Abstract
Broad-range real-time PCR and sequencing of the 16S rRNA gene region is a widely known method for the detection and identification of bacteria in clinical samples. However, because of the need for sequencing, such identification of bacteria is time-consuming. The aim of our study was to develop a more rapid 16S real-time PCR-based identification assay using species- or genus-specific probes. The Gram-negative bacteria were divided into Pseudomonas species, Pseudomonas aeruginosa, Escherichia coli, and other Gram-negative species. Within the Gram-positive species, probes were designed for Staphylococcus species, Staphylococcus aureus, Enterococcus species, Streptococcus species, and Streptococcus pneumoniae. The assay also included a universal probe within the 16S rRNA gene region for the detection of all bacterial DNA. The assay was evaluated with a collection of 248 blood cultures. In this study, the universal probe and the probes targeting Pseudomonas spp., P. aeruginosa, E. coli, Streptococcus spp., S. pneumoniae, Enterococcus spp., and Staphylococcus spp. all had a sensitivity and specificity of 100%. The probe specific for S. aureus showed eight discrepancies, resulting in a sensitivity of 100% and a specificity of 93%. These data showed high agreement between conventional testing and our novel real-time PCR assay. Furthermore, this assay significantly reduced the time needed for identification. In conclusion, using pathogen-specific probes offers a faster alternative for pathogen detection and could improve the diagnosis of bloodstream infections.
Bloodstream infections are a major cause of death in the world and need a thorough and adequate therapeutic strategy. Inadequate antibiotic therapy is associated with higher mortality rates, the appearance of antibiotic resistance, and longer hospitalization lengths (14). Conventional identification and susceptibility testing have several limitations, such as lack of rapidity and sensitivity. The current gold standard, i.e., blood culture, usually requires 6 to 12 h of incubation before growth is detected and a further 24 to 48 h for the definitive identification of the infectious agent and its susceptibility to antibiotics (1, 9). Routine diagnostics already use molecular techniques for the direct detection of viral and bacterial pathogens. However, most in-house assays are targeted against one specific bacterium and/or virus and do not offer broad-range pathogen detection. Recently, several PCR assays have been developed targeting a panel of the most relevant bacterial and fungal bloodstream pathogens, which can be performed directly with blood, such as SeptiFast (Roche Diagnostics GmbH, Mannheim, Germany), SepsiTest (Molzym GmbH & Co. KG, Bremen, Germany), and VYOO (SIRS-Lab GmbH, Jena, Germany), or using positive blood cultures, such as the microarray-based system Prove-it Sepsis (Mobidiag, Helsinki, Finland).
As discussed in our previous work, direct detection in whole blood is hampered by several factors, such as the presence of PCR inhibitors and background DNA, low bacterial load, insufficient sensitivity, and difficulty of establishing an assay capable of detecting a wide range of pathogens (5). In contrast, molecular testing of growth-positive blood cultures does not require highly sensitive assays because of the presence of a high bacterial load. Furthermore, until now, the use of culturing remains essential to determine the microorganism's antimicrobial profile. Therefore, the role of blood cultures remains important for the detection and identification of causative bacterial agents. Molecular testing of blood cultures, possibly in combination with conventional testing, could enable more rapid identification and, consequently, more rapid diagnosis and start of correct therapy. Molecular approaches such as broad-range real-time PCR and sequencing of the 16S rRNA gene region are widely known methods for the detection and identification of bacteria in clinical samples (4, 7, 10, 15). However, because of the need for sequencing, the identification of bacteria is time-consuming.
The aim of our study was to develop a more rapid 16S real-time PCR-based identification assay using species- or genus-specific probes. The assay is particularly intended for the identification of positive blood cultures, for which Gram staining results are known. In this proof-of-concept study, priority was given to the genera or species most frequently found in blood cultures and/or those that could direct the choice of a suitable antibiotic therapy. Therefore, we selected a panel of eight species- or genus-specific probes. The Gram-negative bacteria were divided into Pseudomonas spp., Pseudomonas aeruginosa, Escherichia coli, and other Gram-negative species. Within the Gram-positive species, probes were designed for Staphylococcus spp., Staphylococcus aureus, Enterococcus spp., Streptococcus spp., and Streptococcus pneumoniae. Hence, a first indication about the causative microorganism is given after 2 h, while confirmation and precise identification can be achieved with sequencing. Consequently, multiple species can be detected in samples with polymicrobial infections. The present paper reports a retrospective study performed on blood cultures obtained from patients with suspected bloodstream infections. Results of this new multiprobe assay were compared with conventional blood culture findings.
MATERIALS AND METHODS
Clinical samples.
A total of 248 blood cultures were collected at the Maastricht University Medical Center (MUMC; Maastricht, Netherlands). All samples were analyzed with standard conventional testing. Blood specimens drawn from patients suspected of having bloodstream infections were incubated in blood culture bottles (Plus Aerobic [product no. 442192] and Plus Anaerobic [product no. 442193]; BD Diagnostic Systems, Sparks, MD) and monitored for microbial growth in the Bactec 9240 automated blood culture device (BD). When growth was detected, Gram staining was performed. A small aliquot of each blood culture (1 ml) was requested for the novel molecular assay. Two separate assays were developed for Gram-negative and Gram-positive bacteria. Hence, further analysis was based on the results of Gram staining.
Conventional bacterial identification.
Regarding the Gram-positive cocci (GPCs), to discern Staphylococcus spp. from other GPCs, a catalase test was performed by adding 1 colony to a drop of 3% H2O2. For the identification of Staphylococcus spp., catalase-positive strains were tested for coagulase and DNase production. If both tests were negative, the strain was identified as coagulase-negative Staphylococcus (CoNS). To discern Enterococcus spp. from other catalase-negative GPCs, bile esculin, Tellur diagnostic tablets (Rosco Diagnostica, Taastrup, Denmark), and an API 20 Strep test (bioMérieux SA, Marcy l'Etoile, France) were used, according to the manufacturer's guidelines. Optochin susceptibility (OXOID) was used to differentiate Streptococcus pneumoniae from the other viridans group streptococci, which were further identified by API 20 Strep. In the case of beta-hemolytic streptococci, latex agglutination was performed using the Prolex streptococcal grouping latex kit (product code PL.030; Bio Trading).
Bacterial strains.
Reference strains were used to validate the specificity of the assay, including S. aureus ATCC 25923 and ATCC 29213, Staphylococcus epidermidis ATCC 12228 and ATCC 14990, S. pneumoniae ATCC 49619, P. aeruginosa ATCC 27853, Enterococcus faecalis ATCC 29212, Enterococcus faecium BM 4147, and Escherichia coli ATCC 35218.
Multiprobe assay.
An aliquot (0.1 ml) of blood culture was 1:100 diluted in 0.9% NaCl. Dilutions were centrifuged for 5 min at 12,000 rpm, and the bacterial pellet was resuspended in 100 μl nuclease-free water. The primers and the universal bacterial TaqMan probe have been described previously (8). The probes for P. aeruginosa, Pseudomonas spp., E. coli, Staphylococcus spp., S. aureus, Enterococcus spp., Streptococcus spp., and S. pneumoniae were designed by using the BLAST tool and ClustalW software. Multiple sequence alignments were made and are partly shown in Fig. 1. The designed probe sequences are given in Table 1. All primers and probes were tested for specificity and cross-reactions both manually and with use of the NCBI-BLAST tool (http://www.ncbi.nlm.nih.gov/BLAST). Primer and probe matrices were performed to determine optimal concentrations. Each test contained a 5-μl purified sample and a 20-μl reaction mixture. The reaction mixture contained 12.5 μl of TaqMan environmental master mix 2.0 (Applied Biosystems, Foster City, CA), 0.9 μM forward primer, 0.6 μM reverse primer, and 0.2 μM each probe. There were three separate reactions. The first reaction included the universal probe and the P. aeruginosa probe. The second reaction included the probes targeting E. coli and Pseudomonas spp. The third reaction included the Staphylococcus probe, the S. aureus probe, and the Enterococcus probe, the fourth and final reaction included the Streptococcus probe and the S. pneumoniae probe. Reactions were performed using the ABI Prism 7000 real-time PCR system (Applied Biosystems, Foster City, CA), and optimal thermal cycling conditions used were as follows: 2 min at 50°C, initial denaturation at 95°C for 15 min, 42 cycles of denaturation for 15 s at 95°C, and annealing at 60°C for 1 min. The cycle threshold (CT) value, the cycle number at which amplicon fluorescence exceeded the preset detection threshold, was recorded for all samples. The cutoff value to consider a PCR result as positive was set to a CT value of 35.
FIG. 1.
Multiple sequence alignments of the amplified part of the bacterial 16S rRNA gene. Sequences of the bacteria included in the multiprobe assay are shown. The arrows represent the forward and reverse primer targeting the 16S rRNA gene. The sequences of the probes are in boldface and underlined. a, Staphylococcus probe; b, Enterococcus probe; c, Pseudomonas probe; d, S. aureus probe; e, E. coli probe; f, Streptococcus probe; g, universal probe; h, S. pneumoniae probe; i, P. aeruginosa probe.
TABLE 1.
Probes designed for use in multiplex PCR
| Probe | Sequence (5′-3′)a |
|---|---|
| Pseudomonas species | NED-CCTTCCTCCCAACTTAAAGTGCTT-MGB |
| P. aeruginosa | JOE-CCAAAACTACTGAGCTAGAGTACG-BHQ1 |
| E. coli | JOE-GGAGTAAAGTTAATACCTTTGCTCATT-BHQ1 |
| Staphylococcus species | NED-AATCTTCCGCAATGGGCGAAAGC-MGB |
| S. aureus | FAM-AGATGTGCACAGTTACTTACACATAT-BHQ1 |
| Enterococcus species | JOE-TCCTTGTTCTTCTCTAACAACAGAG-BHQ1 |
| Streptococcus species | NED-CCAGAAAGGGACSGCTAACT-MGB |
| S. pneumoniae | JOE-CCAAAGCCTACTATGGTTAAGCCA-BHQ1 |
NED, fluorescent label (Applied Biosystems); MGB, minor groove binder; JOE, 6-carboxy-4,5-dichloro-2,7-dimethyoxyfluorescein; BHQ1, black hole quencher 1; FAM, 6-carboxyfluorescein.
DNA sequencing.
Samples with discrepant results were further analyzed with sequencing. The DNA was purified using the MSB PCRapace PCR cleanup kit (Invitek, Berlin, Germany) and dissolved in 30 μl NASBA H2O. Cyclic sequencing was performed using BigDye 3.0 (Applied Biosystems, Foster City, CA). The resulting sequence product was purified and separated with the 3730 DNA Analyzer (Applied Biosystems, Foster City, CA).
RESULTS
Design of the species- or genus-specific probes.
The specificity of the probes was evaluated using BLAST. In silico analysis revealed that nearly only cross-reactivity was found with not clinically relevant microorganisms such as Geobacillus spp. and Lactobacillus plantarum. In silico cross-reactivity with clinically relevant microorganisms was tested in vitro with reference strains. For example, the in silico E. coli probe cross-reacted with Acinetobacter spp. and Enterobacter spp. However, in vitro tests showed no positive signals for these microorganisms. Furthermore, cross-reactions were found with Escherichia albertii and Shigella spp. Based on the literature, these microorganisms occur only rarely in blood cultures, and therefore, the risk of false identification was minimal. In a few cases such as with the Streptococcus and the Staphylococcus probes, cross-reactivity with Bacillus cereus was found. The species conferring cross-reactivity in PCR were then sequenced to check for mismatches between the probe-target hybrids. Sequencing results showed that this occurred because of at least two mismatches at the binding site of both probes (data not shown). However, in all these cases, the cross-reacting fluorescent signals generated by the mismatched probes were weak compared to that of the positive template control and uncharacteristically appeared over 5 cycles later than signals from 100% matched probes (data not shown).
Evaluation of the multiprobe assay by testing clinical blood culture samples.
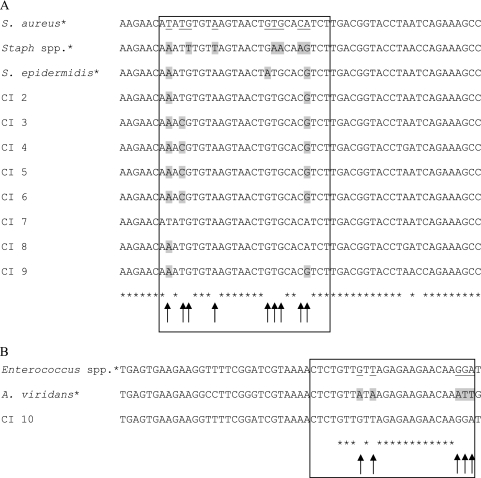
From a total of 248 blood cultures, the presence or absence of bacterial DNA was determined in 232 growth-positive and 16 growth-negative samples using the universal probe, yielding a sensitivity and specificity of 100% (Table 2). Our multiprobe assay was performed after Gram staining of the positive blood cultures. Considering the growth-positive blood cultures, results from the multiprobe assay were in accordance with conventional identification in 222 cases (96%). The specific probes targeting Pseudomonas spp., P. aeruginosa, E. coli, Streptococcus spp., S. pneumoniae, and Staphylococcus spp. all had a sensitivity and specificity of 100%. The majority of Gram-positive blood cultures, 85 of 145 (59%), contained a coagulase-negative Staphylococcus species. In 31 blood cultures, the causative agent was identified as S. aureus. Regarding the Gram-positive staphylococcal blood cultures, the multiprobe assay was in conflict with culture results in nine cases. The nine discrepancies were further analyzed by coagulase testing, specific S. aureus real-time PCR, and sequencing (Table 3; Fig. 2). One out of nine cases, clinical isolate (CI) 7, tested coagulase positive and was positive for two targets specific for S. aureus, i.e., femA and sa442. Sequencing of the PCR product also confirmed the results of the multiprobe assay. The sequences of the eight other clinical isolates showed three mismatches with the reference S. aureus sequence, as shown in Fig. 2A. Hence, the remaining eight cases were confirmed as discordant. Consequently, sensitivity and specificity of 100% and 93%, respectively, and positive and negative predictive values of 79% and 100%, respectively, were achieved when sequencing was considered the gold standard. The probe specific for Enterococcus spp. showed one conflicting result. One blood culture was determined to contain Aerococcus viridans, while our multiprobe assay identified the infectious agent as an Enterococcus sp. (Table 3). Further analysis revealed that the partial sequence of CI 10 was completely similar to the sequence of a reference Enterococcus strain, as shown in Fig. 2B. Comparison of the reference A. viridans and Enterococcus strains showed five mismatches. Consequently, the probe specific for Enterococcus spp. yielded a sensitivity and specificity of 100% when sequencing was considered the gold standard.
TABLE 2.
Bacterial isolates in 232 positive blood cultures and results from universal and specific real-time PCR
| Pathogen | Result of blood culture (no. [%])a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| UNI | PSEU | PSEUAE | ECOLI | STAPH | STAU | ENTE | STREPT | STREPN | |
| Gram-negative pathogens | |||||||||
| Acinetobacter lwoffii/haemolyticus | 1 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Bacteroides fragilis | 2 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Citrobacter koseri | 1 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Enterobacter cloacae | 3 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Escherichia coli | 43 (100) | 0 | 0 | 43 | NA | NA | NA | NA | NA |
| Gram-negative rod | 2 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Klebsiella oxytoca | 5 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Klebsiella pneumoniae | 7 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Moraxella catarrhalis | 1 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Morganella morganii | 1 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Neisseria meningitidis | 1 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Prevotella buccae | 1 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Proteus mirabilis | 2 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Pseudomonas aeruginosa | 10 (100) | 10 | 10 | 0 | NA | NA | NA | NA | NA |
| Pseudomonas oryzihabitans | 1 (100) | 1 | 0 | 0 | NA | NA | NA | NA | NA |
| Serratia marcescens | 4 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Gram-positive pathogens | |||||||||
| Aerococcus viridans | 1 (100) | NA | NA | NA | 0 | 0 | 1 | 0 | 0 |
| Coagulase-negative Staphylococcus spp. | 85 (100) | NA | NA | NA | 85 | 9 | 0 | 0 | 0 |
| Corynebacterium spp. | 2 (100) | NA | NA | NA | 0 | 0 | 0 | 0 | 0 |
| Enterococcus avium | 1 (100) | NA | NA | NA | 0 | 0 | 1 | 0 | 0 |
| Enterococcus faecalis | 3 (100) | NA | NA | NA | 0 | 0 | 3 | 0 | 0 |
| Enterococcus faecium | 4 (100) | NA | NA | NA | 0 | 0 | 4 | 0 | 0 |
| Lactobacillus spp. | 1 (100) | NA | NA | NA | 0 | 0 | 0 | 0 | 0 |
| Propionibacterium acnes | 1 (100) | NA | NA | NA | 0 | 0 | 0 | 0 | 0 |
| Staphylococcus aureus | 31 (100) | NA | NA | NA | 31 | 31 | 0 | 0 | 0 |
| Streptococcus agalactiae | 1 (100) | NA | NA | NA | 0 | 0 | 0 | 1 | 0 |
| Streptococcus group A and/or D | 2 (100) | NA | NA | NA | 0 | 0 | 0 | 2 | 0 |
| Streptococcus milleri | 3 (100) | NA | NA | NA | 0 | 0 | 0 | 3 | 0 |
| Streptococcus oralis | 1 (100) | NA | NA | NA | 0 | 0 | 0 | 1 | 0 |
| Streptococcus pneumoniae | 6 (100) | NA | NA | NA | 0 | 0 | 0 | 6 | 6 |
| Streptococcus pyogenes | 1 (100) | NA | NA | NA | 0 | 0 | 0 | 1 | 0 |
| Streptococcus sanguis | 1 (100) | NA | NA | NA | 0 | 0 | 0 | 1 | 0 |
| Mixed | |||||||||
| Alcaligenes faecalis + Propionibacterium spp. | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Klebsiella oxytoca + Serratia marcescens | 1 (100) | 0 | 0 | 0 | NA | NA | NA | NA | NA |
| Streptococcus salivarius + Streptococcus viridans | 1 (100) | NA | NA | NA | 0 | 0 | 0 | 1 | 0 |
| Totals | |||||||||
| Concordant | 11 | 10 | 43 | 116 | 31 | 8 | 16 | 6 | |
| Discordant | 0 | 0 | 0 | 0 | 9 | 1 | 0 | 0 | |
UNI, universal probe; PSEU, Pseudomonas spp.; PSEUAE, P. aeruginosa; ECOLI, E. coli; STAPH, Staphylococcus spp.; STAU, S. aureus; ENTE, Enterococcus spp.; STREPT, Streptococcus spp.; STREPN, S. pneumoniae; NA, not applicable.
TABLE 3.
Clinical isolates with discordant blood culture and PCR results
| CIa | Result of blood culturea |
|||
|---|---|---|---|---|
| Specific probe(s) | femA/sa442 | Coagulase activity | Sequence | |
| 1 | STAPH, STAU | N/N | N | Unidentified |
| 2 | STAPH, STAU | N/N | N | Staphylococcus hominis |
| 3 | STAPH, STAU | N/N | N | S. hominis |
| 4 | STAPH, STAU | N/N | N | Staphylococcus pasteuri |
| 5 | STAPH, STAU | N/N | N | S. hominis |
| 6 | STAPH, STAU | N/N | N | Staphylococcus schleiferi |
| 7 | STAPH, STAU | P/P | P | S. aureus |
| 8 | STAPH, STAU | N/N | N | S. pasteuri |
| 9 | STAPH, STAU | N/N | N | S. epidermidis |
| 10 | ENTE | NA | NA | Enterococcus spp. |
Clinical isolates (CI) 1 to 9 are CoNS species, while CI 10 is A. viridans. STAPH, Staphylococcus spp.; STAU, S. aureus; ENTE, Enterococcus spp; N, negative; P, positive; NA, not applicable.
FIG. 2.
Partial sequence alignments of the 16S rRNA gene, derived from the clinical isolates with discordant results. (A) Region where the probe specific for S. aureus binds. The sequences of the eight discrepancies are shown in the rectangle. The nucleotides underlined in the rectangle represent the nucleotides which distinguish S. aureus from Staphylococcus (Staph) spp. The sequence alignment of clinical isolate (CI) 7 is the exact reverse complement of the S. aureus probe sequence, which implicates that this isolate, which was identified as CoNS, is in fact S. aureus. CI 1 was not shown because the sequence could not be determined. GenBank accession numbers of reference strains are as follows: S. aureus, FN433596; Staphylococcus spp., GQ222246; and S. epidermidis, GQ911565. (B) Region where the probe specific for Enterococcus spp. binds. The nucleotides underlined in the rectangle represent the nucleotides which distinguish Enterococcus spp. from A. viridans. Conventional testing identified CI 10 as Aerococcus viridans, whereas our assay and sequencing classified it as an Enterococcus sp. GenBank accession numbers of reference strains are as follows: Enterococcus spp., AB489105; A. viridans, M58797. *, reference sequences.
DISCUSSION
New strategies for the detection and identification of bacterial pathogens in blood are continuously under investigation worldwide. Current diagnostic tools are hampered by several factors, such as a lack of sensitivity, a long time to results, the presence of PCR inhibitory compounds in certain sample materials, and the occurrence of fastidious and nonculturable microorganisms. Rapid universal 16S rRNA gene-based PCR is often combined with sequence analysis, and a considerable number of commercial assays have been generated and tested recently, i.e., SeptiFast (Roche Diagnostics GmbH, Mannheim, Germany), Prove-it Sepsis (Mobidiag, Helsinki, Finland), and SepsiTest (Molzym GmbH & Co. KG, Bremen, Germany). The latter includes a universal 16S rRNA gene-based PCR assay combined with sequence analysis, while the two other commercial assays use a panel of probes for the detection of a range of bacterial and mycotic pathogens. Virtually all studies conducted with the SeptiFast and SepsiTest assays found similar results, i.e., an overall agreement of 77 to 83% (2, 3, 6, 12, 13, 15, 16). Both assays are validated for whole-blood samples and can be completed in 4 to 6 h. Tissari et al. performed an observational study comparing conventional culture with Prove-it Sepsis. The assay, based on a microarray platform and performed with blood cultures, had a clinical sensitivity of 94.7% and a specificity of 98.8% (11).
In our study, the 16S rRNA gene was used for the design of eight species- or genus-specific probes. The sequence similarities found in in silico analysis (BLAST) were derived mainly from not clinically relevant microorganisms such as Geobacillus spp., Paenibacillus spp., L. plantarum, and Lactobacillus fermentum. Remaining similarities were, if available, tested in vitro with reference strains. A limited number of microorganisms showed cross-reactivity. For example, streptococcal and staphylococcal probes weakly cross-reacted with Bacillus cereus. In these cases, sequencing determined the correct identification. Additionally, as the assay is always preceded by Gram staining, in most cases, morphology data obtained by Gram staining was in contradiction with these false-positive signals and targeted the samples for confirmatory sequencing.
Our multiprobe assay was designed for use with blood culture material and can be completed within 2 h. Use of whole-blood samples could significantly improve the turnaround time of our assay. However, since culturing still remains essential to determine the microorganism's antimicrobial profile, we chose to use blood cultures instead of whole blood. In this study, we found an overall agreement of 97% between conventional testing and our multiprobe assay. One of the strengths of our approach is that the assay can be extended by adding more probes for other bacterial pathogens to the identification panel. The grouping of Gram-negative and Gram-positive bacteria was based on the identifications needed for antimicrobial susceptibility testing. The assay was designed to use a universal probe for detection instead of Sybr green, because it is known that Sybr green can generate false-positive signals because of the presence of background human DNA or the formation of primer-dimers.
Analysis of the conflicting results showed that, in some cases, our assay was in accordance with sequencing results. The reference sequence derived from a Staphylococcus sp. and a S. epidermidis strain showed seven and three mismatches, respectively, with the reference sequence derived from an S. aureus strain. The sequences derived from the discrepant clinical isolates only showed three or fewer mismatches with the S. aureus sequence. This indicated that within the group of staphylococci, more homologous sequences can cause false positives because of the less efficient binding capacity of the S. aureus-specific probe.
Overall, these results showed strong agreement between conventional testing and our novel, real-time PCR assay. Furthermore, this assay significantly reduced the time needed for identification in comparison to that of routine diagnostics. In conclusion, using pathogen-specific probes offers a faster alternative for pathogen detection and could improve the diagnosis of bloodstream infections. The assay will be implemented in a clinical trial investigating the impact of earlier pathogen identification in combination with susceptibility testing with the choice of therapy.
Footnotes
Published ahead of print on 20 October 2010.
REFERENCES
- 1.Beekmann, S. E., D. J. Diekema, K. C. Chapin, and G. V. Doern. 2003. Effects of rapid detection of bloodstream infections on length of hospitalization and hospital charges. J. Clin. Microbiol. 41:3119-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casalta, J. P., F. Gouriet, V. Roux, F. Thuny, G. Habib, and D. Raoult. 2009. Evaluation of the LightCycler SeptiFast test in the rapid etiologic diagnostic of infectious endocarditis. Eur. J. Clin. Microbiol. Infect. Dis. 28:569-573. [DOI] [PubMed] [Google Scholar]
- 3.Dierkes, C., B. Ehrenstein, S. Siebig, H. J. Linde, U. Reischl, and B. Salzberger. 2009. Clinical impact of a commercially available multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis. BMC Infect. Dis. 9:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan, Q. J., S. Q. Shang, and Y. D. Wu. 2009. Rapid diagnosis of bacterial meningitis in children with fluorescence quantitative polymerase chain reaction amplification in the bacterial 16S rRNA gene. Eur. J. Pediatr. 168:211-216. [DOI] [PubMed] [Google Scholar]
- 5.Hansen, W. L., C. A. Bruggeman, and P. F. Wolffs. 2009. Evaluation of new preanalysis sample treatment tools and DNA isolation protocols to improve bacterial pathogen detection in whole blood. J. Clin. Microbiol. 47:2629-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancini, N., D. Clerici, R. Diotti, M. Perotti, N. Ghidoli, D. De Marco, B. Pizzorno, T. Emrich, R. Burioni, F. Ciceri, and M. Clementi. 2008. Molecular diagnosis of sepsis in neutropenic patients with haematological malignancies. J. Med. Microbiol. 57:601-604. [DOI] [PubMed] [Google Scholar]
- 7.Mignard, S., and J. P. Flandrois. 2006. 16S rRNA sequencing in routine bacterial identification: a 30-month experiment. J. Microbiol. Methods 67:574-581. [DOI] [PubMed] [Google Scholar]
- 8.Nadkarni, M. A., F. E. Martin, N. A. Jacques, and N. Hunter. 2002. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology 148:257-266. [DOI] [PubMed] [Google Scholar]
- 9.Peters, R. P., M. A. van Agtmael, S. A. Danner, P. H. Savelkoul, and C. M. Vandenbroucke-Grauls. 2004. New developments in the diagnosis of bloodstream infections. Lancet Infect. Dis. 4:751-760. [DOI] [PubMed] [Google Scholar]
- 10.Qian, Q., Y. W. Tang, C. P. Kolbert, C. A. Torgerson, J. G. Hughes, E. A. Vetter, W. S. Harmsen, S. O. Montgomery, F. R. Cockerill III, and D. H. Persing. 2001. Direct identification of bacteria from positive blood cultures by amplification and sequencing of the 16S rRNA gene: evaluation of BACTEC 9240 instrument true-positive and false-positive results. J. Clin. Microbiol. 39:3578-3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tissari, P., A. Zumla, E. Tarkka, S. Mero, L. Savolainen, M. Vaara, A. Aittakorpi, S. Laakso, M. Lindfors, H. Piiparinen, M. Maki, C. Carder, J. Huggett, and V. Gant. 2010. Accurate and rapid identification of bacterial species from positive blood cultures with a DNA-based microarray platform: an observational study. Lancet 375:224-230. [DOI] [PubMed] [Google Scholar]
- 12.Varani, S., M. Stanzani, M. Paolucci, F. Melchionda, G. Castellani, L. Nardi, M. P. Landini, M. Baccarani, A. Pession, and V. Sambri. 2009. Diagnosis of bloodstream infections in immunocompromised patients by real-time PCR. J. Infect. 58:346-351. [DOI] [PubMed] [Google Scholar]
- 13.von Lilienfeld-Toal, M., L. E. Lehmann, A. D. Raadts, C. Hahn-Ast, K. S. Orlopp, G. Marklein, I. Purr, G. Cook, A. Hoeft, A. Glasmacher, and F. Stuber. 2009. Utility of a commercially available multiplex real-time PCR assay to detect bacterial and fungal pathogens in febrile neutropenia. J. Clin. Microbiol. 47:2405-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallet, F., S. Nseir, L. Baumann, S. Herwegh, B. Sendid, M. Boulo, M. Roussel-Delvallez, A. V. Durocher, and R. J. Courcol. 18 August 2009. Preliminary clinical study using a multiplex real-time PCR test for the detection of bacterial and fungal DNA directly in blood. Clin. Microbiol. Infect. doi: 10.1111/j.1469-0691.2009.02940.x. [DOI] [PubMed]
- 15.Wellinghausen, N., A. J. Kochem, C. Disque, H. Muhl, S. Gebert, J. Winter, J. Matten, and S. G. Sakka. 2009. Diagnosis of bacteremia in whole-blood samples by use of a commercial universal 16S rRNA gene-based PCR and sequence analysis. J. Clin. Microbiol. 47:2759-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westh, H., G. Lisby, F. Breysse, B. Boddinghaus, M. Chomarat, V. Gant, A. Goglio, A. Raglio, H. Schuster, F. Stuber, H. Wissing, and A. Hoeft. 2009. Multiplex real-time PCR and blood culture for identification of bloodstream pathogens in patients with suspected sepsis. Clin. Microbiol. Infect. 15:544-551. [DOI] [PubMed] [Google Scholar]