Abstract
Female mammals have long been neglected in biomedical research. The NIH mandated enrollment of women in human clinical trials in 1993, but no similar initiatives exist to foster research on female animals. We reviewed sex bias in research on mammals in 10 biological fields for 2009 and their historical precedents. Male bias was evident in 8 disciplines and most prominent in neuroscience, with single-sex studies of male animals outnumbering those of females 5.5 to 1. In the past half-century, male bias in non-human studies has increased while declining in human studies. Studies of both sexes frequently fail to analyze results by sex. Underrepresentation of females in animal models of disease is also commonplace, and our understanding of female biology is compromised by these deficiencies. The majority of articles in several journals are conducted on rats and mice to the exclusion of other useful animal models. The belief that non-human female mammals are intrinsically more variable than males and too troublesome for routine inclusion in research protocols is without foundation. We recommend that when only one sex is studied, this should be indicated in article titles, and that funding agencies favor proposals that investigate both sexes and analyze data by sex.
Keywords: sex bias, animal models, nervous system diseases, estrous cycles, research strategies
1. Introduction
Women and non-human female mammals have been given short shrift in biomedical research, often on the assumption that results from males apply to females, or because of concern that hormonal cycles decrease the homogeneity of study populations and confound effects of experimental manipulations (Wizemann and Pardue, 2001). Some consider males representative of the human species and differences from the male norm as atypical or abnormal; others seek to protect women from adverse effects of drugs (Marts and Keitt, 2004), and generalize findings on males to females without justification.
Epidemiological and clinical studies of men often generate different results from those for women, exemplified by sex differences in response to many drugs (Soldin and Mattison, 2009), in cardiovascular disease (Barrett-Connor, 1997, Berger et al., 2009) and in autoimmune dysfunction (Fish, 2008; Lockshin, 2006; Whitacre, 2001). Widespread prevalence of sex differences in human disease and neglect of women in biological research negatively impacts the health of women (Correa-de-Araujo, 2006). Understanding of these consequences eventually led to the US National Institute of Health Revitalization Act of 1993, which required enrollment of female participants in federally supported phase III clinical trials. A European Union program was designed to provide basic researchers with practical tools and best practice examples regarding sex and gender differences in study design (Klinge, 2008). These interventions contributed to increased inclusion of women in clinical research in the USA, paralleled by similar changes in the European Union and Australia (Wetherington, 2007; Rogers and Ballantyne, 2008; Klinge, 2008; Soldin and Mattison, 2009), but most studies that enrolled both sexes did not provide sex-specific analysis (Hayes and Redberg, 2008). No funding agency stipulations presently require enrollment of females in non-human animal research, nor has there been movement to conduct basic science studies on both sexes (Sandberg and Ji, 2008).
A substantial portion of the NIH budget supports research on animal models, an enterprise that facilitated development of treatments to alleviate depredations of aging, autoimmune diseases, cancer, behavioral dysfunctions, cardiovascular disease, diabetes and other human afflictions (Council report, 1989). What remains in question is whether present-day research on animal models is sufficiently attentive to female subjects, and whether appropriate species are investigated. Previous analyses of a few journals and disciplines revealed a strong male sex bias (e.g., Berkley, 1992; Blanchard et al., 1995; Sechzer et al., 1994).
2. Literature Survey
To gain a contemporary view of sex bias in the human and animal literatures, we systematically sampled journal articles from 2009 across a broad range of disciplines, including several that have not been canvassed for decades, and others never previously surveyed. We also tracked changes in sex bias and species utilization in 5 journals for six or more decades, beginning in 1909, for an historical perspective. Reports were categorized with respect to species studied, sex of subjects, whether both males and females were sampled, and if so whether outcomes were reported by sex or included sex as a covariate in modeling (cf Geller et al., 2006).
2.1 Inclusion criteria
10 major biological disciplines were selected and matched to corresponding subject categories in the ISI Web of Knowledge (Thompson Scientific) to obtain journal lists. Topics surveyed included Pharmacology (Journal Citation Reports® subject category “pharmacology and pharmacy”), Endocrinology (“endocrinology and metabolism”), Animal Behavior (“behavioral sciences”), Behavioral physiology (also “behavioral sciences”), Neuroscience (“neurosciences”), General biology (“biology”), Zoology (“zoology”), Physiology (“physiology”), Reproduction (“reproductive biology”) and Immunology (“immunology”). Within each division, except for reproduction, four journals were chosen from among those with high impact factors, that were not already included in another category, and were representative of the diversity of subtopics (Table 1).
Table 1.
Matrix of journals surveyed by subject area for 2009. Additional 2009 data were gathered for the clinical journals JCEM and JCI.
| Discipline | Journal A | Journal B | Journal C | Journal D |
|---|---|---|---|---|
| General Biology | PLoS Biology | Proceedings of the Royal Society B: Biological Sciences | Nature | Science |
| Immunology | Journal of Immunology | Infection and Immunity | Immunity | Vaccine |
| Neuroscience | Journal of Neuroscience | Neuroscience | The Journal of Comparative Neurology | Nature Neuroscience |
| Physiology | Journal of Physiology (London) | American Journal of Physiology – Renal Physiology | American Journal of Physiology – Gastrointestinal and Liver Physiology | American Journal of Physiology – Heart and Circulatory Physiology |
| Pharmacology | Neuropsycho- pharmacology | Journal of Psychopharma- cology | The Journal of Pharmacology and Experimental Therapeutics | British Journal of Pharmacology |
| Reproduction | Biology of Reproduction | Reproduction | ||
| Endocrinology | European Journal of Endocrinology | Journal of Neuroendo- crinology | Endocrinology | American Journal of Physiology – Endocrinology and Metabolism |
| Behavioral Physiology | Journal of Comparative Psychology | Behavioral Neuroscience | Physiology & Behavior | Hormones and Behavior |
| Behavior | Behavioral Ecology and Sociobiology | Animal Behaviour | Animal Cognition | Behavioral Ecology |
| Zoology | Physiological and Biochemical Zoology | Journal of Comparative Physiology A | Journal of Experimental Zoology | Journal of Mammalogy |
For reproduction, two journals were sampled more extensively. For each journal, we surveyed the first 20 primary research articles in 2009 that reported organismal mammalian work. For two reproductive biology journals and one endocrinology journal, we surveyed 100 articles for 2009. Reviews, brief communications, and reports involving fetal organisms, or those restricted to cell lines were excluded. When journals were arranged by subtopics, articles meeting our inclusion criteria were sampled evenly across the several topics. In journals such as Nature and Science, only biological sciences articles were considered.
For the historical survey, 20 articles were sampled from the first issue(s) from each available decade from 1909 for five journals. Journal of Pharmacology and Experimental Therapeutics (JPET) and the Journal of Physiology (Lond) (J Physiol) were surveyed from 1909–2009; Endocrinology was tracked from 1939–2009. The Journal of Clinical Endocrinology and Metabolism (JCEM) and the Journal of Clinical Investigation (JCI) were surveyed from 1949–2009.
2.2 Coding
Articles were coded for species and sex (i.e., whether the report listed subjects as female, male, both sexes, or left sex unspecified). When different parts of a study utilized different sexes, or specification was incomplete, assessments were biased in favor of inclusivity and rated as “both sexes.” Field studies were categorized as investigating both sexes when this was explicitly stated or could be inferred with confidence. Whether data were analyzed by sex was noted in studies employing both sexes.
Species categories were: human, non-human primate, rat, mouse, guinea pig, hamster, rabbit, cat, dog, cow, sheep, pig; species with low representation were combined in an “other” category.
Additional analyses were conducted in Pub Med and Web of Science with keywords to probe sex distribution in animal models for diseases with central nervous system involvement such as depression, multiple sclerosis, and obesity, each known to be more prevalent or severe in women than men.
3. Contemporary analysis
3.1 Subject sex across fields: animal studies
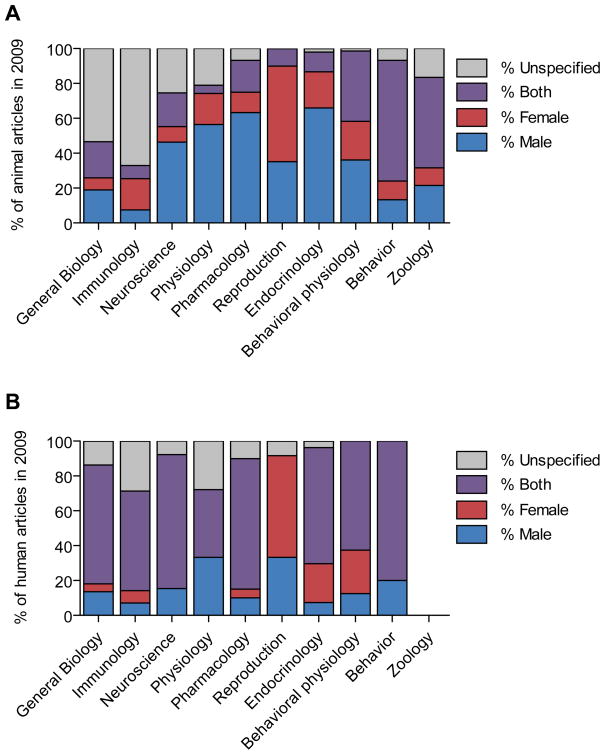
The 2009 survey of research on non-human mammals revealed a male bias in 8 of the 10 fields surveyed, including neuroscience, physiology, pharmacology, endocrinology, zoology and, to a lesser extent, behavioral physiology and behavior (Figure 1A). The ratio of articles reporting on only males versus only females was most skewed in neuroscience (5.5:1), pharmacology (5:1) and physiology (3.7:1). The weakest male biases were in behavior (1.4:1) and behavioral physiology (1.5:1). A female skew was present in reproduction, where females featured 1.6 times more often than males, and in immunology, with females represented 2.2 times more frequently than males among the minority of studies that specified subject sex. Subject sex was omitted in 22–42% of articles in neuroscience, physiology, and interdisciplinary biology journals, and in more than 60% of immunology reports, while fewer than 8% of articles in the behavior, endocrinology, and pharmacology categories failed to specify sex of experimental animals or tissues (Figure 1A).
Figure 1.
Distribution of studies by sex and field in 2009. (A) Percent of articles describing non-human animal research that used male subjects, female subjects, both male and female subjects, or did not specify the sex of the subjects. (B) Percent of articles describing human research in the same categories. The zoology category was excluded because of insufficient use of human subjects in this field to form an accurate estimate.
3.2 Subject sex across fields: human studies
In the same journals, male bias in articles involving humans was evident in fewer fields (interdisciplinary biology, neuroscience, physiology, pharmacology, and behavior) and female bias emerged in others (reproduction, endocrinology, and behavioral physiology; Figure 1B). A higher percentage of articles reported on both sexes in human than non-human animal research (60 versus 26%).
The majority of animal behavior and zoological articles incorporated both males and females (70% and 51%, respectively) and data were analyzed by sex in 75% and 56% of those reports, respectively (Figure 2). Only 12.5% of physiology articles reported data for both sexes, and analysis by sex was performed in only 30% of those cases. Comparably low numbers characterized the neuroscience, immunology, and pharmacology categories (Figure 2).
Figure 2.
Percent of articles in which some portion of the results was analyzed by sex. Data are presented by discipline for articles that utilized both sexes.
3.3 Species use
In 2009, over 85% of articles in neuroscience, pharmacology, immunology and physiology described research on rodents; rats and mice featured in 94% of articles in the field of endocrinology. Much greater species diversity and non-rodent models characterized studies in the behavior, zoology and reproduction categories (Figure 3).
Figure 3.
Species use in animal studies by subject area in 2009. Six fields (general biology, immunology, neuroscience, physiology, pharmacology, and endocrinology) relied on rodents in 80% or more of animal studies.
4. Historical analysis
4.1 By sex: animal studies
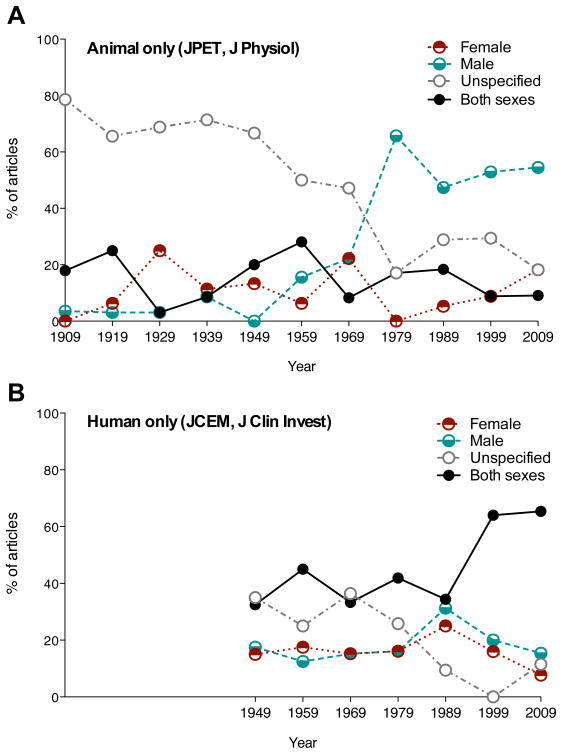
79% of articles dealing with non-human mammals in the early 20th century failed to report subject sex in the Journal of Physiology (London) and the Journal of Pharmacology and Experimental Therapeutics (Figure 4A). The percentage of articles reporting subject sex increased steadily through 1969, without substantially affecting the relative abundance of male and female single-sex studies. A marked increase in male-only reports after 1969 stabilized at around 50% (Figure 4A). Less than one third of articles included both sexes; averaged over the 100-year interval, studies that enrolled both females and males remained relatively stable at 15%. Of such articles, 34% analyzed data separately by sex.
Figure 4.
Historical change in study sex distribution in animal and human literatures. (A) Combined data from two journals publishing primarily non-human animal research: JPET and J Physiol.. Human studies were excluded from consideration for this graph. (B) Combined data from two clinical journals: JCEM and J Clin Invest. JCEM debuted in 1941. Animal studies were excluded from consideration for this graph. In the both animal and human literatures the number of studies in which sex is not specified has declined, but remains close to 20% in the animal literature. In the human literature there has been an increase in percent of studies of both sexes, not echoed in non-human animal research. Animal studies restricted to males alone have become more common in recent years.
4.2 By sex: human studies
In its early years, virtually all reports in Journal of Clinical Investigation (JCI) and Journal of Clinical Endocrinology and Metabolism (JCEM) were restricted to human research, and approximately equal numbers of male-only and female-only articles were published across the entire time span (between 8–31% for each sex, Figure 4B). Reports on both sexes remained stable at 32–45% from 1949–1989, with a marked increase to >60% in 1999 and 2009 (Figure 4B). Articles with sex unspecified exceeded 20% from 1949–1979, but declined to a mean of 7% between 1989–2009.
In JCI the percent of human studies declined from 100% in 1949 to ~60% of the total number published between 1969 and 1989, with a further decrease to 24 and 26% in 1999 and 2009%. This reflects a transition to mouse models (64% of reports published in 1999 and 2009), which exhibited a 2:1 male to female ratio and 25% incidence of sex-unspecified reports.
4.3 By species
Fewer than 10% of animal studies in J Physiol and JPET in the first two decades of the 20th century employed rats and mice; a sustained increase over the next 90 years culminated in 88% of articles investigating rats and mice in 2009 (Figure S1). Between 1909 and 1939 cat and dog use held steady at ~50%, and “other” species comprised about a third of those investigated. The major changes in species utilization occurred between 1969 and 2009, largely owing to increased mouse use.
5. Present state and implications of sex bias
Single sex studies of males still predominate in the biological literature, and neglect of females is widespread in many disciplines, including neuroscience, pharmacology, endocrinology, zoology, and physiology. One cannot assume that beyond the reproductive system, sex differences either do not exist or are irrelevant; despite this, a high proportion of studies failed to specify sex, and in experiments performed on both sexes data often were not analyzed by sex. Reporting of the sex of tissues or cell lines is even more rare (Marts and Keitt, 2004), notwithstanding the fact that every mammalian cell has a sexual signature and basic cell chemistry and organ structure may differ between females and males.
Sexual dimorphism has been documented in the abundance of more than 23,000 mouse transcripts of active genes, ranging from 14% in the CNS to 70% in the liver, with intermediate values in adipose tissue and muscle (Yang et al, 2006; Isensee et al., 2007); many of these genes are implicated in common diseases in which susceptibility is sex-biased. A substantial portion of dimorphic gene expression may be under epigenetic control (Gabory et al., 2009). Some sex differences occur only in certain environments, at specific ages or stages of the reproductive cycle (Becker et al., 2005). Studies limited to a single sex cannot yield a complete understanding of underlying mechanisms (Pessin and Marts, 2005).
Sex differences in immune-mediated diseases are well documented; women are affected far more often than men and exhibit more robust cell-mediated and humoral responses to antigenic challenges (Fish, 2008). Gonadal steroids mediate a number of these sex differences. Remarkably, contemporary non-human animal research in immunology reveals a striking failure in most studies to indicate subject sex (Figure. 2).
A partial list of sexually dimorphic rodent behavioral traits included wheel running behavior, open field activity, aggression, taste preferences, food intake, performance on learning tasks, and responses to brain damage (Goy and McEwen, 1980); this by no means is the complete inventory. In learning and memory studies, females generally outperform males in classical conditioning and most operant tasks, but in other tests males outperform females. Sex differences in learning are associated with differences in neuroanatomy (Dalla and Shors, 2009) detectable at all levels of analysis; Andreano and Cahill (2009) conclude “it is incumbent on investigators who don’t attend to the possibility of sex influences to justify this neglect.”
A trait that does not differ between the sexes nevertheless may be subserved by different mechanisms in females and males (Cahill, 2006). Many results based on a single sex generalize to the other sex (Sechzer, et al., 1994), but without investigation, which findings generalize remains undetermined. The idea that one “can evaluate one sex and learn equally about the other is no longer an option” (Cahill, 2005). Without sex analysis, one risks claiming a general effect where it only applies to one sex, or that no effect exists when there are opposite offsetting effects in the two sexes (Wetherington, 2007).
Sex differences in trait variance?
The tradition of performing behavioral experiments on male rats may be linked to the early discovery of estrous-linked changes in locomotor activity (Wang, 1923), which alerted experimenters to programmed changes in females that might confound analyses of non-reproductive traits. The hoped-for reduction in variance by relying on males did not materialize (Goy and McEwen, 1980). Few systematic studies compare variance in males to that of females tested without regard to the stage of the estrous or menstrual cycle. In a meta-analysis of 40 strains of inbred mice, involving more than 8000 animals, Mogil and Chanda (2005) concluded that “there is simply no reality to the assumption that female mice exhibit more variable responses… in a particular nociceptive assay…” than do males; the long-held assumption that the estrous cycle of female mice renders them more variable than male mice requires reappraisal. Sex differences are, in any case, incompletely explained by actions of sex hormones (Cahill, 2006; Arnold, 2009).
6. Implications for individual diseases
Women are diagnosed with anxiety disorders 2.25 times more often than men in a sample of 8 anxiety subtypes (Bekker et al., 2007), but the majority of animal studies on anxiety and anxiolytic drugs focus on male rats (Palanza, 2001).
Women have more stroke events than men over the course of their lives, with poorer functional outcomes and more depression, but in some age groups stroke is more common in men (Reeves et al., 2008). Women have a lower incidence of hypertension than age-matched men for most of the life span (Sandberg and Ji, 2008), but after menopause hypertension in women equals or exceeds that in men (Reckelhoff, 2001). An earlier study (Anastos et al. 1991) of a US population reported more hypertension in women than men. In reconciling these data, we arbitrarily listed the incidence of these diseases as equal in both sexes; We inspected 40 articles on animal models of stroke in Journal of Stroke and Cerebrovascular Disease and Journal of Cerebral Blood Flow and Metabolism for 2009, with the same criteria as for data in Table 1; 65% of reports described research on males, none were on females, 10% included both sexes, and 25% failing to specify sex. The male bias is striking and probably greater than indicated if the majority of sex unspecified studies were conducted on males.
Multiple sclerosis (MS) is approximately 3 times more prevalent in women than men (reviewed in Gold and Voskuhl, 2009) with disease activity decreasing temporarily in late pregnancy (Voskuhl and Palaszynski, 2001); Inspection of 20 articles in PubMed in three immunology journals with the key phrase “experimental autoimmune encephalitis”— a widely used animal model of MS—indicated that 85% of 2009–10 studies were on female mice or rats, none on males, and 15% left sex unspecified for a striking female bias. In this instance, concentrating on females is justifiable if the goal is to disentangle the mechanisms mediating greater disease susceptibility in women. It is notable that the phase of the ovarian cycle when female mice are infected can have a dramatic impact on anti-virus immunity and disease susceptibility due to preferential activation of T regulatory cells (Huber, 2008).
Pain is among the most well analyzed data sets for animal models and human studies (Mogil and Chanda, 2005); 79% of organismal studies published in the journal Pain during 1996–2005 investigated only males (Mogil and Chanda, 2005), despite women being ~ 1.5 times at greater risk than men for many clinical pain conditions (Fillingim et al., 2009). The efficacy of drug treatment differs between women and men; women have a 1.5–1.7 fold greater risk of adverse reactions to medications (Gandhi et al., 2004). Product descriptions available to patients do not include information about sex differences in adverse reactions to drugs (Franconi et al., 2007). Understanding sex differences in drug responses is essential for safety and effectiveness of treatment (Soldin and Mattison, 2009). In pharmacological interventions targeting immune disorders the absence of sex-based differences in study design and analysis “has effectively led to one drug’ treatment regimens for both men and women” (Fish, 2008); 8 of 10 prescription drugs withdrawn in the U.S. in 2005 were removed because of differences in side effects and health issues in women (Fish, 2008).
Despite well-established sex differences in pharmacokinetics and pharmacodynamics, and “repeated attempts to draw attention to sex-dependent drug effects… the vast majority of rodent researchers continue to use males exclusively in their drug studies (Hughes, 2007).” Investigation of both male and female rodents in a single pharmacology article is very rare, even though a majority of those that did consider both sexes report sex-dependent effects (Hughes, 2007). That fluctuations in female hormone status may affect pharmacokinetics and efficacy is all the more reason to study them in the first place (Stein, 2004).
7. Recommendations
The exclusion of females in much of non-human animal research can and should be corrected. When there is convincing evidence of the absence of sex differences in a particular trait, one can test equal numbers of male and female subjects and combine them into a single group for analysis (Mogil and Chanda, 2005). When the status of sex differences is unknown we recommend explicit comparison the two sexes side by side within studies. This will facilitate the discovery of sex differences and promote elaboration of mechanism of action of the sex-specific factors.
Females can be studied irrespective of estrous or menstrual cycle state without substantial increase in outcome variance for some traits (e.g., Mogil and Chanda, 2005; Meziane et al., 2007). Alternatively, when traits are known to vary as a function of the estrous or menstrual cycle, or one suspects sex differences, comparison of males with two or more groups of females at known estrous cycle stages is a viable and recommended option (Becker et al., 2005).
Incorporation of hormone variations in study design is both important (Johnson et al., 2009) and feasible. A study of brain injury in rats exemplifies this approach; intact females subjected to injury during either proestrous or non-proestrous phases of the cycle were compared to ovariectomized females and males (Bramlett and Dietrich, 2001), revealing reduced damage in the intact females compared to males; damage in the ovariectomized females and males was similar. With appropriate phenotyping, female rodents can be used without confounding effects of the estrous cycle. Meziane et al. (2007) found that “the behavior of C57BL/6J females, with the exception of the tail suspension performance, remained stable across all four phases of the estrous cycle in all of the tests including open field, rotarod, startle reflex and pre-pulse inhibition, tail flick and hot plate”.
When there is substantially greater prevalence of a trait in women than men, as is the case for asthma, anxiety disorders, depression, multiple sclerosis, and osteoporosis, and practical considerations preclude testing of both sexes, then predominantly females should be investigated (c.f., Greenspan et al., 2007) as appears to have happened for multiple sclerosis studies in 2009 (see above). Holdcroft (2007) notes that most females used in non-human animal research are young, undergoing estrous cycles, and have not been pregnant; results from such studies may have limited applicability to human populations in which childbearing is normal.
Despite a chorus of pleas for disclosure of the sex of tissues, cell lines and animals in published research, inclusion of females has not improved. Berkley (1992) in a survey of 4 neuroscience journals noted that 57% of single-sex articles concerned males and 17% females; 17 years later, we found no positive change in the ratio of single-sex male and female animal studies in neuroscience (46% male, 9% female; Figure 1A). Male vertebrate utilization in the journal Behavioral Neuroscience in 1984 and 1991 was stable at 78% and 81%, respectively (Sechzer et al., 1994); in 2009, 65% of single-sex mammalian studies in this journal were concerned with males and 10% with females. Stein (2004), comparing his findings to earlier surveys of neuroscience journals, noted little or no change in studies comparing females to males and a low percentage of studies specifying subject sex when adult human brain tissue was assayed. Our historical analysis revealed a large increase in male only studies since 1969 in research on non-human mammals without improvement in utilization of both sexes; an opposite trend characterizes research on humans, where the majority of studies since 1993 investigate both sexes. This reveals a substantial lag in the translational impact of research on animals.
Mogil and Chanda (2005) concluded that “basic scientists are shirking their responsibilities to half of the human population by avoiding the use of direct animal models of them”. Considering that the female to male ratio for Graves’ disease, systemic lupus erythematosis, and Hashimoto’s thyroiditis ranges from more than 7 to 10:1 and that other autoimmune diseases (e.g., ankylosing spondylitis, Goodpasture syndrome, Reiter syndrome, and vasculitis) occur predominantly in men (Fish, 2008), it is remarkable that 75% of 2009 articles we examined that utilized animal models in the journals Infection and Immunity, Immunity and Journal of Immunology, failed to specify subject sex.
Sechzer et al. (1994) recommended that journal editors and reviewers require specification of the numbers and proportions of males and females studied, and that generalization from single-sex studies should be restricted to the sex investigated. Berkley (1992) similarly encouraged reviewers to ensure that authors state the sex and reproductive state of the species used, and that editors adopt sex specification as part of journal policy.
Few have responded to these exhortations. Editors and reviewers have for the most part neglected these issues. Journal policy does not encourage study of both sexes. History suggests that proclamations lacking teeth will fail to address the problem. We recommend that journal editors adopt a mandatory policy for non-human animal research similar to that described in the instructions to authors in the Journal of the National Cancer Institute. The instructions state in part that “clinical and epidemiological studies should be analyzed to see if there is an effect of sex… If there is no effect, it should be so stated in the results”. At a workshop on sex differences in March 2010, sponsored by the Institute of Medicine of the National Academy of Science, the editor of the Journal of Neurochemistry made similar recommendations (Wald and Wu, 2010). Based on the salutary effect of the 1993 NIH revitalization act in increasing enrollment of women in biomedical research, which by 2008 totaled 60% of NIH supported investigations (National Institutes of Health: Department of Health and Human Services, 2009), we believe that sexual parity and study of both sexes in non-human animal research will be achieved only if funding agencies return without review grant proposals that fail to specify and rationalize subject sex. Positive changes will occur when researchers view them as necessary, feasible, and increasing the quality and impact of their research (Klinge, 2008). An excellent guideline is available in the materials prepared by the NIH (National Institutes of Health, 2001) to promote the inclusion of women in clinical research. That document could be paraphrased for non-human animals as follows:
If male and female animal models are thought to differ in response to an intervention then the study must be designed with adequate sample sizes to answer the question for each sex.
If prior research strongly indicates that there are no significant differences between male and female animals, then sex is not required in subject sex selection, but study of both males and females is both feasible and encouraged.
If information about the existence of sex differences is absent or equivocal then both sexes should be studied in numbers sufficient to permit valid analysis.
Study of mechanisms underlying sex differences should be a high priority.
Outreach training activities offering practical suggestions and additional sources of information should be made available by the NIH to help investigators design studies that fully incorporate female animals.
The review process for extramural funding should treat inclusion of female animals as a matter of scientific merit that affects funding.
8. Benefits of studying multiple species
Scientifically valid comparisons between species and interspecific extrapolations require the use of common analytic procedures and similar dimensions. Meaningful comparisons between animal and human data must be based on functional outcomes, i.e., does the trait under consideration perform the same function in the animal model and human (Beach, 1979). The choice of the best animal models for advancing understanding of normal and abnormal human functions is constrained by disciplinary traditions, expedience, cost, ethical and political consideration, and institutional resources.
Utilization of rodents has increased substantially in the last few decades. A Web of Science citation analysis with the terms rat and mouse in the topic category revealed an increase of 76% in the number of articles per year from 1979–1984 to 2004–2008.
Non-traditional species often offer advantages for investigation of biological problems (the Krogh principle, Krebs, 1975; Jorgenson, 2001). Numerous exciting discoveries emerged from studies of species other than laboratory rats and mice. To cite two examples, social monogamy in some vole species, a trait absent in standard rodent models, yielded understanding of the role of the hormone oxytocin in pair-bond formation (Ross and Young, 2009). The study of seasonal rhythms is controlled by day length and transduced by the hormone melatonin in virtually all mammals studied to date (Prendergast et al., 2002), except for lab rats and mice, which fail to display seasonal rhythms; in 31 of 36 mouse strains the pineal gland fails to secrete melatonin (Goto et al., 1989).
9. Conclusions
Just as the absence of statistical analysis from biological research prior to the 1940’s did not prevent major advances in biology, the neglect of females has not prevented progress in non-human animal research. Several arguments nevertheless can be made for abandoning the status quo ante for biomedical research. To understand the biology of women or develop safe treatments for diseases of women one must do more than study men. The plethora of sex differences at all levels of biological organization suggests that observations on males cannot indiscriminately be generalized to females and vice versa. The extreme male sex bias in non-human animal research on pain is one of many puzzling examples, given that women are more susceptible to pain than men (Greenspan et al., 2007).
The estrous cycles of guinea pigs, rats, and mice, first characterized circa 1920, contributed to the subsequent relegation of female mammals to second-class status by the biomedical community, which sought to avoid perceived complications associated with variations in ovarian hormone secretion. We question the claim reported in a recent news article in which an investigator asserts that in the absence of tracking of estrous cycles of rodents, the data of experiments employing females are uninterpretable (Wald and Wu, 2010). Many studies over the past 90 years established that replicable results are just as likely to emerge from investigation of female as male mammals, including scores that did not track female reproductive cycles (Goy and McEwen, 1980).
Animal researchers over the past few decades have contended and responded appropriately to increased regulatory demands more onerous than a requirement to study females. Some principal investigators will not welcome yet another requirement, but with time and recognition that females are as tractable as males, a culture shift and acceptance of this important corrective could occur. The formation of the Organization for the Study of Sex Differences, which held its first meeting in 2007, and the launch of a new journal, The Biology of Sex Differences, are positive developments that can advance the creation of gender balanced science. The recent appearance of news articles in both Nature (Haden, 2010) and Science (Wald and Wu, 2010) highlighting sex bias in research suggests increasing awareness of these issues. The genetic sex and hormone cycles of female animal models profoundly affect biological processes that remain urgently in need of investigation if we are to fulfill the mission of improving quality of life for women as well as men.
Supplementary Material
Figure S1. Historical trends in animal species distribution. Four journals for which historical data were available were surveyed: JPET, J Physiol Lond., JCEM, and J Clin Invest. Human studies were excluded from this survey. By 2009 nearly all research is conducted on rodents, with a marked rise in the use of mice in the past two decades.
Acknowledgments
We thank the Robert Wood Johnson Foundation Health & Society Scholars program for its financial support. We are grateful to Julia Heiman and Gregory Demas for encouraging us to write this article, to four anonymous reviewers as well as Sabra Klein, Rae Silver, Jill Becker, David Soergel, and Ellen Zucker for suggestions that improved the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anastos K, Charney P, Charon RA, Cohen E, Jones CY, Marte C, Swiderski DM, Wheat ME, Williams S. Hypertension in women: what is really known? The women’s caucus, working group on women’s health of the Society of General Internal Medicine. Ann Intern Med. 1991;115:287–293. doi: 10.7326/0003-4819-115-4-287. [DOI] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- Beach FA. Animal models for human sexuality. Ciba Found Symp. 1978:113–143. doi: 10.1002/9780470720448.ch7. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bekker MH, van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gend Med. 2007;4(Suppl B):S178–193. doi: 10.1016/s1550-8579(07)80057-x. [DOI] [PubMed] [Google Scholar]
- Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA. 2009;302:874–882. doi: 10.1001/jama.2009.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ. Vive la différence! Trends Neurosci. 1992;15:331–332. doi: 10.1016/0166-2236(92)90048-d. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Griebel G, Blanchard RJ. Gender bias in the preclinical psychopharmacology of anxiety: male models for (predominantly) female disorders. J Psychopharm. 1995;9:79–82. doi: 10.1177/026988119500900201. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Cahill L. His brain, her brain. Sci Am. 2005;292:40–47. [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Christensen J, Kjeldsen MJ, Andersen H, Friis ML, Sidenius P. Gender differences in epilepsy. Epilepsia. 2005;46:956–960. doi: 10.1111/j.1528-1167.2005.51204.x. [DOI] [PubMed] [Google Scholar]
- Correa-De-Araujo R. Serious gaps: how the lack of sex/gender-based research impairs health. J Womens Health (Larchmt) 2006;15:1116–1122. doi: 10.1089/jwh.2006.15.1116. [DOI] [PubMed] [Google Scholar]
- Council on Scientific Affairs. Animals in research. JAMA. 1989;261:3602–3606. [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franconi F, Brunelleschi S, Steardo L, Cuomo V. Gender differences in drug responses. Pharmacol Res. 2007;55:81–95. doi: 10.1016/j.phrs.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol. 2009;304:8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- Geller SE, Adams MG, Carnes M. Adherence to federal guidelines for reporting of sex and race/ethnicity in clinical trials. J Womens Health (Larchmt) 2006;15:1123–1131. doi: 10.1089/jwh.2006.15.1123. [DOI] [PubMed] [Google Scholar]
- Gold SM, Voskuhl RR. Estrogen and testosterone therapies in multiple sclerosis. Prog Brain Res. 2009;175:239–251. doi: 10.1016/S0079-6123(09)17516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Goy RW, McEwen BS. Sexual differentiation of the brain: based on a work session of the Neurosciences Research Program. MIT Press; 1980. [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ Consensus Working Group of the Sex, Gender, Pain SIG of the IASP. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haden EC. Sex bias blights drug studies. Nature. 2010;464:332–333. doi: 10.1038/464332b. [DOI] [PubMed] [Google Scholar]
- Hayes SN, Redberg RF. Dispelling the myths: calling for sex-specific reporting of trial results. Mayo Clin Proc. 2008;83:523–525. doi: 10.4065/83.5.523. [DOI] [PubMed] [Google Scholar]
- Holdcroft A. Integrating the dimensions of sex and gender into basic life sciences research: methodologic and ethical issues. Gend Med. 2007;4(Suppl B):S64–74. doi: 10.1016/s1550-8579(07)80048-9. [DOI] [PubMed] [Google Scholar]
- Huber SA. Coxsackievirus B3-induced myocarditis: infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology. 2008;378:292–298. doi: 10.1016/j.virol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN. Sex does matter: comments on the prevalence of male-only investigations of drug effects on rodent behaviour. Behav Pharmacol. 2007;18:583–589. doi: 10.1097/FBP.0b013e3282eff0e8. [DOI] [PubMed] [Google Scholar]
- Isensee J, Ruiz Noppinger P. Sexually dimorphic gene expression in mammalian somatic tissue. Gend Med. 2007;4(Suppl B):S75–95. doi: 10.1016/s1550-8579(07)80049-0. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Greaves L, Repta R. Better science with sex and gender: Facilitating the use of a sex and gender-based analysis in health research. Int J Equity Health. 2009;8:14. doi: 10.1186/1475-9276-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen CB. August Krogh and Claude Bernard on basic principles in experimental physiology. Bioscience. 2001;51:59–61. [Google Scholar]
- Klinge I. Gender perspectives in European research. Pharmacol Res. 2008;58:183–189. doi: 10.1016/j.phrs.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Krebs HA. The August Krogh principle: “For many problems there is an animal on which it can be most conveniently studied”. J Exp Zool. 1975;194:221–226. doi: 10.1002/jez.1401940115. [DOI] [PubMed] [Google Scholar]
- Lockshin MD. Sex differences in autoimmune disease. Lupus. 2006;15:753–756. doi: 10.1177/0961203306069353. [DOI] [PubMed] [Google Scholar]
- Marts SA, Keitt S. Foreward: a historical overview of advocacy for research in sex-based biology. Adv Mol Cell Biol. 2004;34:v–xiii. [Google Scholar]
- Meziane H, Ouagazzal AM, Aubert L, Wietrzych M, Krezel W. Estrous cycle effects on behavior of C57BL/6J and BALB/cByJ female mice: implications for phenotyping strategies. Genes Brain Behav. 2007;6:192–200. doi: 10.1111/j.1601-183X.2006.00249.x. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. NIH policy and guidelines on the inclusion of women and minorities as subjects in clinical research. 2001 Obtained from: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-02-001.html.
- National Institutes of Health: Department of Health and Human Services. Monitoring adherence to the NIH policy on the inclusion of women and minorities as subjects in clinical research. 2009 Obtained from: http://orwh.od.nih.gov/inclusion/inclreports.html.
- Palanza P. Animal models of anxiety and depression: how are females different? Neurosci Biobehav Rev. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Pessin J, Marts SA. Sex, gender, drugs, and the brain. Endocrinology. 2005;146:1649. doi: 10.1210/en.2005-0198. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Nelson RJ, Zucker I. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In: Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R, editors. Hormones, brain and behavior. Academic Press; San Diego: 2002. pp. 93–156. [Google Scholar]
- Reckelhoff JF. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers WA, Ballantyne AJ Australian gender equity in health research group. Exclusion of women from clinical research: myth or reality? Mayo Clin Proc. 2008;83:536–542. doi: 10.4065/83.5.536. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg K, Ji H. Why can’t a woman be more like a man?: Is the angiotensin type 2 receptor to blame or to thank? Hypertension. 2008;52:615–617. doi: 10.1161/HYPERTENSIONAHA.108.115063. [DOI] [PubMed] [Google Scholar]
- Sechzer JA, Rabinowitz VC, Denmark FL, McGinn MF, Weeks BM, Wilkens CL. Sex and gender bias in animal research and in clinical studies of cancer, cardiovascular disease, and depression. Ann N Y Acad Sci. 1994;736:21–48. doi: 10.1111/j.1749-6632.1994.tb12816.x. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Gender differences in psychiatric disorders with a focus on estrogen. In: Legato MJ, editor. Principles of gender-specific medicine. Elsevier Academic Press; Amsterdam: 2004. pp. 137–144. [Google Scholar]
- Soldin OP, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48:143–157. doi: 10.2165/00003088-200948030-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG. Brain trauma, sex hormones, neuronal survival, and recovery of function. In: Legato MJ, editor. Principles of gender-specific medicine. Elsevier Academic Press; Amsterdam: 2004. pp. 104–115. [Google Scholar]
- Voskuhl RR, Palaszynski K. Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neuroscientist. 2001;7:258–270. doi: 10.1177/107385840100700310. [DOI] [PubMed] [Google Scholar]
- Wald C, Wu C. Biomedical research. Of mice and women: the bias in animal models. Science. 2010;327:1571–1572. doi: 10.1126/science.327.5973.1571. [DOI] [PubMed] [Google Scholar]
- Wang GH. The relation between ‘spontaneous’ activity and the oestrus cycle in the white rat. Comp Psychol Monog. 1923;6:1–27. [Google Scholar]
- Wetherington CL. Sex-gender differences in drug abuse: a shift in the burden of proof? Exp Clin Psychopharmacol. 2007;15:411–417. doi: 10.1037/1064-1297.15.5.411. [DOI] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Wizemann TM, Pardue ML. Exploring the biological contributions to human health: does sex matter? National Academy Press; Washington, DC: 2001. [PubMed] [Google Scholar]
- World Health Organization. The SuRF Report 2: Surveillance of chronic disease risk factors. 2005 Obtained from: https://apps.who.int/infobase/surf2/SuRF2.pdf.
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Historical trends in animal species distribution. Four journals for which historical data were available were surveyed: JPET, J Physiol Lond., JCEM, and J Clin Invest. Human studies were excluded from this survey. By 2009 nearly all research is conducted on rodents, with a marked rise in the use of mice in the past two decades.