Abstract
Analysis of overnight carrot broth culture using the BD GeneOhm StrepB assay (carrot broth-enhanced PCR) yields increased sensitivity compared to that of carrot broth culture alone for the detection of Streptococcus agalactiae. We investigated the prospect of reducing the carrot broth incubation time prior to PCR performance. In vitro experimentation demonstrated that carrot broth-enhanced PCR nominally detected 10 CFU S. agalactiae after 4 h of carrot broth incubation with competitive flora. Detection rates improved with inocula of 100 and 1,000 CFU S. agalactiae, with the majority of these aliquots demonstrating detection after 2 h of carrot broth incubation. Carrot broth was prospectively inoculated with clinical vaginal/anorectal swabs, with 500-μl aliquots collected. Early aliquots from 227 specimens were subjected to carrot broth-enhanced PCR (early-aliquot carrot broth-enhanced PCR) in instances of subsequent positive carrot broth culture or positive overnight clinical carrot broth-enhanced PCR. The S. agalactiae detection rate by early-aliquot carrot broth-enhanced PCR (66.1%) exceeded that observed for 227 remnant swabs retrospectively tested by direct swab PCR (56.4%; P = 0.03). Early-aliquot carrot broth-enhanced PCR detection rate differences were most pronounced in aliquots from 83 carrot broth aliquots collected after 6 h (84.3%) compared to detection rates from either direct swab PCR of these samples (51.8%; P < 0.0002) or early-aliquot carrot broth-enhanced PCR of 144 carrot broth aliquots collected after fewer than 6 h of incubation (55.6%; P < 0.0002). Enhanced sensitivity of early-aliquot carrot broth-enhanced PCR versus direct swab PCR suggests that this assay could serve as a surrogate rapid detection method facilitating the prevention of group B streptococcal disease.
Streptococcus agalactiae (beta-hemolytic Streptococcus group B) can impart significant morbidity and mortality to the neonatal demographic (2). Subsequent to evidence that intrapartum antimicrobial chemoprophylaxis can prevent neonatal S. agalactiae colonization, sepsis, and mortality (21), the Centers for Disease Control and Prevention (CDC) published recommendations in 1996 (9) promoting both maternal risk-based strategies and microbiological surveillance toward the goal of identifying candidates for chemoprophylactic intervention. A 65% reduction in early-onset group B streptococcal disease was realized from 1993 to 1998 (29). Schrag et al. (28) subsequently reported that microbiological screening-derived data outperformed risk-based strategies in identifying these at-risk mothers. As a result, revised CDC guidelines published in 2002 (10) advocate universal late-antenatal screening at 35 to 37 weeks of gestation for S. agalactiae colonization.
Van Dyke et al. (35) recently reported results from a 2-year, 10-state surveillance project assessing invasive group B streptococcal disease. While the percentage of women being screened for S. agalactiae increased from 48.1% in 1999 to 85.0% in 2003 to 2004, the overall incidence of disease showed only a nominal decrease. The surveillance showed that term infants contributed to 74.4% of documented early-onset disease cases. Within this cohort, 82.0% of mothers were appropriately screened for S. agalactiae, but only approximately 25% of testing generated a positive result. In another study, Puopolo et al. (23) reported that 82.4% of term infants with early-onset group B streptococcal disease were born to mothers whose S. agalactiae status was determined to be negative.
Taken together, these data can imply that current laboratory modalities for the detection of S. agalactiae are not adequate. Carrot broth, a derivative of Granada medium (26), is a selective and differential medium for the cultivation of S. agalactiae. Culture of primary clinical specimens that generates an orange pigment upon overnight incubation is specific for beta-hemolytic strains of S. agalactiae. Nonpigmented broth following overnight incubation requires terminal subculture to enriched medium and up to an additional 48 h of observation. A commercial PCR assay, validated by our laboratory to utilize carrot broth culture as the substrate, demonstrated 10.4% increased sensitivity over terminally subcultured carrot broth, with all results finalized within 24 h of broth inoculation (4). This carrot broth-enhanced S. agalactiae PCR also exhibited an approximate 40% increase in sensitivity compared to that of a small subset of primary clinical swabs directly subjected to the commercial PCR assay. In this report, we extend the comparison of carrot broth-enhanced PCR to direct swab PCR by using a larger subset of primary clinical swabs. Moreover, carrot broth-enhanced PCR is characterized in a temporal fashion for potential utility in rapid laboratory diagnosis of S. agalactiae colonization.
(Results of this work were previously presented, in part, at the 110th General Meeting of the American Society for Microbiology, San Diego, CA, 23 to 27 May 2010 [15].)
MATERIALS AND METHODS
In vitro experimentation.
Clinical isolates of Streptococcus agalactiae, Escherichia coli, Klebsiella pneumoniae, Serratia marcescens, Enterobacter cloacae, Proteus mirabilis, coagulase-negative Staphylococcus species, Candida albicans, Enterococcus faecalis, viridans group Streptococcus species, Haemophilus influenzae, Gardnerella vaginalis, Neisseria gonorrhoeae, and Bacteroides fragilis were cultivated on appropriate tryptic soy agar with 5% sheep blood, chocolate agar, Sabouraud dextrose agar, or anaerobic (CDC) blood agar (Remel, Incorporated, Lenexa, KS) and incubated 16 to 24 h in appropriate 35°C CO2-enriched or anaerobic-selective conditions. Suspensions of each culture were adjusted with physiological saline to a 4.0 McFarland turbidity equivalent (∼1.0 × 109 CFU/ml). Following 10-fold serial dilution in physiological saline, between 101 and 103 CFU S. agalactiae was delivered in replicate to StrepB Carrot Broth kit tubes (Carrot Broth; Hardy Diagnostics, Santa Maria, CA). Inoculated carrot broth was subsequently challenged with ∼1.0 × 108 CFU each of the remaining organisms in 100-μl volumes. Carrot broth was incubated in 35°C ambient air.
Clinical carrot broth culture for S. agalactiae.
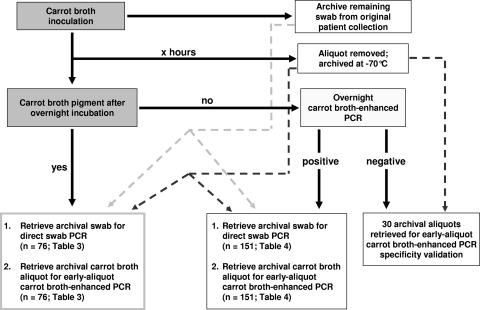
Primary clinical vaginal/anorectal specimens submitted in the dual-swab Copan BBL CultureSwab collection and transport system (BD Diagnostics, Sparks, MD) for routine prenatal S. agalactiae screening were evaluated. One swab was placed into carrot broth. Tubes were incubated in 35°C ambient air. The remnant swab was archived at 4°C pending utilization within the study protocol (Fig. 1). All protocols were approved by the Wheaton Franciscan Healthcare Institutional Review Board.
FIG. 1.
Algorithm for clinical comparison of early-aliquot carrot broth-enhanced PCR to both direct swab PCR and overnight carrot broth-enhanced PCR.
Carrot broth aliquots.
Aliquots (500 μl) of carrot broth inoculated for in vitro experimentation or for clinical protocols (early aliquot) were collected and archived at −70°C pending utilization within the study protocol (Fig. 1). One aliquot was aseptically removed per inoculated tube. Collection intervals were arbitrarily classified as less than 3 h (mean, 2.33 h; range, 1.08 h to 2.97 h), 3.00 to 3.99 h, 4.00 to 4.99 h, 5.00 to 5.99 h, 6.00 to 6.99 h, and ≥7.00 h (mean, 7.99 h; median, 7.34 h).
Overnight carrot broth-enhanced PCR (clinical reference method).
Following one overnight incubation, carrot broth was observed for characteristic pigment. In the absence of color development, fresh 500-μl aliquots of carrot broth were centrifuged (14,000 × g, 5 min) in glass bead lysis tubes that are constituents of the BD GeneOhm StrepB assay (BD Diagnostics, Ste-Foy, Quebec, Canada). Supernatant was discarded and replaced with 50 μl kit-provided Tris-EDTA buffer. Lysis tube contents were then vortexed for 5 min, pulse centrifuged for 5 to 10 s, and subsequently incubated for 2 min on a 95°C heating block. Lysates were cooled for ≥10 min at 2 to 8°C.
Early-aliquot carrot broth-enhanced PCR (investigation method).
S. agalactiae screens yielding a positive result via either pigmented carrot broth or overnight clinical carrot broth-enhanced PCR were subject to investigational PCR. Archived frozen aliquots (early aliquots) were thawed and processed for carrot broth-enhanced PCR as described above. The specificity of early-aliquot carrot broth-enhanced PCR was determined by retrospective analysis of 30 aliquots derived from S. agalactiae screens that yielded a negative result upon clinical overnight carrot broth-enhanced PCR.
Direct swab PCR.
The archived remnant swab from S. agalactiae screens yielding a positive result via either pigmented carrot broth or overnight carrot broth-enhanced PCR was retrospectively processed for the StrepB assay per manufacturer specifications. In brief, swabs were incubated for 5 min in sample buffer, with a 50-μl aliquot subsequently transferred to lysis tubes. The contents of lysis tubes were vortexed for 5 min, pulse centrifuged for 5 to 10 s, and incubated for 2 min on a 95°C heating block. Lysates were cooled for ≥10 min at 2 to 8°C prior to StrepB performance. Direct swab PCR was performed in a routine fashion within 5 days of specimen collection, per manufacturer specifications.
BD GeneOhm StrepB assay.
Aliquots (1.5 μl) of processed lysates were delivered to kit-provided SmartCycler reaction tubes containing lyophilized master mix that was reconstituted with 25 μl of kit-provided diluent [Tris-HCl buffer, (NH4)2SO4, MgCl2]. All reaction tubes were pulse centrifuged for 5 to 10 s, inverted and vortexed for 10 to 15 s, and loaded onto a SmartCycler fluorescence thermal cycler for 37 cycles of PCR, targeting a 154-bp fragment of cfb. Real-time amplicon detection occurred via hybridization of an FAM (6-carboxyfluorescein)- and DABCYL [4-(dimethylaminoazo)benzene-4-carboxylic acid]-based molecular beacon. The validity of each generated PCR result, in the face of possible medium-based inhibitors, was determined by an internal control (134-bp DNA fragment not found in S. agalactiae that is flanked by the sequence of both S. agalactiae-specific primers) contained within the master mix. Following reaction tube setup, lysates were frozen at −20°C.
Follow-up analysis.
Lysates derived from frozen carrot broth aliquots or primary swabs that did not yield a positive result were repeat tested in duplicate. The significance test of proportions was used to determine if detection rate differences were significant. The alpha level was set at 0.05; all P values are two-tailed.
RESULTS
In vitro lower limit of detection for carrot broth-enhanced S. agalactiae PCR.
The incubation of 10 CFU S. agalactiae in carrot broth for 2 h in the presence of ∼1.3 × 109 simulated gastrointestinal or urogenital flora resulted in no detection of S. agalactiae-specific nucleic acid via carrot broth-enhanced PCR (Table 1). The incubation of analogous suspensions for ≥4 h resulted in a 33.3% detection rate of the organism. In contrast, carrot broth-enhanced PCR detected S. agalactiae in 58.3% and 100.0% of competitive cultures incubated for 2 h with 102 and 103 CFU S. agalactiae, respectively. A detection rate of 95.8% (23/24) was yielded when simulated cultures of 102 and 103 CFU S. agalactiae were assessed by carrot broth-enhanced PCR at 6 h of incubation.
TABLE 1.
In vitro temporal estimation of the carrot broth-enhanced PCR lower limit of detectiona
| S. agalactiae inoculum | Carrot broth-enhanced PCR detection rate (%) at: |
||||
|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 12 h | 24 h | |
| 101 CFU | 0.0 | 33.3 | 41.7 | 25.0 | 33.3 |
| 102 CFU | 58.3 | 66.7 | 91.7 | ND | ND |
| 103 CFU | 100.0 | 100.0 | 100.0 | ND | ND |
Twelve replicates were performed per interval for each inoculum. ND, not determined.
Clinical characterization of early-aliquot carrot broth-enhanced S. agalactiae PCR.
Two hundred twenty-seven primary specimens were reported positive for S. agalactiae in routine clinical practice on the basis of either characteristic overnight carrot broth pigmentation or the detection of S. agalactiae-specific nucleic acid via carrot broth-enhanced PCR of overnight nonpigmented carrot broth. One hundred three early aliquots were prospectively collected from carrot broth cultures following incubation periods less than 5 h (Table 2). The mean detection rate of early-aliquot carrot broth-enhanced PCR within these three intervals was 48.5%, with a rate of 51.4% at the 4.00-to-4.99-h interval (Table 2). The detection rate trended 21.8% higher at the 5.00-to-5.99-h interval (P = 0.051) and was significantly elevated at the 6.00-to-6.99-h interval (82.1%; P = 0.005) compared to that of the 4.00-to-4.99-h interval. The specificity of early-aliquot carrot broth-enhanced PCR was demonstrated by 30 negative results being yielded from early aliquots of carrot broth (five from each incubation interval) that subsequently yielded negative results upon overnight clinical carrot broth-enhanced PCR.
TABLE 2.
Temporal comparison of early-aliquot carrot broth-enhanced PCR and retrospective direct swab PCR detection rates for 227 specimens that initially screened positive for S. agalactiae by either overnight carrot broth culture or overnight carrot broth-enhanced PCR
| No. of specimens | Early-aliquot carrot broth-enhanced PCR |
% positive from remnant direct swab PCR | P value | |
|---|---|---|---|---|
| Collection interval (h) | % positive | |||
| 33 | <3 | 54.5 | 66.7 | 0.31 |
| 35 | 3.00-3.99 | 40.0 | 54.3 | 0.23 |
| 35 | 4.00-4.99 | 51.4 | 48.6 | 0.81 |
| 41 | 5.00-5.99 | 73.2 | 65.9 | 0.47 |
| 39 | 6.00-6.99 | 82.1 | 46.2 | 0.0009 |
| 44 | ≥7 | 86.3 | 56.8 | 0.002 |
| Total (227) | 66.1 | 56.4 | 0.03 | |
Comparison of early-aliquot carrot broth-enhanced PCR to retrospective direct swab PCR for the detection of S. agalactiae.
Retrospective direct swab PCR performed on 227 remnant swabs yielded a detection rate of 56.4% (Table 2). In contrast, 66.1% of frozen aliquots prospectively collected from these cultures and subjected to early-aliquot carrot broth-enhanced PCR yielded a positive result (P = 0.03) (Table 2). Detection differences between early-aliquot carrot broth-enhanced PCR and retrospective direct swab PCR were not significant when carrot broth was incubated fewer than 6 h prior to aliquot collection (P ≥ 0.23). However, carrot broth-enhanced PCR on aliquots collected at least 6 h after swab inoculation yielded an increased detection rate compared to that of direct swab PCR (P ≤ 0.002).
Comparison of early-aliquot carrot broth-enhanced PCR to retrospective direct swab PCR for the detection of S. agalactiae on the basis of the carrot broth culture result.
Seventy-six (33.5%) of the 227 specimens yielding a positive result for S. agalactiae produced a pigmented carrot broth. The overall rate of positive early-aliquot carrot broth-enhanced PCR results (85.5%) (Table 3) did not significantly differ from the rate of positive direct swab PCR results (80.3%; P = 0.39). In contrast, early aliquots from 151 specimens yielding a laboratory diagnosis of S. agalactiae colonization solely on the basis of overnight carrot broth-enhanced PCR (Table 4) demonstrated a higher rate of S. agalactiae molecular detection (56.2%) than the corresponding direct swab PCR (44.4%; P = 0.04). This difference was accentuated when aliquots were collected after 6 h of carrot broth incubation (P ≤ 0.01).
TABLE 3.
Temporal comparison of early-aliquot carrot broth-enhanced PCR and retrospective direct swab PCR detection rates for 76 specimens that initially screened positive for S. agalactiae by overnight carrot broth culture
| No. of specimens | Early-aliquot carrot broth-enhanced PCR |
% positive from remnant direct swab PCR | P valuea | |
|---|---|---|---|---|
| Collection interval (h) | % positive | |||
| 12 | <3 | 83.3 | 91.7 | - |
| 12 | 3.00-3.99 | 50.0 | 75.0 | - |
| 10 | 4.00-4.99 | 80.0 | 80.0 | - |
| 19 | 5.00-5.99 | 94.7 | 89.5 | - |
| 13 | 6.00-6.99 | 100.0 | 69.2 | - |
| 10 | ≥7 | 100.0 | 70.0 | - |
| Total (76) | 85.5 | 80.3 | 0.39 | |
-, P value is too small for valid statistical comparison.
TABLE 4.
Temporal comparison of early-aliquot carrot broth-enhanced PCR and retrospective direct swab PCR detection rates for 151 specimens that initially screened positive for S. agalactiae solely by overnight carrot broth-enhanced PCR
| No. of specimens | Early-aliquot carrot broth-enhanced PCR |
% positive from remnant direct swab PCR | P value | |
|---|---|---|---|---|
| Collection interval (h) | % positive | |||
| 21 | <3 | 38.1 | 52.4 | 0.35 |
| 23 | 3.00-3.99 | 34.7 | 43.5 | 0.55 |
| 25 | 4.00-4.99 | 40.0 | 36.0 | 0.77 |
| 22 | 5.00-5.99 | 54.5 | 45.5 | 0.55 |
| 26 | 6.00-6.99 | 73.1 | 34.6 | 0.005 |
| 34 | ≥7 | 82.4 | 52.9 | 0.01 |
| Total (151) | 56.2 | 44.4 | 0.04 | |
DISCUSSION
Proper targeting of the appropriate candidates for intrapartum antimicrobial chemoprophylaxis is essential for the prevention of neonatal invasive group B streptococcal disease. This especially holds true for two demographics for which screening methods are not readily available. Whitney et al. (36) used a multivariate analysis to demonstrate that the incidence of early-onset group B streptococcal disease increased with the number of women who did not receive prenatal care (P = 0.01). African American women, as an example, have a higher-than-average probability of receiving inadequate prenatal care (18), with a large percentage being unsure of their S. agalactiae colonization status (12). Higher rates of S. agalactiae colonization within this race (7, 27, 34) may manifest as a greater likelihood of early-onset group B streptococcal disease (11, 36). In the context of preterm labor, associations have been reported between gestational age and early- and late-onset group B streptococcal disease (5, 32), with early-onset disease mortality rates inversely proportional to gestational age (29).
One means by which a demand for rapid turnaround time of S. agalactiae detection may be met during instances of emergent labor is nucleic acid amplification of primary clinical specimens. Past studies (3, 16) reported the sensitivity of the BD GeneOhm StrepB assay as 94 to 97% for the detection of S. agalactiae. These data appear to conflict with the direct swab PCR sensitivity value of 59% previously reported by our group in a small study set (4) and the 56% value presented in this report (sensitivity data from our laboratory did not differ significantly between studies [P = 0.77]). In addition, a 77% sensitivity of the BD GeneOhm StrepB assay was reported by Smith et al. (33).
The foundation for these disparities may lie in the reference methods employed. Carrot broth-enhanced PCR was previously shown to detect S. agalactiae in approximately 10% more specimens than conventional carrot broth culture (4). These findings were verified by a discrepancy analysis that involved duplicate repeat testing and substantial corroboration by an alternative broth-enhancement PCR method. In our current study, carrot broth-enhanced PCR detected S. agalactiae in 6.6% more specimens than conventional carrot broth culture (P = 0.29 compared to a previous prevalence study in our laboratory [4]). Past reports (20, 30) reveal that terminally subcultured carrot broth is up to 15% more sensitive for the detection of S. agalactiae than LIM broth culture (a past CDC-advocated selective medium [10]). Recently, Church et al. (14) documented terminally subcultured carrot broth detecting five more instances of S. agalactiae colonization than initial LIM broth inoculation in a 279-woman cohort with 18% S. agalactiae prevalence. Scicchitano and Bourbeau (31) reported that the performance of the BD GeneOhm StrepB assay from 18- to 24-h LIM broth enhancement had a sensitivity equivalent to that of the reference culture. Goodrich and Miller (19) determined sensitivity of the StrepB assay at 92.5% for 4-h LIM broth cultures that were subsequently held at 4°C prior to nucleic acid extraction. Finally, a component of the combined reference standard utilized by Smith et al. (33) was a selective solid medium that outperformed a reference agar medium.
When it is considered that past evaluations of direct swab PCR (3, 16) utilized LIM broth culture as the reference standard, one can surmise that sensitivity differences can be attributed to an evolving, more-sensitive carrot broth-enhanced PCR reference method. This paradigm becomes important when analyzing the recent report from Van Dyke et al. (35). Despite chronicling a nearly 40% increase in women being correctly targeted for intrapartum chemoprophylaxis, a less-than-anticipated decrease in group B streptococcal disease resulted. In fact, mathematical modeling suggested that between 38% and 74% more disease was observed than predicted. With screening increasing in such an aggressive fashion, the source of the deficit in disease prevention may actually lie in laboratory diagnosis. Indeed, a false-negative screening rate of 62.4% in one demographic of group B streptococcal disease was noted. An analogous rate of 82.4% was reported by Puopolo et al. on the basis of conventional culture methodology (23). The delineation of diagnostic methods employed in the aforementioned CDC surveillance program (35) was not provided, yet one can speculate upon the effect that sole reliance on LIM broth or direct swab PCR has on accurate S. agalactiae detection.
A third demographic for which highly accurate rapid S. agalactiae detection has a potential application has recently emerged. Van Dyke et al. (35) reported that 74.4% of group B streptococcal disease occurred in term infants. These data extend the findings of Puopolo et al. (23), who reported a rate of 68%. Transient S. agalactiae colonization during antepartum and intrapartum stages may be a contributory factor (6, 38). We therefore sought to determine if carrot broth-enhanced PCR could be further modified to accommodate emergent clinical practice.
In vitro experimentation involved mock inoculation of carrot broth with small quantities of S. agalactiae in the presence of simulated flora, quantities of which were 8 log10 greater than that of S. agalactiae in some instances. B. fragilis, E. coli, and E. cloacae were representative of flora sampled in anorectal collections, while viridans group Streptococcus, H. influenzae (24), and coagulase-negative staphylococci may be encountered in vaginal samplings. Pathogens of the female genital tract, such as G. vaginalis, C. albicans, and N. gonorrhoeae, were also constituents of simulated competitive flora. E. faecalis was included because of past data demonstrating the ability of this organism to suppress the growth potential of S. agalactiae (17). None of the mock cultures described in Table 1 exhibited carrot broth pigmentation, yet carrot broth-enhanced PCR detected an original 101 CFU S. agalactiae inoculum in 33.3% of cultures following 4 h of incubation. With an initial S. agalactiae inoculum of 102 CFU, this percentage increased to 66.7% of cultures at this interval. All mock inoculations with 103 CFU S. agalactiae yielded a positive carrot broth-enhanced PCR result, with inoculations of 102 CFU S. agalactiae yielding a >90% rate of carrot broth-enhanced PCR detection at 6 h of incubation.
The significance of this 6-h time point appeared to mirror findings within the clinical portion of this investigation. While no significant difference was demonstrated between the rate of S. agalactiae detection via early-aliquot carrot broth-enhanced PCR within the first 6 h of carrot broth incubation and the detection rate upon retrospective direct swab PCR analysis (P ≥ 0.23) (Table 2), findings were markedly different following the 6-h time point. The early-aliquot carrot broth-enhanced PCR detection rate following 6.00 to 6.99 h of carrot broth incubation (82.1%) was greater than that derived from remnant direct swab PCR (46.2%; P = 0.0009).
Both the likelihood of early-aliquot carrot broth-enhanced PCR and retrospective direct swab PCR performing in an equivalent fashion at earlier time points and the demonstration of increased detection of S. agalactiae by early-aliquot carrot broth-enhanced PCR at later intervals may be related to the organism burden of the primary specimen. In the subset of 76 specimens that generated pigmented carrot broth following overnight incubation, no significant difference between detection rates of early-aliquot carrot broth-enhanced PCR and direct swab PCR was observed, independent of the carrot broth incubation interval (P = 0.39) (Table 3). In contrast, testing of early aliquots and remnant swabs from specimens that were reported positive for S. agalactiae solely on the basis of highly sensitive overnight carrot broth-enhanced PCR revealed a significantly higher detection rate from incubated carrot broth than from direct swab PCR (P = 0.04) (Table 4). Again, this difference was most significant following 6 h of incubation. Moreover, the percentage of retrospective direct swab PCR detection within the context of specimens yielding a positive carrot broth result (80.3% [Table 3]) was higher than that rate in specimens not yielding a positive overnight carrot broth result (44.4% [Table 4]). Likewise, early-aliquot carrot broth-enhanced PCR demonstrated an increased rate of detection in carrot broth-positive specimens (all P < 0.0002).
While nonhemolytic S. agalactiae strains (14) may be contributory to this phenomenon, our data suggest that the true benefit of early-aliquot carrot broth-enhanced PCR could lie in the rapid detection of S. agalactiae from clinical specimens possessing a low organism burden. Moreover, these data extend previous findings (4) demonstrating that carrot broth-enhanced PCR identifies more positive S. agalactiae screens than either direct swab PCR or conventional carrot broth culture. Rallu et al. (25) previously expressed concern over the utilization of broth-enhanced amplification methods for the detection of S. agalactiae. These authors hypothesized, in part, that the detection of low organism burden (which may be deemed clinically insignificant in some circles) may propagate the overuse of intrapartum chemoprophylaxis, potentiating antibiotic resistance induction. Service-oriented consequences secondary to risk factor-negative, S. agalactiae screen-positive mothers have been described (22). However, at least three studies insinuate that low-level colonization (37) or the purported presence of S. agalactiae that falls below detection limits for direct swab PCR or CDC-advocated culture methods (23, 35) plays a significant role in the onset of group B streptococcal disease, even despite appropriate third-trimester screenings (23, 35).
In summary, evolving reference standards, such as overnight colorimetric broth-enhanced PCR, should have a role of importance in the future identification of candidates for intrapartum chemoprophylaxis. A provisional CDC recommendation (13) discusses optional direct broth testing by molecular modalities. The colorimetric aspect of the medium may allow cost-conscious laboratories to forego molecular testing when a characteristic observation is made. Apart from carrot broth, a variety of colorimetric media has been described (1, 8). The increased sensitivities of some of these media have allowed for their adoption as a matrix for highly sensitive reflex PCR testing (4). Improved detection of S. agalactiae via an early-aliquot carrot broth-enhanced PCR algorithm, compared to direct swab testing, may provide laboratories an additional option for screening expectant mothers in emergent situations. In total, the utilization of these methods provides an increased potential for prevention of neonatal invasive group B streptococcal disease.
Acknowledgments
The authors express their sincere gratitude to Timothy Kramme, Patricia Luedke, Melissa Schlosser, and Kathleen Wynveen for excellent technical assistance. Additional acknowledgment is given to Walt Earhart for illustrative assistance.
Footnotes
Published ahead of print on 27 October 2010.
REFERENCES
- 1.Adler, A., C. Block, D. Engelstein, D. Hochner-Celnikcier, R. Drai-Hassid, and A. E. Moses. 2008. Culture-based methods for detection and identification of Streptococcus agalactiae in pregnant women—what are we missing? Eur. J. Clin. Microbiol. Infect. Dis. 27:241-243. [DOI] [PubMed] [Google Scholar]
- 2.Baker, C. J. 1997. Group B streptococcal infections. Clin. Perinatol. 24:59-70. [PubMed] [Google Scholar]
- 3.Bergeron, M. G., D. Ke, C. Ménard, F. J. Picard, M. Gagnon, M. Bernier, M. Ouellette, P. H. Roy, S. Marcoux, and W. D. Fraser. 2000. Rapid detection of group B streptococci in pregnant women at delivery. N. Engl. J. Med. 343:175-179. [DOI] [PubMed] [Google Scholar]
- 4.Block, T., E. Munson, A. Culver, K. Vaughan, and J. E. Hryciuk. 2008. Comparison of carrot broth- and selective Todd-Hewitt broth-enhanced PCR protocols for real-time detection of Streptococcus agalactiae in prenatal vaginal/anorectal specimens. J. Clin. Microbiol. 46:3615-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyer, K. M., C. A. Gadzala, L. I. Burd, D. E. Fisher, J. B. Paton, and S. P. Gotoff. 1983. Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease. I. Epidemiologic rationale. J. Infect. Dis. 148:795-801. [DOI] [PubMed] [Google Scholar]
- 6.Boyer, K. M., C. A. Gadzala, P. D. Kelly, L. I. Burd, and S. P. Gotoff. 1983. Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease. II. Predictive value of prenatal cultures. J. Infect. Dis. 148:802-809. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, J. R., S. L. Hillier, M. A. Krohn, P. Ferrieri, D. F. Zaleznik, and C. J. Baker. 2000. Group B streptococcal colonization and serotype-specific immunity in pregnant women at delivery. Obstet. Gynecol. 96:498-503. [DOI] [PubMed] [Google Scholar]
- 8.Carvalho, M. G., R. Facklam, D. Jackson, B. Beall, and L. McGee. 2009. Evaluation of three commercial broth media for pigment detection and identification of a group B Streptococcus (Streptococcus agalactiae). J. Clin. Microbiol. 47:4161-4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 1996. Prevention of perinatal group B streptococcal disease: a public health perspective. MMWR Morb. Mortal. Wkly. Rep. 45(RR-7):1-24. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2002. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Morb. Mortal. Wkly. Rep. 51(RR-11):1-22. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2004. Diminishing racial disparities in early-onset neonatal group B streptococcal disease. MMWR Morb. Mortal. Wkly. Rep. 53:502-505. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 2005. Disparities in universal prenatal screening for group B streptococcus—North Carolina, 2002-2003. MMWR Morb. Mortal. Wkly. Rep. 54:700-703. [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. 29 July 2010. Provisional recommendations for the prevention of perinatal group B streptococcal disease. MMWR Morb. Mortal. Wkly. Rep. [Epub ahead of print.] http://www.cdc.gov/groupbstrep/guidelines/downloads/provisional-recommendations-508.pdf.
- 14.Church, D. L., H. Baxter, T. Lloyd, B. Miller, and S. Elsayed. 2008. Evaluation of StrepB carrot broth versus Lim broth for detection of group B streptococcus colonization status of near-term pregnant women. J. Clin. Microbiol. 46:2780-2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culver, A., M. Napierala, K. Munson, and E. Munson. 2010. Temporal characterization of carrot broth-enhanced real-time PCR as an alternative means for rapid detection of Streptococcus agalactiae, abstr. C-1126, p. 106. Abstr. 110th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC. [DOI] [PMC free article] [PubMed]
- 16.Davies, H. D., M. A. Miller, S. Faro, D. Gregson, S. C. Kehl, and J. A. Jordan. 2004. Multicenter study of a rapid molecular-based assay for the diagnosis of group B Streptococcus colonization in pregnant women. Clin. Infect. Dis. 39:1129-1135. [DOI] [PubMed] [Google Scholar]
- 17.Dunne, W. M., Jr., and C. A. Holland-Staley. 1998. Comparison of NNA agar culture and selective broth culture for detection of group B streptococcal colonization in women. J. Clin. Microbiol. 36:2298-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elam-Evans, L. D., M. M. Adams, P. M. Gargiullo, J. L. Kiely, and J. S. Marks. 1996. Trends in the percentage of women who received no prenatal care in the United States, 1980-1992: contributions of the demographic and risk effects. Obstet. Gynecol. 87:575-580. [DOI] [PubMed] [Google Scholar]
- 19.Goodrich, J., and M. B. Miller. 2007. Comparison of culture and 2 real-time polymerase chain reaction assays to detection group B Streptococcus during antepartum screening. Diagn. Microbiol. Infect. Dis. 59:17-22. [DOI] [PubMed] [Google Scholar]
- 20.Hutchens, K. A., and P. C. Schreckenberger. 2006. Comparison of real-time PCR (Cepheid SmartCycler) with standard LIM broth culture and StrepB Carrot Broth for the detection of group B Streptococcus in prenatal vaginal/rectal specimens, abstr. C-138, p. 124-125. Abstr. 106th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 21.Lim, D. V., W. J. Morales, A. F. Walsh, and D. Kazanis. 1986. Reduction of morbidity and mortality rates for neonatal group B streptococcal disease through early diagnosis and chemoprophylaxis. J. Clin. Microbiol. 23:489-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peralta-Carcelen, M., C. A. Fargason, Jr., S. P. Cliver, G. R. Cutter, J. Gigante, and R. L. Goldenberg. 1996. Impact of maternal group B streptococcal screening on pediatric management in full-term newborns. Arch. Pediatr. Adolesc. Med. 150:802-808. [DOI] [PubMed] [Google Scholar]
- 23.Puopolo, K. M., L. C. Madoff, and E. C. Eichenwald. 2005. Early-onset group B streptococcal disease in the era of maternal screening. Pediatrics 115:1240-1246. [DOI] [PubMed] [Google Scholar]
- 24.Quentin, R., J. M. Musser, M. Mellouet, P. Y. Sizaret, R. K. Selander, and A. Goudeau. 1989. Typing of urogenital, maternal, and neonatal isolates of Haemophilus influenzae and Haemophilus parainfluenzae in correlation with clinical course of isolation and evidence for a genital specificity of Haemophilus influenzae biotype IV. J. Clin. Microbiol. 27:2286-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rallu, F., P. Barriga, C. Scrivo, V. Martel-Laferrière, and C. Laferrière. 2006. Sensitivities of antigen detection and PCR assays greatly increased compared to that of the standard culture method for screening for group B streptococcus carriage in pregnant women. J. Clin. Microbiol. 44:725-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosa-Fraile, M., J. Rodriguez-Granger, M. Cueto-Lopez, A. Sampedro, E. B. Gaye, J. M. Haro, and A. Andreu. 1999. Use of Granada medium to detect group B streptococcal colonization in pregnant women. J. Clin. Microbiol. 37:2674-2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schrag, S. J., K. E. Arnold, J. C. Mohle-Boetani, R. Lynfield, E. R. Zell, K. Stefonek, H. Noga, A. S. Craig, L. T. Sanza, G. Smith, and A. Schuchat, for the Active Bacterial Core Surveillance Team. 2003. Prenatal screening for infectious diseases and opportunities for prevention. Obstet. Gynecol. 102:753-760. [DOI] [PubMed] [Google Scholar]
- 28.Schrag, S. J., E. R. Zell, R. Lynfield, A. Roome, K. E. Arnold, A. S. Craig, L. H. Harrison, A. Reingold, K. Stefonek, G. Smith, M. Gamble, and A. Schuchat. 2002. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N. Engl. J. Med. 347:233-239. [DOI] [PubMed] [Google Scholar]
- 29.Schrag, S. J., S. Zywicki, M. M. Farley, A. L. Reingold, L. H. Harrison, L. B. Lefkowitz, J. L. Hadler, R. Danila, P. R. Cieslak, and A. Schuchat. 2000. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N. Engl. J. Med. 342:15-20. [DOI] [PubMed] [Google Scholar]
- 30.Schreckenberger, P., A. Y. Hsiung, C. Marnell, L. Terrile, Y. Soto, M. Miller, E. Ilendo, R. Nachum, L. Fairbanks, G. Abbey, D. Luper, G. Peterson, and J. Hardy. 2005. Evaluation of Strep B Carrot Broth and LIM broth methods for recovery of group B streptococci (GBS). Results of a multicenter trial, abstr. C-109, p. 124. Abstr. 105th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC.
- 31.Scicchitano, L. M., and P. P. Bourbeau. 2009. Comparative evaluation of the AccuProbe group B streptococcus culture test, the BD GeneOhm Strep B assay, and culture for detection of group B streptococci in pregnant women. J. Clin. Microbiol. 47:3021-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel, J. D. 1998. Prophylaxis for neonatal group B streptococcus infections. Semin. Perinatol. 22:33-49. [DOI] [PubMed] [Google Scholar]
- 33.Smith, D., J. D. Perry, L. Laine, A. Galloway, and F. K. Gould. 2008. Comparison of BD GeneOhm real-time polymerase chain reaction with chromogenic and conventional culture methods for detection of group B Streptococcus in clinical samples. Diagn. Microbiol. Infect. Dis. 61:369-372. [DOI] [PubMed] [Google Scholar]
- 34.Stapleton, R. D., J. M. Kahn, L. E. Evans, C. W. Critchlow, and C. M. Gardella. 2005. Risk factors for group B streptococcal genitourinary tract colonization in pregnant women. Obstet. Gynecol. 106:1246-1252. [DOI] [PubMed] [Google Scholar]
- 35.Van Dyke, M. K., C. R. Phares, R. Lynfield, A. R. Thomas, K. E. Arnold, A. S. Craig, J. Mohle-Boetani, K. Gershman, W. Schaffner, S. Petit, S. M. Zansky, C. A. Morin, N. L. Spina, K. Wymore, L. H. Harrison, K. A. Shutt, J. Bareta, S. N. Bulens, E. R. Zell, A. Schuchat, and S. J. Schrag. 2009. Evaluation of universal antenatal screening for group B streptococcus. N. Engl. J. Med. 360:2626-2636. [DOI] [PubMed] [Google Scholar]
- 36.Whitney, C. G., B. D. Plikaytis, W. S. Gozansky, J. D. Wenger, and A. Schuchat, for the Neonatal Group B Streptococcal Disease Study Group. 1997. Prevention practices for perinatal group B streptococcal disease: a multistate surveillance analysis. Obstet. Gynecol. 89:28-32. [DOI] [PubMed] [Google Scholar]
- 37.Yancey, M., K. P. Duff, P. Clark, T. Kurtzer, B. H. Frentzen, and P. Kubilis. 1994. Peripartum infection associated with vaginal group B streptococcal colonization. Obstet. Gynecol. 84:816-819. [PubMed] [Google Scholar]
- 38.Yancey, M. K., A. Schuchat, L. K. Brown, V. L. Ventura, and G. R. Markenson. 1996. The accuracy of late antenatal screening cultures in predicting genital group B streptococcal colonization at delivery. Obstet. Gynecol. 88:811-815. [DOI] [PubMed] [Google Scholar]