Abstract
A 65-year-old woman with a history of gastric bleeding, breast cancer, antineoplastic chemotherapy, and prednisone use presented with a fever, chest pain, a dry cough, hypotension, and prominent pulmonary bronchovascular markings. She was treated with piperacillin-tazobactam and azithromycin and rapidly improved. Six days later, the blood culture grew a pleomorphic Gram-negative bacillus. Initial subculture failed, but the organism was identified as Helicobacter pylori by sequencing the 16S rRNA gene. The bacterium eventually grew on brucella agar upon extended incubation.
CASE REPORT
A 65-year-old woman with a history of metastatic breast cancer presented to the emergency department at M. D. Anderson Cancer Center in February 2003 with a fever, chills, chest pain, a dry cough, and shortness of breath for several hours. The patient's cancer was diagnosed in 1996 and treated with surgery and chemotherapy. However, metastases to bone and cervical lymph nodes were found in 1999, for which she received additional chemotherapy. Metastases to the liver were found in December 2002, and the patient completed 3 cycles of vinorelbine 2 weeks prior to this emergency room visit. She had been receiving prednisone for amelioration of chemotherapy-related side effects and omeprazole for stomach bleeding and discomfort. The stomach problem was presumed to be chemotherapy related and had been present for an uncertain period of time. Because of the overall weakness and cancer burden, the patient was deemed not suitable for further cancer therapy. Her cancer care took place in Spain, her home country, and our institution.
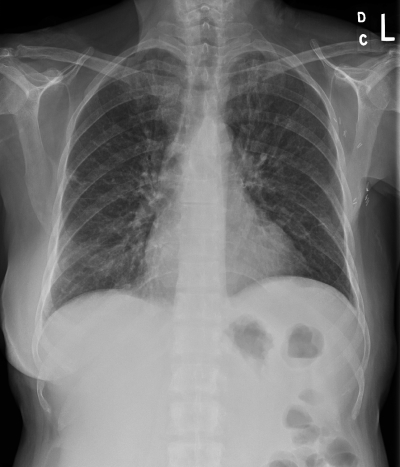
Her vital signs were significant for fever (37.8°C) and hypotension (74/47 mm Hg). On physical examination, the patient was found to have scattered rhonchi and expiratory wheezes, and a chest X-ray demonstrated prominent bilateral bronchovascular markings (Fig. 1). She had no history of chronic respiratory or cardiac problems or symptoms of an acute respiratory viral syndrome, such as rhinorrhea or sneezing. Laboratory examination showed anemia (hemoglobin concentration, 12.5 g/dl) and an increment of blood leukocytes from 3.1 × 109 cells/liter 2 weeks earlier to 6.3 × 109 cells/liter with 89% neutrophils. Together, the symptoms and examination suggested a diagnosis of atypical pneumonia to the emergency department physician, and a blood sample was obtained for culture for microorganisms. The patient was treated empirically with azithromycin and piperacillin-tazobactam for the infection, rehydrated, and admitted to the hospital. Her symptoms and hypotension resolved within 24 h, and she was discharged 2 days later (without a further chest X-ray) with 2 additional weeks of oral azithromycin. The discharge blood leukocyte count further rose to 9.4 × 109 cells/liter with 94% neutrophils to go along with the probable infection.
FIG. 1.
Chest X-ray showing nonspecific pulmonary infiltrates in a 65-year-old woman with Helicobacter pylori bacteremia and respiratory symptoms.
Meanwhile, after 6 days of incubation, the blood culture (Bactec aerobic/F bottle with resins) became positive for a pleomorphic Gram-negative bacillus. Subculture of this organism, however, was unsuccessful despite the use of various media, including sheep blood agar, chocolate II agar, Campy agar, MacConkey agar, buffered charcoal yeast extract agar, brucella agar (with 5% sheep blood, hemin, and vitamin K), Trypticase soy broth, and thioglycolate broth (all from BBL, Becton-Dickinson, Sparks, MD) and under aerobic, anaerobic, and microaerophilic incubation conditions. In an attempt to identify this organism, we performed a PCR to amplify the 16S rRNA gene of this organism using our published method (3). Genomic DNA was extracted from the positive-culture bottle, and highly conserved 16S primers were used. A 515-bp DNA fragment in the middle of the 16S gene was amplified and sequenced directly, and a GenBank query showed a complete (100%) match with Helicobacter pylori. A review of the cellular morphology showed curved cells as well as short and long rods. These features, along with the difficulty of subculturing, were also consistent with H. pylori.
The finding of H. pylori and the patient's history of stomach problems led to further antiulcer treatment with clarithromycin, ampicillin, and omeprazole in her home country. A month after presentation, the results for a follow-up chest X-ray were normal, and a serum sample tested positive for antibodies of the IgA, IgM, and IgG classes against H. pylori, whereas results for antibodies against Mycoplasma and Chlamydia spp., agents commonly causing atypical pneumonia, were negative and positive (from past infection; IgG class), respectively. The patient was lost to long-term follow-up for cancer.
Despite the subculture failure, the positive blood culture mixture was stored at −70°C. Years later, attempts were made again to isolate the organism. Indeed, the strain (MDA-1397) grew on a brucella agar plate (with 5% sheep blood, hemin, and vitamin K) upon incubation for 5 days at 37°C with 5% CO2. On further subculture, fine growth became noticeable after 3 days and translucent colonies reached about 0.5 mm in size by 5 days but barely reached 1 mm on further incubation. Gram staining showed relatively long curved rods with faint safranin staining. Limited biochemical tests included positive catalase, oxidase, and urease reactions. The DNA from the isolated organism was amplified and sequenced again, and the 16S rRNA gene (1,449 bp) matched 99.7% with several H. pylori strains in GenBank, whereas matches with other Helicobacter spp. were 98.2% or less. It is known that minor heterogeneity of the 16S genes exists among H. pylori strains, so we consider the identity of this strain to be confirmed as H. pylori. We also tested the growth of this strain again under microaerobic incubation (GasPak; BBL) at 42°C and 37°C on brucella agar. The organism did grow at 37°C in the GasPak (slightly better than in the aerobic chamber) but not at 42°C, unlike Campylobacter jejuni.
Discussion.
H. pylori, previously known as Campylobacter pylori, is a curved or spiral flagellated Gram-negative bacillus that colonizes the gastric mucosa, a highly specialized niche (1, 7). H. pylori is a well-known cause of gastritis and peptic ulcer. It also plays an etiologic role in metaplasia and dysplasia of gastric mucosa, gastric adenocarcinoma, and non-Hodgkin lymphoma of the stomach (2). Levels of serologic evidence for past or current H. pylori infection are high, from 10% in the United States to nearly 100% in developing countries (2). The organism can be cultured from gastric biopsy specimens.
To our knowledge, H. pylori bacteremia has been reported only once (6). The 83-year-old woman in that report also presented with dyspnea and fever; however, these manifestations were confounded by enlarged paratracheal lymph nodes with lymphoma involvement (as a possible cause of dyspnea) and urinary tract Escherichia coli infection (as possible cause of fever). The blood culture bottle (Bactec NR730) showed growth after 5 days of incubation, and H. pylori was isolated using CampyPak Plus under microaerophilic incubation and identified by biochemical tests. The patient did not receive anti-H. pylori treatment, because her fever resolved prior to the culture result. She did not have symptoms or signs suggestive of peptic ulcer and died of lymphoma a few weeks later.
Our patient had suffered from gastric bleeding and discomfort, which in retrospect suggested the differential diagnosis of peptic ulcer. However, it was masked by the side effects of antineoplastic chemotherapy until the episode of H. pylori bacteremia. Thus, in view of the high prevalence of H. pylori, prolonged stomach complaints in a cancer patient may warrant investigation, such as endoscopy. In the absence of severe neutropenia, a well-known risk for bacteremia, our patient's use of prednisone, with its immunosuppressive activity, might have aided the invasion of H. pylori from the gastric ulcer into the blood circulation. The eventual subculture of H. pylori with an optimal medium (brucella agar) and extended incubation (5 days or more) allowed isolation of H. pylori, which we did not satisfy initially.
The prominent bronchovascular markings in the chest radiograph in our case were thought to reflect “atypical pneumonia” by the emergency department physician. However, in retrospect, these almost certainly reflected early pulmonary edema as part of a sepsis syndrome induced by the H. pylori bacteremia. A repeat chest radiograph during the hospitalization was not obtained to confirm the rapid resolution of the prominent markings, but the patient's prompt symptomatic improvement after the administration of antibiotics and intravenous fluids, the pertinent negative findings, and the lack of serologic evidence of recent infection by Mycoplasma or Chlamydia spp. make atypical pneumonia unlikely. The piperacillin and azithromycin that were administered in our case likely both contributed the rapid clinical improvement.
So far, H. pylori has not been reported to be associated with atypical pneumonia or recovered from a lower respiratory specimen, even among patients with severe comorbidities. On the other hand, current culture methods for respiratory specimens do not include brucella agar or extended incubation, which precludes recovery of H. pylori even if this organism is present. A single study reported the possible detection of H. pylori by Warthin-Starry staining and microscopy in tracheal secretions for patients under intensive care to raise the possibility of aspiration of gastric contents (5). We have shown previously that oral Campylobacter species, curved Gram-negative rods closely related to H. pylori, may be present in aspiration pneumonia (4).
In summary, H. pylori bacteremia may occur as a rare complication in patients with undiagnosed gastric infection. It is helpful that clinical microbiology laboratories are aware of the cultivation methods for this highly fastidious organism.
Nucleotide sequence accession number.
The 16S rRNA gene of this H. pylori strain, MDA-1397, has been deposited in GenBank under accession number HQ 266659.
Acknowledgments
This work was supported in part by National Institutes of Health grant CA16672 for the M. D. Anderson Cancer Center DNA Sequencing Core Facility.
We thank Kevin McEnery and Reginald Munden for helpful discussions and the medical team for caring for the patient. We have no conflicting interest.
Footnotes
Published ahead of print on 22 September 2010.
REFERENCES
- 1.Goodwin, C. S., T. Armstrong, T. Chilvers, M. Peters, M. D. Collins, L. Sly, W. McConnell, and W. E. S. Harper. 1989. Transfer to Campylobacter pylori and Campylobacter mustelae to Helicobacter gen. nov. as Helicobacter pylori comb. nov. and Helicobacter mustelae comb. nov. respectively. Int. J. Syst. Bacteriol. 39:397-405. [Google Scholar]
- 2.Graham, D. Y., and J. J. Y. Sung. 2006. Helicobacter pylori, p. 1049-1066. In M. Feldman, L. S. Friedman, and L. J. Brandt (ed.), Gastrointestinal and liver disease, 8th ed. Saunders, Philadelphia, PA.
- 3.Han, X. Y., A. S. Pham, J. J. Tarrand, P. K. Sood, and R. Luthra. 2002. Rapid and accurate identification of mycobacteria by sequencing hypervariable regions of the 16S ribosomal RNA gene. Am. J. Clin. Pathol. 118:796-801. [DOI] [PubMed] [Google Scholar]
- 4.Han, X. Y., J. J. Tarrand, and D. C. Rice. 2005. Oral Campylobacter species involved in extraoral abscess: a report of three cases. J. Clin. Microbiol. 43:2513-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitz, H. S., and S. S. Farber. 1993. Demonstration of Helicobacter pylori in tracheal secretions. J. Am. Osteopath. Assoc. 93(1):87-91. [PubMed] [Google Scholar]
- 6.Ndawula, E. M., R. J. Owen, G. Mihr, P. Borman, and A. Hurtado. 1994. Helicobacter pylori bacteraemia. Eur. J. Clin. Microbiol. Infect. Dis. 13(7):621. [DOI] [PubMed] [Google Scholar]
- 7.Warren, J. R., and B. J. Marshall. 1983. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet i(8336):1273-1275. [PubMed] [Google Scholar]