. [DOI] [PubMed] [Google Scholar]
- 111.Hueber AJ, Asquith DL, Miller AM, et al. Mast cells express IL-17A in rheumatoid arthritis synovium. J Immunol. 2010;184:3336–3340. doi: 10.4049/jimmunol.0903566. [DOI] [PubMed] [Google Scholar]
- 112.MacLeod H, Wetzler LM. T cell activation by TLRs: a role for TLRs in the adaptive immune response. Sci STKE. 2007:pe48. doi: 10.1126/stke.4022007pe48. [DOI] [PubMed] [Google Scholar]
- 113.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 114.Lee J, Ho WH, Maruoka M, et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 115.Hurst SD, Muchamuel T, Gorman DM, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 116.Ikeda K, Nakajima H, Suzuki K, et al. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–3596. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 117.Kang CM, Jang AS, Ahn MH, et al. Interleukin-25 and interleukin-13 production by alveolar macrophages in response to particles. Am J Respir Cell Mol Biol. 2005;33:290–296. doi: 10.1165/rcmb.2005-0003OC. [DOI] [PubMed] [Google Scholar]
- 118.Kleinschek MA, Owyang AM, Joyce-Shaikh B, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dolgachev V, Petersen BC, Budelsky AL, Berlin AA, Lukacs NW. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived Th2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. J Immunol. 2009;183:5705–5715. doi: 10.4049/jimmunol.0901666. [DOI] [PubMed] [Google Scholar]
- 120.Wang YH, Angkasekwinai P, Lu N, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Angkasekwinai P, Park H, Wang YH, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zaph C, Du Y, Saenz SA, et al. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. J Exp Med. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sonobe Y, Takeuchi H, Kataoka K, et al. Interleukin-25 expressed by brain capillary endothelial cells maintains blood-brain barrier function in a protein kinase Cepsilon-dependent manner. J Biol Chem. 2009;284:31834–31842. doi: 10.1074/jbc.M109.025940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tamachi T, Maezawa Y, Ikeda K, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 125.Yu HS, Angkasekwinai P, Chang SH, Chung Y, Dong C. Protease allergens induce the expression of IL-25 via Erk and p38 MAPK pathway. J Korean Med Sci. 2010;25:829–834. doi: 10.3346/jkms.2010.25.6.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goswami S, Angkasekwinai P, Shan M, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nat Immunol. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Siegle JS, Hansbro N, Herbert C, et al. Early-life viral infection and allergen exposure interact to induce an asthmatic phenotype in mice. Respir Res. 2010;11:14. doi: 10.1186/1465-9921-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shi Y, Ullrich SJ, Zhang J, et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem. 2000;275:19167–19176. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- 129.Tian E, Sawyer JR, Largaespada DA, Jenkins NA, Copeland NG, Shaughnessy JD., Jr Evi27 encodes a novel membrane protein with homology to the IL17 receptor. Oncogene. 2000;19:2098–2109. doi: 10.1038/sj.onc.1203577. [DOI] [PubMed] [Google Scholar]
- 130.Rickel EA, Siegel LA, Yoon BR, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 131.Caruso R, Stolfi C, Sarra M, et al. Inhibition of monocyte-derived inflammatory cytokines by IL-25 occurs via p38 Map kinase-dependent induction of Socs-3. Blood. 2009;113:3512–3519. doi: 10.1182/blood-2008-08-172767. [DOI] [PubMed] [Google Scholar]
- 132.Terashima A, Watarai H, Inoue S, et al. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med. 2008;205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stock P, Lombardi V, Kohlrautz V, Akbari O. Induction of airway hyperreactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J Immunol. 2009;182:5116–5122. doi: 10.4049/jimmunol.0804213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Neill DR, Wong SH, Bellosi A, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Saenz SA, Siracusa MC, Perrigoue JG, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Price AE, Liang HE, Sullivan BM, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Angkasekwinai P, Chang SH, Thapa M, Watarai H, Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang YH, Ito T, Homey B, et al. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 139.Liu YJ. Thymic stromal lymphopoietin: master switch for allergic inflammation. J Exp Med. 2006;203:269–273. doi: 10.1084/jem.20051745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 141.Dardalhon V, Awasthi A, Kwon H, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(−) effector T cells. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pan G, French D, Mao W, et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 143.Kim MR, Manoukian R, Yeh R, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 144.Xu G, Zhang L, Wang DY, et al. Opposing roles of IL-17A and IL-25 in the regulation of TSLP production in human nasal epithelial cells. Allergy. 2010;65:581–589. doi: 10.1111/j.1398-9995.2009.02252.x. [DOI] [PubMed] [Google Scholar]
- 145.Ballantyne SJ, Barlow JL, Jolin HE, et al. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 146.Huber M, Heink S, Grothe H, et al. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol. 2009;39:1716–1725. doi: 10.1002/eji.200939412. [DOI] [PubMed] [Google Scholar]
- 147.Hamada H, Garcia-Hernandez Mde L, Reome JB, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martin-Orozco N, Muranski P, Chung Y, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.You Z, DuRaine G, Tien JY, Lee C, Moseley TA, Reddi AH. Expression of interleukin-17B in mouse embryonic limb buds and regulation by BMP-7 and bFGF. Biochem Biophys Res Commun. 2005;326:624–631. doi: 10.1016/j.bbrc.2004.11.087. [DOI] [PubMed] [Google Scholar]
- 150.Kokubu T, Haudenschild DR, Moseley TA, Rose L, Reddi AH. Immunolocalization of IL-17A, IL-17B, and their receptors in chondrocytes during fracture healing. J Histochem Cytochem. 2008;56:89–95. doi: 10.1369/jhc.7A7223.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Moore EE, Presnell S, Garrigues U, et al. Expression of IL-17B in neurons and evaluation of its possible role in the chromosome 5q-linked form of Charcot-Marie-Tooth disease. Neuromuscul Disord. 2002;12:141–150. doi: 10.1016/s0960-8966(01)00250-4. [DOI] [PubMed] [Google Scholar]
- 152.Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 153.Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol. 2002;169:642–646. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- 154.Croxford AL, Kurschus FC, Waisman A. Cutting edge: an IL-17F-CreEYFP reporter mouse allows fate mapping of Th17 cells. J Immunol. 2009;182:1237–1241. doi: 10.4049/jimmunol.182.3.1237. [DOI] [PubMed] [Google Scholar]
- 155.Kawaguchi M, Kokubu F, Huang SK, et al. The IL-17F signaling pathway is involved in the induction of IFN-gamma-inducible protein 10 in bronchial epithelial cells. J Allergy Clin Immunol. 2007;119:1408–1414. doi: 10.1016/j.jaci.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 156.Michel ML, Keller AC, Paget C, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang YH, Angkasekwinai P, Lu N, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McHenga SS, Wang D, Li C, Shan F, Lu C. Inhibitory effect of recombinant IL-25 on the development of dextran sulfate sodium-induced experimental colitis in mice. Cell Mol Immunol. 2008;5:425–431. doi: 10.1038/cmi.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Caruso R, Sarra M, Stolfi C, et al. Interleukin-25 inhibits interleukin-12 production and Th1 cell-driven inflammation in the gut. Gastroenterology. 2009;136:2270–2279. doi: 10.1053/j.gastro.2009.02.049. [DOI] [PubMed] [Google Scholar]