Abstract
Radiographic assessment of the biliary tract is often essential in patients who have undergone liver transplantation. T- or straight-tube cholangiography, percutaneous transhepatic cholangiography, and endoscopic retrograde cholangiography all may be used. A total of 264 cholangiograms in 79 adult liver transplant patients (96 transplants) was reviewed. Normal radiographic features of biliary reconstructive procedures, including choledochocholedochostomy and choledochojejunostomy, are demonstrated. Complications diagnosed by cholangiography included obstruction, bile leaks, and tube problems, seen in eight, 24, and 12 transplants respectively. Stretching and incomplete filling of intrahepatic biliary ducts were frequently noted and may be associated with rejection and other conditions. Transhepatic biliary drainage, balloon catheter dilatation of strictures, replacement of dislodged T-tubes, and restoring patency of obstructed T-tubes using interventional radiologic techniques were important in avoiding complications and additional surgery in selected patients.
The first human liver transplant was performed by Starzl in 1963 [1]. One hundred eighty-four patients received transplants by his Denver surgical team over the 18-year period up to 1980 [2]. As survival rates have improved, the number of liver transplants performed each year has steadily increased. At the University Health Center of Pittsburgh, 175 patients received 226 orthotopic liver transplants from 1981 to 1984.
During the early years of liver transplantation, biliary drainage was usually achieved by cholecystoduodenostomy without T-tube [3]. Postoperative cholangiography was infrequently performed because of a lack of easy tube access to the biliary tree. Moreover, percutaneous transhepatic cholangiography (PTC) was little used before the introduction of the Chiba needle, and endoscopic retrograde cholangiography (ERC) was not widely available.
Hyperbilirubinemia and elevated liver function tests in the posttransplant patient were often assumed to be due to liver rejection [4]. Treatment consisted of increased immunosuppressive therapy. However, hepatic dysfunction was often later found to be the result of biliary obstruction. Thus, in the Denver series, 25% of the first 59 transplanted patients with a cholecystoduodenostomy were found to be obstructed [4]. Unfortunately, obstruction was usually diagnosed only at delayed reoperation or autopsy.
Today, a biliary tract complication is one of the primary initial diagnostic considerations in the liver transplant patient with an abnormal postoperative course. The current practice of using T or straight tubes in biliary reconstruction and the ready availability of PTC and ERC have resulted in cholangiography studies being one of the first steps in the evaluation of such patients. Cholangiography permits the easy identification of several biliary complications, including obstruction, bile leaks, and tube malfunction, which may require early surgical intervention. In selected cases, therapeutic interventional radiologic techniques may be used, obviating additional surgical procedures. We report our experience with operative and postoperative cholangiograms and interventional biliary radiologic procedures in normal and abnormal adult liver transplant patients.
Subjects and Methods
During the 3-year period between February 1981 and February 1984, 106 adult patients received 133 orthotopic liver transplantations at the Presbyterian-University Hospital of Pittsburgh. Twenty-three patients received two transplants each, and two patients received three transplants each. There were 49 men and 57 women aged 16–56 years.
Cholangiograms were available in 79 patients (96 transplants). Patients not having cholangiography included some who died intra-operatively and others who had internal biliary stents, where routine cholangiograms were not performed. A total of 264 cholangiograms was analyzed, which included 141 by T-tube cholangiography, 29 by tube cholangiography, nine by PTC, five by ERC, and 80 operative studies. Some of the cholangiograms were obtained during various biliary interventional procedures, including transhepatic catheter drainage, dilatation of strictures, biliary tube replacement, and restoration of lumen patency of obstructed T-tubes.
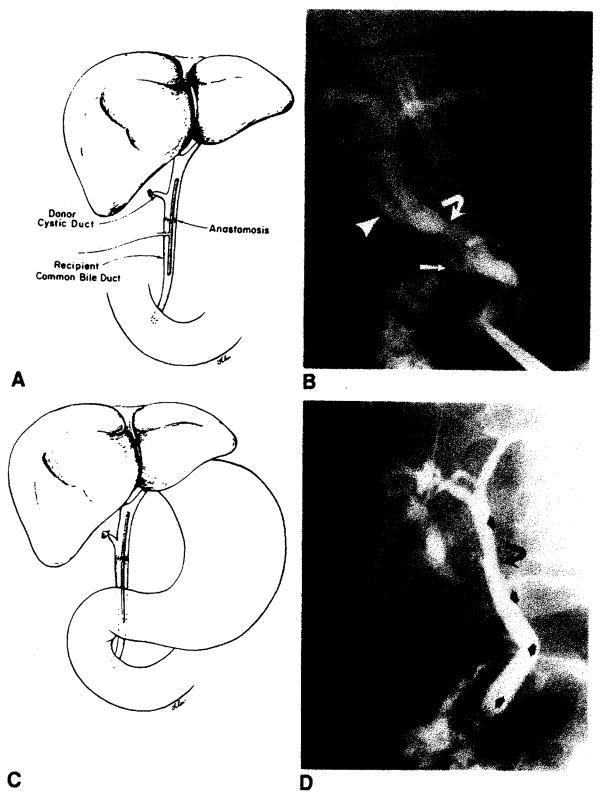
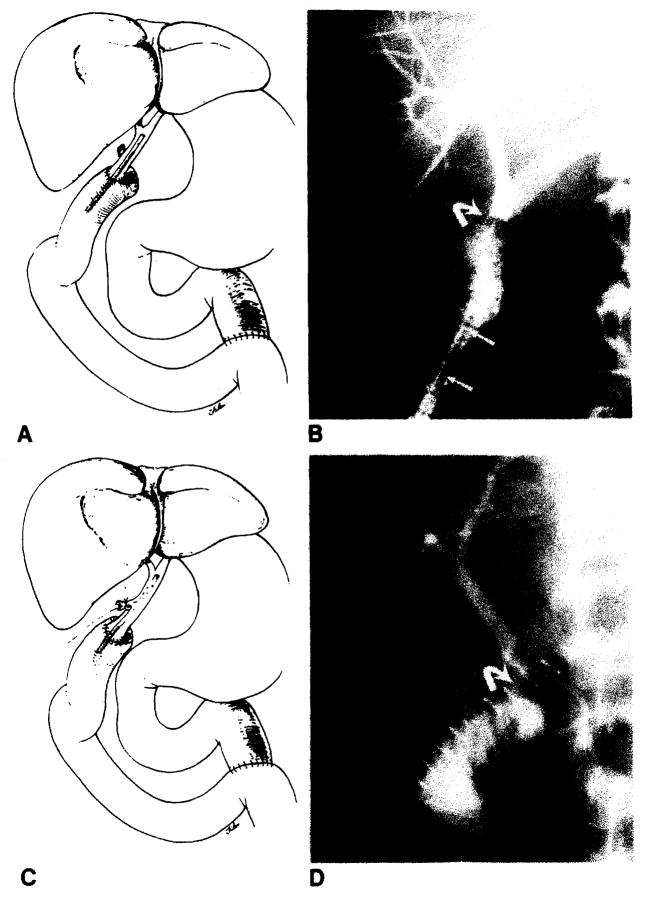
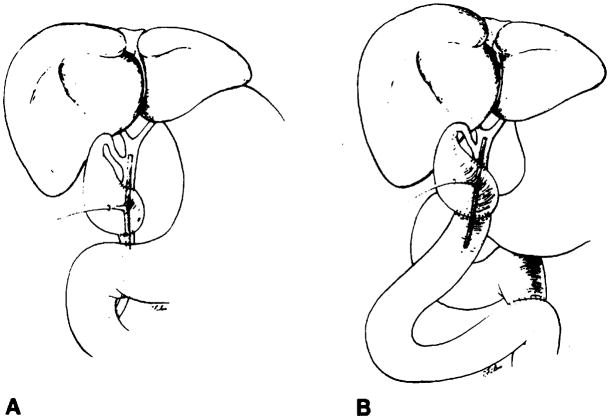
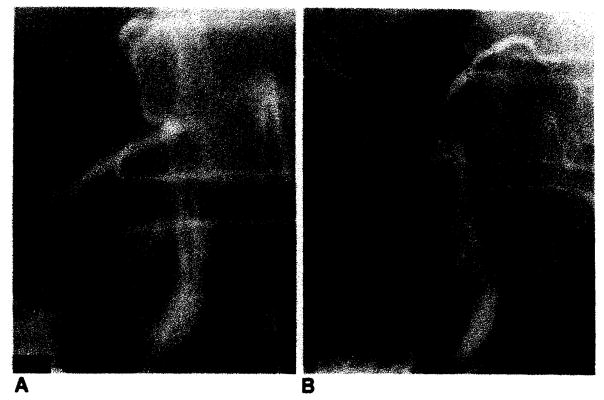
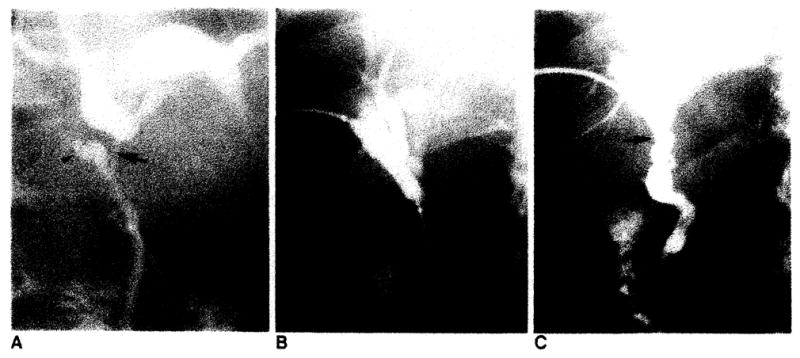
Bile duct reconstruction was accomplished by one of several methods in these adult transplant patients. In 83 transplants, an end-to-end choledochocholedochostomy was performed, 80 with T-tube stent and three with straight internal stent (fig. 1). Eight transplants had biliary reconstruction by choledochojejunostomy in Roux-en-Y, three with T-tube stent and five with straight internal stent (fig. 2). In two transplants, the donor gallbladder was used as a pedicle graft for biliary reconstruction. In one of these patients, it was used between the donors and the recipients common bile ducts (fig. 3A), and in the other patient, between the donor’s common bile duct and the recipient’s jejunum in Roux-en-Y (fig. 3B). Three transplants did not have internal biliary drainage established at the time of original transplantation; in two a cholecystostomy tube was placed, and in one a tube choledochostomy was used for external biliary drainage.
Fig. 1.
Normal choleclochochotedochostomy. A, Schematic diagram demonstrates T-tube stent, donor cystic duct remnant, and anastomosis. T-tube enters choledochotomy in recipient’s common bile duct. B, T-tube cholangiogram demonstrates donor’s (arrowhead) and recipient’s (arrow) cystic duct remnants and anastomosis (curved arrow). This anastomosis between donor’s common bile duct and recipient’s common hepatic duct is less frequently performed than common bile duct-to-common bite duct anastomosis shown in A. C, Schematic diagram demonstrates straight internal stent across biliary anastomosis. D, PTC demonstrates anastomosis (curved arrow) and position of stent (arrows).
Fig. 2.
Normal choledochojejunostomy in Roux-en-Y. A, Schematic diagram shows straight internal stent across biliary anastomosis. B, PTC shows biliary anastomosis (curved arrow). Note migration of stent (arrows) into Roux limb of jejunum. C, Schematic diagram shows T-tube stent which enters donors cystic duct remnant. D, T-tube cholangiogram shows biliary anastomosis (curved arrow).
Fig. 3.
Schematic diagrams demonstrate biliary reconstruction using donor’s gallbladder as pedicle graft. There are two anastomoses. In both A and B. proximal anastomosis is between Hartmann’s pouch and donor’s common bile duct. Distal anastomosis is between fundus of gallbladder and either recipient’s common bile duct (A) or recipient’s jejunum in Roux-en-Y (B).
Results
Cholangiography
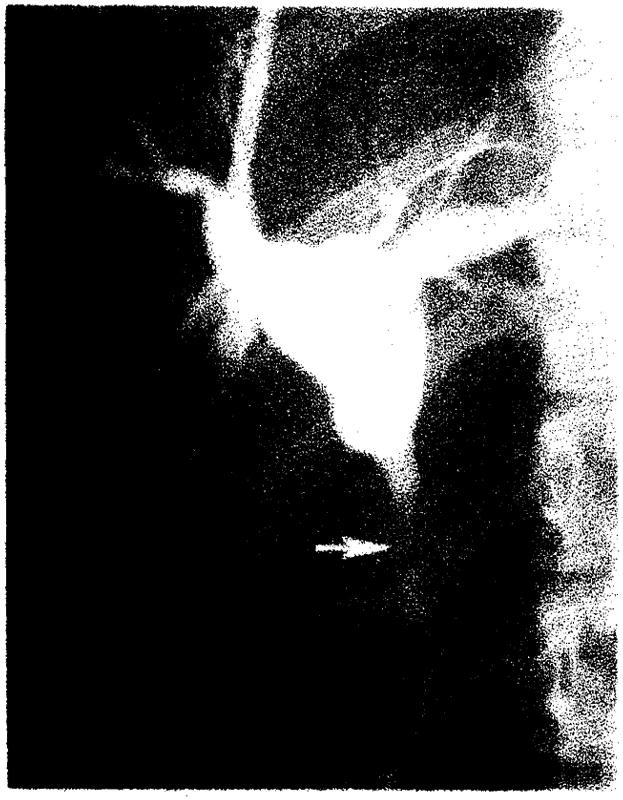
Complications observed at cholangiography were obstruction, bile leakage, and malpositioned and obstructed T-tubes. Biliary duct obstruction was observed in eight transplants (eight patients). A stricture developed in the donor’s common hepatic duct (above the anastomosis) in two patients at 3 and 7 weeks after operation (fig. 4A). Anastomotic strictures developed at a choledochojejunostomy in one patient and at the choledochocholedochostomy in two patients (fig. 5). These occurred postoperatively at 2 weeks, 7 months, and 16 months, respectively. Six months after operation, another patient developed biliary obstruction secondary to a stricture in the native distal common bile duct below the anastomosis, which was successfully treated by hepaticojejunostomy. Two patients developed functional obstruction due to the T-tube blocking the anastomosis in one and the ampulla of Vater in another. Both responded to removal of the tubes.
Fig. 4.
Treatment of common hepatic duct stricture by transhepatic balloon dilatation. A. T-tube cholangiogram 3 weeks after transplantation demonstrates biliary obstruction due to stricture in donor’s common hepatic duct (arrow). Note donor’s and recipient’s cystic duct remnants (arrowheads). B, Transhepatic dilatation with 8-mm balloon. C. Catheter cholangiogram 6 weeks after dilatation demonstrates patent donor’s common hepatic duct (arrow). Catheter was removed. Patient was asymptomatic in 20-month follow-up.
Fig. 5.
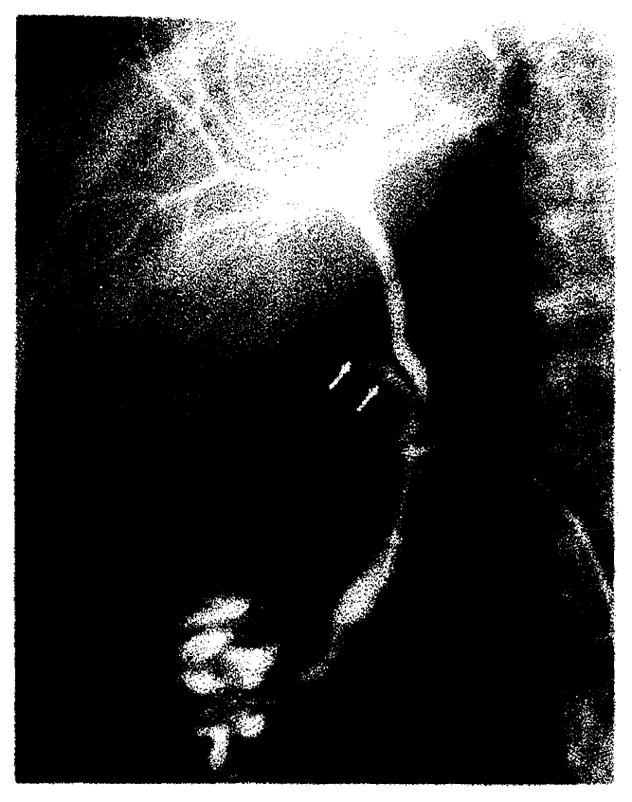
Anastomotic stricture (arrow) at choledochocholedochostomy, which proved to be cholangiocarcinoma by transcatheter brush biopsy. PTC 16 months after liver transplantation. Patient was treated by iridium-192 wire through transhepatic drainage catheter. Another patient (not shown) with benign anastomotic stricture had similar cholangiography findings and was treated by transhepatic balloon dilatation.
Leakage of contrast material at the time of cholangiography was observed in 24 transplants, 10 intraoperatively and 14 during postoperative evaluation. In seven postoperative leaks, the leakage was minimal and occurred at the choledochotomy and along the T-tube tract. In the other seven patients, the leakage was more significant, usually arising from the anastomosis and occasionally from the choledochotomy site, and resulted in an abnormal periductal or subhepatic collection (fig. 6).
Fig. 6.
Bile leak from choledochocholedochostomy resulting in both periductal (A, curved arrow) and subhepatic bile collections (B, arrows). B is later film of same patient shown in A.
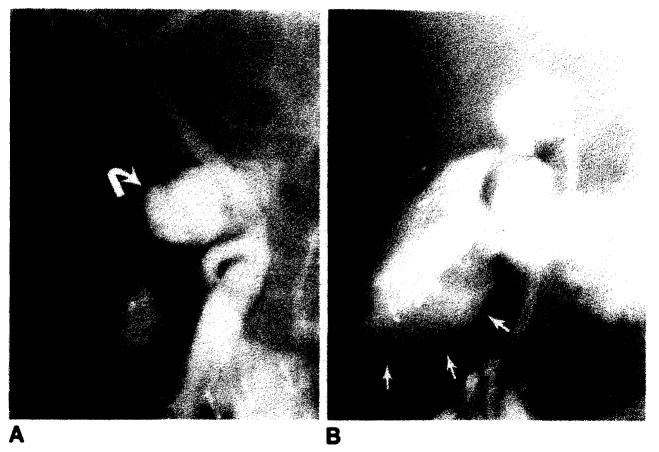
Tube problems were observed in 12 transplants. The most common finding was abnormal placement of the proximal limb of the T-tube in the donor’s cystic duct remnant (fig. 7). Others included T-tubes outside the duct (fig. 8A) and tubes folded on themselves. Occasionally T-tubes became obstructed, presumably by sludge and encrustation of bile.
Fig. 7.
Abnormal position of proximal limb of T-tube in donor’s cystic duct remnant (arrows) demonstrated on operative cholangiogram.
Fig. 8.
Biliary tube replacement after distodgment of T-tube. A, T-tube cholangiogram demonstrates T-tube outside choledochotomy. B, Cholangiogram after straight tube was replaced into common hepatic duct for external biliary drainage.
In 50 (63%) of the operative cholangiograms reviewed, marked thickening of the duodenal folds were observed. In those patients who later had follow-up postoperative cholangiograms, fold thickness returned to normal in all cases.
Poor filling, stretching, and attenuation of the intrahepatic ducts and occasionally liver enlargement were frequently noted (figs. 9 and 10). These findings were present in a moderate to marked degree in 18 transplants and to a lesser degree in several others.
Fig. 9.
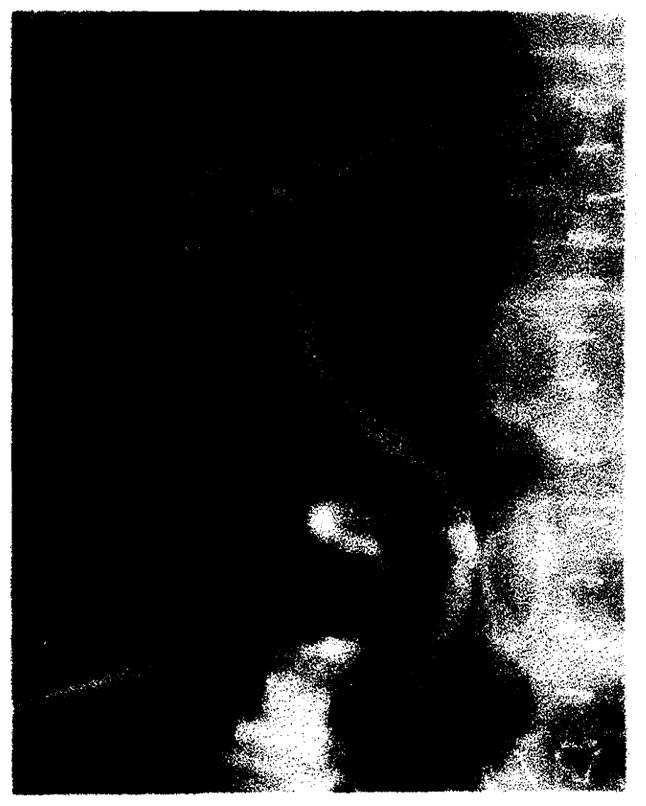
Clinical rejection. Poor filling, stretching, and mild attenuation of intrahepatic biliary tree.
Fig. 10.
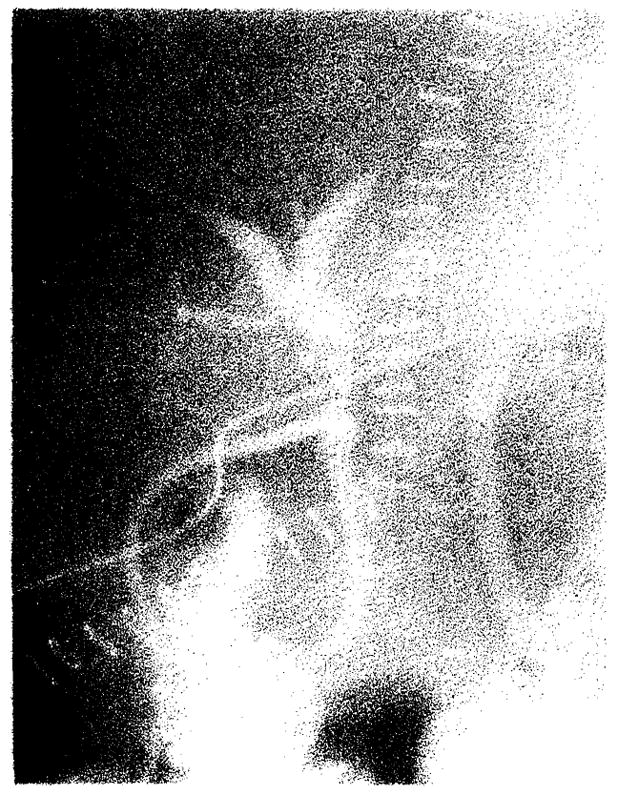
Clinical rejection. Marked attenuation and essentially no filling of intrahepatic biliary tree.
Interventional Radiology
Transhepatic catheter drainage was initially used to treat five of the eight patients with biliary obstruction. This included the two patients with a stricture in the donor’s common hepatic duct. Both were further successfully treated by transhepatic balloon catheter dilatation (fig. 4B). They continue to do well 8 and 20 months after treatment. One patient with a choledochocholedochostomy stricture was also treated by balloon dilatation. In another patient with a choledochocholedochostomy stricture whose original transplant was done for cholangiocarcinoma, a transcatheter brush biopsy was first performed, which was positive for adenocarcinoma (fig. 5). This patient was recently treated by an iridium-192 wire inserted through the transhepatic catheter. The patient with a choledochojejunostomy stricture who initially was treated by transhepatic catheter drainage subsequently underwent successful surgical revision of the anastomosis.
In two patients, the T-tube was outside the choledochotomy (fig. 8A). There was fear of bile leakage and peritonitis because both patients were recently postoperative with immature T-tube tracts. In each patient, a guide wire was inserted through the tube and the choledochotomy into the biliary tree. A straight tube was then inserted for external biliary drainage (fig. 8B). After maturation of the tract, the tube was removed and both patients did well.
T-tube malfunction due to blockage by biliary sludge occurred in two patients. Under fluoroscopic guidance, passage of guide wires through the tubes easily reestablished patency.
Discussion
The increased success rate of liver transplantation over the last 20 years is due to many factors. Improved surgical techniques, changes in patient selection, the use of the immunosuppressive drug cyclosporine, and better postoperative care are among the most important [5, 6]. One element of postoperative care that has evolved is the frequent use of cholangiography [3, 7, 8].
Indications for cholangiography after transplantation are failure of liver function tests to return to normal, a rise in bilirubin or liver function test levels, obstructive symptoms associated with clamping the T-tube, bile drainage from the tube insertion site, wound, or surgical drains, and signs and symptoms of cholangitis. In addition, routine T-tube cholangiograms are generally obtained before tube removal.
At our center, the procedure of choice for biliary reconstruction in patients with normal native bile ducts is an end-to-end anastomosis of the donor’s and recipient’s common bile ducts (choledochocholedochostomy). Both gallbladders are removed. Occasionally, the biliary anastomosis is performed between the donor’s common bile duct the recipient’s common hepatic duct. Cholangiography in these patients often demonstrates the two cystic duct remnants (fig. 1B). The anastomosis may appear as a minor ringlike narrowing (fig. 1B) or a change in caliber between two dissimilar-sized ducts, or may not be radiographically evident.
Depending on the size and quality of the bile ducts, a T or straight tube is used to stent the anastomosis (fig. 1). A T-tube stent is preferred because it permits direct monitoring of the quality and quantity of the bile output and provides easy access to evaluate the biliary tree. A T-tube stent is usually inserted through a choledochotomy in the recipient’s common bile duct (fig. 1A), to avoid possible interference with the donor duct blood supply. Rarely, the T-tube is inserted through the recipients or donor’s cystic duct remnant. In patients with straight internal stents, evaluation of the biliary tree is accomplished by PTC or ERC (fig. 1D).
In cases where the recipient’s duct is not available or is abnormal (sclerosing cholangitis, cholangiocarcinoma), an end-to-side anastomosis of the donor’s common bile duct to a Roux-en-Y loop of jejunum is performed (chotedochojejunostomy). In most cases, an internal straight tube is placed to stent the anastomosis (fig. 2A). Evaluation of the biliary tree in these patients can be accomplished only by PTC (fig. 2B). A T-tube through a choledochotomy is not routinely used in this setting, to avoid possible interruption of the donor duct blood supply. In some patients, however, the T-tube can be inserted directly through a widely patent cystic duct remnant (fig. 2C). Cholangiography is then easily performed directly through the T-tube (fig. 2D).
An alternative surgical approach for biliary reconstruction consists of using the donor’s gallbladder as a pedicle graft between the donor’s and the recipient’s common bile ducts [9] (fig. 3A). In this way, the largest possible anastomoses can be fashioned from the obliquely cut duct ends. This technique may also be used where there is a marked disparity in size of the two ducts or where the ducts are too short to permit direct duct-to-duct anastomosis. In patients without a normal common bile duct (e.g., sclerosing cholangitis), the distal anastomosis is made with a Roux limb of jejunum (fig. 3B). A T-tube is inserted through a cholecystotomy with the T-tube limbs stenting the proximal and distal anastomoses. At our center, biliary reconstruction by a gallbladder pedicle graft is not routinely performed.
In patients whose operation is complicated by excessive blood loss, technical difficulties, or instability of the patient, biliary reconstruction may be postponed [10]. Instead, temporary external biliary drainage is established by tube cholecystostomy or choledochostomy. Final biliary reconstruction is generally accomplished at a second operation.
If biliary obstruction is diagnosed by cholangiography, prompt treatment is initiated. The preferred initial step in our five patients with bile duct strictures was transhepatic catheter drainage. Two patients with common hepatic duct strictures were further successfully treated by balloon catheter dilatation. These strictures occurred in the donor’s biliary tree above the anastomosis. The cause is speculative but is thought most likely to have been ischemia. As demonstrated in one of our patients, the late development of a biliary stricture in a patient receiving a transplant because of cholangiocarcinoma should be evaluated for possible recurrent carcinoma.
Bile leakage can be an especially serious clinical problem as there is a significantly higher risk of infection in immuno-suppressed patients. Leakage generally occurs at the choledochotomy or at the bile duct anastomosis. Most leaks were minimal, usually extending from the choledochotomy along the T-tube tract, and resolved without intervention. However, in one febrile patient with a minimal choledochotomy leak on cholangiography, a CT scan demonstrated a large subhepatic fluid collection consistent with an abscess. The collection was drained percutaneously and proved to be an infected biloma. Because of the nature and extent of the fluid collection, surgical drainage was performed. Thus, most small leaks are innocuous. However, where the clinical course is not consistent with the cholangiographic findings, the use of other imaging techniques, such as CT or sonography, should be considered.
In transplants with larger leaks demonstrated at cholangiography, abdominal exploration and repair of the site of leakage was generally required (fig. 6). In some patients, bile leakage was secondary to necrosis of the distal donor duct, and revision of the anastomosis was necessary. Major bile leaks have been associated with complete donor bile duct necrosis secondary to hepatic artery thrombosis. In these patients, retransplantation, rather than biliary reconstruction, is always necessary. Significant bile leakage noted on operative cholangiography at time of transplantation was ordinarily corrected immediately.
Malpositioned T-tubes have not generally resulted in serious clinical problems. Abnormal placement of the proximal limb of the T-tube in the donor cystic duct has been the most frequently observed finding at cholangiography. It is our impression that tubes so positioned do not function optimally. If noted during the operative study (fig. 7), the proximal limb is moved and repositioned into the common duct.
An almost universal finding on operative cholangiograms with contrast material in the duodenum is enlargement of the duodenal folds. The reasons for this are probably two: First, the portal vein is cross-clamped for about 20 min while the anastomosis is performed. During this period of occlusion, there is marked swelling of the intestine [8]. Second, because of massive fluid requirements during operation, due in part to the significant Wood loss that generally occurs, there are probably compartmental fluid shifts, which contribute to fold thickening. Postoperative cholangiograms typically demonstrate the folds to have returned to normal.
The diagnosis of rejection is generally made by exclusion. Elevation in serum bilirubin and liver enzyme levels can be due to rejection, but other causes such as ischemic damage, biliary obstruction, vascular thrombosis, and viral infection must be excluded [11]. Biliary obstruction can be excluded by cholangiography; vascular thrombosis can be ruled out by angiography. A number of patients in our series demonstrated poor filling, stretching, and attenuation of the intrahepatic biliary ducts (figs. 9 and 10). Many of these patients carried a clinical and/or pathologic diagnosis of rejection at the time of cholangiography. After successful treatment of rejection, the cholangiographic findings often return to normal. The pathophysiologic mechanism for these intrahepatic biliary changes and their correlation with the severity of rejection are unknown. One speculation is that these findings are caused, in part, by lymphocytic infiltration occurring in the portal tracts during the rejection process. The radiographic manifestations of rejection and other hepatic parenchymal abnormalities are currently under investigation and will be the subject of a later report.
Acknowledgments
We thank Donna Scahill for manuscript preparation and Ron Filer for artwork.
Footnotes
Presented at the annual meeting of the American Roentgen Ray Society, Las Vegas, April 1984.
References
- 1.Starzl TE, Marchioro TL, Von Kaulla KN, Hermann G, Brittain RS, Waddell WR. Homotransplantation of the liver in humans. Surg Gynecol Obstet. 1963;117:659–676. [PMC free article] [PubMed] [Google Scholar]
- 2.Iwatsuki S, Klintmalm GBG, Starzl TE. Total hepatectomy and liver replacement (orthotopic liver transplantation) for primary hepatic malignancy. World J Surg. 1982;6:81–85. doi: 10.1007/BF01656377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE. Liver transplantation. Johns Hopkins Med J. 1978;143:73–83. [PubMed] [Google Scholar]
- 4.Martineau G, Porter KA, Corman J, et al. Delayed biliary duct obstruction after orthotopic liver transplantation. Surgery. 1972;72:604–610. [PMC free article] [PubMed] [Google Scholar]
- 5.Starzl TE, Koep U, Halgrimson CG, et al. Fifteen years of clinical liver transplantation. Gastroenterology. 1979;77:375–388. [PMC free article] [PubMed] [Google Scholar]
- 6.Calne RY, Williams R, Lindop M, et al. Improved survival after orthotopic liver grafting. Br Med J. 1981;283:115–118. doi: 10.1136/bmj.283.6284.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starzl TE, Putnam CW, Ishikawa M, et al. Current policies in hepatic transplantation: candidacy of patients with alcoholic liver disease or preformed antidonor antibodies and a reappraisal of biliary duct reconstruction. NY Acad Sci. 1975;252:145–158. doi: 10.1111/j.1749-6632.1975.tb19151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Starzl TE, Iwatsuki S, Van Thiel DH, et al. Evolution of liver transplantation. Hepatology. 1982;2:614–636. doi: 10.1002/hep.1840020516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calne RY. A new technique for biliary drainage in orthotopic liver transplantation utilizing the gallbladder as a pedicle graft conduit between the donor and recipient common bile ducts. Ann Surg. 1976;184:605–609. doi: 10.1097/00000658-197611000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwatsuki S, Shaw BW, Jr, Starzl TE. Biliary tract complications in liver transplantation under cyclosporin-steroid therapy. Transplant Proc. 1983;15:1288–1291. [PMC free article] [PubMed] [Google Scholar]
- 11.Pichlmayr R, Brölsch C, Neuhaus P, et al. Report on 68 human orthotopic liver transplantations with special reference to rejection phenomena. Transplant Proc. 1983;15:1279–1283. [Google Scholar]