Abstract
We developed a novel multiplex PCR assay using dual-priming oligonucleotide primers targeting the RD1 gene for simultaneous identification of the Mycobacterium tuberculosis complex and M. bovis bacillus Calmette-Guérin (BCG). This assay would be useful both for detection of the M. tuberculosis complex and for differentiation of M. bovis BCG from pathogenic M. tuberculosis complex species.
Tuberculosis is a major global public health problem. This disease, mainly caused by the Mycobacterium tuberculosis complex, is still found in areas of endemicity in developing countries and has reemerged in developed countries with the increase in the incidence of AIDS and other immunocompromising conditions (6). In South Korea, 95 to 99% of children are vaccinated with M. bovis bacillus Calmette-Guérin (BCG) (12). However, complications have been reported, including M. bovis BCG dissemination, osteomyelitis, abscesses, and lymphadenitis (5). In addition, M. bovis BCG has been used for the treatment of bladder cancer. For these patients, complications that have included pneumonitis, hepatitis, and noncaseating granulomas have also been reported (9). It has been reported that susceptibilities to antituberculosis drugs differ between M. tuberculosis and M. bovis BCG (17). Therefore, it is important for clinicians to distinguish M. bovis BCG from other members of the M. tuberculosis complex, especially for patients who have a history of M. bovis BCG vaccination and treatment.
In this study, to develop a novel multiplex PCR assay for the detection and discrimination of the M. tuberculosis complex and M. bovis BCG, the RD1 sequence, which is absent in M. bovis BCG but is present in the M. tuberculosis complex (12, 14), was selected for analysis using dual-priming oligonucleotide (DPO) primers (4).
To validate the novel multiplex PCR assay, we tested the following isolates: (i) reference strain isolates from the American Type Culture Collection (ATCC), the Korean Type Culture Collection (KTCC), and the Korean Institute of Tuberculosis (KIT) of the Korean National Tuberculosis Association, including two M. tuberculosis isolates (ATCC 25177 and ATCC 27294), 13 isolates representing nontuberculous mycobacteria (NTM; M. abscessus ATCC 19977, M. avium ATCC 25291, M. fortuitum KTCC 1122, M. intracellulare KIT 41105, M. kansasii KTCC 9515, M. margeritense ATCC 700351, M. marinum ATCC 927, M. mucogenicum KTCC 19088, M. nonchromogenicum ATCC 19530, M. peregrinum KTCC 9615, M. scrofulaceum KTCC 9519, M. septicum ATCC 700731, and M. szulgai KTCC 9520), and four isolates representing nonmycobacterial species (Escherichia coli ATCC 25922, Haemophilus influenzae ATCC 9007, Klebsiella pneumoniae ATCC 700603, and Staphylococcus aureus ATCC 29213); (ii) isolates from two patients with M. bovis BCG infections (BCG-oma or BCG osteitis) previously proven using in-house, real-time PCR with two hydrolysis probes (3); and (iii) extracts from two commercial M. bovis BCG vaccines, the Tokyo 172 BCG vaccine (Japan BCG Manufacturing Corporation, Tokyo, Japan) and the Pasteur 1173p2 vaccine (Korean National Tuberculosis Association, Seoul, South Korea).
DNA was extracted from the reference strain isolates and M. bovis BCG by a heating method; each colony was isolated from culture media and suspended in 500 μl of 1× Tris-EDTA buffer. The solution was heated to 100°C for 20 min, and after centrifugation at 15,000 rpm for 5 min, the supernatant was used for PCR. DNA was extracted from commercial BCG vaccines by the use of a QIAamp blood Mini kit (Qiagen, Hilden, Germany) or a viral RNA kit (Qiagen).
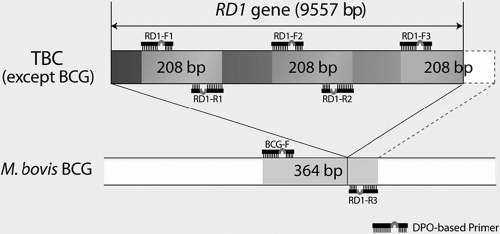
The four pairs of DPO primers for amplification of the RD1 gene were designed to generate amplicons of different sizes from the M. bovis BCG genome or from the M. tuberculosis complex (Fig. 1). The BCG-F and RD1-R3 primers, which are complementary to sequences flanking the deleted region, produced a 364-bp PCR amplicon from M. bovis BCG strains from which the RD1 region had been deleted. No PCR product was generated from the M. tuberculosis complex by this pair of primers, because the two primers are too far apart to amplify efficiently the entire 9,557-bp region in the M. tuberculosis complex. In mycobacterial species with the RD1 region present in the genome, a copy of the 208-bp product was produced by primers RD1-F3 (which is complementary to the RD1 sequence) and RD1-R3. Two copies of the amplicons were also generated using primer pair RD1-F1 and RD1-R1 and primer pair RD1-F2 and RD1-R2; these amplicons are complementary to different sequences within RD1 but amplify regions that are all the same size (208 bp). To confirm proper amplification, this multiplex PCR included amplification of the cellulose synthase 3 gene from Arabidopsis thaliana, which generates a 720-bp internal control band. PCR was performed using a 20-μl reaction mixture containing 1× Master Mix, 1× primer mix, and 3 μl of DNA. All cycling profiles incorporated an initial denaturation at 94°C for 15 min; 40 amplification cycles of 30 s at 94°C, 30 s at 60°C, and 30 s at 72°C; a final extension at 72°C for 5 min; and then a hold at 4°C. PCR products were visualized by 2.0% agarose gel electrophoresis and ethidium bromide staining. To reduce the risk of contamination and carryover, we observed universal standard precautions for nucleic acid amplification and included negative controls in each run.
FIG. 1.
Design of a novel multiplex PCR: a schematic representation of the RD1 gene and of the locations of the novel dual-priming oligonucleotide (DPO) primers and their PCR amplicons. TBC, M. tuberculosis complex.
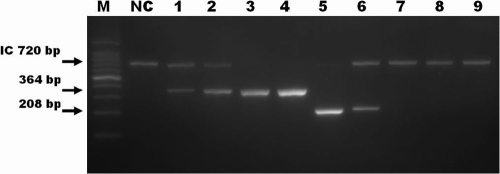
Our novel assay with DPO primers reliably produced 208-bp products from the M. tuberculosis complex samples, and no false positives were seen with the four nonmycobacterial species (data not shown). The specificity of the novel multiplex assay was also determined using NTM isolates that are frequently found in human specimens. Among the isolates from 13 reference strains of NTM, no isolate produced a 208-bp PCR product from RD1 (Fig. 2). To evaluate the discriminatory power of the novel multiplex PCR, the following samples were tested and the results compared: clinical isolates from patients diagnosed with BCG-oma or BCG osteitis (12); extracts from the M. bovis BCG vaccines BCG Pasteur 1173p2 and BCG Tokyo 172; M. tuberculosis isolates; and NTM isolates (M. intracellulare, M. avium, and M. abscessus). In contrast to the 208-bp amplicons produced from the M. tuberculosis complex isolates, a single 364-bp amplicon was observed in all specimens of M. bovis BCG. In samples containing DNA from NTM, neither the 364-bp nor the 208-bp product was observed after amplification (Fig. 3).
FIG. 2.
The specificity of the novel assay using species of nontuberculous mycobacteria (NTM). Each lane contains a PCR product amplified from DNA of one of the following mycobacterial species: M. tuberculosis (lanes 1, 9, 10, and 18), M. avium (lane 2), M. abscessus (lanes 3 and 4), M. intracellulare (lane 5), M. fortuitum (lane 6), M. kansasii (lane 7), M. mucogenicum (lane 8), M. peregrinum (lane 11), M. scrofulaceum (lane 12), M. septicum (lane 13), M. marinum (lane 14), M. szulgai (lane 15), M. margeritense (lane 16), and M. nonchromogenicum (lane 17). Lane NC represents a negative control, and lane M contains molecular size markers (100-bp ladder). The expected sizes of the PCR products are marked on the left, and the 720-bp internal control (IC) amplicon was detected in each test sample.
FIG. 3.
Detection and discrimination of M. tuberculosis complex species and M. bovis bacillus Calmette-Guérin (BCG) by the use of a novel assay. Each lane contains a PCR product amplified from DNA of the following mycobacteria: BCG Pasteur, isolated from a patient (lane 1); BCG Tokyo, isolated from a patient (lane 2); BCG Pasteur 1173p2, vaccine (lane 3); BCG Tokyo 172, vaccine (lane 4); and isolates of M. tuberculosis (lanes 5 and 6), M. intracellulare (lane 7), M. avium (lane 8), and M. abscessus (lane 9). Lane NC represents a negative control, and lane M contains molecular size markers (100-bp ladder). The expected sizes of the PCR products are marked on the left, and the 720-bp internal control (IC) amplicon was detected in each test sample.
Confirmation of M. bovis BCG infection is difficult with molecular tests. 16S rRNA sequences have been used for the identification of bacteria other than the M. tuberculosis complex, but these sequences are highly homologous among M. tuberculosis complex species. Thus, this sequence is not suitable for differentiating M. bovis BCG from M. tuberculosis complex species (10). Another frequently targeted sequence used to detect the M. tuberculosis complex is the insertion sequence IS6110, which is present in the M. tuberculosis complex genome in various copy numbers (7). However, the use of IS6110 for the detection and differentiation of the M. tuberculosis complex and M. bovis BCG has some limitations. The use of IS6110 alone cannot differentiate M. bovis BCG (1 to 2 copies of IS6110) from M. bovis (1 to 6 copies) (15). Moreover, the copy numbers of IS6110 are variable among species within the M. tuberculosis complex (8, 11, 18, 19, 20). Therefore, alternative targets have been investigated for the detection and differentiation of M. bovis BCG and the M. tuberculosis complex.
In this study, the RD1 gene was selected as the target of the novel assay to detect and discriminate the M. tuberculosis complex and M. bovis BCG by the use of single-round amplification. Previous studies of M. bovis BCG substrains have elucidated more than 100 genomic regions that are absent in M. bovis BCG but present in M. tuberculosis (2, 13). These deleted sequences are referred to as regions of difference (RD), and RD1 is absent from all BCG substrains and from the related live vaccine strain of M. microti but is present in virulent M. bovis and M. tuberculosis (2, 14). The RD1 region is not shared by most nontuberculous species (1, 16). Therefore, we developed a multiplex PCR for the detection and discrimination of M. tuberculosis and M. bovis BCG based on amplification of the RD1 sequence and the use of DPO primers. A DPO contains two different priming regions (a stabilizer and a determiner) joined with polydeoxyinosine. The polydeoxyinosine linker prevents formation of secondary structures and effectively eliminates nonspecific priming, thus decreasing nonspecific products in the PCR. The linker enables the more specific detection of the target pathogen (4). DPO in conjunction with amplification of the RD1 sequence is a novel tool for the molecular discrimination of highly homologous mycobacterium species.
In conclusion, our novel multiplex PCR is a sensitive and reliable assay for differential diagnosis of tuberculosis. This novel assay would be useful in clinical situations of suspected tuberculosis, not only for the rapid detection of the M. tuberculosis complex in clinical specimens but also for the differentiation of M. bovis BCG from other pathogenic M. tuberculosis complex species.
Acknowledgments
This study was supported by a grant (06-2007-009) from the Seoul National University Bundang Hospital Research Fund.
We gratefully acknowledge Yun-Jee Kim and Dae-Hoon Lee for technical assistance.
Footnotes
Published ahead of print on 13 October 2010.
REFERENCES
- 1.Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. [DOI] [PubMed] [Google Scholar]
- 2.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 3.Chang, H. E., S. R. Heo, K. C. Yoo, S. H. Song, S. H. Kim, H. B. Kim, K. U. Park, J. Song, J. H. Lee, S. S. Park, and E. C. Kim. 2008. Detection of Mycobacterium tuberculosis complex using real-time polymerase chain reaction. Korean J. Lab. Med. 28:103-108. [DOI] [PubMed] [Google Scholar]
- 4.Chun, J. Y., K. J. Kim, I. T. Hwang, Y. J. Kim, D. H. Lee, I. K. Lee, and J. K. Kim. 2007. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. 35:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deeks, S. L., M. Clark, D. W. Scheifele, B. J. Law, M. Dawar, N. Ahmadipour, W. Walop, C. E. Ellis, and A. King. 2005. Serious adverse events associated with bacillus Calmette-Guerin vaccine in Canada. Pediatr. Infect. Dis. J. 24:538-541. [DOI] [PubMed] [Google Scholar]
- 6.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher, H. A. 2001. Molecular epidemiology of tuberculosis: recent developments and applications. Curr. Opin. Pulm. Med. 7:154-159. [DOI] [PubMed] [Google Scholar]
- 8.Fomukong, N., M. Beggs, H. el Hajj, G. Templeton, K. Eisenach, and M. D. Cave. 1997. Differences in the prevalence of IS6110 insertion sites in Mycobacterium tuberculosis strains: low and high copy number of IS6110. Tuber. Lung. Dis. 78:109-116. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez, O. Y., D. M. Musher, I. Brar, S. Furgeson, M. R. Boktour, E. J. Septimus, R. J. Hamill, and E. A. Graviss. 2003. Spectrum of bacille Calmette-Guerin (BCG) infection after intravesical BCG immunotherapy. Clin. Infect. Dis. 36:140-148. [DOI] [PubMed] [Google Scholar]
- 10.Harmsen, D., S. Dostal, A. Roth, S. Niemann, J. Rothganger, M. Sammeth, J. Albert, M. Frosch, and E. Richter. 2003. RIDOM: comprehensive and public sequence database for identification of Mycobacterium species. BMC Infect. Dis. 3:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanduma, E., T. D. McHugh, and S. H. Gillespie. 2003. Molecular methods for Mycobacterium tuberculosis strain typing: a user's guide. J. Appl. Microbiol. 94:781-791. [DOI] [PubMed] [Google Scholar]
- 12.Kim, S. H., S. Y. Kim, B. W. Eun, W. J. Yoo, K. U. Park, E. H. Choi, E. C. Kim, and H. J. Lee. 2008. BCG osteomyelitis caused by the BCG Tokyo strain and confirmed by molecular method. Vaccine 26:4379-4381. [DOI] [PubMed] [Google Scholar]
- 13.Majlessi, L., P. Brodin, R. Brosch, M. J. Rojas, H. Khun, M. Huerre, S. T. Cole, and C. Leclerc. 2005. Influence of ESAT-6 secretion system 1 (RD1) of Mycobacterium tuberculosis on the interaction between mycobacteria and the host immune system. J. Immunol. 174:3570-3579. [DOI] [PubMed] [Google Scholar]
- 14.Mostowy, S., A. G. Tsolaki, P. M. Small, and M. A. Behr. 2003. The in vitro evolution of BCG vaccines. Vaccine 21:4270-4274. [DOI] [PubMed] [Google Scholar]
- 15.Oettinger, T., M. Jorgensen, A. Ladefoged, K. Haslov, and P. Andersen. 1999. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber. Lung. Dis. 79:243-250. [DOI] [PubMed] [Google Scholar]
- 16.Pai, M. 2005. Alternatives to the tuberculin skin test: interferon-gamma assays in the diagnosis of Mycobacterium tuberculosis infection. Indian J. Med. Microbiol. 23:151-158. [DOI] [PubMed] [Google Scholar]
- 17.Riska, P. F., W. R. Jacobs, Jr., and D. Alland. 2000. Molecular determinants of drug resistance in tuberculosis. Int. J. Tuberc. Lung. Dis. 4:S4-S10. [PubMed] [Google Scholar]
- 18.van Soolingen, D., P. W. Hermans, P. E. de Haas, D. R. Soll, and J. D. van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren, R. M., T. C. Victor, E. M. Streicher, M. Richardson, G. D. van der Spuy, R. Johnson, V. N. Chihota, C. Locht, P. Supply, and P. D. van Helden. 2004. Clonal expansion of a globally disseminated lineage of Mycobacterium tuberculosis with low IS6110 copy numbers. J. Clin. Microbiol. 42:5774-5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuen, L. K., B. C. Ross, K. M. Jackson, and B. Dwyer. 1993. Characterization of Mycobacterium tuberculosis strains from Vietnamese patients by Southern blot hybridization. J. Clin. Microbiol. 31:1615-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]