Abstract
The microscopic observation drug susceptibility assay (MODS) is a novel and promising test for the early diagnosis of tuberculosis (TB). We evaluated the MODS assay for the early diagnosis of TB in HIV-positive patients presenting to Pham Ngoc Thach Hospital for Tuberculosis and Lung Diseases in southern Vietnam. A total of 738 consecutive sputum samples collected from 307 HIV-positive individuals suspected of TB were tested by smear, MODS, and the mycobacteria growth indicator tube method (MGIT). The diagnostic sensitivity and specificity of MODS compared to the microbiological gold standard (either smear or MGIT) were 87 and 93%, respectively. The sensitivities of smear, MODS, and MGIT were 57, 71, and 75%, respectively, against clinical gold standard (MODS versus smear, P < 0.001; MODS versus MGIT, P = 0.03). The clinical gold standard was defined as patients who had a clinical examination and treatment consistent with TB, with or without microbiological confirmation. For the diagnosis of smear-negative patients, the sensitivities of MODS and MGIT were 38 and 45%, respectively (P = 0.08). The median times to detection using MODS and MGIT were 8 and 11 days, respectively, and they were 11 and 17 days, respectively, for smear-negative samples. The original bacterial/fungal contamination rate of MODS was 1.1%, while it was 2.6% for MGIT. The cross-contamination rate of MODS was 4.7%. In conclusion, MODS is a sensitive, specific, and rapid test that is appropriate for the detection of HIV-associated TB; its cost and ease of use make it particularly useful in resource-limited settings.
It is estimated by the World Health Organization (WHO) that there were 9.4 million new cases of tuberculosis (TB) in 2008 (24). Of these, 1.4 million (15%) were in HIV-positive patients, and 23% of all HIV-related deaths are estimated to be attributable to TB (23).
Vietnam is a high-TB-burden country with steeply rising rates of HIV-TB coinfection (21); 8.1% of newly diagnosed TB patients are now HIV infected (24). These cases are the most urgently in need of diagnosis because they have the highest morbidity and mortality, and yet the diagnosis of TB among HIV-infected individuals is difficult. Screening algorithms based on clinical symptoms alone show high sensitivity but low specificity (5, 25). The microscopy smear method, while simple, specific, and widely available in high-burden settings, has particularly low sensitivity in HIV-infected patients and cannot be used to rule out a diagnosis of TB (13, 20). Microbiological confirmation remains desirable and allows investigation of drug susceptibility profiles. Commercial rapid liquid culture techniques have been endorsed by the WHO (27), show higher sensitivity, and are more rapid than traditional solid-medium-based techniques such as Lowenstein-Jensen culture. However, their high cost and biosafety infrastructure requirements limit their applicability in many high-burden settings. Rapid molecular line-probe assays, also endorsed for use in low-resource settings by the WHO (28), allow simultaneous identification of Mycobacterium tuberculosis and resistance to rifampin or isoniazid but are currently only recommended for smear-positive samples and positive cultures. In addition, they are expensive and require molecular expertise, which is often not available in low-resource settings.
Recent evaluations of a novel diagnostic test for TB, the microscopic observation drug susceptibility assay (MODS), have shown it to be economical and rapid, with a turnaround time of 7 days, making it ideal for use in high-burden, low-resource settings (2, 3, 14). MODS has been shown effective in the identification of TB in HIV-infected patients (2, 18). The increasing number of HIV-positive pulmonary TB suspects presenting to Pham Ngoc Thach Hospital, a referral TB hospital in the south of Vietnam, has led to an urgent need for a rapid and sensitive test to detect TB for this population. Here, we evaluated MODS as a promising method for TB detection. We assessed the sensitivity, specificity, negative predictive value, positive predictive value, contamination rate, and turnaround time of MODS against the clinical gold standard and the microbiological gold standard methods.
MATERIALS AND METHODS
Enrollment.
All HIV-positive individuals suspected of having TB who presented to the HIV/TB ward at Pham Ngoc Thach Hospital from May to November 2008 were enrolled into the study unless they had received >8 days of TB therapy. The data on socioeconomic and demographic features, TB history, TB contact history, HIV status, and presenting clinical features were prospectively collected on a standard case report form. Samples were collected as per routine care as deemed appropriate by the treating physician (usually three samples in accordance with WHO recommendations). No additional samples were collected as part of the present study, and only sputum samples were evaluated. The definition of TB was based on microbiological confirmation by either the smear method or the mycobacteria growth indicator tube method (MGIT), intention to treat, treatment management, and outcome. TB was defined as “confirmed TB” if the patient had clinical symptoms consistent with TB (22) and the either smear or MGIT was positive in any sample, including samples that were collected before the enrollment started. These samples were not included in the sensitivity comparison, but patients with prior samples positive in this illness episode by either smear or MGIT were classified in the “confirmed TB” group. A positive MODS culture was not considered part of the definition of “confirmed TB” because this was the test under evaluation.
The patient was defined as “probable TB” on “intention to treat” if the patient had clinical symptoms consistent with TB (22) but had no microbiological confirmation, received no alternative diagnosis, and initiated TB treatment and transferred to a District Tuberculosis Unit for treatment and follow-up. Patients who satisfied the first two characteristics of “probable TB” but self-discharged prior to treatment were also classified in this group if the clinician intended to treat the patient for TB. It was impossible to either rule out or confirm TB in this group due to the lack of microbiological confirmation.
Patients were defined as “TB unlikely” if they recovered without TB treatment, had TB treatment but deteriorated, or received an alternative diagnosis and treatment. It was impossible to rule out TB in these patients completely because clinical deterioration on therapy may have been due to undetected drug-resistant TB.
Ethics.
The protocol was approved by the Institutional Review Board (IRB) at Pham Ngoc Thach Hospital and the Health Services of Ho Chi Minh City. Individual informed consent was not sought because the study was conducted on routine samples only and did not involve any intervention, additional samples, or change in patient management. A patient consent waiver was approved by the IRB of Pham Ngoc Thach Hospital.
Sample collection.
All sputum samples were collected and transferred to the microbiology department on the same day (or the following day if they were collected after 4 p.m.). The samples were then submitted for smear, MGIT, and MODS culture. The number of specimens per patient was decided by the treating physician.
Sample processing.
Sputum samples were homogenized and decontaminated by Sputaprep (NaOH-2% N-acetyl-l-cysteine [NALC]) (Nam Khoa Company, Viet Nam) prior to testing. The kit contains Mucoprep (0.5 M NaOH and 0.05 sodium citrate), NALC, and phosphate buffer (PO4, 10×, 0.67 M). Phosphate buffer (1×), homogenization buffer, and decontamination buffer were then prepared from the kit for sample processing. In brief, a 3- to 5-ml sample was added to 3 to 5 ml of HDB contained in a 50-ml Falcon tube. The tube was shaken lightly by automated shaker and left at room temperature for 20 min. After that, 35 to 39 ml of 1× phosphate buffer was added to the mixture. The mixture was shaken by hand and then centrifuged at 3,000 × g at 4°C for 30 min. The supernatant was then discarded, and a 0.5-ml pellet at the bottom was resuspended with 2 ml of distilled water. The deposit was then aliquoted into three parts for smear, MGIT culture, and MODS.
Homogenous smear.
Two drops of pellet from each sample were put onto a slide for homogenous smear preparation. The smears were then stained by the Ziehl-Neelsen method according to the WHO standard protocol (26).
MGIT culture.
Processed samples were subjected to MGIT culture according to the protocol of Becton Dickinson (BACTEC MGIT 960 mycobacterial detection system). In brief, 0.1 ml of PANTA (Becton Dickinson), 0.5 ml of oleic acid-albumin-dextrose-catalase (OADC), and 0.5 ml of each processed sample were added into an MGIT medium tube. The mixture was inversely mixed by hand and then inoculated and incubated at 37°C in the MGIT machine. Positive results were reported automatically by the MGIT system. A smear from an MGIT-positive culture was made to confirm acid-fast bacilli.
MODS technique.
The MODS culture was conducted in a biosafety cabinet class I that was placed in a room separate from the sample processing room, the smear preparation room, and the MGIT culture room. The MODS method was performed as described in Park et al. (15) using the minor modifications described by Caws et al. (6). Briefly, MODS medium was prepared with 5.9 g of Middlebrook 7H9 broth (Difco, Sparks, MD), 3.1 ml of glycerol, and 1.25 g of Bacto Casitone (Difco) in 880 ml of sterile-distilled water. The medium was autoclaved and stored in 22-ml aliquots at 4°C. Each new batch was tested for sterility by incubating one aliquot at 37°C for 1 week. Before use, OADC and PANTA were added into each tube to final concentrations of 5.5 and 0.22% to make working MODS media. One 48-well MODS plate (Becton Dickinson) was set up each day. Portions (750 μl) of working MODS media were aliquoted into each well, and 250 μl of processed sample was added. One positive control (H37Rv) and one negative control well (sterile-distilled water) were inoculated into each plate. The samples were inoculated into alternate wells to reduce cross-contamination. Empty wells contained MODS media. To prevent cross-contamination from evaporation and ensure safety, plate seals (optical films; Bio-Rad) were used. The plate was further sealed with sticky tape, placed inside a Tupperware box, and incubated at 37°C; the plate was examined every alternate day after 5 days of inoculation for evidence of growth. Contamination was recorded if there was any growth or turbidity in any negative control well.
Subculture on LJ medium.
All cultures determined to be positive by MODS or MGIT were subcultured on Lowenstein-Jensen (LJ) medium (Becton Dickinson) in duplicate and incubated at 37°C for several weeks. These isolates were then subjected to standard biochemical identification tests, DNA extraction (10), and archiving.
Spoligotyping.
Spoligotyping was performed according to the standard international spoligotyping protocol (11) for all cultures determined to be positive by MODS (n = 396). If MODS was contaminated during subculture from MODS to LJ medium for DNA extraction (n = 20) or MODS was negative but MGIT was positive (n = 55), cultures positive by MGIT were used for spoligotyping. Multiple isolates from the same patient were compared to identify discrepant spoligotypes. If a single positive culture was obtained from a patient, samples processed on the same plate were compared to identify probable cross-contamination. Cross-contamination of MGIT was not addressed here due to resource limitations.
Statistical methods.
Accuracy measures of the three tests were calculated for two different definitions of the “gold standard” reference test: (i) microbiological confirmation (confirmed group) or (ii) “clinical diagnosis” (clinical gold standard, including the probable and the confirmed groups). In addition, we analyzed data on a per-patient or a per-sample basis.
For the per-patient analysis, the data were aggregated to provide one result per patient, i.e., the per-patient test was regarded as positive if at least one sample yielded a positive test result. The reported confidence intervals (CIs) for accuracy measures (sensitivities, specificities, and positive and negative predictive values) were calculated according to the method of Pearson and Clopper. Comparisons of accuracies between tests were done by using the McNemar test.
In the per-sample analysis we used binary marginal generalized linear regression models with an identity link function for all analyses. These models are very flexible, allow for the inclusion of covariates, and account for the fact that results of multiple samples from the same patient or test results of different tests on the same sample may be dependent (16). Specifically, we used a marginal regression model to calculate the CIs for accuracy measures; to compare the sensitivities of smear, MGIT, and MODS; and to assess the impact of the duration of TB treatment on the sensitivity of MODS.
For the per-sample analysis, we also calculated time-dependent sensitivity curves for MGIT and MODS. A test result was considered as positive by time t if the respective test was positive overall and reached the positive value at most t days after sample collection. Time-dependent sensitivity curves were estimated with the Kaplan-Meier method and samples without a positive test result were formally regarded as censored on day “infinity.” The time-dependent sensitivities of MGIT and MODS by days 7 and 14, respectively, were compared by using a marginal regression model as described above. In addition, the times to positive MGIT and MODS result, respectively, were compared in samples in which both tests reached positivity with the Cox proportional-hazards regression model. Robust sandwich estimators of the standard errors were used to adjust for the possible dependence of multiple samples from the same patient or test results of different tests on the same sample.
Comparison of the demographic and clinical features of patients between TB diagnoses (definite, probable, or unlikely) were made using the Fisher exact test for categorical data and the Kruskal-Wallis test for continuous data.
All reported CIs are two-sided 95% CIs, and P values of ≤0.05 were regarded as statistically significant. All analyses and graphs were generated with Stata version 9 (Statacorp, Texas).
RESULTS
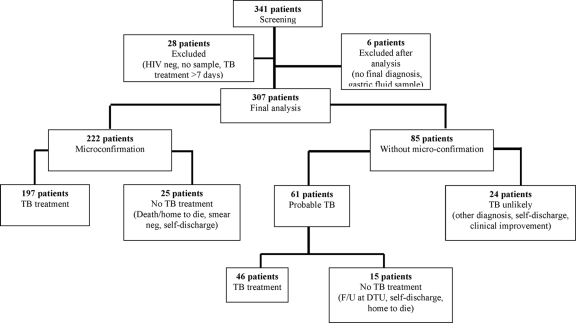
A total of 341 HIV-positive individuals were screened for pulmonary TB (Fig. 1). Of these, 8.2% (28/341) patients were excluded because (i) they subsequently tested as HIV negative (3 cases), (ii) no samples were collected (24 cases), or (iii) they had already received TB treatment for more than 8 days (1 case). Thus, 313 patients were eligible for the analysis. However, six additional patients were excluded after clinical and laboratory analysis because insufficient information was collected prior to self-discharge of the patient (four cases) or an inappropriate sample (gastric fluid) was collected (two cases). Thus, data from 307 patients were analyzed and reported in the present study.
FIG. 1.
Flow chart of patient recruitment and groups of patient based on micro-confirmation (smear or MGIT), TB treatment, and outcome. F/U, follow-up; DTU, district tuberculosis unit.
A total of 738 sputum samples were collected from these 307 patients. A total of 222 (72% [222/307]) patients had microbiological confirmation by a method other than MODS. This group also included six patients with microbiological confirmation by smear or MGIT based on samples collected prior to study enrolment. A total of 61 patients (20% [61/307]) were classified as “probable TB” and 24 patients (8% [24/307]) were classified as “TB unlikely.”
Demographics and clinical features.
More than 90% (301/307) of the study population was male, with a median age of 29. Almost 60% (182/307) of the patients had BCG vaccination determined by a BCG scar. Only 13% (42/307) were on antiretroviral (ARV) therapy. Twenty percent (61/307) of the patients had previously been diagnosed with TB once in their medical history. Table 1 shows demographic characteristics of the study population and comparisons of the three groups. Table 2 shows clinical features of the 307 HIV-associated TB suspects. Cough, fever, and weight loss were the most frequent symptoms with the majority of patients having a history of illness between 30 and 59 days. Lymphadenopathy was present in 43% of patients.
TABLE 1.
Demographic characteristics of patients
| Characteristic | Total population (n = 307) |
TB status determinationa |
|||
|---|---|---|---|---|---|
| No. of patients (%) | Pc | Microconfirmation (n = 222) | Probable TB (n = 61) | TB unlikely (n = 24) | |
| Gender and age | |||||
| Male | 301 (98.1) | 1.000 | 217 (97.8) | 60 (98.4) | 24 (100.0) |
| Median age in yr (IQR) | 29 (26-33) | 0.801 | 29 (26-33) | 30 (26-33) | 30 (27-35) |
| BCG vaccinationb | 0.858 | ||||
| Yes | 182 (59.3) | 130 (58.7) | 39 (63.9) | 13 (54.2) | |
| No | 117 (38.1) | 85 (38.3) | 21 (34.4) | 11 (45.8) | |
| Unknown | 8 (2.6) | 7 (3.2) | 1 (1.7) | 0 | |
| TB history | <0.001 | P1 = 0.52 | P2 = 0.001 | P3 < 0.01 | |
| Yes | 61 (19.9) | 36 (16.2) | 12 (19.7) | 13 (54.2) | |
| No | 245 (79.8) | 186 (83.8) | 48 (78.7) | 11 (45.8) | |
| Unknown | 1 (0.3) | 0 | 1 (1.7) | 0 | |
| ARV therapy | 0.116 | ||||
| Yes | 42 (13.7) | 29 (13.1) | 10 (16.4) | 3 (12.5) | |
| No | 261 (85.0) | 192 (86.5) | 49 (80.3) | 20 (83.3) | |
| Unknown | 4 (1.3) | 1 (0.5) | 2 (3.3) | 1 (4.2) | |
| TB contact | 0.758 | ||||
| Yes | 12 (3.9) | 10 (4.5) | 1 (1.6) | 1 (4.2) | |
| No | 281 (91.5) | 203 (91.4) | 56 (91.8) | 22 (91.7) | |
| Unknown | 14 (4.6) | 9 (40.1) | 4 (6.6) | 1 (4.1) | |
Data are presented as the number of patients (%) except as noted for the median age in column 1. Microconfirmation, microbiological confirmation.
That is, the presence of a BCG scar.
P refers to the P value of a (global) comparison of all three groups. Where P was <0.05, separate pairwise comparisons P1, P2, and P3 were also determined (as indicated in the respective columns): P1, confirmed TB versus probable TB; P2, probable TB versus TB unlikely; and P3, TB unlikely versus confirmed TB.
TABLE 2.
Clinical features of 307 TB/HIV suspects
| Characteristic | Total population (n = 307) |
TB status determinationa |
|||
|---|---|---|---|---|---|
| No. of patients (%) | Pb | Microconfirmation TB (n = 222) | Probable TB (n = 61) | TB unlikely (n = 24) | |
| History of illness | 0.107 | ||||
| ≤29 days | 74 (24.10) | 52 (23.4) | 12 (19.7) | 10 (41.7) | |
| 30-59 days | 185 (60.3) | 130 (58.6) | 42 (68.9) | 13 (54.2) | |
| ≥60 days | 48 (15.7) | 40 (18.02) | 7 (11.5) | 1 (4.2) | |
| Cough | 297 (96.7) | 0.465 | 216 (97.30) | 58 (95.1) | 23 (95.8) |
| Fever | 294 (95.8) | <0.001 | 216 (97.30) (P1 = 0.63) | 60 (98.4) (P2 < 0.001) | 18 (75.00) (P3 < 0.001) |
| Night sweat | 245 (79.80) | 0.106 | 174 (78.4) | 54 (88.5) | 17 (70.8) |
| Wt loss | 292 (95.1) | 0.201 | 212 (95.50) | 59 (96.7) | 21 (87.50) |
| Lymphadenopathy | 138 (44.9) | 0.031 | 106 (47.8) (P1 = 0.08) | 24 (39.3) (P2 = 0.89) | 8 (33.3) (P3 = 0.25) |
Data are presented as the number of patients (%) except as noted otherwise in footnote b. Microconfirmation, microbiological confirmation.
P refers to the P value of a (global) comparison of all three groups. Where P was <0.05, separate pairwise comparisons P1, P2, and P3 were also determined (as indicated in the respective columns): P1, confirmed TB versus probable TB; P2, probable TB versus TB unlikely; and P3, TB unlikely versus confirmed TB.
Accuracy of MODS. (i) Accuracy against microbiological confirmation as the gold standard.
The microbiological gold standard was defined as patients whose samples were determined to be positive by either smear or MGIT. MODS detected 87.4% of these cases with a specificity of 93%. The accuracy of MODS against microbiological gold standard, by patient and sample analysis, is detailed in Table 3.
TABLE 3.
Accuracy of MODS versus microbiological confirmation as the gold standard
| Parametera | Accuracy of MODS |
|||
|---|---|---|---|---|
| By patients |
By samples |
|||
| % (no./total no.) | 95% CI | % (no./total no.) | 95% CI | |
| Sensitivity | 87.4 (194/222) | 82.3-95.1 | 81.0 (431/523) | 76.3-85.7 |
| Specificity | 93.0 (79/85) | 85.3-97.4 | 97.0 (200/206) | 94.8-99.3 |
| PPV | 97.0 (194/200) | 93.6-98.9 | 98.6 (431/437) | 97.5-99.7 |
| NPV | 73.8 (79/107) | 64.5-81.9 | 66.4 (200/301) | 58.5-74.4 |
PPV, positive predictive value; NPV, negative predictive value.
(ii) Accuracy against the clinical gold standard.
The clinical gold standard was defined as patients satisfying the definition of the “microbiological confirmation” group (n = 222 patients) or “probable TB” group (n = 61 patients). In all, 283 patients and 684 samples were classified as TB using the clinical gold standard. Table 4 present the sensitivities and negative predictive value of the MODS, smear, and MGIT methods versus the clinical gold standard categorized by patient and by sample analyses. MODS was significantly more sensitive than the smear method (71% versus 57% [P < 0.001] by patient analysis and 64% versus 54% [P < 0.001] by sample analysis) but less sensitive than MGIT (75% [P = 0.03] by patient analysis and 70% [P < 0.001] by sample analysis). The specificity and positive predictive value of all methods were 100%.
TABLE 4.
Sensitivities and negative predictive values of the MODS, smear, and MGIT methods versus the clinical gold standard
| Parameter, subject groupa | Comparison type (n) | % (no./total no.) [95% CI] |
P (95% CI of difference [%])c |
|||
|---|---|---|---|---|---|---|
| MODS | Smear | MGIT | MODS vs smear | MODS vs MGIT | ||
| Sensitivity, all subjects | By patient (283) | 71 (200/283) [64.9-75.9] | 57 (161/283) [50.9-62.7] | 75 (212/283) [69.4-79.8] | <0.001 (8.6, 18.9) | 0.03 (-8.1, -0.4) |
| By sample (684) | 64 (437/684) [58.5- 69.2] | 54 (369/684) [48.2-59.7] | 70 (473/684) [63.9-74.4] | <0.001 (6.9, 12.9) | <0.001 (-7.7, -2.8) | |
| Sensitivity, smear-negative subjects | Patient (122) | 38 (46/122) [29.1-46.9] | NAb | 45 (55/122) [36.1-54.3] | NA | 0.078 (-15.4, 0.7) |
| By sample (315) | 29 (92/315) [22.4-36.0] | NA | 36 (114/315) [28.8-43.6] | NA | 0.003 (-11.5, -2.4) | |
| NPV, all subjects | By patient (283) | 22.4 (24/107) [14.9-31.5] | 16.4 (24/146) [10.8-23.5] | 25.3 (24/95) [16.9-35.2] | 0.323 (-12.7, 4.2) | 0.711 (-7.9, 11.6) |
| By sample (684) | 18 (54/301) [11.3-24.6] | 14.6 (54/369) [9.1-20.2] | 20.4 (54/265) [12.9-27.8] | <0.001 (1.6, 5.1) | 0.002 (0.9, 3.9) | |
| NPV, smear-negative subjects | By patient (122) | 24.0 (24/100) [16.0-33.6] | NA | 26.4 (24/91) [17.7-36.7] | NA | 0.770 (-8.6, 11.7) |
| By sample (315) | 19.50 (54/277) [12.3-26.7] | NA | 21.2 (54/255) [13.5-28.9] | NA | 0.009 (0.42, 2.9) | |
NPV, negative predictive value.
NA, not applicable.
95% CI of difference, 95% confidence interval of the difference between the means.
MODS in the diagnosis of smear-negative HIV-associated TB.
A total of 122 patients with 315 samples were diagnosed with confirmed or probable TB, but all of their smear samples were negative. Of these 122 patients, 15 (12%) were determined to be positive by MGIT only, 40 of 122 (33%) patients were determined to be positive by both MODS and MGIT, and 6 of 122 (5%) patients were determined to be positive by MODS only. MODS detected 72.8% (40/55) of culture positive-smear negative TB cases. Comparisons of the sensitivity and negative predictive value of MODS and MGIT are detailed in Table 4. The sensitivity of MODS tended to be lower than MGIT in the “by patient” analysis (38% versus 45%, P = 0.078). Conversely, MGIT was significantly more sensitive than MODS (36% versus 29%, P = 0.003) in the “by sample” analysis. The specificity and positive predictive value of these tests in smear-negative patients and samples were 100%.
Of 122 smear-negative TB cases, 30 cases did not have TB therapy at Pham Ngoc Thach Hospital because of death, self-discharge, or referral to District Tuberculosis Units to start TB treatment and follow-up. Ten of these thirty cases (33%) did not receive TB treatment because the patient was discharged following a negative smear before the culture results were available.
TB treatment-dependent sensitivity.
A total of 684 samples from 283 patients with a clinical TB diagnosis were analyzed. Of these, 14% (97/684) of the samples were from patients not on TB treatment. A total of 540 of 587 (92%) samples were collected from patients who had been on TB treatment for ≤3 days, and 47 of 587 (8%) samples were from patients who had been on TB treatment for >4 days. The sensitivities of the MODS, smear, and MGIT approaches versus the clinical gold standard in patients receiving TB treatment for ≤3 days or for ≥4 days were compared. The sensitivity of the MODS and smear methods were significantly decreased among samples collected after 4 days of TB treatment compared to earlier samples (53% versus 70% [P = 0.035] for MODS and 45% versus 61% [P = 0.034] for smear); The sensitivity of MGIT also tended to be lower for longer TB treatment duration but the result did not achieve statistical significance (74% versus 60% [P = 0.053]).
Time to positive.
“Time to positive” was defined as the number of days from sample processing (day 1) to result available. Smear results were available on day 2 (routine procedure at Pham Ngoc Thach Hospital). In samples positive by either MODS (437/684) or MGIT (473/684), the median time to detection of MODS and MGIT were 8 days (interquartile range [IQR], 6 to 10 days) and 11 days (IQR, 8 to 10 days), respectively. Among smear-negative samples, the median time to detection of MODS and MGIT were 11 days (IQR, 9 to 16 days) and 17 days (IQR, 13 to 21 days).
Time-dependent sensitivity.
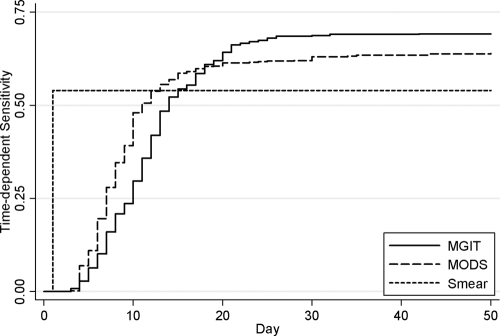
The time-dependent sensitivities of MODS and MGIT are presented in Fig. 2. In samples determined to be positive by both MODS and MGIT, MODS was faster than MGIT in 70% (289/418) samples, with a median time difference of 2 days (IQR, 0 to 5 days, P < 0.01). In smear-negative samples, of 79 samples positive by both MODS and MGIT, the MODS results were available 4 days earlier than MGIT (IQR, 0 to 7 days, P < 0.01). MODS also yielded a higher sensitivity than MGIT by day 7 (28% versus 16%, P < 0.001) and day 14 (57% versus 52%, P = 0.009) after inoculation.
FIG. 2.
Time-dependent sensitivity of MODS, smear, and MGIT analyses. The sensitivities of the MODS method were higher than those of the MGIT method by day 7 (P < 0.001) or by day 14 (P = 0.001).
Contamination and spoligotyping.
In all, 738 samples were cultured by both MODS and MGIT. We assessed the contamination in terms of fungi or other bacteria and cross-contamination between samples for the MODS assay.
In terms of fungal contamination, the original contamination rate of MODS in samples was 1.1% (8/738), while it was 2.6% (19/738) for MGIT. All MGIT contaminated samples were decontaminated again and reinoculated into MGIT medium. The final fungal contamination rate of MGIT was 1.8% (13/738). Reprocessing for sample contaminated by MODS was not attempted because of low volume (total of 1 ml for each well). Contamination with fungi was also observed in eight negative control wells.
To assess cross-contamination of MODS with TB bacteria, spacer oligonucleotide typing (spoligotyping) was applied to all available MODS isolates (437/478). Serial positive cultures from individual patients were compared for discrepancies in spoligotype. A positive MGIT culture (n = 41) was used for comparison if the MODS culture yielded a negative spoligotype (n = 21) or subculture was contaminated from MODS to LJ medium (n = 20). A total of 412 of 437 (94%) samples had defined spoligotypes, while the remaining 25 of 437 did not because of a negative MGIT culture (n = 3), a negative spoligotype (n = 3), or DNA not available (n = 19). Spoligotypes were deemed as indicating possible cross-contamination if serial isolates from an individual patient were discrepant or if an isolate was H37Rv (the positive control isolate).
Eight samples from eight patients (1.1% [8/738]) were determined to be positive by MODS with H37Rv, the positive control strain. An additional 27 MODS isolates were identified as probable instances of MODS cross-contamination due to multiple strains isolated from one patient. It is impossible to rule out infection with multiple strains in these patients, but the maximum cross-contamination rate of MODS with TB bacteria was 4.7% (35/738). All false-positive MODS cultures were in “confirmed” or “probable” TB groups.
DISCUSSION
We have shown MODS to be a sensitive and rapid method for diagnosis of TB in HIV-infected patients. Although MODS was slightly less sensitive than MGIT (71% versus 75%, P = 0.03), MODS is faster than MGIT in samples positive by both methods with a 2-day difference (P < 0.001). In smear-negative TB cases, although MODS tended to be less sensitive than MGIT (38% versus 45%, P = 0.078), MODS detected more cases than MGIT by day 7 (4.4% versus 0.6%, P = 0.027) and day 14 (21% versus 12%, P < 0.001). MODS detected 72.8% (40/55) culture-positive, smear-negative TB cases.
Therefore, MODS is an appropriate microbiological method for the early detection of paucibacillary TB, especially for HIV/TB patients.
Delays in diagnosis result in poor outcomes, increased morbidity, and ongoing transmission (4). MODS detected significantly more TB cases at day 7 (4.4% versus 0.6%) and day 14 (21% versus 12%) than commercial rapid liquid culture, similar to findings comparing MODS and Lowenstein-Jensen methods in previous studies (2, 9, 14); this is crucial for early diagnosis of TB in immunocompromised patients. More than 30% of the smear-negative TB cases in our study did not receive TB treatment because the MGIT culture result was not available at the time of discharge. This underlies the need for a rapid diagnostic test in HIV/TB cases. Suspected TB cases who are smear negative are generally prescribed 7 to 14 days of treatment with broad-spectrum antibiotics to exclude other possible causes of community-acquired pneumonia before being retested for TB, in accordance with WHO policy (25).
Contamination is an issue with all microbiological techniques, and evaluation of contamination is of importance for the wide application of MODS. We have shown the fungal contamination rate to be 1.1%. The probable cross-contamination of MODS was 4.7%, which is within the expected contamination range of MGIT culture (3 to 8.5%) (7, 8, 12, 19). Cross-contamination is difficult to evaluate effectively in TB culture techniques because genotyping techniques have relatively low discriminatory power in settings where TB is endemic, and it is difficult to rule out TB infection in symptomatic patients in a high-prevalence setting. The median cross-contamination rate of TB laboratories is approximately 3% (1), but it can be much higher (17).
In conclusion, MODS is an alternative method that is rapid, sensitive, specific, inexpensive, and feasible for the diagnosis of paucibacillary TB in high burden and low resource countries.
Acknowledgments
The Wellcome Trust of Great Britain funded this work.
We also thank the staff and patients of Pham Ngoc Thach Hospital for their contribution to this study.
Footnotes
Published ahead of print on 6 October 2010.
REFERENCES
- 1.Alonso, V., R. Paul, L. Barrera, and V. Ritacco. 2007. False diagnosis of tuberculosis by culture. Medicina 67:287-294. (In Spanish.) [PubMed] [Google Scholar]
- 2.Arias, M., F. C. Mello, A. Pavon, A. G. Marsico, C. Alvarado-Galvez, S. Rosales, C. L. Pessoa, M. Perez, M. K. Andrade, A. L. Kritski, L. S. Fonseca, R. E. Chaisson, M. E. Kimerling, and S. E. Dorman. 2007. Clinical evaluation of the microscopic-observation drug-susceptibility assay for detection of tuberculosis. Clin. Infect. Dis. 44:674-680. [DOI] [PubMed] [Google Scholar]
- 3.Brady, M. F., J. Coronel, R. H. Gilman, and D. A. Moore. 2008. The MODS method for diagnosis of tuberculosis and multidrug resistant tuberculosis. J. Vis. Exp. 11:pii:845. doi.10.3791/845. [DOI] [PMC free article] [PubMed]
- 4.Cain, K. P., T. Anekthananon, C. Burapat, S. Akksilp, W. Mankhatitham, C. Srinak, S. Nateniyom, W. Sattayawuthipong, T. Tasaneeyapan, and J. K. Varma. 2009. Causes of death in HIV-infected persons who have tuberculosis in Thailand. Emerg. Infect. Dis. 15:258-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cain, K. P., K. D. McCarthy, C. M. Heilig, P. Monkongdee, T. Tasaneeyapan, N. Kanara, M. E. Kimerling, P. Chheng, S. Thai, B. Sar, P. Phanuphak, N. Teeratakulpisarn, N. Phanuphak, H. D. Nguyen, T. Q. Hoang, H. T. Le, and J. K. Varma. 2009. An algorithm for tuberculosis screening and diagnosis in people with HIV. N. Engl. J. Med. 362:707-716. [DOI] [PubMed] [Google Scholar]
- 6.Caws, M., T. M. Dang, E. Torok, J. Campbell, D. A. Do, T. H. Tran, V. C. Nguyen, T. C. Nguyen, and J. Farrar. 2007. Evaluation of the MODS culture technique for the diagnosis of tuberculous meningitis. PLoS One 2:e1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chew, W. K., R. M. Lasaitis, F. A. Schio, and G. L. Gilbert. 1998. Clinical evaluation of the mycobacteria growth indicator tube (MGIT) compared with radiometric (Bactec) and solid media for isolation of Mycobacterium species. J. Med. Microbiol. 47:821-827. [DOI] [PubMed] [Google Scholar]
- 8.Chien, H. P., M. C. Yu, M. H. Wu, T. P. Lin, and K. T. Luh. 2000. Comparison of the BACTEC MGIT 960 with Lowenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Int. J. Tuberc. Lung Dis. 4:866-870. [PubMed] [Google Scholar]
- 9.Ha, D. T., N. T. Lan, M. Wolbers, T. N. Duong, N. D. Quang, T. T. V. Thinh, L. T. H. Ngoc, N. T. N. Anh, T. V. Quyet, N. T. B. Tuyen, V. T. Ha, J. Day, H. T. T. Hang, V. S. Kiet, N. T. Nho, D. V. Hoa, N. H. Dung, N. H. Lan, J. Farrar, and M. Caws. 2009. Microscopic observation drug susceptibility assay (MODS) for early diagnosis of tuberculosis in children. PLoS One 4:e8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honore-Bouakline, S., J. P. Vincensini, V. Giacuzzo, P. H. Lagrange, and J. L. Herrmann. 2003. Rapid diagnosis of extrapulmonary tuberculosis by PCR: impact of sample preparation and DNA extraction. J. Clin. Microbiol. 41:2323-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremer, K., D. van Soolingen, R. Frothingham, W. H. Haas, P. W. Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley, M. A. Yakrus, J. M. Musser, and J. D. van Embden. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levidiotou, S., D. Papamichael, E. Gessouli, S. Golegou, S. Anagnostou, E. Galanakis, C. Papadopoulou, and G. Antoniadis. 1999. Detection of mycobacteria in clinical specimen using the mycobacteria growth indicator tube (MGIT) and the Lowenstein-Jensen medium. Microbiol. Res. 154:151-155. [DOI] [PubMed] [Google Scholar]
- 13.Monkongdee, P., K. D. McCarthy, K. P. Cain, T. Tasaneeyapan, H. D. Nguyen, T. N. Nguyen, T. B. Nguyen, N. Teeratakulpisarn, N. Udomsantisuk, C. Heilig, and J. K. Varma. 2009. Yield of acid-fast smear and mycobacterial culture for tuberculosis diagnosis in people with human immunodeficiency virus. Am. J. Respir. Crit. Care Med. 180:903-908. [DOI] [PubMed] [Google Scholar]
- 14.Moore, D. A., C. A. Evans, R. H. Gilman, L. Caviedes, J. Coronel, A. Vivar, E. Sanchez, Y. Pinedo, J. C. Saravia, C. Salazar, R. Oberhelman, M. G. Hollm-Delgado, D. LaChira, A. R. Escombe, and J. S. Friedland. 2006. Microscopic-observation drug susceptibility assay for the diagnosis of TB. N. Engl. J. Med. 355:1539-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park, W. G., W. R. Bishai, R. E. Chaisson, and S. E. Dorman. 2002. Performance of the microscopic observation drug susceptibility assay in drug susceptibility testing for Mycobacterium tuberculosis. J. Clin. Microbiol. 40:4750-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pepe, M. S. 2004. The statistical evaluation of medical tests for classification and prediction. Oxford University Press, Oxford, United Kingdom.
- 17.Ramos, M., H. Soini, G. C. Roscanni, M. Jaques, M. C. Villares, and J. M. Musser. 1999. Extensive cross-contamination of specimens with Mycobacterium tuberculosis in a reference laboratory. J. Clin. Microbiol. 37:916-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy, K. P., M. F. Brady, R. H. Gilman, J. Coronel, M. Navincopa, E. Ticona, G. Chavez, E. Sanchez, C. Rojas, L. Solari, J. Valencia, Y. Pinedo, C. Benites, J. S. Friedland, and D. A. Moore. 2010. Microscopic observation drug susceptibility assay for tuberculosis screening before isoniazid preventive therapy in HIV-infected persons. Clin. Infect. Dis. 50:988-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somoskovi, A., and P. Magyar. 1999. Comparison of the mycobacteria growth indicator tube with MB redox, Lowenstein-Jensen, and Middlebrook 7H11 media for recovery of mycobacteria in clinical specimens. J. Clin. Microbiol. 37:1366-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steingart, K. R., A. Ramsay, and M. Pai. 2007. Optimizing sputum smear microscopy for the diagnosis of pulmonary tuberculosis. Expert Rev. Anti-Infect. Ther. 5:327-331. [DOI] [PubMed] [Google Scholar]
- 21.Tran, N. B., R. M. Houben, T. Q. Hoang, T. N. Nguyen, M. W. Borgdorff, and F. G. Cobelens. 2007. HIV and tuberculosis in Ho Chi Minh City, Vietnam, 1997-2002. Emerg. Infect. Dis. 13:1463-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. 2004. TB/HIV: a clinical manual, 2nd ed. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/who_htm_tb_2004_329/en/index.html.
- 23.World Health Organization. 2009. Global tuberculosis control 2009: epidemiology, strategy, and financing. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/global_reprt/2009//pdf/full_report.pdf.
- 24.World Health Organization. 2009. Global tuberculosis control 2009: epidemiology, strategy, and financing (update). World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/global_reprt/2009/update/tbu_9.pdf.
- 25.World Health Organization. 2007. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource constrained settings. World Health Organization, Geneva, Switzerland. http://whqlibdoc.who.int/hq/2007/WHO_HTM_TB_2007.379_eng.pdf.
- 26.World Health Organization. 2010. Laboratory service in tuberculosis control. II. Microscopy. WHO/TB/98.258. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/1998/en/index2.html.
- 27.World Health Organization. 2007. Use of liquid TB culture and drug susceptibility testing (DST) in low- and middle-income countries. Summary report of the expert group meeting on the use of liquid culture media. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/dots/laboratory/Use%20of%20Liquid%20TB%20Culture_Summary%20Report.pdf.
- 28.World Health Organization. 2008. WHO policy statement: molecular line probe assays for rapid screening of patients at risk of multidrug-resistant tuberculosis. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/dots/laboratory/line_probe_assays/en/.