Abstract
We describe a point-of-care immunochromatographic test for the simultaneous detection of both nontreponemal and treponemal antibodies in the sera of patients with syphilis that acts as both a screening and a confirmatory test. A total of 1,601 banked serum samples were examined by the dual test, and the results were compared to those obtained using a quantitative rapid plasma reagin (RPR) test and the Treponema pallidum passive particle agglutination (TP-PA) assay. Compared to the RPR test, the reactive concordance of the dual test nontreponemal line was 98.4% when the RPR titers of sera were ≥1:2 and the nonreactive concordance was 98.6%. Compared to the TP-PA assay, the reactive and nonreactive concordances of the treponemal line were 96.5% and 95.5%, respectively. These results indicate that the dual test could be used for the serological diagnosis of syphilis in primary health care clinics or resource-poor settings and therefore improve rates of treatment where patients may fail to return for their laboratory results.
Syphilis is a sexually transmitted infection caused by the spirochete bacterium Treponema pallidum. The World Health Organization estimates that more than 12 million new cases of adult syphilis occur worldwide each year and that the disease can be transmitted congenitally and affects 500,000 or more infants annually (11). The diagnosis of syphilis is based on clinical manifestations or on serological testing. The serological diagnosis of syphilis requires the detection of two distinct antibodies, heterophile or nontreponemal antibodies (reagin) directed against lipoidal material released from damaged host cells and from the treponemes themselves and antibodies directed against T. pallidum antigens. The nontreponemal antibodies are indicators of active infection since a significant reduction in titer can be used to suggest success of therapy, while a significant increase can indicate a possible relapse or reinfection. The nontreponemal antigen is a mixture of cardiolipin, lecithin, and cholesterol, which are the components of the Venereal Disease Research Laboratory (VDRL) (5) and rapid plasma reagin (RPR) tests for syphilis (8). The treponemal antibodies are directed against specific surface antigens of T. pallidum or recombinants such as the 15-, 17-, and 47-kDa proteins. Treponemal tests such as the T. pallidum passive particle agglutination (TP-PA) assay, the fluorescent treponemal antibody absorption (FTA-ABS) (6) test, and enzyme immunoassays have been used as confirmatory tests (3, 7, 9). The only commercially available point-of-care (POC) serological tests for syphilis detect treponemal antibodies (4, 10, 13, 16). These tests do not indicate active infection that requires treatment, since they measure lifetime exposure to syphilis. One thousand six hundred one serum samples originally submitted to the Georgia Public Health Laboratory in Atlanta for serological testing for syphilis were obtained for this study. All identifiers were removed prior to shipment to the CDC, and the samples were numbered sequentially. The patterns of reactivity were determined at the CDC by using the quantitative RPR test (Becton Dickinson, Sparks, MD) and the TP-PA assay (Fujirebio, Tokyo, Japan). In addition, a panel of 105 clinical serum samples from patients with known stages of syphilis were included. Of these, 7 had primary untreated, 14 had primary treated, 6 had secondary untreated, 28 had secondary treated, 5 had latent untreated, and 45 had latent treated disease. In addition, 14 sera exhibited an RPR-positive, TP-PA-negative pattern and were classified as biologically false positive, and 179 sera obtained from patients with diseases other than syphilis were also tested.
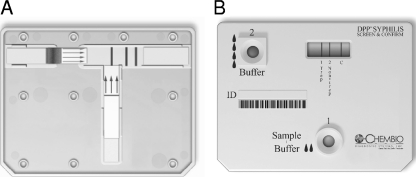
The immunochromatographic device used in these studies was manufactured by Chembio Diagnostics Systems Inc., Medford, NY. The device is based on the principle of a dual-path platform (DPP) comprising a plastic cassette (5 by 7 cm) containing two nitrocellulose membrane strips perpendicular to each other in a T formation (Fig. 1). This allows independent delivery of the test sample and the detecting conjugate reagent. One strip receives the sample and running buffer via well 1. The diluted sample migrates toward the second strip, on which are striped two test lines and a control line (C). The recombinant T. pallidum antigen (T1) and synthetic nontreponemal antigen (A. R. Castro, U.S. patent application 60/693,120) (T2) are bound to the membrane's solid phase. The third line serves as a procedural control. After 5 min, additional buffer is added to the second strip via well 2, which hydrates colloidal gold particles conjugated to protein A and anti-human IgM antibody. The conjugate migrates along the second strip to the test area. If antibodies to treponemal and nontreponemal antigens are present in the serum sample, they will form visible red/magenta-colored lines within 15 min.
FIG. 1.
Structure of the Chembio dual POC test for syphilis showing the locations of the antigen lines. A, dissected view following testing of reactive serum; B, complete cassette following testing of reactive serum.
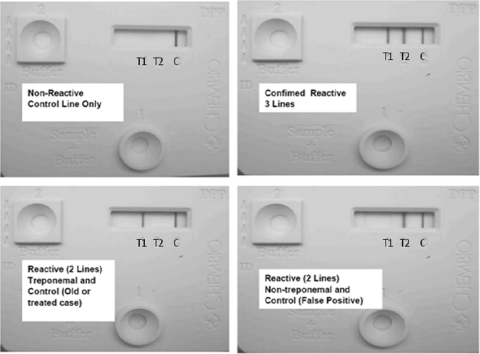
Confirmed reactivity in the dual POC test was characterized by the appearance of three red/magenta lines in the window of the device, namely, a treponemal line (T1), a nontreponemal line (T2), and a control line (C). A visible treponemal line (T1) and control line (C) with no visible nontreponemal line (T2) was interpreted as probably due to an old or previously treated case of syphilis. A visible nontreponemal line (T2) and a control line (C) with no visible treponemal line (T1) were interpreted as a false-reactive nontreponemal test. A nonreactive result was demonstrated by the appearance of only one red/magenta control line (C) (Fig. 2). This test is designed to be used for serum, plasma, or whole blood. The device is stable at room temperature for 1 year.
FIG. 2.
Dual POC syphilis tests showing patterns of reactivity and their interpretations.
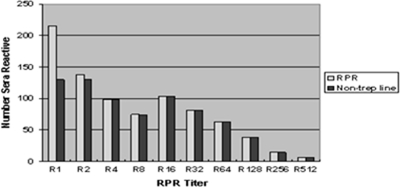
Of the 1,601serum samples tested, 834 were reactive in both the RPR and TP-PA assays, while 173 were reactive in the TP-PA assay alone. The remaining 594 sera were nonreactive in both the treponemal and nontreponemal reference tests. A comparison of the results obtained in the dual POC test and those of the RPR test gave a reactive concordance of 98.4% when the RPR titers of sera were ≥1:2 and 98.6% for the nontreponemal line of the dual test. Compared to the TP-PA assay, the reactive and nonreactive concordances of the treponemal line were 96.5% and 95.5%, respectively (Tables 1 and 2). The 834 serum samples reactive in both the RPR test and the TP-PA assay were tested quantitatively using the RPR test and the distribution of RPR titers, and rates of reactivity with the nontreponemal line of the dual POC test are shown in Fig. 3. The results of testing 105 sera from patients with known stages of syphilis by the dual POC and comparator tests are shown in (Table 3). Complete concordance between the dual rapid test and the comparator tests was obtained in all cases of primary and untreated secondary disease. The dual POC test detected nontreponemal antibody in all cases of untreated disease but was unable to detect this antibody in one case of treated secondary disease and six cases of treated latent disease where the RPR reactivity could only be detected in undiluted serum or when diluted 1:2. The dual POC test was also unable to detect treponemal antibody in three cases of treated and one case of untreated latent disease. One hundred seventy-nine serum samples obtained from patients with diseases other than syphilis were also tested using the RPR test, the TP-PA assay, and the dual POC test. One sample, from a patient with pinta (a nonvenereal treponematosis), was found to be reactive in both lines of the DPP POC test, as well as the comparator RPR and TP-PA assays. One serum sample from a patient with malaria was reactive in the RPR test but nonreactive with the nontreponemal line in the dual POC test. However, four serum samples from malaria patients were reactive with the nontreponemal line in the dual POC test but nonreactive in the RPR test. Since these same samples also proved nonreactive with the treponemal line in the POC test and in the TP-PA assay, these patients would not have received treatment for syphilis, having been classified as biologically false positive. Similarly, 6 of 22 sera from patients with leprosy were found to be reactive with the nontreponemal line of the dual POC test but nonreactive in the RPR test. All of these sera were nonreactive in all of the confirmatory treponemal tests (Table 4).
TABLE 1.
Performance characteristics of the dual POC syphilis test (nontreponemal line) compared to that of the RPR test
| Dual test nontreponemal line result | No. (%) of samples tested with RPR test |
||
|---|---|---|---|
| Reactive | Nonreactive | Total | |
| Reactive | 739 (46.2) | 11 (0.7) | 750 (46.8) |
| Nonreactive | 95a (5.9) | 756 (47.2) | 851 (53.2) |
| Total | 834 (52.1) | 767 (47.9) | 1,601 (100.0) |
Of the 95 samples found nonreactive with the dual POC test, 85 had an RPR titer of 1:1. With an RPR titer of ≥1:2, the concordance with the POC test was 98.4% and the concordance with the nonreactive RPR test was 98.6%.
TABLE 2.
Performance characteristics of the dual POC syphilis test (treponemal line) compared to those of the comparator TP-PA test
| Dual test treponemal line | No. (%) of samples tested with TP-PA assaya |
||
|---|---|---|---|
| Reactive | Nonreactive | Total | |
| Reactive | 972 (60.7) | 27 (1.7) | 999 (62.4) |
| Nonreactive | 35 (2.2) | 567 (35.4) | 602 (37.6) |
| Total | 1,007 (62.9) | 594 (37.1) | 1,601 (100.0) |
With the TP-PA assay, the reactive and nonreactive concordances of the POC test were 96.5 and 95.5%, respectively.
FIG. 3.
Distribution of RPR titers among RPR-positive, TP-PA-positive sera obtained from the Georgia Public Health Laboratories and rates of seroreactivity of the nontreponemal line in the dual POC test.
TABLE 3.
Documented cases of syphilis tested with the dual POC test and the comparator RPR and TP-PA tests
| Syphilis category | Total no. of samples | Dual rapid teste |
Comparatore |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Trepa |
Trepb |
RPR test |
TP-PA assay |
||||||
| Rc | NRd | R | NR | R | NR | R | NR | ||
| Primary | |||||||||
| Untreated | 7 | 7 | 0 (100) | 7 | 0 (100) | 7 | 0 (100) | 7 | 0 (100) |
| Treated | 14 | 9 | 5 (62.8) | 13 | 1 (92.8) | 9 | 5 (62.8) | 13 | 1 (92.8) |
| Secondary | |||||||||
| Untreated | 6 | 6 | 0 (100) | 6 | 0 (100) | 6 | 0 (100) | 6 | 0 (100) |
| Treated | 28 | 22 | 6 (78.5) | 28 | 0 (100) | 26 | 2 (92.8) | 28 | 0 (100) |
| Latent | |||||||||
| Untreated | 5 | 3 | 2 (60) | 4 | 1 (80) | 3 | 2 (60) | 5 | 0 (100) |
| Treated | 45 | 35 | 10 (77.7) | 44 | 1 (97.7) | 41 | 4 (91.1) | 45 | 0 (100) |
| Total | 105 | 82 | 23 | 102 | 3 | 92 | 13 | 104 | 1 |
Non-Trep, nontreponemal line.
Trep, treponemal line.
R, reactive.
NR, nonreactive.
Shown is the number of serum samples reactive (% concordance).
TABLE 4.
Serum samples of patients with diseases other than syphilis tested with the dual POC test and the comparator RPR and TP-PA tests
| Sample category | Total no. of samples | Dual rapid test |
Comparator |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Trepa |
Trepb |
Non-Trep |
Trep |
||||||
| Rc | NRd | R | NR | R | NR | R | NR | ||
| Pinta | 1 | 1 | 0 (100)e | 1 | 0 (100) | 1 | 0 (100) | 1 | 0 (100) |
| Lyme disease | 10 | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) |
| Hepatitis A | 10 | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) |
| Hepatitis B | 10 | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) |
| Hepatitis C | 10 | 1 | 9 (90) | 1 | 9 (90) | 1 | 9 (90) | 1 | 9 (90) |
| HTLV I/II | 10 | 0 | 10 (100) | 2 | 8 (80) | 0 | 10 (100) | 2 | 8 (80) |
| HIV | 10 | 0 | 10 (100) | 1 | 9 (90) | 0 | 10 (100) | 1 | 9 (90) |
| Hypergammaglobulinemia | 10 | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) |
| Rheumatoid factor | 10 | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) |
| Malaria | 20 | 4 | 16 (80) | 0 | 20 (100) | 1 | 19 (95) | 0 | 20 (100) |
| Leprosy | 22 | 6 | 16 (72.7) | 0 | 22 (100) | 0 | 22 (100) | 0 | 22 (100) |
| Mumps | 1 | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) |
| Toxoplasmosis | 1 | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) |
| Rubeola | 1 | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) | 0 | 1 (100) |
| Mononucleosis | 11 | 0 | 11 (100) | 1 | 10 (90) | 0 | 11 (100) | 1 | 10 (90.9) |
| Varicella-zoster virus infection | 10 | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) | 0 | 10 (100) |
| Herpes simplex virus infection | 21 | 0 | 21 (100) | 2 | 19 (90.4) | 0 | 21 (100) | 2 | 19 (90.4) |
| Cytomegalovirus infection | 11 | 0 | 11 (100) | 0 | 11 (100) | 0 | 11 (100) | 0 | 11 (100) |
| Total | 179 | 12 | 157 | 8 | 171 | 3 | 176 | 8 | 171 |
Non-Trep, nontreponemal line.
Trep, treponemal line.
R, Reactive.
NR, nonreactive.
Shown is the number of serum samples reactive (% concordance).
Of 14 RPR-positive, TP-PA-negative (classified as biologically false positive) sera, 7 were found to be reactive with the nontreponemal line of the dual POC test. All were nonreactive with the treponemal line.
Serological testing remains the method most frequently used to establish a diagnosis of syphilis, both when there are clinical indications of the disease and when screening for asymptomatic infection. Routine serological screening of women during pregnancy and treatment of those found to be positive is also recognized as a cost-effective intervention to prevent congenital infection (15). Serological testing for syphilis is performed in laboratory settings, requiring appropriate equipment and trained personnel to interpret results that may not be available for several days after the sample has been collected. In developing countries, treatment is often not provided owing to the failure of patients to return for results of their laboratory tests. In these cases, not only does the patient remain untreated, but there are opportunities for further spread of disease. However, the results of the POC test for syphilis could aid clinicians in making an immediate judgment on the necessity for treatment and thereby greatly increase the effectiveness of syphilis control efforts. At present, the only commercially available POC tests for syphilis detect antibodies directed against treponemal antigens. While treponemal POC tests have been shown to be useful tools to screen for individuals who have been exposed to syphilis, their use as the sole diagnostic test inevitably results in significant rates of overtreatment (1, 2). Ongoing use of these tests, particularly in high-prevalence settings, although cost-effective (12, 14), would also result in repeated treatment and counseling of individuals who are no longer infected. In contrast, the use of the dual POC test described here would result in the ability to both screen and confirm the serological status of patients within 15 min and give a better indication of active disease. It would then permit the clinician to initiate treatment and partner notification activities at the consultation site. The dual POC test exhibits remarkable performance characteristics compared to the standard laboratory-based testing algorithm. When sera have RPR titers of ≥1:8, the dual POC test has a sensitivity of 99.7%.
The dual POC test is designed for use with serum, plasma, and whole blood. While this evaluation was conducted with archived serum samples, the test's utility will be dependent upon its performance at nonlaboratory sites using finger stick blood specimens. Clinical trials are currently being planned in both industrialized and developing country settings. The results of the dual POC test were read visually. In order to overcome the subjectivity associated with visual reading, the manufacturer has developed a reader that measures the density of the test lines. The reader can indicate either a positive or a negative result or provide a numerical readout of the line densities. Preliminary data indicate that the reader appears to be slightly more sensitive than visual reading in detecting very low titers, thus increasing the overall sensitivity of the assay. In addition, these data also indicate that the density of the nontreponemal line correlates with RPR titers and that the system could be the basis of a new quantitative nontreponemal assay.
Acknowledgments
We thank the Georgia Department of Health Laboratories for supplying serological specimens for this study.
Footnotes
Published ahead of print on 29 September 2010.
REFERENCES
- 1.Blandford, J. M., T. L. Gift, S. Vasaikar, D. Mwesigwa-Kayongo, P. Dlali, and R. N. Bronzan. 2007. Cost-effectiveness of on-site antenatal screening to prevent congenital syphilis in rural eastern Cape Province, Republic of South Africa. Sex. Transm. Dis. 34(7 Suppl.):S61-S66. [DOI] [PubMed] [Google Scholar]
- 2.Bronzan, R. N., D. C. Mwesigwa-Kayongo, D. Narkunas, G. P. Schmid, G. A. Neilsen, R. C. Ballard, P. Karuhije, J. Ddamba, E. Nombekela, G. Hoyi, P. Dlali, N. Makwedini, H. G. Fehler, J. M. Blandford, and C. Ryan. 2007. On-site rapid antenatal syphilis screening with an immunochromatographic strip improves case detection and treatment in rural South African clinics. Sex. Transm. Dis. 34(7 Suppl.):S55-S60. [DOI] [PubMed] [Google Scholar]
- 3.Deacon, W. E., and E. F. Hunter. 1962. Treponemal antigens as related to identification of syphilis serology. Proc. Soc. Exp. Biol. Med. 110:352-356. [DOI] [PubMed] [Google Scholar]
- 4.Goel, N., M. Sharma, N. Gupta, and R. Sehgal. 2005. Rapid immunochromatographic test for syphilis. Indian J. Med. Microbiol. 23:142-143. [DOI] [PubMed] [Google Scholar]
- 5.Harris, A., A. A. Rosenberg, and E. R. del Vecchio. 1948. The VDRL slide flocculation test for syphilis; a supplementary report. J. Vener. Dis. Inf. 29:72-75. [PubMed] [Google Scholar]
- 6.Hunter, E. F., W. E. Deacon, and P. E. Meyer. 1964. An improved FTA test for syphilis: the absorption procedure (FTA-ABS). Public Health Rep. 79:410-412. [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter, E. F., R. M. McKenney, S. E. Madison, and D. D. Cruce. 1979. Double-staining procedure for the fluorescent treponemal antibody absorption (FTA-ABS) test. Br. J. Vener. Dis. 55:105-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen, S. A., V. Pope, and R. E. Johnson. 1998. Manual of test for syphilis. 9th edition. American Public Health Association, Washington, DC.
- 9.Larsen, S. A., B. M. Steiner, and A. H. Rudolph. 1995. Laboratory diagnosis and interpretation of tests for syphilis. Clin. Microbiol. Rev. 8:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nessa, K., A. Alam, F. A. Chawdhury, M. Huk, S. Nahar, G. Salauddin, S. Khursheed, S. Rahman, E. Gurley, R. F. Breiman, and M. Rahman. 2008. Field evaluation of simple rapid tests in the diagnosis of syphilis. Int. J. STD AIDS 19:316-320. [DOI] [PubMed] [Google Scholar]
- 11.Peeling, R. W., and H. Ye. 2004. Diagnostic tools for preventing and managing maternal and congenital syphilis: an overview. Bull. World Health Organ. 82:439-446. [PMC free article] [PubMed] [Google Scholar]
- 12.Terris-Prestholt, F., D. Watson-Jones, K. Mugeye, L. Kumaranayake, L. Ndeki, H. Weiss, J. Changalucha, J. Todd, F. Lisekie, B. Gumodoka, D. Mabey, and R. Hayes. 2003. Is antenatal syphilis screening still cost effective in sub-Saharan Africa. Sex. Transm. Infect. 79:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Dommelen, L., A. Smismans, V. J. Goossens, J. Damoiseaux, C. A. Bruggeman, F. H. Van Tiel, and C. J. Hoebe. 2008. Evaluation of a rapid one-step immunochromatographic test and two immunoenzymatic assays for the detection of anti-Treponema pallidum antibodies. Sex. Transm. Infect. 84:292-296. [DOI] [PubMed] [Google Scholar]
- 14.Vickerman, P., R. W. Peeling, F. Terris-Prestholt, J. Changalucha, D. Mabey, D. Watson-Jones, and C. Watts. 2006. Modelling the cost-effectiveness of introducing rapid syphilis tests into an antenatal syphilis screening programme in Mwanza, Tanzania. Sex. Transm. Infect. 82(Suppl. 5):v38-v43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson-Jones, D., J. Changalucha, B. Gumodoka, H. Weiss, M. Rusizoka, L. Ndeki, A. Whitehouse, R. Balira, J. Todd, D. Ngeleja, D. Ross, A. Buvé, R. Hayes, and D. Mabey. 2002. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. J. Infect. Dis. 186:940-947. [DOI] [PubMed] [Google Scholar]
- 16.Zarakolu, P., I. Buchanan, M. Tam, K. Smith, and E. W. Hook. 2002. Preliminary evaluation of an immunochromatographic strip test for specific Treponema pallidum antibodies. J. Clin. Microbiol. 40:3064-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]