Abstract
Spectra VRE (Remel, Lenexa, KS) is a chromogenic medium designed to recover and differentiate vancomycin-resistant Enterococcus faecium and Enterococcus faecalis (VRE). This medium was compared to bile esculin azide agar (BEAV) and was 98.2% sensitive and 99.3% specific compared to BEAV, which was 87.6% sensitive and 87.1% specific at 24 h.
Two Enterococcus species, E. faecalis and E. faecium, cause the majority of human enterococcal infections (1). The rapid strain identification for patients colonized in the gastrointestinal tract with vancomycin-resistant strains of these species (VRE) is critical, as infection with these organisms can result in endocarditis, urinary tract, bloodstream, and wound infections with reduced therapeutic options (3). Screening for VRE is essential for the proper implementation of isolation precautions, as asymptomatic carriers serve as reservoirs for VRE infection or transmission (4, 7, 9). Successful identification of patients colonized with VRE requires rapid and accurate screening tests that are easily interpretable. The purpose of this multicenter study was to compare the performance of a new chromogenic medium, Spectra VRE, to bile esculin azide agar supplemented with 6 μg vancomycin/ml (BEAV; Remel) as a means of screening stool specimens for VRE colonization.
Stool specimens were collected from inpatients in sterile containers for Clostridium difficile testing and stored at 4°C for up to 3 days. Specimens were plated with a sterile Dacron swab to BEAV and Spectra VRE and streaked for isolation by the quadrant technique. Inoculated plates were incubated at 35°C in ambient air and examined for growth at 18, 24, and 48 h. Pink, purple, or dark blue colonies on Spectra VRE were presumptively identified as vancomycin-resistant E. faecium. Light blue colonies on Spectra VRE were presumptively identified as vancomycin-resistant E. faecalis. Presumed VRE colonies on BEAV appeared dark brown or black.
Presumptive VRE from Spectra VRE and BEAV were subcultured to tryptic soy agar plates (TSA; Remel) and incubated at 35°C for 24 h. Catalase-negative, Gram-positive cocci positive for l-pyrrolidonyl-β-naphthylamide (PYR; Remel) were further identified using methyl-α-d-glucopyranoside (MDG; Remel), motility test medium (Remel), PB arabinose (Remel), and colony morphology on blood agar. VRE isolates were identified based on the following performance characteristics: E. faecalis, MDG-negative, nonmotile, arabinose-negative, white colonies; E. faecium, MDG-negative, nonmotile, arabinose-positive, gray colonies. The Etest (bioMérieux, Durham, NC) was performed to determine the vancomycin MIC of each isolate. Breakpoints for VRE were interpreted as defined by CLSI guidelines (2). Confirmatory identification and antimicrobial susceptibility testing were also performed with the Vitek 2 (bioMérieux). All confirmed VRE isolates from Spectra VRE agar were identified by 16S rRNA gene sequencing using the MicroSeq 500 protocol (Applied Biosystems, Carlsbad, CA). Generated sequences were analyzed using the Basic Local Assignment Search Tool (BLAST), a search engine provided by the National Center for Biotechnology Information (NCBI).
Appropriately colored colonies growing on either medium, confirmed as VRE by sequencing and vancomycin MIC ≥ 32 mg/ml, served as the gold standard for this study. The combined sensitivity and specificity of Spectra VRE were 91.2% and 99.7% following 18 h of incubation, 98.2% and 99.3% at 24 h, and 99.1% and 96.5% at 48 h, respectively (Table 1). Two of the three false positives reported at site A were attributed to the growth of single light blue colonies that were both identified as vancomycin-sensitive E. faecalis. The remaining false-positive isolate produced multiple pink colonies and was identified as a Lactobacillus sp. Four of the seven false positives reported at site B produced pink colonies that were identified as Gram-positive bacilli. Two of the seven false positives produced blue colonies that were identified as Pediococcus spp. The remaining false-positive isolate, which produced blue-green colonies, was identified as Enterococcus durans. In addition, one false-negative result was reported from site B in which vancomycin-resistant E. faecium was recovered on BEAV at 24 h and demonstrated no growth on Spectra VRE after 48 h of incubation.
TABLE 1.
Analysis of Spectra VRE and BEAV for the detection of VRE from 399 fecal specimens at 18, 24, and 48 h (combined data)
| Incubation period (h) | Medium | No. ofa: |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | ||||||
| 18 | Spectra VRE | 103 | 1 | 285 | 10 | 91.2 | 99.7 | 99.0 | 96.6 |
| BEAV | 83 | 28 | 258 | 30 | 73.5 | 90.2 | 74.8 | 89.6 | |
| 24 | Spectra VRE | 111 | 2 | 284 | 2 | 98.2 | 99.3 | 98.2 | 99.3 |
| BEAV | 99 | 37 | 249 | 14 | 87.6 | 87.1 | 72.8 | 94.7 | |
| 48 | Spectra VRE | 112 | 10 | 276 | 1 | 99.1 | 96.5 | 91.8 | 99.6 |
| BEAV | 106 | 79 | 207 | 7 | 93.8 | 72.4 | 57.3 | 96.7 | |
True-positive results (TP) are defined as pink-, purple-, or blue-pigmented colonies on Spectra VRE or brown-black colonies on BEAV that were identified as VRE with supplemental testing and confirmed with the vancomycin Etest (≥32 μg/ml). FP, false-positive results; TN, true-negative results; FN, false-negative results.
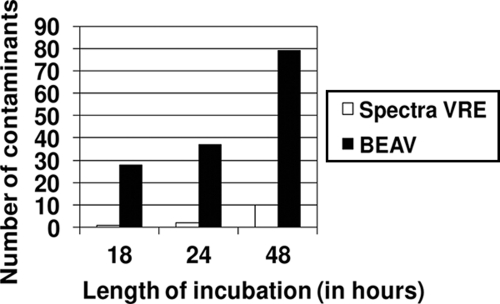
By comparison, the combined sensitivity and specificity of BEAV for the detection of VRE at 18, 24, and 48 h were 73.5% and 90.2%, 87.6% and 87.1%, and 93.8% and 72.4%, respectively (Table 1). The reduced specificity of BEAV agar stemmed from the growth of Lactobacillus spp., Lactococcus spp., Pediococcus spp., and Enterococcus spp. other than E. faecalis or E. faecium at both trial sites (Fig. 1). The combined positive predictive value (PPV) and negative predictive value (NPV) of both media are shown in Table 1.
FIG. 1.
Growth of contaminants on each medium at 18, 24, and 48 h. Contaminants are classified as potential false-positive isolates. Colonies demonstrating any hue of pink, purple, or blue were classified as potentially false positive on Spectra VRE; isolates with a brown-black hue were classified as potentially false positive on BEAV.
Colonies recovered from site A on Spectra VRE were differentiated based on pink, purple, dark, or light blue pigmentation. A total of 51 vancomycin-resistant E. faecium isolates were recovered on Spectra VRE. The sensitivity of Spectra VRE based on colony pigmentation alone at 18, 24, and 48 h was 94.1%, 98.0%, and 100.0%, respectively. For site B, the process for colony selection and identification was similar, but this group did not differentiate between dark and light blue colonies. A total of 61 vancomycin-resistant E. faecium isolates and 1 vancomycin-resistant E. faecalis isolate were recovered on Spectra VRE at site B. The sensitivity of Spectra VRE at site B (based on colony pigmentation) was 88.7% at 18 h and 98.4% at 24 and 48 h. Independent clinical trial site analyses of Spectra VRE and BEAV are shown in Table 2. Differences in sensitivity of BEAV at 18 and 24 h at sites A and B may have been due to reading technique or different circulating clones of VRE; regardless, data from both sites highlight the lack of sensitivity and specificity when using BEAV to screen for VRE.
TABLE 2.
Analysis of Spectra VRE and BEAV for the detection of VRE from fecal specimens at 18, 24, and 48 h (individual clinical trial site results)
| Clinical site and incubation period (h) | Medium | No. ofa: |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| TP | FP | TN | FN | ||||||
| Clinical site A | |||||||||
| 18 | Spectra VRE | 48 | 1 | 114 | 3 | 94.1 | 99.1 | 98.0 | 97.4 |
| BEAV | 30 | 11 | 104 | 21 | 58.8 | 90.4 | 73.2 | 83.2 | |
| 24 | Spectra VRE | 50 | 2 | 113 | 1 | 98.0 | 98.3 | 96.2 | 99.1 |
| BEAV | 40 | 14 | 101 | 11 | 78.4 | 87.8 | 74.1 | 90.2 | |
| 48 | Spectra VRE | 51 | 3 | 112 | 0 | 100.0 | 97.4 | 94.4 | 100.0 |
| BEAV | 45 | 31 | 84 | 6 | 88.2 | 73.0 | 59.2 | 93.3 | |
| Clinical site B | |||||||||
| 18 | Spectra VRE | 55 | 0 | 171 | 7 | 88.7 | 100.0 | 100.0 | 96.1 |
| BEAV | 53 | 17 | 154 | 9 | 85.5 | 90.1 | 75.7 | 94.5 | |
| 24 | Spectra VRE | 61 | 0 | 171 | 1 | 98.4 | 100.0 | 100.0 | 99.4 |
| BEAV | 59 | 23 | 148 | 3 | 95.2 | 86.5 | 72.0 | 98.0 | |
| 48 | Spectra VRE | 61 | 7 | 164 | 1 | 98.4 | 95.9 | 89.7 | 99.4 |
| BEAV | 61 | 48 | 123 | 1 | 98.4 | 71.9 | 56.0 | 99.2 | |
True-positive results (TP) are defined as pink-, purple-, or blue-pigmented colonies on Spectra VRE or brown-black colonies on BEAV that were identified as VRE with supplemental testing and confirmed with the vancomycin Etest (≥32 μg/ml). FP, false-positive results; TN, true-negative results; FN, false-negative results.
VRE isolates recovered on Spectra VRE were sequenced for identification. Two discrepancies were observed between the Vitek 2, biochemical testing, and sequence analyses. Several pink colonies recovered at 24 h of incubation were identified as E. faecium by the Vitek 2 and biochemical testing but were most closely related to E. faecalis by sequence analysis (99% identification). Similarly, several light blue colonies observed at 18 h of incubation were identified by the Vitek 2 and biochemical testing as E. faecalis but by sequencing as E. faecium (98% identification).
The rapid detection of VRE colonization is critical for proper infection control. Traditional culture methods that rely on colony morphology, biochemical characteristics, and susceptibility testing may take up to 5 days, thus prolonging effective patient management (8). Several chromogenic media have been developed to date, including chromID VRE (bioMérieux) (6) and CHROMagar VRE (BD, Baltimore, MD) (5), that detect VRE rapidly with high sensitivity and specificity. The data presented in this report demonstrate that Spectra VRE can also rapidly identify VRE with high sensitivity and specificity following 24 h of incubation, regardless of the ability to differentiate to species, when distinguishing VRE from common stool flora based on pink-, purple-, or blue-pigmented colonies.
Footnotes
Published ahead of print on 13 October 2010.
REFERENCES
- 1.Cetinkaya, Y., P. Falk, and G. C. Mayhall. 2000. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 13:686-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2009. Performance standards for antimicrobial susceptibility testing; 19th informational supplement M100-S19. NCCLS, Wayne, PA.
- 3.Harbarth, S. Cosgrove, and Y. Carmeli. 2002. Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 46:1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh, J. Y., W. G. Lee, and H. Y. Jin. 2006. Molecular characterization on vancomycin-resistant enterococci from clinical and surveillance specimens. Infect. Control Hosp. Epidemiol. 27:1076-1080. [DOI] [PubMed] [Google Scholar]
- 5.Kallstrom, G., C. D. Doern, and W. M. Dunne. 2010. Evaluation of a chromogenic agar under development to screen for VRE colonization. J. Clin. Microbiol. 48:999-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledeboer, N. A., K. Das, M. Eveland, C. Roger-Dalbert, S. Mailler, S. Chatellier, and W. M. Dunne. 2007. Evaluation of a novel chromogenic agar medium for isolation and differentiation of vancomycin-resistant Enterococcus faecium and Enterococcus faecalis isolates. J. Clin. Microbiol. 45:1556-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostrowsky, B. E., W. E. Trick, A. H. Sohn, S. B. Quirk, S. Holt, L. A. Carson, B. C. Hill, M. J. Arduino, M. J. Kuehnert, and W. R. Jarvis. 2001. Control of vancomycin-resistant enterococcus in health care facilities in a region. N. Engl. J. Med. 344:1427-1433. [DOI] [PubMed] [Google Scholar]
- 8.Palladino, S., I. D. Kay, J. P. Flexman, I. Boehm, A. G. Costa, E. J. Lambert, and K. J. Christiansen. 2003. Rapid detection of vanA and vanB genes directly from clinical specimens and enrichment broths by real-time multiplex PCR assay. J. Clin. Microbiol. 41:2483-2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trick, W. E., S. M. Paule, S. Cunningham, R. L. Cordell, M. Lankford, V. Stosor, S. L. Solomon, and L. R. Peterson. 2004. Detection of vancomycin-resistant enterococci before and after antimicrobial therapy: use of conventional culture and polymerase chain reaction. Clin. Infect. Dis. 38:780-786. [DOI] [PubMed] [Google Scholar]