Abstract
In examining neural processing specific to the self, primarily by contrasting self-related stimuli with non-self-related stimuli (i.e., self vs. other), neuroimaging studies have activated a consistent set of regions, including medial prefrontal cortex (MPFC), precuneus, and right and left inferior parietal cortex. However, criticism has arisen that this network may not be specific to self-related processing, but instead reflects a more general aspect of cortical processing. For example, it is almost identical to the active network of the resting state, the “default” mode, when the subject is free to think about anything at all. We tested the self-specificity of this network by using transcranial magnetic stimulation (TMS) to briefly disrupt local cortical processing while subjects rated adjectives as like or unlike themselves or their best friend. Healthy volunteers show a self-reference effect (SRE) in this task, in which performance with self-related items is superior to that with other-related items. As individual adjectives appeared on a monitor, single-pulse TMS was applied at five different times relative to stimulus onset (SOA: stimulus onset asynchrony) ranging from 0 to 480 ms. In 18 subjects, TMS to left parietal cortex suppressed the SRE from 160 to 480 ms. SRE suppression occurred at later SOA with TMS to the right parietal cortex. In contrast, no effects were seen with TMS to MPFC. Together with our previous work, these results provide evidence for a self-specific processing system in which midline and lateral inferior parietal cortices, as elements of the default network, play a role in ongoing self-awareness.
Keywords: TMS, Self, Parietal cortex, Default network
Introduction
The concept of the “default” network was developed over the last decade after it was recognized, initially by Raichle (1998), that there was a common pattern to the deactivations occurring in imaging contrasts during PET or fMRI over a wide spectrum of tasks when images obtained during the task conditions were contrasted with resting or control conditions (Binder et al. 1999; Fransson 2006; Gould et al. 2006; Greicius et al. 2003; Gusnard et al. 2001; Gusnard and Raichle 2001; Mason et al. 2007; Mazoyor et al. 2001; McKiernan et al. 2003). This pattern has almost invariably included medial prefrontal cortex (MPFC), precuneus/posterior cingulate, and left and right lateral parietal cortex. It was suggested that these areas were a default network that the brain returned to when not engaged in specific responses to the outside world. While the functions of this network are not known, a number of hypotheses have emerged, from ongoing neural maintenance to keep the brain in a responsive state (Raichle et al. 2001) to a system for reviewing past knowledge, planning future behavior and supporting self-consciousness (Cavanna and Trimble 2006).
Over the same period, a number of neuroimaging studies have attempted to identify regions in the brain specific to processing the self, primarily by contrasting self-related stimuli with non-self-related stimuli (i.e., self vs. other). For example, studies have been conducted in which a subject’s own face is distinguished from other faces (Kircher et al. 2000, 2001; Platek et al. 2004), or his or her own name from other names (Perrin et al. 2005; Sugiura et al. 2006). Subjects have also been asked to recall personal vs. impersonal information (Maguire and Mummery 1999; Vinogradov et al. 2006; Nunez et al. 2005) or to assess their own vs. another’s personality traits, appearance, attitudes, or feelings (Craik et al. 1999; Gusnard et al. 2001; Kircher et al. 2000, 2001; Kelley et al. 2002; Johnson et al. 2002; Kjaer et al. 2002; Fossati et al. 1995; Schmitz et al. 2004; Ochsner et al. 2005), including our own work using PET in a task deciding whether visually presented adjectives described Self and Best Friend (Lou et al. 2004). A very consistent set of cortical regions—including MPFC, precuneus/posterior cingulate, and left and right lateral parietal cortex—has been activated in these studies, across this wide variety of self-related tasks, that looks remarkably identical to the default network.
The striking similarity of the network derived from self/other contrasts and the default network has led to speculation concerning the role of self-related processing in the default network (e.g., Lou et al. 2004), but interpretations have varied. Two recent reviews have in fact come to opposite conclusions. Schilbach et al. (2008) built upon ideas that the default network may be a neural correlate for a “sense of self” (Gusnard 2005; Wicker et al. 2003). They argued that this network may indeed support the integration of self-referential information. Schilbach et al. made the case that self-consciousness has a social dimension and that self-processing occurs within a context of social cognition. Social cognition according to these authors is the set of related processes by which self/other distinctions are made and which mediate engagement of self/other interactions. They suggested that this processing of self-relevant information is performed in the default network and that, when in an unconstrained (resting) state, humans are predisposed to entertain social thoughts about oneself or other people. On the other hand, another recent review of many of the same imaging studies came to the conclusion that the network found in self/other contrasts is not related to self-specific processing (Ruby and Legrand 2008). They noted that the regions comprising the “self” network are quite similar both to those activated in the “other” conditions of self/other tasks, as seen in contrasts with other control conditions (e.g., Calder and Wicker 2002) as well as to those in the default network. Given that the same network appears to be activated for representation of self-referential content, for representation of another’s mind, and for “operational” processing going on in the solitary and undisturbed brain at rest, Ruby and Legrand concluded that it is not activated by self-specific content. They offered an alternative explanation of a non-specific cerebral network involved in a reasoning process of hypothesis generation and selection using information available to the subject in the surrounding context and also recalled from memory. In this context, the greater brain activation in self-specific conditions of self/other tasks occurs because of their greater salience to the subject and the greater depth of processing or number of pathways to memory that stimuli related to the subject’s own person afford.
That opposing conjectures can arise in the interpretation of the same imaging results reflects the fact that brain imaging techniques are correlative and can only suggest brain/behavior relationships but cannot prove causal relationships. On the other hand, transcranial magnetic stimulation (TMS), a non-invasive means of temporarily modulating neural function, offers a way of testing whether direct causal relationships link brain with behavior (for a review, see Luber et al. 2007a). In the present case, modulation of performance in a self/other task caused by TMS applied to a node of the default network could demonstrate its involvement with self-specific processing. In a previous study, we were able to provide such a demonstration (Lou et al. 2004). Subjects rated adjectives as like or unlike themselves or their best friend. They consistently performed faster and more accurately when responding to adjectives in relation to themselves than to their best friend. It is typical to find such a self-reference effect (SRE) when processing stimuli related to the self compared to another (see Gillihan and Farah 2005 for a review). SREs may indicate a functionally distinct cognitive system for self-knowledge, much like the distinct systems proposed for language and face perception (e.g., Rogers et al. 1977). Guided by PET activation results, we tested the involvement of mid-line cortical regions in the default network by using TMS to briefly disrupt local cortical self-processing while subjects performed our task. Single-pulse TMS was applied to mid-line prefrontal cortex, precuneus, and midline occipital cortex at various times ranging from 0 to 480 ms after the onset of the visually presented adjective. We found a latency- and site-specific effect: while judgments about best friends were not affected by TMS, TMS did disrupt performance for self-judgments with stimulation of precuneus at 160 ms after stimulus onset, but not with stimulation of pre-frontal or occipital cortex at any latency. Since the TMS pulses did not affect responses involving the best friend as well as one’s self, it is unlikely they were disrupting a general memory retrieval system. Therefore, these results provide evidence that at least one cortical region included in the default network might process information in a self-specific way.
Left and right lateral parietal regions are also activated in most self/other tasks and are prominent elements of the default network. TMS to right parietal cortex has disrupted discrimination of one’s own face from another’s (Uddin et al. 2006). While in our previous study, we concentrated on midline sites of the network; in this study, we applied single-pulse TMS to the left and right inferior parietal regions (as well as MPFC) while subjects performed the adjective task. At each site, we tested for TMS effects on self-specific processing by looking for a nullification of the SRE at each of the applied latencies. Best friend performance would be expected to be disrupted at the same times the SRE was if TMS was affecting a general memory system, but should be unaffected if the network processed self-specific information. Our study had two objectives. First, we wished to see whether additional areas of the self/other network besides the precuneus could be implicated in self-specific processing. Second, we wished to see whether changes in the temporal pattern of TMS disruption at different sites could provide information about the role various regions play in self-specific processing and as part of the default network.
Methods
Subjects
Eighteen healthy subjects (seven female) with a mean age of 27.8 ± 8.8 (SD) years were recruited and provided written informed consent for the study, which was approved by the New York State Psychiatric Institute IRB. Subjects were required to have normal or corrected-to-normal vision. All subjects were screened with psychiatric, physical and neurological examinations, urine drug screens, and pregnancy tests for women of childbearing capacity. Potential subjects were excluded if they had a history of current or past Axis I psychiatric disorder (including substance abuse/dependence) as determined by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-NP), a history of neurological disease, or seizure risk factors.
Adjective task
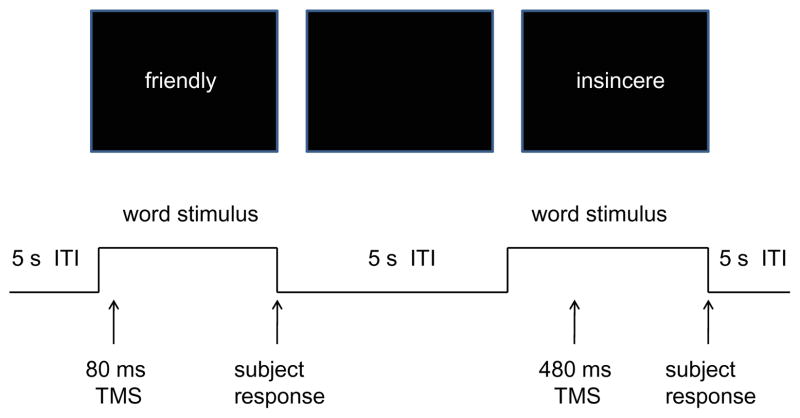
A set of 555 adjectives describing personality characteristics were obtained from Anderson (1968). From this set, six 90-word lists were randomly chosen (without replacement) for each subject to be used in the experimental session. The last 15 words were used to practice the subject on the task. Subjects were seated in a cushioned chair in the middle of the testing room, facing a computer monitor 100 cm away, with his/her head resting in a chin rest. Each trial began with an adjective presented in the center of the computer monitor (Fig. 1). The adjective remained on the screen for a maximum of 4 s and disappeared when the subject responded. As a list of adjectives was presented, subjects were asked to judge the applicability of each adjective to one’s self or, in separate blocks, his/her best friend, on a six-point scale by pressing a number from 1 to 6 on a computer keyboard (1 = extremely uncharacteristic, 6 = extremely characteristic). Subjects were asked to proceed at their own pace. After approximately 5 min following the end of a 90-word list, each word was presented again, one at a time, with the requirement for the subject to indicate with a yes/no button press whether or not the adjective had been judged to describe him/her self (or the best friend). During this second presentation of the list, subjects were asked to respond as quickly as possible. Reaction times and accuracy (match/mismatch in responses between the two list presentations) were recorded. A match occurred when the subject responded with a “yes” button press if he/she had chosen 4–6 in the first list presentation, or responded with a “no” if 1–3 had previously been chosen.
Fig. 1.
Schematic depiction of two trials of the TMS portion of the adjective task. Word stimuli are presented until the subject responds with a button push, or until 4 s have elapsed. A blank screen follows for an intertrial interval (ITI) of 5 s. In each trial, a TMS pulse occurs at one of five times relative to stimulus onset—in the figure, at 80 and 480 ms
TMS application
Single-pulse TMS was applied using a figure 8 coil (9 cm diameter) powered by a Magstim 200 stimulator (Magstim Co., Whitland, South West Wales, UK). TMS stimulus intensity was set at 150% of resting motor threshold of the left hemisphere (a group mean of 59.1 ± 11.2% of maximum stimulator output), which was defined as the lowest intensity needed to evoke motor potentials of at least 50 uV recorded via EMG from the first dorsal interosseus muscle (FDI) in at least 5 out of 10 stimulations (Rossini et al. 2007). The motor threshold was performed with the figure 8 coil placed laterally to the vertex over a site producing the largest EMG response to TMS pulses, tangentially over the scalp, and with the handle of the coil facing toward the back of the head, rotated clockwise 45° in the tangential plane. Selection of the cortical areas targeted for stimulation was based on sites activated in self/other contrasts of PET images using the adjective task (Lou et al. 2004). Three cortical sites were selected for stimulation: left and right lateral parietal cortex (angular gyrus) and midline prefrontal cortex. Order of stimulation in the session was counterbalanced between subjects. The sites were identified using high-resolution structural MRI scans obtained for each subject. The coil was positioned and accuracy of placement continuously monitored during task performance using Brainsight, a computerized frameless stereotaxic system (Rogue Research, Montreal, Canada). The system made it possible to track and correct online deviations from the target site to within several millimeters. We could not obtain structural images for five subjects; thus, the coil was positioned for these subjects using the International 10/20 system placements corresponding to the target cortical sites (Homan et al. 1987): P3 and P4 for the parietal sites, and 1 cm anterior to Fz for the prefrontal target. The focality of stimulation using a 150% intensity pulse from a Magstim 200 device (assuming a resting motor threshold of 40% stimulator output: Pitcher et al. 2003) was calculated using a finite-element analysis of a realistic head model (Deng et al. 2008, 2009). Such pulses will produce an electric field strong enough to produce suprathreshold neuronal depolarization over a cortical region with a radius of about 1.8 cm. Based on the PET imaging of the adjective task from our previous study (Lou et al. 2004), this is an appropriate focality for the midline targets used. However, while the estimated stimulated region remained within the lateral parietal cortex, it was somewhat larger than the regions activated in the PET study. For this reason, we have limited our discussion of results to lateral parietal cortex (rather than any of its subregions).
For each trial of a list’s second presentation, a single pulse of TMS was delivered with a stimulus onset asynchrony (SOA) between the onset of an adjective and the TMS pulse of 0, 80, 160, 240, or 480 ms. Choice of SOA was randomized for each trial, with the constraint that eighteen trials of each SOA occurred during each 90 trial block and no more than four trials in a row had the same SOA. A TMS block was performed for Self and Best Friend conditions, counterbalanced across subjects, at each of the three stimulation sites. Each session lasted approximately 2.5 h.
Analysis
Median reaction time (RT) and mean accuracy (% correct) measures were determined for each subject. Although accuracy and RT may represent different aspects of task processing, in the adjective task there is considerable confounding of the two measures in the form of speed/accuracy trade-offs. In our previous study (Lou et al. 2004), we successfully developed an efficiency score to characterize overall performance and counteract speed/accuracy trade-offs. The efficiency score is defined as the velocity (i.e., 1/RT) corrected by a factor defined by an accuracy term set to be 0 with chance performance and 1 with perfect performance: Efficiency = [(% accuracy − 50)/50]/(RT(in seconds)). All three measures (efficiency, accuracy, and RT) are reported and analyzed, but in keeping with our previous published work, the results are interpreted through the efficiency measure.
Eighteen subjects participated in the study, and all completed the blocks of TMS at the two parietal sites. Three subjects felt discomfort during prefrontal TMS and discontinued stimulation to that site. Omnibus repeated measures ANOVAs were run on the three performance measures for the 15 subjects who completed all three sites, with factors of Site (frontal, left and right parietal), Self/Best Friend, and SOA (0, 80, 160, 240, and 480 ms). These ANOVAs were repeated with the two parietal sites (removing the frontal site) for the 18 subjects that completed these sites.
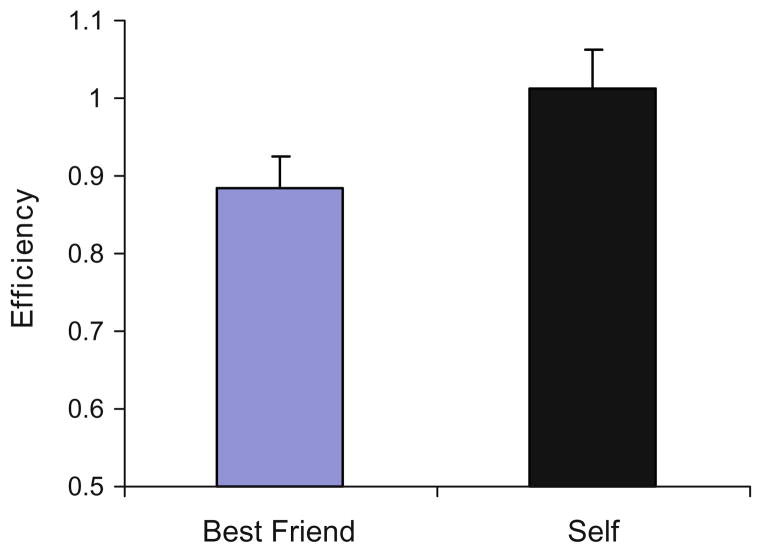
A main effect of Self/Best Friend was generally expected if TMS did not affect processing, reflecting the SRE previously found with the adjective task. In our own pilot work, 18 of 23 subjects (none from the present study) doing the task without TMS performed with greater efficiency when making self-judgments than for best friend, with mean group efficiency scores of 1.01 (±0.05) for self and 0.89 (±0.04) for best friend (t22 = 3.5, P < .001; Fig. 2). A limitation of a no-stimulation condition is that it does not include the superficial effects of TMS (auditory click, scalp sensations, etc.), which can alter how a subject approaches a task. We therefore did not use a no-TMS condition in the design of our present study, relying on the observation that the SRE we found in subjects with no TMS was replicated in the presence of TMS in our previously published TMS study (Lou et al. 2004) at all SOAs with prefrontal stimulation, and at all SOAs but one (at 160 ms) with midline parietal TMS (see Fig. 4 of Lou et al. 2004).
Fig. 2.
Mean group efficiency scores (and standard error bars) for Self and Best Friend conditions for a separate sample of 23 subjects performing the adjective task without TMS
Given an overall SRE (reflected in a main effect of Self/Best Friend in omnibus ANOVAs), TMS effects are observable in effects on SOA and on Site. With significant outcomes involving SOA in the omnibus ANOVAs, site-specific ANOVAs were run, with factors Self/Best Friend and SOA. When a significant interaction of Self/Best Friend and SOA was found (indicating a possible self-specific TMS effect), post hoc paired t-tests were performed, comparing the means of Self and Best Friend efficiency for all five SOAs. For these tests, the 0.05 alpha level was Bonferroni-corrected to 0.01. Here, in the most specific case, we looked for a temporally sensitive nullification of the SRE (i.e., when self-performance is not significantly greater than best friend in a one-tailed t-test) as the expected sign of the influence of TMS in disturbing self-related neural processing. In interpreting these results, we were able to rely on the data from our pilot work involving the task with no TMS, as well as from cases in our previous study (Lou et al. 2004) when the SRE remained undisturbed by TMS. For both these groups of subjects, when no effect was caused by TMS, efficiency in the Self condition ranged from the low 0.9’s to low 1.0’s, while in the Best Friend condition, ranged from low to high 0.8’s (e.g., Fig. 2). Thus, for example, in cases where t-tests showed no difference in Self/Best Friend performance, if efficiency dropped in the Self condition from its normal range while Best Friend performance remained undisturbed, then a conclusion of self-specific processing, disrupted by TMS, could be suggested.
Results
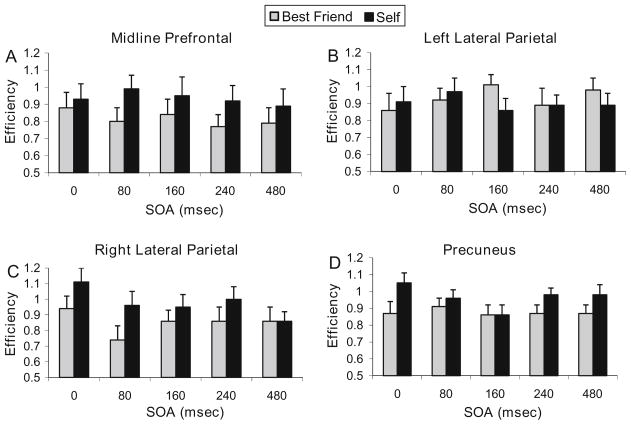
Group mean efficiency, accuracy, and RT are shown in Table 1 and efficiency scores in Fig. 3 for Self and Best Friend conditions at the five SOAs for each of the three sites. Omnibus ANOVAs for the efficiency measure showed a main effect of Self/Best Friend (F1,14 = 13.4, P < .003), a Site × Self/Best Friend interaction (F2,13 = 5.2, P < .025), and an interaction of SOA and Self/Best Friend (F4,11 = 4.1, P < .03). ANOVA for accuracy produced similar results, with a main effect of Self/Best Friend (F1,14 = 10.7, P < .006), a Site × Self/Best Friend interaction (F2,13 = 5.0, P < .025), and an interaction of SOA and Self/Best Friend (F4,11 = 3.5, P < .05). ANOVA for RT showed a main effect of SOA (F4,11 = 11.3, P < .001).
Table 1.
Group mean performance scores for three stimulation sites and self/best friend conditions at each SOA
| SOA (ms) | Midline prefrontal |
Left parietal |
Right parietal |
|||
|---|---|---|---|---|---|---|
| Best friend | Self | Best friend | Self | Best friend | Self | |
| Efficiency | ||||||
| 0 | 0.88 ± 0.09 | 0.93 ± 0.09 | 0.86 ± 0.10 | 0.91 ± 0.09 | 0.94 ± 0.08 | 1.11 ± 0.09 |
| 80 | 0.80 ± 0.08 | 0.99 ± 0.08 | 0.92 ± 0.07 | 0.97 ± 0.08 | 0.74 ± 0.09 | 0.96 ± 0.09 |
| 160 | 0.84 ± 0.09 | 0.95 ± 0.11 | 1.01 ± 0.06 | 0.86 ± 0.07 | 0.86 ± 0.07 | 0.95 ± 0.08 |
| 240 | 0.77 ± 0.07 | 0.92 ± 0.09 | 0.89 ± 0.10 | 0.89 ± 0.06 | 0.86 ± 0.09 | 1.00 ± 0.08 |
| 480 | 0.79 ± 0.09 | 0.89 ± 0.10 | 0.98 ± 0.07 | 0.89 ± 0.07 | 0.86 ± 0.09 | 0.86 ± 0.06 |
| RT (ms) | ||||||
| 0 | 877 ± 45 | 844 ± 73 | 866 ± 52 | 856 ± 53 | 873 ± 74 | 809 ± 60 |
| 80 | 956 ± 90 | 909 ± 69 | 929 ± 68 | 892 ± 54 | 913 ± 74 | 815 ± 60 |
| 160 | 930 ± 57 | 898 ± 59 | 900 ± 56 | 891 ± 49 | 874 ± 61 | 913 ± 80 |
| 240 | 897 ± 50 | 916 ± 90 | 896 ± 62 | 896 ± 54 | 922 ± 81 | 935 ± 82 |
| 480 | 979 ± 97 | 926 ± 75 | 903 ± 61 | 915 ± 55 | 944 ± 78 | 943 ± 74 |
| Accuracy (% correct) | ||||||
| 0 | 86.0 ± 2.5 | 86.3 ± 2.3 | 83.6 ± 2.6 | 86.4 ± 2.6 | 86.7 ± 1.9 | 90.7 ± 1.7 |
| 80 | 84.1 ± 2.4 | 91.8 ± 1.4 | 89.5 ± 2.0 | 90.1 ± 1.6 | 80.6 ± 2.8 | 86.7 ± 2.7 |
| 160 | 86.3 ± 2.6 | 88.5 ± 3.1 | 92.9 ± 1.2 | 86.1 ± 2.2 | 85.2 ± 2.1 | 89.5 ± 2.1 |
| 240 | 83.0 ± 2.3 | 87.0 ± 1.5 | 85.8 ± 2.3 | 87.7 ± 1.7 | 84.9 ± 3.0 | 92.0 ± 1.4 |
| 480 | 84.4 ± 3.0 | 86.3 ± 3.0 | 91.0 ± 1.6 | 88.6 ± 2.2 | 85.8 ± 2.7 | 87.7 ± 1.5 |
Fig. 3.
Mean group efficiency scores (with SE error bars) between Self and Best Friend conditions. Scores are shown for each SOA, for TMS to a midline prefrontal cortex, b left lateral parietal cortex, and c right lateral parietal cortex. The plot in d shows the change in efficiency with TMS to precuneus from data collected in a different group (Lou et al. 2004)
ANOVAs for parietal sites alone for the efficiency measure also showed Site × Self/Best Friend (F1,17 = 14.8, P < .002) and SOA and Self/Best Friend (F4,14 = 3.5, P < .035) interactions, as well as an interaction of Site and SOA (F4,14 = 3.1, P < .05). For accuracy, there were also interactions of Site × Self/Best Friend (F1,17 = 11.2, P < .004) and Site and SOA (F4,14 = 4.7, P < .02), as well as a main effect of SOA (F4,14 = 9.9, P < .0005). For RT, there was an interaction for SOA and Self/Best Friend (F4,14 = 3.2, P < .05), and a trend for an interaction of Site and SOA (F4,14 = 2.9, P < .06).
Given the interactions of SOA with Self/Best Friend in these tests (indicative of an effect of TMS differential for self-processing), ANOVAs were run to examine the pattern of effects at each site.
At the midline prefrontal site, an SRE was clearly apparent, with performance in the Self condition more efficient and accurate than Best Friend at all SOAs, and RTs faster at all but one SOA (Table 1). The SRE can be observed in the difference of efficiency scores displayed in Fig. 4a and was reflected in the repeated measures ANOVA for efficiency, where a main effect of Self/Best Friend (F1,14 = 8.7, P < .01) was found with no main effect of SOA or interaction. The ANOVA for accuracy had the same result, with just a main effect of Self/Best Friend (F1,14 = 8.9, P < .01). RT did show a main effect of SOA (F4,11 = 5.0, P < .02).
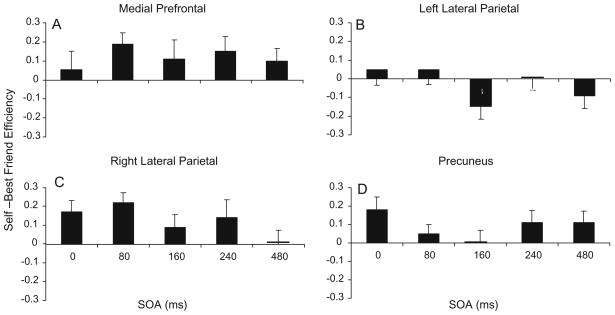
Fig. 4.
Change in efficiency scores between Self and Best Friend conditions. A positive value indicates an SRE. The error bars for each SOA represent the standard error of the difference between Self and Best Friend. Scores are shown for each SOA, for TMS to a midline prefrontal cortex, b left lateral parietal cortex, and c right lateral parietal cortex. The plot in d shows the change in efficiency with TMS to precuneus from data collected in a different group (Lou et al. 2004) and included for purposes of comparison. The change score was zero at 240 ms with TMS to left lateral parietal cortex, at 480 ms for right lateral parietal cortex, and at 160 ms for precuneus
At the right parietal site, the SRE was nullified at the highest latency SOA (480 ms), with an SRE occurring only at the earlier latencies (Fig. 4c). The disappearance of the SRE appeared to be attributable to a drop in efficiency during the Self condition from the values seen at the other latencies (and to those seen in the task with no TMS), while Best Friend efficiency remained unchanged across SOAs (see Table 1). In the repeated measures ANOVA for efficiency, there was a main effect of Self/Best Friend (F1,17 = 12.64, P < .003) with no main effect of SOA, and a significant interaction of Self/Best Friend and SOA (F4,14 = 3.55, P < .04). In post hoc analyses, performance in Self conditions was more efficient than Best Friend at latencies of 0 ms (t17 = 2.74, P < .007) and 80 ms (t17 = 4.02, P < .0004), but not at 160, 240, or 480 ms. In the ANOVA for accuracy, there was a main effect of Self/Best Friend (F1,17 = 7.9, P < .02), while RT showed a main effect for SOA (F4,14 = 6.5, P < .004) and an interaction of Self/Best Friend and SOA (F4,14 = 3.4, P < .04).
At the left parietal site, the SRE was nullified at all but the earliest SOAs. In addition, there was a reversal of the effect at SOA of 160 ms (and to a lesser degree at 480 ms), with efficiency for Best Friend exceeding that of Self for the first time (Fig. 4b). The loss of the SRE was evident in the ANOVAs for efficiency, accuracy, and RT, as there were no main effects or interactions at the left parietal site. However, there was a trend for the Self/Best Friend × SOA interaction (F4,14 = 2.1, P < .14) for efficiency. In post hoc analyses, Best Friend efficiency was greater than Self at 160 ms (t17 = 2.22, P < .02). As can be seen in Table 1, efficiency in the Self condition dropped to a value usually seen in the Best Friend condition, while Best Friend efficiency increased to a value usually only seen in the Self condition.
Discussion
In this study, self-specific effects on performance in the adjective task were found using TMS at both lateral parietal sites. Specifically, using disruption of the SRE (here, Self-performance superiority in efficiency over Best Friend) in the task as the indicator of an effect of TMS, the results at the prefrontal site replicated the finding of our previous study, where TMS had no effect on performance at any SOA in 13 subjects (Lou et al. 2004). Similar to TMS to the precuneus in Lou et al. (2004), stimulation over the right parietal site had a latency-dependent effect beginning at the same time (160 ms), but continuing for a longer period. At the left parietal site, TMS nullified the SRE at all latencies tested and may have reversed the SRE at 160 ms, such that efficiency for Best Friend was better than for Self. As discussed below, these findings provide support for parietal membership in a self-specific processing system and that the function of the default network is related to self-specific processing (Lou et al. 2004); Schilbach et al. 2008).
TMS effects on the SRE provide evidence for a distinct self-specific system
Initially, in non-physiological experiments contrasting self-related and non-self-related stimuli, SREs in performance were usually obtained and thought to be indicative of a functionally distinct cognitive system for self-knowledge, much like the distinct systems proposed for language and the perception of faces (e.g., Rogers et al. 1977). However, a number of follow-up studies found that SREs could be diminished by certain factors (reviewed in Symons and Johnson 1997). For example, the SRE could be substantially diminished if self-judgments were made relative to another well-differentiated individual, such as an immediate family member. Findings such as these led Symons and Johnson to conclude that SREs are not evidence for a specialized self-processing system. Instead, they proposed that they occur because “the self is a well-developed and often-used construct that promotes elaboration and organization of encoded information.” Gillihan and Farah (2005), noting these studies, suggested that much of what has been reported as physiological evidence for self-specific systems in the brain might show diminished effects if confounding factors were controlled, such as affect and the degree a person has knowledge of another relative to one’s self.
In the present study, attempts were made to control for elaborative and affective differences between self and other conditions in three ways. First, the same set of stimulus words were used for both self and other conditions, and the stimulus lists were equated for positive and negative characteristics. Second, the “other” compared with self was someone who was strongly differentiated in the subject’s memory: his or her best friend. Third, each list was presented a second time immediately after the first. Thus primed, the benefits of added pathways for self-information in memory searches was expected to be diminished. Our results appear to bear out the efficacy of these manipulations and to provide evidence that the observed SRE indicates a functionally distinct system for processing self-specific information. If TMS were disrupting a memory system where the differences were just a matter of degree of differentiation, then with a drop in efficiency in the Self condition, a drop in best friend condition should also occur, although perhaps to a lesser degree. This was not observed in our results, although we did not directly test change against a baseline in this study. However, at all SOAs when TMS nullified the SRE (right parietal cortex at 480 ms, left parietal cortex at 240 ms, and, in our previous study, precuneus at 160 ms: Fig. 1), stimulation caused the efficiency in the Self condition to drop to the level of the efficiency for Best Friend. In our pilot work with 23 subjects not receiving TMS, and our previous study (Lou et al. 2004) and in the prefrontal performance in the present study where there was no evidence of a TMS effect (see Table 1; Fig. 2), efficiency in the Self condition ranged from the low 0.9’s to low 1.0’s, while in the best friend condition, from low to high 0.8’s. In the cases, where the SRE vanished, the Self-efficiency dropped into the performance range usually found with Best Friend, while the efficiency for Best Friend remained within its same range.
The effect of TMS at left parietal cortex also argues against the memory elaboration explanation for the SRE. There, efficiency at 160 and 480 ms (Table 1; Fig. 2) in the Best Friend condition improved to levels seen in the Self condition when the task is performed without TMS, while efficiency in the Self condition with stimulation at those SOAs dropped to levels seen in the Best Friend condition. The facilitated performance in the Best Friend condition and disrupted performance in the Self condition at the same SOAs argue against a memory elaboration explanation, which would predict a drop in performance in either condition. Indeed, these effects suggest the disruption of a self-specific processing system. In this scenario, the disruption occurs when neural processing which normally interferes with task performance is disturbed by TMS, thus resulting in an improvement through subtraction of irrelevant processing. This sort of facilitation effect has been reported with TMS to parietal cortex in visual attention (Walsh et al. 1999) and verbal working memory (Luber et al. 2007a, b) and in the Stroop task with TMS to prefrontal cortex (Hayward et al. 2004). For example, in the study by Walsh et al. (1999) TMS applied to a superior occipital location, which analyzes direction of motion resulted in an improvement in performance in a visual search task when stimuli were moving but direction of motion was irrelevant. In the present study, a left parietal self-specific system may become active when one is attempting to recall characteristics of a best friend. However, being self-specific, this activity may have more to do with aspects of the self/best friend relationship (e.g., how the best friend having a given trait has impacted the subject) and may in fact complicate a decision about a given trait a best friend may or may not possess. Disrupting this self-related processing with left parietal TMS may thus simplify the decision process about best friend traits occurring elsewhere, reducing less relevant input, thus allowing a faster route to action.
TMS effects on SRE demonstrate a role for parietal cortex in episodic memory
In the present study, the sites at which TMS nullified the SRE were over left and right lateral parietal cortex, and our previous study showed this effect at midline parietal cortex. These parietal locations might be surprising, as the adjective task used relies on retrieval of autobiographical episodic memories, and traditionally, parietal cortex is thought to be involved with sensorimotor integration and spatial attention. However, a recent fMRI study has demonstrated that the parietal cortex supports an episodic memory network that is anatomically and functionally distinct from a network involved in sensorimotor integration and spatial attention (Vincent et al. 2006). Spontaneous activity in resting fMRI measurements in right and left lateral parietal areas and precuneus were correlated with activity in left and right hippocampal formation. These areas, which correspond to the same parietal regions found in the default network and stimulated in our studies with TMS, were also found to be active, along with hippocampus, in event-related fMRI measures of episodic memory. Moreover, Vincent et al. found that spontaneous activity in medial temporal areas known to be part of a temporoparietal network involved with sensorimotor integration and spatial attention were correlated with activity in parietal regions spatially segregated from those parietal areas involved with hippocampus and episodic retrieval.
These findings support a rather extensive literature supporting the role of the parietal cortex in episodic retrieval. The precuneus, linked with cingulate and prefrontal regions, has long been implicated in episodic processes (for a review, see Cavanna and Trimble 2006) going back to some of the earliest work in PET, involving recognition of sentences heard 24 h previously (Tulving et al. 1994) and in paired associate learning of words (Shallice et al. 1994). Specifically, autobiographical material activates precuneus as well in episodic memory tasks (Gilboa et al. 2004), as well as left and right lateral parietal cortex (Addis et al. 2004; Lundstrom et al. 2005; Nunez et al. 2005). Our finding of a stronger SRE cancellation on the left was most likely related to the verbal nature of the task, as this region has recently been linked to major anatomical pathways involving language and implicated in semantic processing (Catani et al. 2005).
TMS effects suggest a role for parietal cortex and the default network in autonoetic consciousness
Although there is a great deal of evidence that parietal cortex is related to episodic, and especially autobiographical, memory, that relationship may be indirect. That is, default network regions in the parietal cortex may rely on material retrieved from episodic memory stores located elsewhere in order to review past knowledge, plan future behavior and support self-consciousness (Cavanna and Trimble 2006) or maintain information for interpreting, responding to and predicting external demands (Gusnard 2005). Evidence for this hypothesis was reported in a recent study in which TMS to left and right dorsolateral prefrontal cortex disrupted performance in a non-verbal episodic memory task, but did not do so with stimulation to left and right lateral parietal cortices (Rossi et al. 2006). As discussed above, in the present study, cancellation of the SRE with TMS without affecting performance in the Best Friend condition does not appear to be indicative of a direct effect on a general memory retrieval system, where TMS should presumably disrupt retrieval of Best Friend information as well as Self. Instead, the effects of TMS on performance in the adjective task suggest disruption of a system processing self-specific information.
There have been other suggestions for functions of parietal cortex that may call upon episodic memory, especially given its role as an element of the default network (for a review, see Cavanna and Trimble 2006). For example, Gusnard and Raichle (2001) suggested that midline and lateral parietal cortex participate in conscious awareness, with the precuneus a tonically active region that continuously evaluates the external, and possibly internal, context. Schilbach et al. (2008) proposed that the context being evaluated includes the individual’s social world. In a PET study examining episodic retrieval and the resting state, Andreasen et al. (1995) suggested activations of midline prefrontal and parietal regions might represent a network through which personal identity and past experiences are interlinked with each other, permitting human beings to experience personal identity, consciousness, and self-awareness. This self-awareness, termed “autonoetic consciousness,” is thought to emerge by retrieval of episodic memory and has its basis in the capacity to place events in time and to reference them to oneself (Gardiner 2001).
The neurobiological basis for self-awareness has been inferred mainly by imaging studies. The medial prefrontal and parietal regions are anatomically connected directly via the cingulate gyrus, the cingulum tract, and the superior frontooccipito fasciculus, and indirectly via the medial pulvinar nucleus of thalamus (van den Heuvel et al. 2009; Mufson and Mesulam 1984; Beer et al. 2002), forming a loop of reciprocal cortico-cortical and corticothalamic connections (Tononi and Edelman 2000). The loop is functional in the interaction between prefrontal/anterior cingulate and precuneus/posterior cingulate regions activated by retrieval of episodic memory (Lou et al. 2004 2008). Being recurrent and stabilized by feedback, it allows continuing re-activation, facilitating stimuli to cross duration and intensity thresholds for emergence of consciousness (Tononi and Edelman 2000; Libet et al. 1991). Such organization is ideal for a sustained conscious state of self-awareness. Each of the structures in the loop is tightly connected with polymodal association regions such as the bilateral parietal cortices, and the left anterolateral temporal region, and also with the cerebellum (Mesulam et al. 1977; Trojanowski and Jacobson 1974; Barbas and Mesulam 1981). The need for this relatively newly developed polymodal association region (Chugani 1998; Clancy et al. 2001) is consistent with self-reflection as a phylogenetically (Baars 2005) and ontogenetically (Zelazo 2004) recently developed human capacity. This is in contrast to, for instance, the minimal conceptual self-awareness, which has been more associated with differential activation of the precuneus (Andreasen et al. 1995; Cavanna and Trimble 2006). Polymodal sensory regions may act as “meeting places” for sensory information and retrieved memory information into a temporal and personal perspective. Single-cell studies and local cooling experiments in animals support such a role (Koch and Fuster 1989; Fuster 2000). It may be speculated that the paralimbic loop offers a venue for the distribution of such integrated information across the brain to compete for access to consciousness with self-reference to achieve a sense of unity. The paralimbic loop is therefore a good candidate for the neural substrate of the working space of consciousness as proposed by Baars (2002) and further developed by Dehaene et al. (2003).
Single-pulse TMS demonstrated some of the dynamic processing in the adjective task necessary for consciousness. The left parietal region affected from an early latency of 160 ms (probably due to the verbal nature of the task) and continuing through the latest time tested, while right parietal cortex only showed later processing, primarily at 480 ms is consistent with a recurrent re-activation of cortico-cortical and cortico-thalamic interaction in consciousness as suggested by Tononi and Edelman (2000). The long duration of effective neural activity inferred from our results suggests a sufficient time frame for consciousness to emerge, according to the experiments by Libet et al. (1991), approximately 500 ms.
TMS to midline prefrontal cortex did not disrupt SREs
The lack of TMS effects with stimulation to midline prefrontal cortex in this study was not unexpected, since this result replicated our earlier study (Lou et al. 2004). However, given the universal prominence of activity in this region in self/other contrasts in imaging experiments, the lack of effects requires some consideration. One possibility may be related to the lower tolerability of prefrontal TMS compared to stimulation of the posterior scalp. In the present study, three subjects chose stop the frontal stimulation, but were able to tolerate parietal TMS. The distraction of unpleasant superficial sensations can add to variability in the response (altering reaction time and/or accuracy) reducing effect size. Another possibility is that a single TMS pulse may not be adequate to disrupt processing in the task in this area with the SOAs used. In future studies, other SOAs could be used, perhaps based on ERP studies. It should be noted that the full dynamic range of self-related processing based on ERP evidence was not completely investigated in the present study, which covered early- to mid-latency SOAs. ERP differences have been observed in contrasts of self and other faces (Keyes et al. 2010), familiar and unfamiliar objects (Miyakoshi et al. 2007), and in retrieval of memories concerning self and best friend (Magno and Allan 2007). In all of these studies, ERP components sensitive to self/other conditions occurred within the latencies tested in our studies (i.e., between 0 and 480 ms), but later differences were also observed. For example, one study used a procedure with many similarities to the present study, with subjects shown words and asked to retrieve memories about themselves or a good friend related to the words (Magno and Allan 2007). ERPs exhibiting self/friend differences were seen over both anterior and posterior scalp sites in a 100–400 ms window after the onset of the cue word, and also between 800 and 1,700 ms. This suggests that employment of SOAs later than those used in the present study might be effective in producing frontal effects. Repetitive TMS, in which a train of magnetic stimuli is used, might also serve as a more powerful disruptive technique. A third possibility is that the sort of processing related to the adjective task that might occur in midline PFC could be different than in parietal cortex. For example, it could be that PFC self-specific processing might be more evaluative and affect-laden, and not contribute to the SRE in the task, leaving the source of the SRE to other areas. Of interest in this regard, others have been able to produce a midline PFC TMS effect using the adjective task (Kwan et al. 2007). The adjectives in the task included both desirable and undesirable traits (50% of each). They found subjects were biased toward agreeing with more desirable traits and not agreeing with less undesirable traits when describing themselves as opposed to their best friend. In Kwan et al., single-pulse TMS to medial prefrontal cortex at 500 ms SOA, but not to SMA or precuneus, diminished this bias, i.e. the affective component of the task. This outcome suggests that the midline PFC portion of the default network does process self-specific information, albeit specialized for different functions than parietal cortex.
Conclusion
The parietal effects of the present study, combined with the effects on precuneus in our previous study (Lou et al. 2004) and on medial prefrontal cortex in the study by Kwan et al. (2007), demonstrate that four (out of four) brain regions common to networks observed in self/other tasks and the default network are sensitive to the processing of the self-specific content of the adjective task. These findings support the interpretations of Lou et al. (2004) and Schilbach et al. (2008) that the function of the default network is related to self-specific processing. They also demonstrate the value of TMS generally in testing the function of neural networks implicated by neuroimaging.
Acknowledgments
Dr. Lisanby has received research support, for topics not presented here, from Magstim Company, Neuronetics, Cyberonics, and ANS. Columbia University has applied for a patent for novel TMS technology developed in Dr. Lisanby’s Laboratory, for work unrelated to the topic presented here.
Contributor Information
Hans C. Lou, Email: hl@ipm.hosp.dk, Division of Brain Stimulation and Therapeutic Modulation, New York State Psychiatric Institute, New York, NY, USA. Center for Functionally Integrative Neuroscience, Aarhus University Hospital, 8000 Aarhus, Denmark
Bruce Luber, Division of Brain Stimulation and Therapeutic Modulation, New York State Psychiatric Institute, New York, NY, USA. Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA.
Arielle Stanford, Division of Brain Stimulation and Therapeutic Modulation, New York State Psychiatric Institute, New York, NY, USA. Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA.
Sarah H. Lisanby, Division of Brain Stimulation and Therapeutic Modulation, New York State Psychiatric Institute, New York, NY, USA. Department of Psychiatry, Columbia University College of Physicians and Surgeons, New York, NY, USA
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. NeuroImage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. J Pers Soc Psychol. 1968;9:272–279. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Boles Ponto LL, Hichwa RD. Remembering the past: two facets of episodic memory explored with positron emission tomography. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- Baars BJ. The conscious access hypothesis: origins and recent evidence. Trends Cogn Sci. 2002;6:47–52. doi: 10.1016/s1364-6613(00)01819-2. [DOI] [PubMed] [Google Scholar]
- Baars BJ. Subjective experience is probably not limited to humans: the evidence from neurobiology and behavior. Conscious Cogn. 2005;14:7–21. doi: 10.1016/j.concog.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam M-M. Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J Comp Neurol. 1981;200:407–431. doi: 10.1002/cne.902000309. [DOI] [PubMed] [Google Scholar]
- Beer J, Blakemore C, Previc FH, Liotti M. Areas of the human brain activated by ambient visual motion, indicating three kinds of self movement. Exp Brain Res. 2002;143:78–88. doi: 10.1007/s00221-001-0947-y. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;17:905–917. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffyche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Prev Med. 1998;27:184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Clancy B, Darlington RB, Finlay BL. Translating developmental time across mammalian species. Neuroscience. 2001;105:7–17. doi: 10.1016/s0306-4522(01)00171-3. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovich M, Stuss DT, Winocur G, Tulving E, Kapur S. In search of the self: a positron emission tomography study. Psychol Sci. 1999;10:26–34. [Google Scholar]
- Dehaene S, Sergent C, Changeux J-P. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci USA. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Z-D, Peterchev AV, Lisanby SH. Coil design considerations for deep-brain transcranial magnetic stimulation (dTMS). Conference Proceedings of IEEE Engineering in Medicine and Biology Society; 2008. pp. 5675–5679. [DOI] [PubMed] [Google Scholar]
- Deng Z-D, Lisanby SH, Peterchev AV. Effect of anatomical variability on neural stimulation strength and focality in electro-convulsive therapy (ECT) and magnetic seizure therapy (MST). Conference Proceedings of IEEE Engineering in Medicine and Biology Society; 2009. pp. 682–688. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an fMRi study using positive and negative emotional words. Am J Psychiatry. 1995;160:1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44:2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Cortical dynamics of memory. Int J Psychophysiol. 2000;35:155–164. doi: 10.1016/s0167-8760(99)00050-1. [DOI] [PubMed] [Google Scholar]
- Gardiner JM. Episodic memory and autonoetic consciousness: a first person approach. Philos Trans R Soc Lond B Biol Sci. 2001;356:1351. doi: 10.1098/rstb.2001.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull. 2005;131:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, Bullmore ET, Howard RJ. Task-induced deactivations during successful paired-associates learning: an effect of age but not Alzheimer’s disease. NeuroImage. 2006;31:818–831. doi: 10.1016/j.neuroimage.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA. Being a self: considerations from functional imaging. Conscious Cogn. 2005;14:679–697. doi: 10.1016/j.concog.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward G, Goodwin GM, Harmer CJ. The role of the anterior cingulate cortex in the counting Stroop task. Exp Brain Res. 2004;154:355–358. doi: 10.1007/s00221-003-1665-4. [DOI] [PubMed] [Google Scholar]
- Homan RW, Herman J, Purdy P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr Clin Neurophysiol. 1987;66:376–382. doi: 10.1016/0013-4694(87)90206-9. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Keyes H, Brady N, Reilly RB, Foxe JJ. My face or yours? Event-related potential correlates of self-face processing. Brain Cogn. 2010;72:244–254. doi: 10.1016/j.bandc.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, et al. Towards a functional neuroanatomy of self processing: effects of faces and words. Cogn Brain Res. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Kircher TTJ, Senior C, Phillips ML, Rabe-Hesketh S, Benson PJ, Bullmore ET, et al. Recognizing one’s own face. Cognition. 2001;78:B1–B15. doi: 10.1016/s0010-0277(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17:1080–1086. [PubMed] [Google Scholar]
- Koch KW, Fuster JM. Unit activity in monkey parietal cortex related to haptic perception and temporary memory. Exp Brain Res. 1989;76:292–306. doi: 10.1007/BF00247889. [DOI] [PubMed] [Google Scholar]
- Kwan VS, Barrios V, Ganis G, Gorman J, Lange C, Kumar M, Shepard A, Keenan JP. Assessing the neural correlates of self-enhancement bias: a transcranial magnetic stimulation study. Exp Brain Res. 2007;182:379–385. doi: 10.1007/s00221-007-0992-2. [DOI] [PubMed] [Google Scholar]
- Libet B, Pearl DK, Morledge DM, Gleason CA, Hosobuchi Y, Barbaro NM. Control of the transition from sensory detection to sensory awareness in man by the duration of a thalamic stimulus. The cerebral time-on factor. Brain. 1991;114:1731–1757. doi: 10.1093/brain/114.4.1731. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, Keenan J, Nowak M, Kjaer T, Sackeim H, Lisanby SH. Parietal cortex and representation of the mental self. Proc Natl Acad Sci USA. 2004;101(17):6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luber B, Peterchev A, Nguyen T, Sporn A, Lisanby SH. Application of TMS in psychophysiological studies. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. Cambridge University Press; New York: 2007a. [Google Scholar]
- Luber B, Kinnunen LH, Rakitin BC, Ellsasser R, Stern Y, Lisanby SH. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: frequency and time-dependent effects. Brain Res. 2007b;1128:120–129. doi: 10.1016/j.brainres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. NeuroImage. 2005;27:824–834. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Magno E, Allan K. Self-reference during explicit memory retrieval. An event-related potential analysis. Psychol Sci. 2007;18:672–677. doi: 10.1111/j.1467-9280.2007.01957.x. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Mummery CJ. Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus. 1999;9:54–61. doi: 10.1002/(SICI)1098-1063(1999)9:1<54::AID-HIPO6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Mason MF, Norton ML, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyor B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M, Van Hoesen GW, Pandya DN, Geschwind N. Limbic and sensory connections of the inferior parietal lobule (area PG) in the rhesus monkey: a study with a new method for horseradish peroxidase histochemistry. Brain Res. 1977;136:393–414. doi: 10.1016/0006-8993(77)90066-x. [DOI] [PubMed] [Google Scholar]
- Miyakoshi M, Nomura M, Ohira H. An ERP study on self-relevant object recognition. Brain Cogn. 2007;63:182–189. doi: 10.1016/j.bandc.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J Comp Neurol. 1984;227:109–120. doi: 10.1002/cne.902270112. [DOI] [PubMed] [Google Scholar]
- Nuňez JM, Casey BJ, Egner T, Harre T, Hirsch J. Intentional false responding shares neural substrates with response conflict and cognitive control. NeuroImage. 2005;25:267–277. doi: 10.1016/j.neuroimage.2004.10.041. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Perrin F, Maquet P, Peigneux P, et al. Neural mechanisms involved in the detection of our first name: a combined ERP and PET study. Neuropsychologia. 2005;43:12–19. doi: 10.1016/j.neuropsychologia.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input–output characteristics. J Physiol. 2003;546(2):605–613. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Keenan JP, Gallup GG, Mohamed FB. Where am I? The neurological correlates of self and other. Cogn Brain Res. 2004;19:114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA. 1998;95(3):765–772. doi: 10.1073/pnas.95.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TB, Kuiper NA, Kirker WS. Self-reference and the encoding of personal information. J Pers Soc Psychol. 1977;35:677–688. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Rossi S, Pasqualetti P, Zito G, Vecchio F, Cappa SF, Miniussi C, Babiloni C, Rossini PM. Prefrontal and parietal cortex in human episodic memory an interference study by repetitive transcranial magnetic stimulation. Eur J Neurosci. 2006;23:793–800. doi: 10.1111/j.1460-9568.2006.04600.x. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68:484–488. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- Ruby P, Legrand D. Neuro imaging the self? In: Haggard P, Rosetti Y, Kawato M, editors. Sensorimotor foundations of higher cognition. Attention and performance XXII. Oxford University Press; London: 2008. [Google Scholar]
- Schilbach L, Eickhoff SB, Rotarska-Jagiela A, Fink GR, Vogeley K. Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious Cogn. 2008;17:457–467. doi: 10.1016/j.concog.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage. 2004;22:1168–1177. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Shallice T, Fletcher P, Frith CD, Grasby RS, Frackowiack RSJ, Dolan RJ. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994;368:633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Watanabe J, et al. Cortical mechanisms of person representation: recognition of famous and personally familiar names. NeuroImage. 2006;31:853–860. doi: 10.1016/j.neuroimage.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Symons CS, Johnson BT. The self-reference effect in memory: a meta-analysis. Psychol Bull. 1997;121:371–394. doi: 10.1037/0033-2909.121.3.371. [DOI] [PubMed] [Google Scholar]
- Tononi G, Edelman GM. Schizophrenia and the mechanism of conscious integration. Brain Res Rev. 2000;31:391–400. doi: 10.1016/s0165-0173(99)00056-9. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Jacobson J. Medial pulvinar afferents to frontal eye fields in rhesus monkey demonstrated by horseradish peroxidase. Brain Res. 1974;80:395–411. doi: 10.1016/0006-8993(74)91025-7. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Markovitsch HJ, Craik FIM, Habib R, Houle S. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc Natl Acad Sci USA. 1994;91:2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Molnar-Szakacs I, Zaidel E, Iacoboni M. rTMS to the right parietal lobule disrupts self-other discrimination. Soc Cogn Affect Neurosci. 2006;1:65–71. doi: 10.1093/scan/nsl003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–3141. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Luks TL, Simpson GV, Schulman BJ, Glenn S, Wong AE. Brain activation patterns during memory of cognitive agency. NeuroImage. 2006;31:896–905. doi: 10.1016/j.neuroimage.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Walsh V, Ellison A, Ashbridge E, Cowey A. The role of the parietal cortex in visual attention-hemispheric asymmetries and the effects of learning: a magnetic stimulation study. Neuropsychologia. 1999;37:245–251. doi: 10.1016/s0028-3932(98)00099-2. [DOI] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and the self in the brain? Brain Res Rev. 2003;43:224–230. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Zelazo PD. The development of conscious control in childhood. Trends Cogn Sci. 2004;8:12–17. doi: 10.1016/j.tics.2003.11.001. [DOI] [PubMed] [Google Scholar]