Abstract
We analyzed a collection of 60 Salmonella enterica 4,5,12:i:- phage type U302 multidrug-resistant monophasic variant strains, isolated in Spain between 2000 and 2007. Most strains showed resistance to ampicillin (A), chloramphenicol (C), sulfamethoxazole (Su), gentamicin (G), streptomycin (S), tetracycline (T), and co-trimoxazole (SxT) (an ACSuGSTSxT resistance pattern). Only one pulsed-field gel electrophoresis (PFGE) type was detected, with 19 subtypes (Simpson's index of diversity [SID] = 0.89). Multiple-locus variable-number tandem-repeat analysis (MLVA) showed more variability, with 32 profiles (SID = 0.97), but only showed diversity at the STTR5 and STTR6 loci. PCR and sequencing demonstrated all strains contained the same allantoin-glyoxylate pathway deletion. Four types of deletions were detected in the fljAB operon, all starting at the same position, at the STM2758 gene, and followed by an IS26 insertion. Furthermore, a representative set of strains of the four deletion types harbored plasmids with IS26. We propose that a Salmonella enterica serotype Typhimurium U302 multidrug-resistant (ACSuGSTSxT) strain, defective for the allantoin-glyoxylate pathway and containing IS26 at plasmid pU302L, could be the ancestor of the variant in Spain.
In 1997 the Spanish National Salmonella Reference Laboratory (SNSRL) detected that a Salmonella enterica subsp. enterica multidrug-resistant strain had emerged with an atypical antigenic formula (4,5,12:i:-) and phage type U302 (7). Several studies found this strain to be a monophasic variant of S. enterica serovar Typhimurium (7, 8). Compared to the S. Typhimurium LT2 strain, the monophasic variant contained five major deletions in the allantoin-glyoxylate operon, the fljAB operon, and three prophages, Fels-1, Fels-2, and Gifsy-1 (9). Similar Salmonella monophasic strains have increasingly been detected in human clinical cases and food-related sources around the world (4, 17). The aim of this research is to determine the molecular characterization and genetic evolution of a representative collection of isolates of this Spanish monophasic Salmonella variant.
MATERIALS AND METHODS
To determine the genetic evolution of this monophasic variant, we studied 60 strains of Salmonella enterica 4,5,12:i:- phage type U302, voluntarily submitted to the SNSRL over an 8-year period (2000 to 2007), and the first food isolate from 1998 (Fig. 1). Five S. Typhimurium strains, including the LT2 strain (Spanish Culture Cell Type CECT722) and an S. enterica serovar Enteritidis strain, were used as controls.
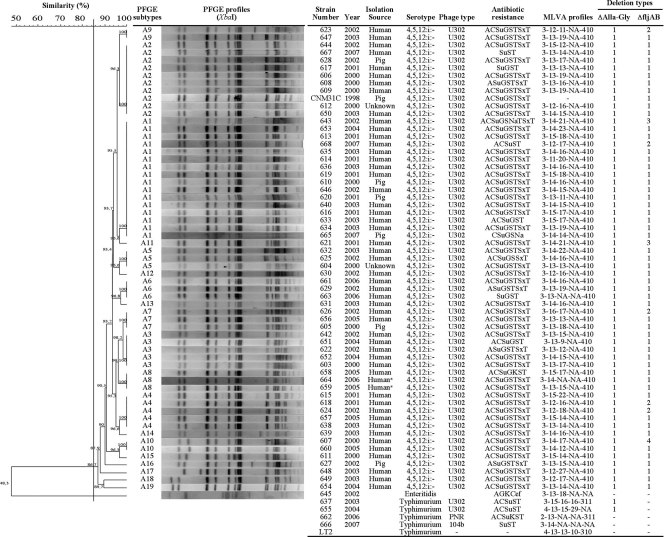
FIG. 1.
Results of PFGE typing, characteristics of the strains used in this study, and type assignment by different typing methods. PFGE dendrogram, types, and profiles obtained with XbaI restriction enzyme following the PulseNet International protocol using Dice and UPGMA (optimization, 0.50%; tolerance, 1.5%). Strain numbers from Salmonella laboratory of Faculty of Pharmacy, UPV/EHU, Vitoria-Gasteiz, Spain. CNM3IC, strain code of Microbiology National Centre (CNM, Majadahonda, Madrid); LT2, Salmonella LT2 strain obtained from the Spanish Type Culture Collection (CECT no. 722); *, human blood source; other human isolates were obtained from stools. Antibiotic resistance: A, ampicillin; C, chloramphenicol; Su, sulfamethoxazole; G, gentamicin; K, kanamycin; S, streptomycin; Cef, cephalothin; Na, nalidixic acid; T, tetracycline; SxT, cotrimoxazole. MLVA profiles are composed of five numbers indicating repeat unit for each locus, with the following order: STTR9-STTR5-STTR6-STTR10-STTR3 (11). Deletion type assigned by PCR and sequencing of allantoine-glyoxylate (ΔAlla-Gly) operon or fljAB (ΔfljAB) operon deletions.
The phage type was defined according to Anderson et al. (2). The susceptibilities to 13 antimicrobial agents were determined by the disk diffusion method according to the 2007 guidelines of the Clinical and Laboratory Standards Institute (CLSI; http://www.clsi.org/). Pulsed-field gel electrophoresis (PFGE) was performed following the PulseNet International protocol (http://www.pulsenetinternational.org/protocol/pfge.asp) using the XbaI restriction enzyme. Dice index and UPGMA were used to obtain a dendrogram (Fig. 1). Multiple-locus variable-number tandem-repeat analysis (MLVA) was carried out by following the protocol described for S. Typhimurium (12, 13). Simpson's index of diversity (SID) was used to calculate the discrimination of techniques (11). PCR and sequencing were used to study the allantoin-glyoxylate and fljAB operon deletions. The allantoin-glyoxylate operon deletion was studied using previously described primers (9). Two contiguous PCRs were designed to delineate the fljAB operon deletions. The first PCR amplified the region from the STM2757 gene to the IS26 insertion sequence (1). The second PCR amplified the region from IS26 (5′-TTG CAA ATA GTC GGT GGT GA-3′) to the iroC gene (5′-CCC AGG GGA TCA CCA TAA TA-3′) or from IS26 (5′-CGC CTG GTA AGC AGA GTT TT-3′) to the STM2815 gene (5′-CAC CCC GAA AGA GGT GAT AA-3′). PFGE-S1 nuclease and hybridization assays were performed to detect whether the monophasic strains contained large plasmids with IS26 (3).
RESULTS AND DISCUSSION
The Salmonella enterica 4,5,12:i:- U302 strains analyzed in this study had very similar antibiotic resistance profiles, which indicates the homogeneity of this group. Although there was some variation compared to the typical “Spanish clone,” the main pattern was still resistance to ampicillin (A), chloramphenicol (C), sulfamethoxazole (Su), gentamicin (G), streptomycin (S), tetracycline (T), and co-trimoxazole (SxT) (ACSuGSTSxT) (Fig. 1). The PFGE-XbaI profiles showed 12 to 15 bands, with no more than 4 band differences. The 60 Salmonella 4,5,12:i:- U302 strains all had the same PFGE type, A, and 19 different subtypes (A1 to A19) were detected (Fig. 1), with a SID value of 0.89. Over the 8 years these strains were isolated from all over the country, only a few changes accumulated, which demonstrates the high clonality of these strains in Spain, a finding which is consistent with previous reports (10, 17). MLVA showed 32 profiles, although only the STTR5 and STTR6 loci presented variability (6 and 16 allele numbers, respectively) (Fig. 1), with a SID value of 0.97. These results are consistent with observations by others that the MLVA method is more discriminative than PFGE and a possible alternative to PFGE for genotyping highly clonal groups of bacteria (12, 13). Based on PFGE and MLVA techniques, the Spanish Salmonella enterica 4,5,12:i:- phage type U302 strains studied are more homogeneous than groups isolated in other countries (17), supporting a clonal origin.
Two of the major deletions previously detected in the Spanish monophasic strains, in the allantoin-glyoxylate and fljAB operons (9), were studied by PCR and sequencing. We confirmed the deletion of the allantoin-glyoxylate operon in all Salmonella 4,5,12:i:- strains and in the two S. Typhimurium U302 strains. Sequencing revealed that the deletion always spanned the STM0517 and STM0529 genes (GenBank accession no. EU265823.1, EU265824.1, and EU265825.1). A similar deletion in this gene region was described by Reen et al. (15) for S. Typhimurium.
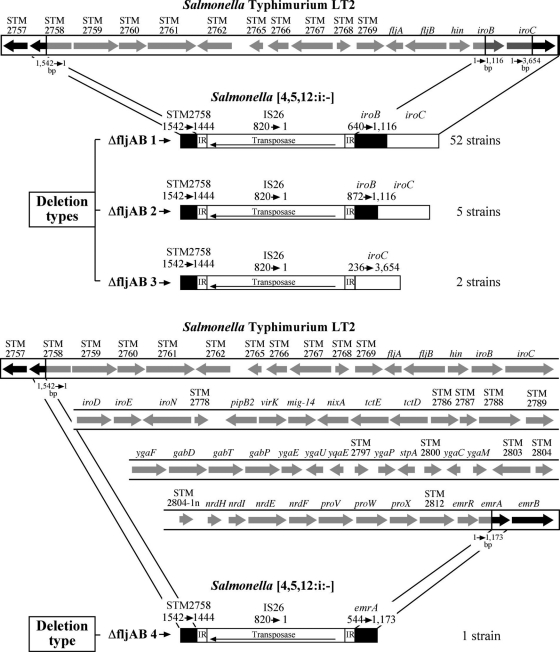
In the present study, we detected variations in the fljAB operon deleted gene region (Fig. 2). The start of the deletion was well conserved in all 60 Salmonella monophasic strains studied. This deletion affected 93.6% of the STM2758 gene and began at the same nucleotide in all cases (Fig. 2). The end of the deletion showed more variability. We identified four different deletion types: ΔfljAB1, ΔfljAB2, ΔfljAB3, and ΔfljAB4 (Fig. 2). ΔfljAB1 was the most common deletion, ending at nucleotide 639 of the iroB gene (STM2773); this sequence is available from GenBank (accession no. GQ402163.1). The ΔfljAB2 deletion ended at nucleotide 871 of the iroB gene (accession no. GQ402164.1); the ΔfljAB3 deletion ended at nucleotide 235 of the iroC gene (STM2774) (accession no. GU939595.1); and ΔfljAB4 was a larger deletion, which ended at nucleotide 543 of the emrA gene (STM2814) (accession no. HM595411) (Fig. 2). No amplification occurred with the control strains.
FIG. 2.
Structure of the different fljAB operon deletion types and the insertion of IS26 detected in Spanish S. enterica serovar [4,5,12:i:-] monophasic variant strains observed by PCR and sequencing, compared with the Salmonella enterica serovar Typhimurium LT2 genome (GenBank accession no. AE006468.1).
Previous studies revealed IS26 was inserted in the fljAB operon deleted region (9); this finding was confirmed here. Other authors (6) have shown similar mutations in the rbs operon of Escherichia coli, with the end point of the deletion always precisely located at the end of IS150 and with different extents of the deletion. In our study, all the deletion types of the genes were replaced by an inverted IS26 sequence. IS26 is a member of a family of transposable elements with a possibly wide host range and of considerable importance for horizontal gene transfer between microorganisms (14). IS26 has been described for many bacteria, including the S. Typhimurium U302 G8430 strain, which harbors a large plasmid (pU302L) with 5 copies of this insertion sequence (5, 14). To detect whether the monophasic strains in our study contained large plasmids with IS26, a selection of 13 strains with different ΔfljAB deletion types were assayed by PFGE-S1 and hybridization. The result was positive in all strains. IS26 was present in the chromosomally deleted region and in the large plasmid, which could involve a replicative transposition event (18). Furthermore, when analyzing the start and end of the deleted regions, we detected a short sequence of 14 nucleotides (5′-AAG ACG CTC TGA AT-3′) in the sequence, left-adjacent to the conserved fragment of the STM2758 gene in the chromosome, upstream from the start of all ΔfljAB-type deletions. Interestingly, we detected the complementary inverted sequence of these 14 nucleotides flanking the end of the third copy of IS26 on the pU302L plasmid (14). The homology in this short sequence could explain the upstream conserved insertion of IS26 in this region at the same position in all the deletion types. Analysis of nucleotide sequences downstream of the different deletions detected no homology with the end of inverted IS26, suggesting rearrangements are associated with the initial transposition event or represent recombination with other IS26s transposed downstream. In agreement with the findings of Cooper et al. (6) and Schneider et al. (16), these different genetic events (transpositions and recombinations) could take place simultaneously or successively. The mechanism and the drive to continue deleting in this region are unknown. However, it is possible for the deletions to be related to each other as a nested series.
Based on the results obtained by PFGE, MLVA, PCR, and sequencing, the Salmonella monophasic strains of this study seem to have maintained great homogeneity over the years. A recent study carried out with some Salmonella 4,5,12:i:- isolates from the United States and Spain concluded that this strain most likely represents multiple clones with distinct geographical distributions that emerged through independent deletion events (17). Our data support this hypothesis and expands the information given by Soyer et al. (17) about Spanish monophasic strains, as we studied a larger number of strains and sequenced the fragments flanking the fljAB deletions. From our data, there is not a clear link between the location of the strains, source, or year of isolation and the different profiles of resistance, fljAB deletion type, and PFGE or MLVA patterns; yet, considering we analyzed only a representative collection of strains of the Spanish clone, we cannot reject these relationships.
In summary, we hypothesize that a Salmonella enterica serotype Typhimurium, phage type U302, multidrug-resistant (ACSuGSTSxT) strain, defective for the allantoin-glyoxylate pathway and harboring the plasmid pU302L, could be the ancestor of the monophasic variant strains assayed in this report. However, more studies are necessary to ascertain if the ΔfljAB1 deletion type could be the origin of the other variations or, to the contrary, if the four genetic defects arose independently.
Acknowledgments
This study was supported by Subvención General a Grupos de Investigación grant (GIU0820) from UPV/EHU and Consolidated Research Group Grant (IT343-10) and Saiotek Grant (S-PC09UN0) from Basque Government. L. Laorden and I. Martínez were supported by a Beca de Investigación Predoctoral grant from the UPV/EHU of Spain.
Footnotes
Published ahead of print on 13 October 2010.
REFERENCES
- 1.Alvarez, J., M. Sota, A. B. Vivanco, I. Perales, R. Cisterna, A. Rementeria, and J. Garaizar. 2004. Development of a multiplex PCR technique for detection and epidemiological typing of Salmonella in human clinical samples. J. Clin. Microbiol. 42:1734-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, E. S., L. R. Ward, M. J. De Saxe, and J. D. H. De Sa. 1977. Bacteriophage-typing designations of Salmonella typhimurium. J. Hyg. (Lond.) 78:297-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton, B. M., G. P. Harding, and A. J. Zuccarelli. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235-240. [DOI] [PubMed] [Google Scholar]
- 4.Bone, A., H. Noel, S. Le Hello, N. Pihier, C. Danan, M. E. Raguenaud, S. Salah, H. Bellali, V. Vaillant, F. X. Weill, and Jourdan-da Silva. 2010. Nationwide outbreak of Salmonella enterica serotype 4,12:i:- infections in France, linked to dried pork sausage, March-May 2010. Euro Surveill. 15(24):pii=19592. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19592. [PubMed] [Google Scholar]
- 5.Chen, C.-Y., G. W. Nace, B. Solow, and P. Fratamico. 2007. Complete nucleotide sequences of 84.5- and 3.2-kb plasmids in the multi-antibiotic resistant Salmonella enterica serovar Typhimurium U302 strain G8430. Plasmid 57:29-43. [DOI] [PubMed] [Google Scholar]
- 6.Cooper, V. S., D. Schneider, M. Blot, and R. E. Lenski. 2001. Mechanisms causing rapid and parallel losses of ribose catabolism in evolving populations of Escherichia coli B. J. Bacteriol. 183:2834-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echeita, M. A., A. Aladueña, S. Cruchaga, and M. A. Usera. 1999. Emergence and spread of an atypical Salmonella enterica subsp. enterica serotype 4,5,12:i:- strain in Spain. J. Clin. Microbiol. 37:3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Echeita, M. A., S. Herrera, and M. A. Usera. 2001. Atypical, fljB-negative Salmonella enterica subsp. enterica strain of serovar 4,5,12:i:- appears to be a monophasic variant of serovar Typhimurium. J. Clin. Microbiol. 39:2981-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garaizar, J., S. Porwollik, A. Echeita, A. Rementeria, S. Herrera, R. M.-Y. Wong, J. Frye, M. A. Usera, and M. McClelland. 2002. DNA microarray-based typing of an atypical monophasic Salmonella enterica serovar. J. Clin. Microbiol. 40:2074-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerra, B., I. Laconcha, S. M. Soto, M. A. Gonzalez-Hevia, and M. C. Mendoza. 2000. Molecular characterisation of emergent multiresistant Salmonella enterica serotype [4,5,12:i:-] organisms causing human salmonellosis. FEMS Microbiol. Lett. 190:341-347. [DOI] [PubMed] [Google Scholar]
- 11.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson, J. T., M. Torpdahl, R. F. Petersen, G. Sorensen, B. A. Lindstedt, and E. M. Nielsen. 2009. Development of a new nomenclature for Salmonella Typhimurium multilocus variable number of tandem repeats analysis (MLVA). Euro Surveill. 14(15):pii=19174. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19174. [PubMed] [Google Scholar]
- 13.Lindstedt, B. A., T. Vardund, L. Aas, and G. Kapperud. 2004. Multiple-locus variable-number tandem-repeats analysis of Salmonella enterica subsp. enterica serovar Typhimurium using PCR multiplexing and multicolour capillary electrophoresis. J. Microbiol. Methods 59:163-172. [DOI] [PubMed] [Google Scholar]
- 14.Mollet, B., S. Iida, J. Shepherd, and W. Arber. 1983. Nucleotide sequence of IS26, a new prokaryotic mobile genetic element. Nucleic Acids Res. 11:6319-6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reen, F. J., E. F. Boyd, S. Porwollik, B. P. Murphy, D. Gilroy, S. Fanning, and M. McClelland. 2005. Genomic comparisons of Salmonella enterica serovar Dublin, Agona, and Typhimurium strains recently isolated from milk filters and bovine samples from Ireland, using a Salmonella microarray. Appl. Environ. Microbiol. 71:1616-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider, D., E. Duperchy, E. Coursange, R. E. Lenski, and M. Blot. 2000. Long-term experimental evolution in Escherichia coli. IX. Characterization of insertion sequence-mediated mutations and rearrangements. Genetics 156:477-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soyer, Y., A. Moreno, M. A. Davis, J. Maurer, P. L. McDonough, D. J. Schoonmaker-Bopp, N. B. Dumas, T. Root, L. D. Warnick, Y. T. Gröhn, and M. Wiedmann. 2009. Salmonella enterica serotype 4,5,12:i:-, an emerging Salmonella serotype that represents multiple distinct clones. J. Clin. Microbiol. 47:3546-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turlan, C., and M. Chandler. 1995. IS1-mediated intramolecular rearrangements: formation of excised transposon circles and replicative deletions. EMBO J. 14:5410-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]