Abstract
Nerve growth factor (NGF) induces auto-phosphorylation and downstream pro-growth and pro-survival signaling from the receptor tyrosine kinase TrkA. Overexpression or activating mutation of TrkA has been described in human acute myeloid leukemia (AML) cells. In the present studies, we demonstrate the chaperone association of TrkA with heat shock protein (hsp) 90 and the inhibitory effect of the hsp90 inhibitor 17-DMAG on TrkA levels and signaling in cultured and primary myeloid leukemia cells. Treatment with 17-DMAG disrupted the binding of TrkA with hsp90 and the co-chaperone cdc37, resulting in polyubiquitylation, proteasomal degradation and depletion of TrkA. Exposure to 17-DMAG inhibited NGF-induced p-TrkA, p-AKT and p-ERK1/2 levels, as well as induced apoptosis of K562, 32D cells with ectopic expression of wild type (wt) TrkA or the constitutively active mutant Δ TrkA, and of primary myeloid leukemia cells. Additionally, 17-DMAG treatment inhibited NGF-induced neurite formation in the rat pheochromocytoma PC-12 cells. Co-treatment with 17-DMAG and K-252a, an inhibitor of TrkA-mediated signaling, induced synergistic loss of viability of cultured and primary myeloid leukemia cells. These findings demonstrate that TrkA is an hsp90 client protein, and inhibition of hsp90 depletes TrkA and its pro-growth and pro-survival signaling in myeloid leukemia cells. These findings also support further evaluation of the combined activity of an hsp90 inhibitor and TrkA antagonist against myeloid leukemia cells.
Keywords: 17-DMAG, TrkA, leukemia, Hsp90 inhibitors
Introduction
TrkA is a transmembrane, glycosylated receptor tyrosine kinase, which is encoded by the NTRK1 gene (1). Binding of TrkA to its ligand, nerve growth factor (NGF) induces autophosphorylation and activation of TrkA (1). TrkA mediates NGF-induced signaling for differentiation in neuronal cells, e.g., neurite formation, and sympathetic neuron-like phenotype in PC-12 cells (2,3). Complete NGF withdrawal or pharmacological inhibition of TrkA activity attenuates p-TrkA levels and ERK1/2 and AKT activity in PC-12 cells (2). Besides involvement in tumors of neuronal origin, Trk mutations and translocations have been reported in breast and pancreatic cancer cells as well as in lymphoma and multiple myeloma cells (4–10). A TrkA mutation conferring ligand-independent pro-growth and pro-survival activity has been documented in AML (11, 12). In this mutation, a seventy five amino acid deletion of TrkA was identified, also designated as Δ TrkA. This mutation is strongly leukemogenic and transforms hematopoietic stem cells by activating the PI3K-mTOR pathways (11, 12). A recent study has demonstrated that AML cells co-express at least one or more isoforms of the Trk receptors (13). Here, a retrovirus-mediated co-expression of TrkA and its ligand NGF in 32D cells resulted in leukemia when the cells were transplanted into mice (13). TrkA mRNA and protein expression has been shown to be highly up-regulated in human AML expressing AML1-ETO (14). CD34+ cells expressing AML-ETO were demonstrated to respond to NGF and IL-3 stimulation by expanding in liquid culture. (14). Additionally, recent studies have demonstrated the role of neurotrophin-induced TrkA signaling in non-Hodgkin lymphoma and diffuse large B-cell lymphoma cells (15).
Heat shock protein 90 (hsp90) is abundantly expressed and stress-inducible, homo-dimeric, ATP-dependent molecular chaperone (16). Hsp90 forms the core of a super chaperone machine, which is required for maintaining a number of signaling protein kinases and transcription factors, known as hsp90 client proteins, into their functionally mature and active conformation (16,17). ATP binding to the hydrophobic N-terminus pocket also alters hsp90 conformation, promoting the interaction of hsp90 with a set of co-chaperones, e.g., p23 and cdc37, that fold the metastable signaling client proteins into their active conformation (16–19). In transformed cells, hsp90 client onco-proteins include several unmutated and mutated protein kinases, e.g., Bcr-Abl, FLT-3, c-KIT, c-Raf and AKT (16,17,20–24). The hsp90 antagonist geldanamycin and its more soluble analogue 17-DMAG bind to the N-terminus ATP-binding pocket of hsp90, replacing the nucleotide and inhibiting the chaperone function of hsp90 (16,17,25–27). Binding of 17-DMAG to hsp90 shifts it from a refolding chaperone complex to the one that promotes degradation of client proteins (16,17,28). The misfolded client protein is then directed to a covalent linkage with polyubiquitin by an E3 ubiquitin ligase, and subsequently degraded by the 26S proteasome (16,17,28). Thus, 17-DMAG treatment promotes polyubiquitylation and proteasomal degradation of the misfolded hsp90 client proteins, including Bcr-Abl, FLT-3, c-Raf, AKT, CDK4 and c-Kit (20–24, 29). Recently, among the Trk receptor family members, TrkB was shown to interact with hsp90 in retinal ganglion cells (30). Additionally, in tumor cells, Brain Derived Neurotrophic factor (BDNF)-mediated activation of TrkB was shown to be dependent on hsp90 (31). In the present studies, we demonstrate that TrkA is an hsp90 client protein, and treatment with 17-DMAG depletes the levels and signaling mediated by TrkA in cultured and primary human myeloid leukemia cells. Furthermore, co-treatment with 17-DMAG and a TrkA antagonist was noted to exert synergistic activity against cultured and primary human myeloid leukemia cells.
Materials and methods
Cell culture
Human CML-BC K562 cells were obtained from American Type Culture Collection (Manassas, VA) and maintained in culture in RPMI medium containing 10% fetal bovine serum, MEM-NEAA and penicillin-streptomycin. (21,24). HS-5 cells were obtained from ATCC and maintained in DMEM containing, 10% FBS, 1% MEM-NEAA and 1% penicillin-streptomycin. Co-cultures of HS-5 and leukemic cells were carried out as described previously (32,33). The rat pheochromocytoma PC-12 cells were obtained from ATCC and maintained in F-12K medium supplemented with 10% fetal bovine serum, 5% horse serum, MEM-NEAA, and penicillin-streptomycin. 32D cells ectopically overexpressing wild-type TrkA (32D/wtTrkA) or mutant TrkA (32D/Δ TrkA) were created and maintained in culture, as previously described (14,15). Human cancer cell lines obtained from the American Type Culture Collection were maintained according to guidelines (http://www.atcc.org and http://www.aacr.org). Logarithmically growing cells were used for all experiments.
Reagents and antibodies
17-DMAG was obtained from National Cancer Institute’s and Kosan Biosciences (South San Francisco, CA). K-252a, an inhibitor of TrkA signaling (34,35), was purchased from Calbiochem (San Diego, CA). Monoclonal anti-TrkA antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). p-TrkA, p-AKT and AKT antibodies were purchased from Cell Signaling Technology (Beverly, MA). Antibodies for c-Raf were obtained from BD Biosciences (San Jose, CA). Ubiquitin antibody was obtained from Covance (FL, USA). ERK1/1 and p-ERK1/2 antibodies were obtained from Invitrogen (Carlsbad, CA).
Primary leukemia blasts
Primary AML and chronic myeloid leukemia (CML) cells were obtained with informed consent as part of a clinical protocol approved by the Institutional Review Board of the Medical College of Georgia. As previously described, bone marrow and/or peripheral blood samples were collected in heparinized tubes, and mononuclear cells were separated using Lymphoprep (Axis-Shield, Oslo, Norway), as previously described (36). Cells were counted prior to their use in experiments.
Immunoprecipitation of TrkA, hsp90 and immunoblot analyses
Following the designated treatments, cells were lysed in thelysis buffer (20 mM Tris [pH 8], 150 nM sodium chloride, 1% Nonidet P-40 (NP-40), 0.1 M sodium fluoride, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM sodium orthovanadate, 2.5 μg/mL leupeptin, 5 μg/mL aprotinin) for 30 minutes on ice, and the lysate was cleared by centrifugation, as previously described (24). Cell lysates were incubated with the hsp90 or TrkA monoclonal antibody for 1 hour at 4°C. To this, washed Protein G agarose beads were added and incubated overnight at 4°C. The immunoprecipitates were washed 3 times with lysis buffer and proteins were eluted with sodium dodecyl sulfate (SDS) sample loading buffer prior to the immunoblot analyses with specific antibodies against hsp90, TrkA, anti-cdc37 or anti-ubiquitin antibody (24).
Western analyses of proteins
Western analyses were performed using specific antisera or monoclonal antibodies according to previously reported protocols, and the horizontal scanning densitometry was performed on Western blotsas previously described (24,27).
RT-PCR to detect TrkA mRNA levels
Primers were designed to detect wild-type TrkA and Δ TrkA. These primers were: TrkA Forward, 5’-TCCCGGCCAGTGTGCAGCTG-3’, and TrkA Reverse 5’-AGGGATGGGGTCCTCGGGGTTGAA-3’. Following drug treatments total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). Two micrograms of total RNA were reverse transcribed with a Superscript First strand synthesis kit (Invitrogen, Carlsbad,CA). The resulting cDNA was used to amplify the 326-bp wtTrkA or the 101-bp Δ TrkA by PCR. Primers designed against β-actin were used as an internal loading control. These primers were β-actin for: 5 ‘-CTACAATGAGCTGCGTGTGG-3 ‘ and β-actinrev: 5 ‘-AAGGAAGGCTGGAAGAGTGC-3’. The resulting PCR products were separated on a 1% agarose gel and imaged with a UV transilluminator.
Apoptosis assessment by Annexin V/Propidium iodide (PI) staining and assessment of non-viable cells by PI staining
After drug treatments, cells were washed with PBS, resuspended in 100 μL of Annexin V staining solution (containing Annexin V-FITC conjugate and PI in a HEPES buffer). Annexin V-FITC was obtained from BD PharMingen (San Diego, CA). Following incubationat room temperature for 15 minutes, cells were analyzed by flowcytometry using BD FacsCalibur (24,27). Alternatively, following exposure to drugs, cells were washed free of drugs and stained with PI. The percentage of non-viable cells was determined by flow cytometry. Synergism defined as a more than expected additive effect was assessed using the median dose effect of Chou-Talalay and the combination index (CI) for each drug combination was obtained using the commercially available software Calcusyn (Biosoft, Ferguson, MO). CI < 1, CI = 1, and CI > 1 represent synergism, additivity, and antagonism between two agents, respectively. CI values between 0.1–0.3 represent strong synergism, 0.3–0.7 represent synergism and 0.7–0.9 represent moderate to slight synergism. Fa or the fraction affected by the treatments is the percentage of apoptotic cells.
Immunofluorescent staining and confocal microscopy
K562 cells were exposed to 17-DMAG and fixed with 4% paraformaldehyde for 10 minutes. Following this, the slides were blocked with 3% BSA for 30 minutes and incubated with anti-TrkA and anti–ubiquitin antibody (Enzo Lifesciences International Inc, PA). After3 washes with PBS, the slides were incubated in anti-mouse Alexa Fluor 488 and anti-rabbit Alexa Fluor 594 secondary antibodies (Molecular Probes,Invitrogen, Eugene, OR) for 1 hour at 1:3000 dilution. After3 washes with PBS, the cells were counterstained with DAPI using Vectashield mountant containing DAPI and imaged using Zeiss LSM510 confocal microscope (Carl Zeiss, Heidelberg, Germany), as previouslydescribed (27).
Statistical analysis
Significant differences between valuesobtained in a population of leukemia cells treated with differentexperimental conditions were determined using the Student’st test.
Results
17-DMAG depletes the protein levels and induces proteasomal degradation of TrkA in human leukemia cells
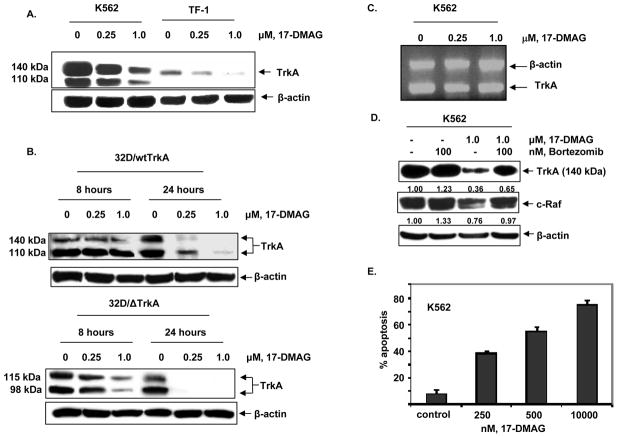
We first determined the effects of 17-DMAG on the levels of TrkA in the cultured CML blast crisis K562 and acute myeloid leukemia TF-1 cells. Figure 1A demonstrates that treatment with 17-DMAG dose-dependently decreased the levels of unglycosylated and glycosylated forms of TrkA (11). We next determined the effects of exposure to 17-DMAG for 8 or 24 hours on the myeloid progenitor cell line 32D overexpressing either wild-type (32D/wtTrkA) or mutant (32D/Δ TrkA) TrkA (Figure 1B). Similar to K562, treatment with 17-DMAG dose-dependently depleted the levels of wild-type and mutant TrkA in 32D cells, although 17-DMAG was more potent and effective in depleting the mutant versus the wild-type TrkA (Figure 1B). We next determined the effects of 17-DMAG on the mRNA levels of TrkA in K562 cells. Treatment of K562 cells with 17-DMAG did not alter the mRNA levels of TrkA, suggesting that the effect of 17-DMAG in depleting TrkA was post-transcriptional (Figure 1C). Consistent with the observation that inhibition of hsp90 directs the hsp90 client oncoproteins to proteasomal degradation (16,17,28), we also determined that co-treatment with the proteasome inhibitor bortezomib restored 17-DMAG-mediated depletion of TrkA and c-Raf levels in K562 cells (Figure 1D). This suggested a chaperone association of TrkA with hsp90 in human leukemia cells that is disrupted by treatment with 17-DMAG. Finally, we demonstrate that treatment of K562 cells with 17-DMAG results in a dose-dependent increase in apoptosis, which likely ensues as a consequence of the abrogation of chaperone association of hsp90 with pro-survival signaling proteins including c-Raf and AKT (Figure 1E).
Figure 1. Treatment with 17-DMAG depletes the levels and induces proteasomal degradation of TrkA.
A. K562 cells and TF-1 cells were treated with the indicated concentrations of 17-DMAG for 8 hours. Following this, total cell lysates were harvested and probed for TrkA and β-actin by Western blot analyses. B. 32D/wtTrkA and 32D/Δ TrkA cells were treated with the indicated concentrations of 17-DMAG for 8 or 24 hours. Following this, total cell lysates were harvested and probed for TrkA and β-actin by Western blot analyses. C. K562 cells were treated with the indicated concentrations of 17-DMAG for 24 hours. Following this, total RNA was extracted and RT-PCR analysis was performed for TrkA. The levels of β-actin served as the loading control. D. K562 cells were treated with the indicated concentrations of 1.0 μM of 17-DMAG and/or 100 nM bortezomib for 4 hours. Following this, total cell lysates were immunoblotted for determining TrkA, c-Raf. and β-actin levels. E. K562 cells were exposed to indicated concentrations of 17-DMAG for 48 hours and the percentage of apoptotic cells were determined by Annexin V/PI staining followed by flow cytometry.
17-DMAG inhibits chaperone association of TrkA with hsp90, promoting polyubiquitylation of TrkA
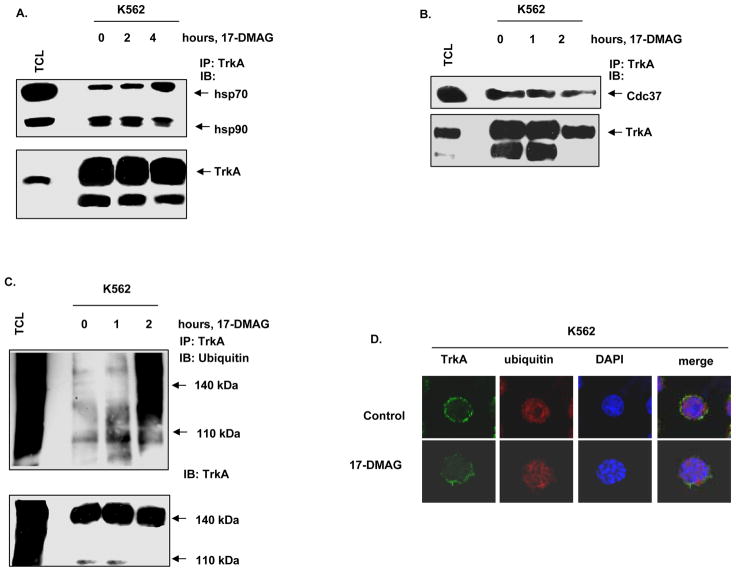
Treatment with an hsp90 inhibitor is known to decrease the chaperone association of the client proteins with hsp90 with simultaneous increase in binding to hsp70. As shown in Figure 2A, treatment with 17-DMAG led to a time-dependent decrease in binding of TrkA with hsp90 and a reciprocal increase in the binding of TrkA to hsp70 (Figure 2A). We next determined the effects of 17-DMAG on the association of TrkA with hsp90 co-chaperone cdc37, that is involved in the loading of kinase client proteins onto hsp90 (18, 19, 35). Figure 2B demonstrates that, in K562 cells, following treatment with 17-DMAG for an interval as short as one hour TrkA binding to cdc37 was reduced, with a further decline in binding of TrkA to cdc37 by two hours. Treatment with 17-DMAG also inhibited the association of hsp90 with the co-chaperone p23 (data not shown). We next determined whether inhibition of chaperone association of hsp90 with TrkA would induce polyubiquitylation of TrkA. Treatment with 17-DMAG increased the intracellular levels of polyubiquitylated TrkA within two hours without a reduction in the total TrkA levels (Figure 2C). The effects of 17-DMAG on the intracellular localization of TrkA was determined by immunofluorescence microscopy. In untreated K562 cells, TrkA was predominantly localized to the cell surface membrane (Figure 2D, first panel). In contrast, following treatment with 0.25 μM of 17-DMAG, the cell surface expression of TrkA was decreased (Figure 2D, second panel). Taken together, these results indicate that 17-DMAG treatment inhibits the chaperone association of TrkA with hsp90, followed by polyubiquitylation, proteasomal degradation and reduced membrane localization of TrkA.
Figure 2. 17-DMAG inhibits the chaperone association of TrkA with hsp90 and promotes polyubiquitylation of TrkA.
A. K562 cells were treated with 1.0 μM of 17-DMAG for the indicated time intervals. Following this, total cell lysates were harvested, and TrkA was immunoprecipitated. The immunoprecipitates were resolved by SDS PAGE and immunoblotted for hsp70, hsp90 and TrkA. B. K562 cells were treated with 1.0 μM, 17-DMAG for the indicated exposure intervals. Following this, TrkA immunoprecipitates were resolved by SDS PAGE and immunoblotted with anti-cdc37 and anti-TrkA to assess binding of cdc37 to TrkA. C. K562 cells were treated with 1.0 μM, 17-DMAG for the indicated time intervals. Following this, TrkA was immunoprecipitated from the total cell lyastes and the immunoprecipitates were resolved by SDS PAGE. Polyubiquitylation of TrkA was determined by immunoblot analysis using an anti-ubiquitin antibody. D. K562 cells were exposed to DMSO or 0.25 μM, 17-DMAG for 24 hours. Cells were then fixed, permeabilized and immunostained for TrkA (green) and Ubiquitin (red). Nuclei were stained with DAPI. The slides were imaged with a LSM 510 meta confocal microscope using a 63×/1.2W lens.
Treatment with 17-DMAG and/or K-252a attenuates the NGF-mediated autophosphorylation of TrkA and downstream signaling
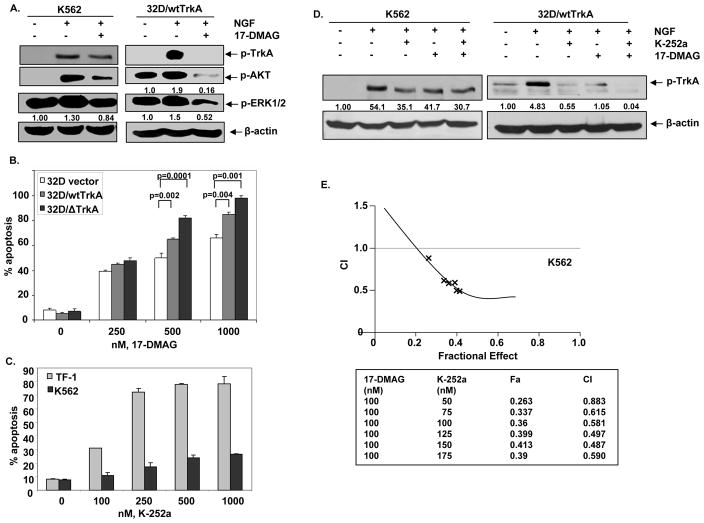
NGF is known to bind TrkA and induces downstream signaling involving auto-phosphorylation of TrkA (pY490), AKT and ERK1/2 (2,4,5). To determine the effects of hsp90 inhibition on NGF-induced signaling, K562 and 32D/wtTrkA cells were treated with NGF alone or with the combination of NGF and 17-DMAG. NGF treatment induced rapid autophosphorylation of TrkA and increased p-AKT and ERK1/2 in both K562 and 32D cells with endogenous and exogenous expression of TrkA, respectively (Figure 3A). Co-treatment with 17-DMAG inhibited NGF mediated increase in p-TrkA, p-AKT, and p-ERK1/2 (Figure. 3A). The decline in p-TrkA and p-AKT levels was more pronounced than in p-ERK1/2 levels. Previous studies have demonstrated that 32D cells expressing Δ TrkA survive and grow in IL-3 depleted culture conditions, as well as exhibit increased levels of phosphorylation of Y490 on TrkA, p-ERK1/2 and p-AKT and induce AML in mice (11, 12). In the present studies, we observed that treatment with 17-DMAG induced significantly more apoptosis of 32D cells expressing either wild type TrkA or Δ TrkA than 32D cells transfected with vector alone (Figure 3B).
Figure 3. Treatment with 17-DMAG and/or K252a attenuates NGF-mediated autophosphorylation of TrkA and downstream signaling in K562 cells and 32D/wtTrkA cells.
A. K562 and 32D/wtTrkA cells were serum starved for 2 hours, then treated with 100 ng/ml NGF and/or 1.0 μM, 17-DMAG for 12 minutes. Following this, Western blot analysis of p-TrkA, p-AKT and p-ERK1/2 was performed on the total cell lysates. The levels of β-actin served as the loading control. B. 32D cells expressing vector, wt/TrKA or Δ TrkA were exposed to indicated concentrations of 17-DMAG for 48 hours and the percentage of apoptotic cells were assessed by Annexin V and PI staining, followed by flow cytometry. C. TF-1 and K562 cells were exposed to indicated concentrations of K-252a for 48 hours and the percentage of apoptotic cells were determined by Annexin V/PI staining followed by flow cytometry. D. K562 and 32D/wtTrkA cells were serum starved for 2 hours and treated with 100 ng/ml of NGF alone or co-treated with 100 ng/ml of NGF and 17-DMAG or 150 nM of K-252a for K562 cells and 75 nM K-252a for 32D/wtTrkA or NFG, 17-DMAG and K-252a for 12 minutes. Following this, Western blot analysis of p-TrkA was performed on the total cell lysates. The levels of β-actin served as the loading control. E. K562 cells were exposed to 100 nM, 17-DMAG and indicated concentrations of K-252a for 48 hours and percentage of apoptotic cells were measured by Annexin V and PI staining followed by flow cytometry. Isobologram analysis was performed to obtain combination index values for each fraction using Calcusyn software. Combination index values <1.0 correspond to synergistic interactions. The fraction of cells affected (Fa) by the drug combination is presented.
We next determined the effects 17-DMAG and/or TrkA specific signaling inhibitor K-252a in human leukemia cells. As shown in Figure 3C, treatment with K-252a induces a dose-dependent increase in apoptosis of TF-1 more than K562 cells. We then determined the effect of inhibiting TrkA signaling in K562 cand 32D/wtTrkA cells. As previously reported, while exposure to K-252a inhibited NGF-induced p-TrkA levels (34,35), co-treatment with 17-DMAG and K-252a produced further decline in the NGF-induced phosphorylation of TrkA (Figure 3D). A similar effect of 17-DMAG and K-252a co-treatment was also observed on p-AKT levels (data not shown). Consistent with these observations, combined treatment with K-252a and 17-DMAG exerted a superior anti-apoptotic effect against K562 cells. (Figure 3E). Analysis of the dose effect relationship for 17-DMAG (100 nM) and K-252a (50– 175 nM) in K562 cells was performed according to the median dose effect method of Chou and Talalay. Following this, the combination index (CI) values were calculated using the % apoptotic cells (fraction affected or Fa) by the co-treatment of the two agents. As can be observed, the combined treatment of 17-DMAG and K-252a results in a synergistic increase in the fraction of apoptotic cells (Fa ranging from 0.2 to 0.4) with the CI values ranging from 0.8 to 0.4, respectively. These observations suggest that, as compared to each agent alone, co-treatment with K-252a and 1-DMAG more potently abrogates TrkA-mediated survival signaling and induces cell death of human leukemia cells.
Activity of 17-DMAG is not affected by co-culture with bone marrow stromal cells (BMSC)
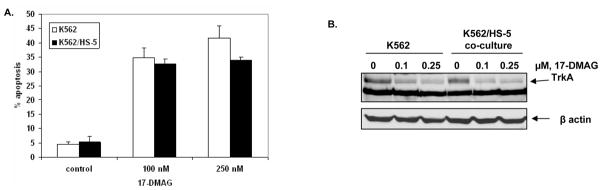
Co culture with the HS-5 BMSC and NGF produced by these cells has been shown to promote survival of TrkA expressing leukemia cells (33,37,38). We next determined whether 17-DMAG would induce apoptosis of leukemia cells co-cultured with HS-5 cells. Our findings demonstrate that 17-DMAG treatment induced similar rate of apoptosis in K562 cells with or without co-culture with HS-5 cells (Figure 4A). Additionally, treatment with 17-DMAG attenuated the levels of TrkA to a similar extent in K562 cells with or without co-culture with BMSC (Figure 4B).
Figure 4. Treatment with 17-DMAG results in the induction of apoptosis in K562 cells and K562/HS-5 co-cultures.
A. K562 cells were cultured in the presence or absence of HS-5 cells and exposed to indicated-concentrations of 17-DMAG for 48 hours. The percentage of apoptotic cells were assessed by Annexin V and PI staining, followed by flow cytometry. B. K562 cells were cultured in the presence or absence of HS-5 cells and treated with indicated concentrations of 17-DMAG for 8 hours. The resulting cell pellets were lysed and the expression of TrkA was assessed by immunoblotting. The levels of β-actin served as loading control.
Treatment with 17-DMAG attenuates the levels of TrkA and inhibits NGF-mediated differentiation of PC-12 cells
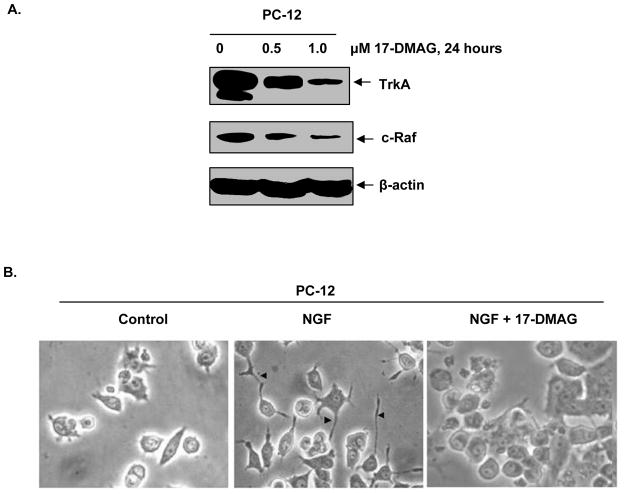
PC-12 cells differentiate and form neurites following exposure to NGF and TrkA-induced signaling (39,40). We next determined the effect of 17-DMAG on TrkA levels and NGF mediated neurite formation and differentiation in PC12 cells. As shown in Figure 5A, treatment with 17-DMAG dose-dependently decreased the levels of TrkA with concomitant decline in c-Raf levels, a known hsp90 client protein. Additionally, treatment with 17-DMAG inhibited NGF-induced neurite formation and differentiation of PC-12 cells (Figure 5B). Collectively, these data demonstrate that 17-DMAG abrogates NGF-induced, TrkA mediated signaling for differentiation in cells derived from neuroectoderm, in addition to inhibiting pro-growth and pro-survival signaling in myeloid leukemia cells.
Figure 5. 17-DMAG attenuates the levels of TrkA and inhibits NGF-mediated neurite formation in PC-12 cells.
A. PC-12 cells were treated with indicated concentrations of 17-DMAG for 24 hours. Following this, total cell lysates were prepared and Western blot analyses were performed for TrkA and c-Raf. The levels of β-actin served as loading control. B. PC-12 cells were serum starved for 24 hours. Cells were then treated with 100ng/ml NGF or co-treated with NGF and 1.0 μM, 17-DMAG for 24 hours. Following this, neurite formation was evaluated utilizing light microscopy and images were acquired with digital camera.
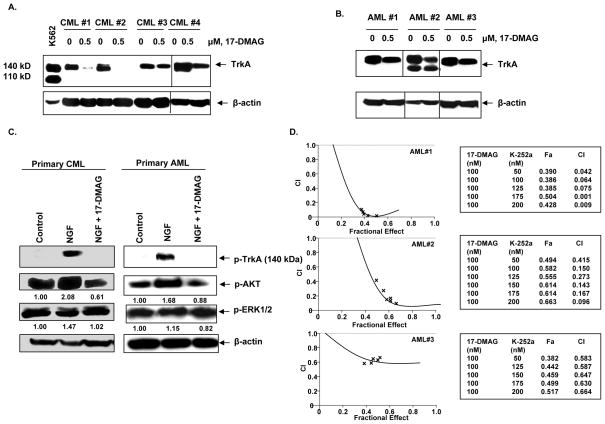
17-DMAG attenuates TrkA levels and NGF-induced signaling in primary CML and AML cells
We next determined the effects of 17-DMAG on the levels of TrkA and NGF-induced p-AKT and p-ERK1/2 levels in primary CML and AML cells. Peripheral blood mononuclear cells from three primary AML and four CML samples were treated with 17-DMAG for 24 hours. 17-DMAG treatment depleted TrkA levels to a varying extent in the primary CML and AML mononuclear cells (Figure 6A & 6B). As was noted in the cultured leukemia cells, exposure to NGF rapidly increased the phosphorylation of TrkA, AKT, and ERK1/2 in the primary AML and CML cells. The effect on a representative sample of each primary cell-type is shown in Figure 6C. Co-treatment with 17-DMAG attenuated NGF-induced levels of p-TrkA, p-AKT and p-ERK1/2 (Figure 6C). The inhibitory effect of 17-DMAG on NGF-induced p-TrkA levels was pronounced. Furthermore, co-treatment with K-252a and 17-DMAG resulted in synergistic loss of viability in the three primary AML samples, with the combination indices ranging from 0.001 to 0.5 (Figure 6D), while the lethal effects of the combination were sub-additive in the primary CML mononuclear cells (data not shown). This suggests that in the primary CML cells the survival signaling is predominantly mediated by BCR-ABL and less by TrkA. The findings also indicate that targeting TrkA-mediated pro-survival signaling by 17-DMAG sensitizes primary AML cells to K-252a.
Figure 6. 17-DMAG depletes TrkA in primary CML and AML cells.
A and B. Primary CML and AML mononuclear cells were treated with the indicated concentrations of 17-DMAG for 24 hours. Following this, Western blot analysis of TrkA was performed on the total cell lysates. The levels of β-actin served as loading control. C. Primary CML and AML mononuclear cells were serum starved for 2 hours, followed by treatment with 100 ng/ml NGF and/or 1.0 μM 17-DMAG for 12 minutes. Western blot analyses of p-TrkA, p-AKT and p-ERK1/2 were performed on the total cell lysates. The levels of β-actin served as the loading control. D. Mononuclear cells isolated from primary AML samples were exposed to 100 nM of 17-DMAG and the indicated concentrations of K-252a for 48 hours. Following this, the percentage of non-viable cells was estimated by PI staining followed by flow cytometry. Isobologram analysis for determining synergistic interaction between 17-DMAG (100 nM) and K-252a (50 to 200 nM) was performed, utilizing the Calcusyn software. CI values less than 1.0 represent synergistic interaction of the two drugs. The percentage of cells affected by the combination (Fa) of the two agents is presented.
Discussion
Here, we report for the first time that the chaperone association of TrkA with hsp90 is inhibited by treatment with 17-DMAG. This leads to depletion of TrkA and inhibition of downstream signaling through p-AKT and p-ERK1/2, resulting in apoptosis of myeloid leukemia cells with endogenous or ectopic expression of the unmutated TrkA or constitutively active Δ TrkA. These findings are consistent with a recent report demonstrating that TrkAI and its oncogenic alternative TrkAIII splice variant exhibit geldanamycin-sensitive interactions with hsp90 in human neuroblastoma cells. (41). However, in our studies we further show that the geldanamycin analogue 17-DMAG, which is clinically active against human AML (42), simultaneously reduced the binding of TrkA to hsp90 and cdc37. The latter is an hsp90 co-chaperone associated with the loading of client protein kinases to the hsp90 chaperone complex (18,19). Reduced binding of TrkA to hsp90 and cdc37 was associated with a concomitant increase in the binding of TrkA to hsp70, resulting in polyubiquitylation and proteasomal degradation of TrkA. Following NGF treatment, the monoubiquitylation of TrkA has been shown to be involved in its endosomal sorting and trafficking (43). In contrast, polyubiquitylation of TrkA leads to its degradation by the proteasome. Although following NGF treatment lysosomes may also be involved in the degradation of polyubiquitylated TrkA (40,43), our studies demonstrate that 17-DMAG treatment mediated degradation of TrkA is primarily through the proteasome. This is supported by the observation that co-treatment with 17-DMAG and bortezomib causes accumulation of TrkA in the detergent insoluble fraction (figure 1D and data not shown). Collectively these observations indicate that TrkA is a bona fide hsp90 client protein and is degraded by the proteasome, following inhibition of hsp90 function with 17-DMAG.
The role of neurotrophins and their receptors in promoting growth and survival of tumors of neuronal and non-neuronal origin is well established (3,4,11–14). For example, Trk family of receptors is expressed not only in neuroblastoma, but also in the solid tumors, lymphoma and leukemia (4–10). In neuroblastoma, TrkB-BDNF expression has been correlated with resistance to DNA-damaging agents by activating the pro-survival PI3K/AKT pathway (44). TrkA expression has also been implicated in leukemogenesis, thereby highlighting the need for targeting TrkA for the therapy of myeloid leukemia (3,4,11–14). Here, we demonstrate that 17-DMAG treatment inhibited activated TrkA and its downstream signaling through p-AKT and p-ERK1/2, resulting in apoptosis of cultured and primary human AML and CML cells. In primary and cultured myeloid leukemia cells, 17-DMAG also inhibited NGF-induced p-TrkA and downstream p-AKT and p-ERK1/2 levels. Similar effects of 17-DMAG were also observed in the mouse myeloid 32D cells overexpressing wild-type TrkA or the mutant Δ TrkA. 17-DMAG treatment caused more depletion of Δ TrkA compared to wtTrkA, associated with more apoptosis of 32D-Δ TrkA versus 32D-wtTrkA cells. This is consistent with the observations that, for maintaining their active conformation, the mutant forms of some of the oncoprotein kinases, e.g., BCR-ABL and FLT-3, are more dependent on their chaperone association with hsp90, hence more susceptible to depletion following treatment with an hsp90 inhibitor (20,22,45). In addition, 17-DMAG was effective in inducing apoptosis of K562 cells with or without the co-culture with the bone marrow stromal HS-5 cells. This is important, since NGF produced by HS-5 cells is known to improve the survival of AML cells, as well as inhibit apoptosis induced by chemotherapeutic agents (33,38). Co-culture of Non-Hodgkin’s lymphoma cells with HS-5 cells also resulted in the activation of NF-κ B pathway, thereby promoting the survival of lymphoma cells (32). Therefore, the ability of 17-DMAG to induce apoptosis of myeloid leukemia cells regardless of co-culture with HS-5 cells suggest that 17-DMAG treatment may override this resistance mechanism in human myeloid leukemia cells.
Following treatment with NGF, rat adrenal pheochromocytoma PC-12 cells produce neurite projections as a phenotypic marker of differentiation (2,39). Treatment with the TrkA-specific inhibitor K252a inhibits NGF-induced neurite extensions of PC-12 cells (34,35) . We observed that 17-DMAG treatment depleted TrkA and c-Raf, inhibited NGF-induced p-TrkA, p-AKT and p-ERK1/2 levels, as well as inhibited NGF-induced neurite formation and differentiation in PC-12 cells. Whether, NGF and TrkA mechanistically regulate not only growth and survival but also the differentiation arrest of myeloid leukemia cells has not been elucidated, and was not the focus of the present study. Our findings also demonstrate that treatment with K-252a and 17-DMAG alone inhibited NGF-induced p-TrkA, p-AKT and p-ERK1/2 levels in myeloid leukemia cells. Importantly, co-treatment with 17-DMAG and K-252a exerted synergistic lethal activity against cultured and primary myeloid leukemia cells. Although the precise mechanistic basis of this synergy is not clear, it may be due to a greater attenuation of p-TrkA and its downstream signaling, or due to attenuation mediated by 17-DMAG of the other collateral survival signaling proteins, e.g, NFκ B and Pim1 (46,47). These findings suggest that combined treatment with an hsp90 inhibitor and a TrkA specific inhibitor would be a promising novel therapy for myeloid leukemia that show oncogenic ‘addiction’ to the activating mutation or overexpression of TrkA, an hsp90 client protein, as well as non-oncogenic addiction to the heat shock response (48).
Acknowledgments
K.N.B. is a Georgia Cancer Coalition Distinguished Cancer Scholar Award recipient. The current study was also supported in part by NCI R01 CA123207 (K.N.B.) and NCI R01 CA116629 (K.N.B).
References
- 1.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurol. 2000;10:381–91. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 2.Chang JH, Mellon E, Schanen NC, Twiss JL. Persistent TrkA activity is necessary to maintain transcription in neuronally differentiated PC12 cells. J Biol Chem. 2003;278:42877–85. doi: 10.1074/jbc.M308155200. [DOI] [PubMed] [Google Scholar]
- 3.Thiele CJ, Li Z, McKee AE. On Trk--the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res. 2009;15:5962–7. doi: 10.1158/1078-0432.CCR-08-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruttgen A, Schneider I, Weis J. The dark side of the NGF family: neurotrophins in neoplasias. Brain Pathol (Zurich, Switzerland) 2006;16:304–10. doi: 10.1111/j.1750-3639.2006.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagadec C, Meignan S, Adriaenssens E, et al. TrkA overexpression enhances growth and metastasis of breast cancer cells. Oncogene. 2009;28:1960–70. doi: 10.1038/onc.2009.61. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawara A, Arima-Nakagawara M, Scavarda NJ, Azar CG, Cantor AB, Brodeur GM. Association between high levels of expression of the TRK gene and favorable outcome in human neuroblastoma. N Engl J Med. 1993;328:847–54. doi: 10.1056/NEJM199303253281205. [DOI] [PubMed] [Google Scholar]
- 7.McGregor LM, McCune BK, Graff JR, et al. Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci U S A. 1999;96:4540–5. doi: 10.1073/pnas.96.8.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005;105:4429–36. doi: 10.1182/blood-2004-08-3096. [DOI] [PubMed] [Google Scholar]
- 9.Renne C, Minner S, Kuppers R, Hansmann ML, Brauninger A. Autocrine NGFbeta/TRKA signalling is an important survival factor for Hodgkin lymphoma derived cell lines. Leuk Res. 2008;32:163–7. doi: 10.1016/j.leukres.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Okada Y, Eibl G, Guha S, Duffy JP, Reber HA, Hines OJ. Nerve growth factor stimulates MMP-2 expression and activity and increases invasion by human pancreatic cancer cells. Clin Exp Metastasis. 2004;21:285–92. doi: 10.1023/b:clin.0000046131.24625.54. [DOI] [PubMed] [Google Scholar]
- 11.Reuther GW, Lambert QT, Caligiuri MA, Der CJ. Identification and characterization of an activating TrkA deletion mutation in acute myeloid leukemia. Mol Cell Biol. 2000;20:8655–66. doi: 10.1128/mcb.20.23.8655-8666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer J, Rhein M, Schiedlmeier B, et al. Remarkable leukemogenic potency and quality of a constitutively active neurotrophin receptor, delta TrkA. Leukemia. 2007;21:2171–80. doi: 10.1038/sj.leu.2404882. [DOI] [PubMed] [Google Scholar]
- 13.Chevalier S, Praloran V, Smith C, et al. Expression and functionality of the trkA proto-oncogene product/NGF receptor in undifferentiated hematopoietic cells. Blood. 1994;83:1479–85. [PubMed] [Google Scholar]
- 14.Mulloy JC, Jankovic V, Wunderlich M, et al. AML1-ETO fusion protein up-regulates TRKA mRNA expression in human CD34+ cells, allowing nerve growth factor-induced expansion. Proc Natl Acad Sci U S A. 2005;102:4016–21. doi: 10.1073/pnas.0404701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sniderhan LF, Garcia-Bates TM, Burgart M, Bernstein SH, Phipps RP, Maggirwar SB. Neurotrophin signaling through tropomyosin receptor kinases contributes to survival and proliferation of non-Hodgkin lymphoma. Exp Hematol. 2009;37:1295–309. doi: 10.1016/j.exphem.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–72. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 17.Pearl LH, Prodromou C, Workman P. The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J. 2008;410:439–53. doi: 10.1042/BJ20071640. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan CK, Mollapour M, Smith JR, et al. Hsp90-dependent activation of protein kinases is regulated by chaperone-targeted dephosphorylation of Cdc37. Mol Cell. 2008;31:886–95. doi: 10.1016/j.molcel.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JR, Clarke PA, de Billy E, Workman P. Silencing the cochaperone CDC37 destabilizes kinase clients and sensitizes cancer cells to HSP90 inhibitors. Oncogene. 2009;28:157–69. doi: 10.1038/onc.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao Q, Nishiuchi R, Li Q, Kumar AR, Hudson WA, Kersey JH. FLT3 expressing leukemias are selectively sensitive to inhibitors of the molecular chaperone heat shock protein 90 through destabilization of signal transduction-associated kinases. Clin Cancer Res. 2003;9:4483–93. [PubMed] [Google Scholar]
- 21.Nimmanapalli R, O’Bryan E, Bhalla K. Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res. 2001;61:1799–804. [PubMed] [Google Scholar]
- 22.Gorre ME, Ellwood-Yen K, Chiosis G, Rosen N, Sawyers CL. BCR-ABL point mutants isolated from patients with imatinib mesylate-resistant chronic myeloid leukemia remain sensitive to inhibitors of the BCR-ABL chaperone heat shock protein 90. Blood. 2002;100:3041–4. doi: 10.1182/blood-2002-05-1361. [DOI] [PubMed] [Google Scholar]
- 23.Fumo G, Akin C, Mecalfe DD, Neckers L. 17-Allylamino-17-demethoxygeldanamycin (17-AAG) is effective in down-regulating mutated, constitutively activated KIT protein in human mast cells. Blood. 2004;103:1078–84. doi: 10.1182/blood-2003-07-2477. [DOI] [PubMed] [Google Scholar]
- 24.Rao R, Fiskus W, Yang Y, et al. HDAC6 inhibition enhances 17-AAG--mediated abrogation of hsp90 chaperone function in human leukemia cells. Blood. 2008;112:1886–93. doi: 10.1182/blood-2008-03-143644. [DOI] [PubMed] [Google Scholar]
- 25.Prodromou C, Roe SM, O’Brien R, Ladbury JE, Piper PW, Pearl LH. Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell. 1997;90:65–75.24. doi: 10.1016/s0092-8674(00)80314-1. [DOI] [PubMed] [Google Scholar]
- 26.Jez JM, Chen JC, Rastelli G, Stroud RM, Santi DV. Crystal structure and molecular modeling of 17-DMAG in complex with human Hsp90. Chem Bio. 2003;10:361–8. doi: 10.1016/s1074-5521(03)00075-9. [DOI] [PubMed] [Google Scholar]
- 27.Rao R, Lee P, Fiskus W, et al. Co-treatment with heat shock protein 90 inhibitor 17-dimethylaminoethylamino-17-demethoxygeldanamycin (DMAG) and vorinostat: a highly active combination against human mantle cell lymphoma (MCL) cells. Cancer Biol Ther. 2009;8:1273–80. doi: 10.4161/cbt.8.13.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider C, Sepp-Lorenzino L, Nimmesgern E, et al. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci U S A. 1996;93:14536–41. doi: 10.1073/pnas.93.25.14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahalingam D, Swords R, Carew JS, Nawrocki ST, Bhalla KN, Giles FJ. Targeting HSP90 for cancer therapy. Br J Cancer. 2009;100:1523–9. doi: 10.1038/sj.bjc.6605066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernstein SL, Russell P, Wong P, Fishelevich R, Smith LE. Heat shock protein 90 in retinal ganglion cells: association with axonally transported proteins. Vis Neurosci. 2001;18:429–36. doi: 10.1017/s0952523801183094. [DOI] [PubMed] [Google Scholar]
- 31.Yang ZF, Ho DW, Lam CT, et al. Identification of brain-derived neurotrophic factor as a novel functional protein in hepatocellular carcinoma. Cancer Res. 2005;65:219–25. [PubMed] [Google Scholar]
- 32.Lwin T, Hazlehurst LA, Li Z, et al. Bone marrow stromal cells prevent apoptosis of lymphoma cells by upregulation of anti-apoptotic proteins associated with activation of NF-kappaB (RelB/p52) in non-Hodgkin’s lymphoma cells. Leukemia. 2007;21:1521–31. doi: 10.1038/sj.leu.2404723. [DOI] [PubMed] [Google Scholar]
- 33.Garrido SM, Appelbaum FR, Willman CL, Banker DE. Acute myeloid leukemia cells are protected from spontaneous and drug-induced apoptosis by direct contact with a human bone marrow stromal cell line (HS-5) Exp Hematol. 2001;29:448–57. doi: 10.1016/s0301-472x(01)00612-9. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto S. K-252a, a potent protein kinase inhibitor, blocks nerve growth factor-induced neurite outgrowth and changes in the phosphorylation of proteins in PC12h cells. J Cell Biol. 1988;107:1531–9. doi: 10.1083/jcb.107.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–6. [PubMed] [Google Scholar]
- 36.Fiskus W, Buckley K, Rao R, et al. Panobinostat treatment depletes EZH2 and DNMT1 levels and enhances decitabine mediated de-repression of JunB and loss of survival of human acute leukemia cells. Cancer Biol Ther. 2009;8:939–50. doi: 10.4161/cbt.8.10.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Labouyrie E, Dubus P, Groppi A, et al. Expression of neurotrophins and their receptors in human bone marrow. Am J Pathol. 1999;154:405–15. doi: 10.1016/S0002-9440(10)65287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–24. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 39.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Natl Acad Sci U S A. 1976;73:2424–8. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jullien J, Guili V, Derrington EA, Darlix JL, Reichardt LF, Rudkin BB. Trafficking of TrkA-green fluorescent protein chimerae during nerve growth factor-induced differentiation. J Biol Chem. 2003;278:8706–16. doi: 10.1074/jbc.M202401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farina AR, Tacconelli A, Cappabianca L, et al. The neuroblastoma tumour-suppressor TrkAI and its oncogenic alternative TrkAIII splice variant exhibit geldanamycin-sensitive interactions with Hsp90 in human neuroblastoma cells. Oncogene. 2009;28:4075–94. doi: 10.1038/onc.2009.256. [DOI] [PubMed] [Google Scholar]
- 42.Lancet JE, Gojo I, Burton M, et al. Phase I study of the heat shock protein 90 inhibitor alvespimycin (KOS-1022, 17-DMAG) administered intravenously twice weekly to patients with acute myeloid leukemia. Leukemia. 2010 doi: 10.1038/leu.2009.292. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Grimes ML, Zhou J, Beattie EC, et al. Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci. 1996;16:7950–64. doi: 10.1523/JNEUROSCI.16-24-07950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho R, Eggert A, Hishiki T, et al. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462–6. [PubMed] [Google Scholar]
- 45.George P, Bali P, Cohen P, et al. Cotreatment with 17-allylamino-demethoxygeldanamycin and FLT-3 kinase inhibitor PKC412 is highly effective against human acute myelogenous leukemia cells with mutant FLT-3. Cancer Res. 2004;64:3645–52. doi: 10.1158/0008-5472.CAN-04-0006. [DOI] [PubMed] [Google Scholar]
- 46.Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require cdc37 and hsp90. Mol Cell. 2002;9:401–10. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- 47.Shay KP, Wang Z, Xing PX, McKenzie IF, Magnuson NS. Pim-1 kinase stability is regulated by heat shock proteins and the ubiquitin-proteasome pathway. Mol Cancer Res. 2005;3:170–81. doi: 10.1158/1541-7786.MCR-04-0192. [DOI] [PubMed] [Google Scholar]
- 48.Luo J, Solimini NL, Elledge SJ. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell. 2009;136:823–37. doi: 10.1016/j.cell.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]