Abstract
Hypoxia is a well-recognized stimulus for pulmonary blood vessel remodeling and pulmonary hypertension development. One mechanism that may account for these effects is the direct action of hypoxia on the expression of specific genes involved in vascular smooth muscle cell (SMC) proliferation. Previous studies demonstrated that the serotonin (5-hydroxytryptamine; 5-HT) transporter (5-HTT) mediates the mitogenic activity of 5-HT in pulmonary vascular SMCs and is overexpressed during hypoxia. Thus, 5-HT-related mitogenic activity is increased during hypoxia. Here, we report that mice deficient for 5-HTT (5-HTT–/–) developed less hypoxic pulmonary hypertension and vascular remodeling than paired 5-HTT+/+ controls. When maintained under normoxia, 5-HTT–/–-mutant mice had normal hemodynamic parameters, low blood 5-HT levels, deficient platelet 5-HT uptake, and unchanged blood levels of 5-hydroxyindoleacetic acid, a metabolite of 5-HT. After exposure to 10% O2 for 2 or 5 weeks, the number and medial wall thickness of muscular pulmonary vessels were reduced in hypoxic 5-HTT–/– mice as compared with wild-type paired controls. Concomitantly, right ventricular systolic pressure was lower and right ventricle hypertrophy less marked in the mutant mice. This occurred despite potentiation of acute hypoxic pulmonary vasoconstriction in the 5-HTT–/– mice. These data further support a key role of 5-HTT in hypoxia-induced pulmonary vascular SMC proliferation and pulmonary hypertension.
Introduction
Exposure to chronic hypoxia leads to the development of pulmonary hypertension (PH) owing to persistent vasoconstriction and structural remodeling of pulmonary vessels. Proliferation of vascular smooth muscle cells (SMCs) is an important component of pulmonary vessel remodeling that results in increased thickness of the medial muscular coat in normally muscular arteries and in extension of muscle into smaller and more peripheral arteries (1). The mechanism by which hypoxia induces pulmonary SMCs’ proliferation, however, is not well understood. One current hypothesis is that hypoxia may directly affect the expression of specific genes involved in pulmonary vascular SMC growth (2).
The serotonin (5-hydroxytryptamine; 5-HT) transporter (5-HTT) in pulmonary vascular SMCs has many attributes suggesting that it may be a key determinant of pulmonary vessel remodeling. In addition to contributing to the uptake and subsequent inactivation of 5-HT passing through the lung, 5-HTT mediates the proliferation of pulmonary vascular SMCs through its ability to internalize indoleamine (3, 4). The requirement of 5-HTT as a mediator of 5-HT mitogenic activity appears specific for pulmonary vascular SMCs, since it has not been reported in other cell types (5). Moreover, exposure of pulmonary vascular SMCs to hypoxia increases 5-HTT expression and activity (3, 6), an effect associated with potentiation of the mitogenic action of 5-HT (6). Increased 5-HTT gene expression also occurs in remodeled pulmonary vessels of rats during PH development associated with chronic exposure to hypoxia (6). Because 5-HTT is a target for drugs that recently have been shown to increase the risk of PH development in humans (7), it may be of clinical relevance; therefore elucidation of its role in pulmonary vascular SMC proliferation is of interest. However, direct evidence for a role of 5-HTT in vessel remodeling during PH is lacking.
The purpose of this study was to investigate whether 5-HTT deficiency affects the development of pulmonary vascular remodeling and PH during chronic hypoxia. We used mice with targeted disruption of the 5-HTT gene (5-HTT–/–) (8) and investigated their hemodynamics and circulating 5-HT. We then examined 5-HTT expression, PH development, and pulmonary vessel muscularization in 5-HTT–/– mice and wild-type controls exposed to chronic hypoxia. To determine whether 5-HTT deficiency could be associated with changes in the extent of hypoxic pulmonary vasoconstriction, we also examined the pressure response to acute hypoxia in both 5-HTT–/– and 5-HTT+/+ mice.
Methods
Mice.
Mice lacking 5-HTT (5-HTT–/–) were generated by homologous recombination on the C57B6/SV129 genetic background. The wild-type 5-HTT+/+ and mutant homozygous 5-HTT–/– mice used in these studies were siblings (8–10 weeks of age) obtained by breeding heterozygous mutants. Pups were typed by Southern blot analysis of tail biopsies as described previously (8). Responses to either acute or chronic hypoxia were then examined in male 5-HTT+/+ and 5-HTT–/– littermates. For the study of chronic hypoxia effects, mice were randomly divided into two groups, one of which was maintained in room air and the other exposed to hypoxia. All animal care and procedures were in accordance with institutional guidelines.
Hemodynamic response of normoxic mice to acute hypoxia.
Mice 8–10 weeks of age, weighing approximately 20–30 g were anesthetized with intraperitoneal ketamine (6 mg/100 g) and xylazine (1 mg/100 g). The trachea was cannulated, and the lungs were ventilated with room air at a tidal volume of 0.2 mL and a rate of 90 breaths per minute. A 26-gauge needle was then introduced percutaneously into the right ventricle via the subxiphoid approach. Right ventricular systolic pressure (RVSP) was measured using a Gould P10 EZ pressure transducer connected to pressure modules and a Gould TA 550 recorder. Right ventricular systolic pressure and heart rate were recorded first while the animal was ventilated with room air and then after 5 minutes of ventilation with the hypoxic gas mixture (8% O2, 92% N2). The heart rate under these conditions was between 300 and 500 beats per minute (bpm). If the heart rate fell below 300 bpm, measurements were excluded from analysis.
Exposure to chronic hypoxia.
Mice were exposed to chronic hypoxia (10% O2) in a ventilated chamber (500-L volume; Flufrance, Cachan, France) as described previously (9). To establish the hypoxic environment, the chamber was flushed with a mixture of room air and nitrogen, and the gas was recirculated. The chamber environment was monitored using an oxygen analyzer (Servomex OA150; Servomex, Crowborough, United Kingdom). Carbon dioxide was removed by soda lime granules, and excess humidity was prevented by cooling of the recirculation circuit. The chamber temperature was maintained at 22–24°C. The chamber was opened every other day for 1 hour to clean the cages and replenish food and water supplies. Normoxic mice were kept in the same room, with the same light-dark cycle.
Assessment of pulmonary hypertension.
Mice exposed previously to hypoxia or room air for 2 or 5 weeks were anesthetized and ventilated with room air as described above. After incision of the abdomen and diaphragm, a 26-gauge needle connected to a pressure transducer was inserted into the right ventricle, and RVSP was recorded immediately. Next, blood was sampled for hematocrit determination and 5-HT measurements. Finally, after an intraperitoneal injection of sodium pentobarbital (40 mg/kg) and exsanguination, the thorax was opened and the lungs and heart were removed. The right ventricle (RV) was dissected from the left ventricle plus septum (LV+S), and these dissected samples were weighed.
The lungs were fixed by intratracheal infusion of 4% aqueous buffered formalin at a pressure of 23 cmH2O. The entire specimen was placed in a bath of the same fixative for 1 week. A midsagittal slice of the right lung, including the apical, azygous, and diaphragmatic lobes, was processed for paraffin embedding. Sections (5 μm thick) were cut for use in light microscopy and stained with hematoxylin-phloxine-saffron and orcein-picroindigo-carmine.
In each mouse, a total of 50–60 intraacinar vessels accompanying either alveolar ducts or alveoli were analyzed by an observer blinded to the genotype and treatment. Each vessel was categorized as nonmuscular (no evidence of any vessel wall muscularization), partially muscular (SMCs identifiable in less than three-fourths of the vessel circumference), or fully muscular (SMCs in more than three-fourths of the vessel circumference). Muscularization was defined as the presence of typical SMCs stained red with phloxine, exhibiting an elongated shape and square-ended nucleus, and bound by two orcein-stained elastic laminae. The percentage of pulmonary vessels in each muscularization category was determined by dividing the number of vessels in that category by the total number counted in the same experimental group.
For fully muscular vessels, video images were obtained and arterial diameters were measured using computerized image analysis device. Percent of wall thickness was then calculated as the diameter of the external elastic lamina minus the diameter of the internal lamina divided by the diameter of the external elastic lamina.
Measurement of systemic arterial pressure in normoxic mice.
To assess systemic arterial pressure in 5-HTT+/+ and 5-HTT–/– mice, animals were anesthetized with intraperitoneal ketamine (6 mg/100 g) and xylazine (1 mg/100 g). The right carotid was exposed, and a polyethylene catheter (PE10) was inserted into the carotid artery.
Platelet [3H]5-HT uptake.
The protocol used to measure platelet [3H]5-HT uptake in normoxic mice of each genotype was adapted from Prina et al. (10). Briefly, blood was collected by cardiac puncture in anesthetized mice and centrifuged at 600 g for 10 minutes in 13 mM of citrate to obtain platelet-rich plasma (PRP). The platelets were counted, and assays were performed by incubating 100 μL of PRP (107 platelets) with 0.2 μM of 5-hydroxy[G-3H]tryptamine creatinine sulfate ([3H]5-HT, 15–16 Ci/mmol) (Amersham International, Amersham, United Kingdom). Under these conditions, [3H]5-HT uptake by platelets was linear for at least 5 minutes. Assays were performed for 1 minute at 37°C with or without the specific 5-HTT inhibitor fluoxetine (10–5 M). The reaction was stopped by adding 2 mL of cold 0.9% NaCl containing 0.4% EDTA, and the samples were centrifuged at 3,600 g for 10 minutes. The platelets were washed three times using 2 mL of the same buffer, then centrifuged. The platelet-free supernatant was discarded, and the platelet pellet was lysed by addition of 1 mL of 0.1 N NaOH. Lysate radioactivity was counted using liquid scintillation spectrometry. Specific [3H]5-HT uptake calculated as the difference between total uptake and uptake in the presence of fluoxetine was expressed in femtomoles of [3H]5-HT taken up per milligram of protein measured using the method of Lowry et al. (11), with BSA as the standard.
Blood 5-HT and 5-HIAA concentrations.
The amine and its metabolite 5-hydroxyindoleacetic acid (5-HIAA) were determined in whole blood using HPLC coupled to electrochemical detection, as described in detail elsewhere (12).
Lung 5-HTT immunoblotting.
Immediately after removal, the lungs were quickly frozen in liquid nitrogen. After thawing at 0°C, the tissues were sonicated in 0.1 mM PBS containing antiproteases (1 μM leupeptin and 1 μM pepstatin A). The homogenates were subjected to SDS-PAGE. Proteins in the gel were transferred to a nitrocellulose membrane by electroblotting in a transblot BioRad transfer apparatus for 12 hours at 4°C. Before the transfer, the gels, Whatman filter paper, and nitrocellulose membrane were soaked in electroblotting buffer (25 mM Tris-HCl; 193 mM glycine; 20% methanol, pH 8.0) for 15 minutes. After transfer, the membrane was blocked using 1× TBST (0.15 M NaCl; 10 mM Tris-HCl, pH 8.0; 0.05% Tween-20; and 5% BSA) for 1 hour at room temperature. The 5-HTT protein was detected by incubating the membrane overnight at 4°C with goat polyclonal anti–5-HTT (Santa Cruz Biotechnology, Santa Cruz, California, USA) diluted 1:1000. The membrane was washed three times in 1× TBST. Specific protein was detected using a horseradish peroxidase–conjugated secondary antibody and enhanced chemiluminescence (ECL) reagents (Amersham). The 5-HTT immunoreactivity was quantified using a semiautomated image analysis device (National Institutes of Health image 1.52) that quantifies both the area and the intensity of immunoreactive bands using a ScanJet II scanner with DeskScan II software (Hewlett Packard, Palo Alto, California, USA). Results are reported in arbitrary units.
Immunohistochemical analysis.
Paraffin sections (5 μm thick) were mounted on Superfrost Plus slides (Fisher Scientific. Illkirch, France). For 5-HTT immunostaining, the slides were dewaxed in 100% toluene, and the sections were then rehydrated by successive immersion first in decreasing ethanol concentrations (100%, 95%, and 70%) then in water. Endogenous peroxidase activity was blocked using H2O2 in methanol (0.3% vol/vol) for 10 minutes. After three PBS washes, sections were preincubated in PBS supplemented with 3% (wt/vol) BSA for 30 minutes, then incubated overnight at 4°C with goat polyclonal anti–5-HTT antibodies (Santa Cruz Biotechnology) diluted to 1:2000 in 1× PBS, 0.02% BSA. Next, the sections were exposed for 1 hour to biotin-labeled anti-goat secondary antibodies (DAKO Corp., Trappes, France) diluted 1:200 in the same buffer. Peroxidase staining of the slides incubated in streptavidin-biotin horseradish peroxidase solution was carried out using 3,3′-diaminobenzidine tetrahydrochloride dihydrate (DAB; Sigma-Aldrich Chemie S.a.r.l., St. Quentin Fallavier, France) and hydrogen peroxide. Finally, the sections were stained with hematoxylin and eosin.
Statistical analysis.
All results are reported as mean plus or minus SEM. The nonparametric Mann-Whitney test was used to compare 5-HTT+/+ and 5-HTT–/– normoxic mice. Two-way ANOVA was used to assess, in 5-HTT+/+ and 5-HTT–/– mice, the effects of normoxia or hypoxia of various duration on hemodynamics, hematocrit, the right ventricular hypertrophy index, and muscularization. When ANOVA indicated an interaction between exposure conditions and 5-HTT expression, 5-HTT+/+ and 5-HTT–/– mice were further compared under each condition using an unpaired nonparametric test. To compare the degree of pulmonary vessel muscularization between the two genotypes under each condition, the nonparametric Mann-Whitney test was performed after ordinal classification of pulmonary vessels as nonmuscular, partially muscular, or muscular.
Results
Platelet [3H]5-HT uptake, blood 5-HT, and 5-HIAA concentrations in 5-HTT–/– versus 5-HTT+/+ normoxic mice.
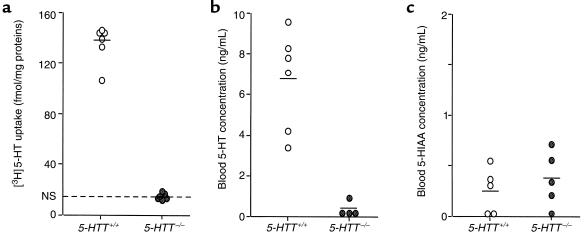
As expected, 5-HTT gene knockout resulted in a dramatic reduction in [3H]5-HT uptake by platelets in the 5-HTT–/– mutants (n = 6) as compared with wild-type animals (n = 6) (Figure 1). Furthermore, [3H]5-HT accumulation in platelets from wild-type mice was almost completely inhibited (–90%) by 10 μM fluoxetine, whereas uptake measured in platelets from 5-HTT–/– mutants was insensitive to selective 5-HTT inhibitor (Figure 1).
Figure 1.
Individual and mean (horizontal line) values of platelet [3H]5-HT uptake (a) and whole blood concentrations of 5-HT (b) and 5-HIAA (c) in normoxic 5-HTT+/+ and 5-HTT–/– mutant mice. NS, nonspecific uptake in the presence of 10 μM fluoxetine.
Whole blood 5-HT levels were also dramatically reduced in 5-HTT–/– mice (n = 6) as compared with wild-type mice (n = 6) (Figure 1), whereas blood 5-HIAA levels did not differ between the two groups (n = 5 in each group) (Figure 1).
Hemodynamic response to acute hypoxia.
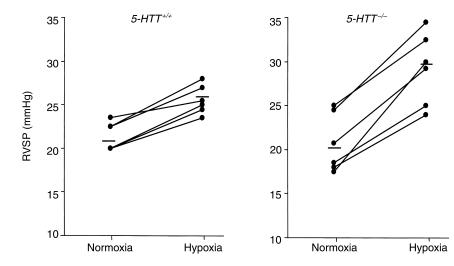
The effect of an acute hypoxic challenge on RVSP was examined in normoxic mice. Whereas under ventilation with room air, RVSP and heart rate did not differ between 5-HTT+/+ and 5-HTT–/– mice, exposure to 8% O2 elicited a larger increase in RVSP (Figure 2) in 5-HTT–/– than in 5-HTT+/+ mice (ΔRVSP, 8.5 ± 1.2 mmHg, n = 6, vs. 4.2 ± 0.5 mmHg, n = 6, respectively; P < 0.01) despite similar heart rates.
Figure 2.
Individual and mean (horizontal line) values of RVSP in normoxic 5-HTT+/+ and 5-HTT–/– mice under ventilation with room air (normoxia) and after 5 minutes of ventilation with a hypoxic gas mixture (hypoxia). Baseline RVSP under normoxic ventilation did not differ between 5-HTT+/+ and 5-HTT–/– mice. The RVSP increase during acute hypoxia was significantly larger in 5-HTT–/– than in 5-HTT+/+ mice (P < 0.01).
Development of pulmonary hypertension and vascular remodeling.
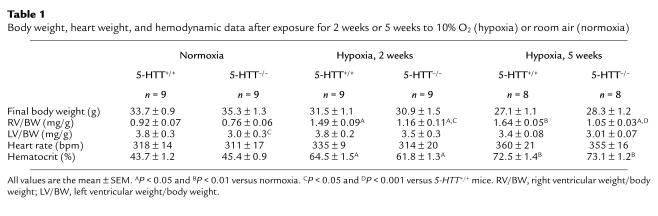
Under normoxic conditions, body weight (BW), RV weight/BW, hematocrit, and heart rate were similar in 5-HTT+/+ and 5-HTT–/– mice (n = 9 in each group) (Table 1). Neither did mean systemic arterial pressure differ significantly between 5-HTT+/+ and 5-HTT–/– mice (160 ± 3.5 mmHg, n = 5, and 148 ± 4 mmHg, n = 5, respectively). However, LV weight/BW was lower in the 5-HTT–/– mutants than in the wild-type mice (Table 1).
Table 1.
Body weight, heart weight, and hemodynamic data after exposure for 2 weeks or 5 weeks to 10% O2 (hypoxia) or room air (normoxia)
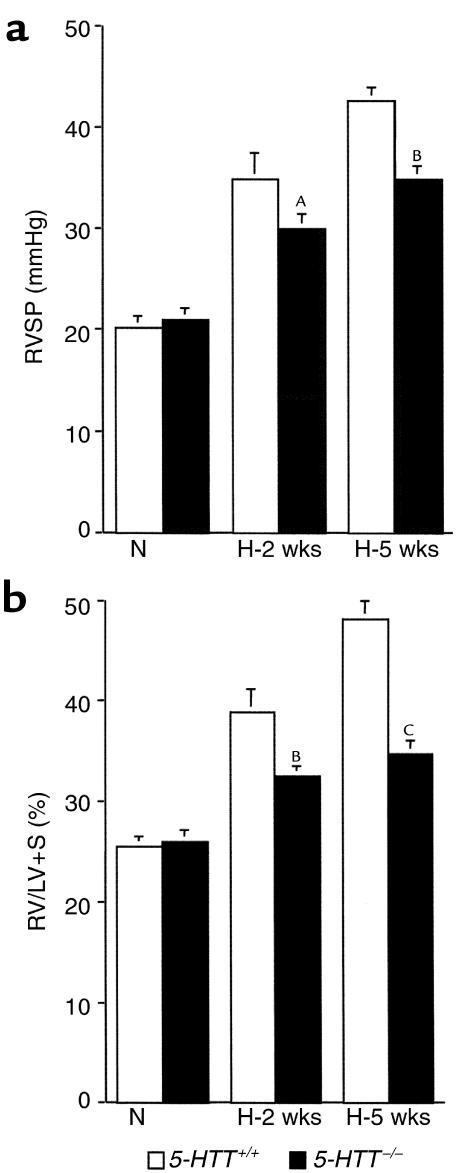
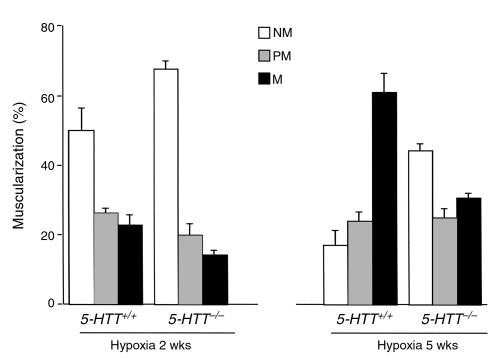
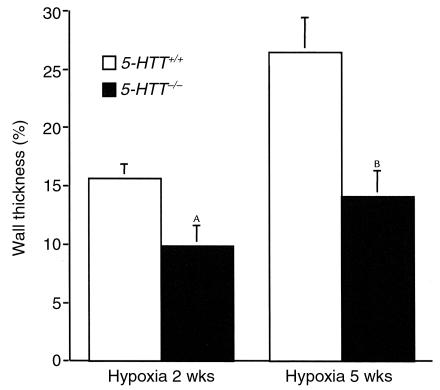
As compared with mice maintained under normoxic conditions, mice exposed to 10% dioxygen exhibited gradual increases in RVSP (Figure 3) and hematocrit (Table 1) such that at 5 weeks (n = 8 in each group) values were higher than at 2 weeks (n = 9 in each group) in mice of either genotype. However, only in 5-HTT+/+ mice was RV/LV+S significantly higher after 5 weeks than after 2 weeks of hypoxia (P < 0.01). Whereas after exposure to hypoxia for similar durations, BW, hematocrit, and heart rate did not differ between either genotype, RVSP was significantly lower and right ventricular hypertrophy less severe in 5-HTT–/– mutants than in 5-HTT+/+ mice (Figure 3, Table 1). Furthermore, distal pulmonary vessel muscularization, which also increased with the duration of hypoxia exposure, was less marked at both the alveolar duct and the alveolar wall levels in 5-HTT–/– than in 5-HTT+/+ mice (P < 0.01 and P < 0.001 after 2 weeks and 5 weeks of hypoxia, respectively; Figure 4). After exposure to hypoxia for similar durations, the normalized wall thickness of muscular arteries (diameter < 100 μm) was smaller in 5-HTT–/– than in 5-HTT+/+ mice (Figure 5).
Figure 3.
RVSP (a) and right ventricle/left ventricle plus septum weight (RV/LV+S) (b) in 5-HTT+/+ and 5-HTT–/– mutant mice exposed to normoxia (N) (n = 9 in each genotype) or 10% O2 for 2 weeks (H-2 weeks) (n = 9 of each genotype) or 5 weeks (H-5 weeks) (n = 8 of each genotype). AP < 0.05, BP < 0.01, and CP < 0.001 as compared with corresponding values in 5-HTT+/+ mice under similar conditions.
Figure 4.
Distribution of vessels according to the accompanying airway. Fifty to 60 intraacinar vessels were analysed in each lung from mice of each genotype after exposure to hypoxia for 2 weeks (n = 9 of each genotype) or 5 weeks (n = 8 of each genotype). Percentages of nonmuscular (NM), partially muscular (PM), or fully muscular (M) vessels determined separately at the alveolar duct and alveolar wall levels differed significantly between 5-HTT+/+ and 5-HTT–/– mice after exposure to hypoxia for either 2 weeks (P < 0.01) or 5 weeks (P < 0.001).
Figure 5.
Normalized wall thickness measured in fully muscular arteries in lungs from 5-HTT+/+ and 5-HTT–/– mice exposed to chronic hypoxia over 2 weeks (n = 9 mice of each genotype) or 5 weeks (n = 8 mice of each genotype). AP < 0.05, BP < 0.01 as compared with values in 5-HTT+/+ mice exposed to hypoxia of the same duration.
Lung 5-HTT expression and immunolocalization in response to hypoxia.
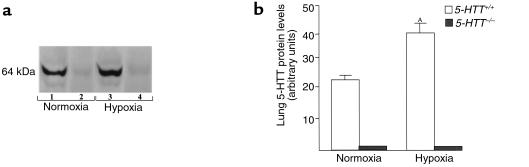
To evaluate the consequences of exposure to hypoxia on lung 5-HTT expression, the levels of 5-HTT protein in lung tissues were estimated using Western blot analysis. A single immunoreactive band (64 kDa) was observed in lungs from wild-type mice (Figure 6). The density of this band increased after two weeks of exposure to hypoxia (40.2 ± 4 OD vs. 23.6 ± 0.7 OD arbitrary units in lungs from hypoxic and normoxic mice, respectively; n = 5 in each group). In contrast, the 5-HTT immunoreactive band was not detected in lungs from 5-HTT–/– mice exposed to either normoxia or hypoxia.
Figure 6.
Western blot of 5-HTT protein in lungs from 5-HTT+/+ and 5-HTT–/– mutant mice at the end of a 2-week exposure to normoxia or hypoxia. (a) The 5-HTT immunoreactivity in lungs from 5-HTT+/+ mice (lanes 1, 3) and 5-HTT–/– mice (lanes 2, 4). The specific antibodies recognized a protein with an apparent molecular weight of 64 kDa. (b) Quantitation of the 5-HTT signal in lungs from normoxic and chronically hypoxic 5-HTT+/+ and 5-HTT–/– mice (n = 5 in each group). AP < 0.05 as compared with 5-HTT levels in normoxic 5-HTT+/+ mice.
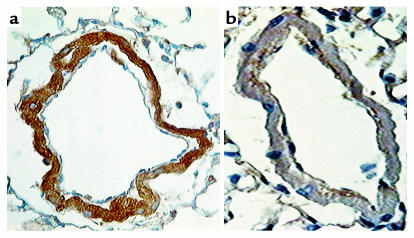
Immunohistochemical studies showed 5-HTT–like immunostaining in endothelial and SMCs of pulmonary vessels from wild-type mice exposed to hypoxia for 2 weeks (Figure 7). In contrast, only a very low level of background staining was observed in arteries from 5-HTT–/– mice exposed to hypoxia of the same duration (Figure 7).
Figure 7.
Immunohistochemical localization of 5-HTT in distal pulmonary vessels from a 5-HTT+/+ mouse (a) and a 5-HTT–/– mutant mouse (b) exposed to hypoxia for 2 weeks. In the pulmonary vessels from wild-type mice, dense immunostaining is visible in the smooth muscle cells. In contrast, only a very low level of background staining is observed in vessels from 5-HTT–/– mice. Scale bar, 25 μm.
Discussion
The present results demonstrate that the 5-HTT is a major determinant of pulmonary vascular remodeling during exposure to chronic hypoxia. Thus, in mice lacking the 5-HTT gene and exposed to hypoxia for two or five weeks, the number and wall thickness of muscular pulmonary vessels were decreased as compared with wild-type controls. Concomitantly, RVSP was lower and the RV was less hypertrophied in hypoxic 5-HTT–/– mutants than in hypoxic wild-type mice. In contrast, the increase in RVSP elicited by an acute hypoxic challenge was larger in 5-HTT–/– than in 5-HTT+/+ normoxic mice. Therefore, the attenuation of PH development and vascular remodeling in the 5-HTT–deficient mice cannot be explained by decreased pulmonary vasoreactivity to hypoxia. These data strongly support a key role for 5-HTT in vascular SMC proliferation and in the consequent development of PH in response to hypoxia.
Hypoxia is a well-recognized stimulus for pulmonary blood vessel remodeling. One mechanism that may account for this effect is a direct action of hypoxia on the expression of specific genes involved in SMC proliferation. A recent study has shown that mice partially deficient in hypoxia-inducible factor-1 (HIF-1), an essential mediator of transcriptional responses to decreased dioxygen availability, have decreased development of hypoxic PH with reduced muscularization of distal pulmonary vessels (2). This is evidence that specific genes expressed under the control of HIF-1 during exposure to hypoxia are involved in pulmonary vascular SMC proliferation. Among vasoactive molecules or growth factors that have been implicated in PH, inducible nitric oxide synthase (13), heme oxygenase 1 (14), VEGF (15), VEGF receptor 1 (16), and endothelin 1 (ET-1) (17) are known to be expressed by hypoxia-inducible genes that contain functionally important HIF-1 binding sites. However, these molecules exhibit little or no growth-promoting effects on pulmonary SMCs, and none of them demonstrate selective effects on the pulmonary circulation.
We reported recently that the mitogenic action of 5-HT on rat-cultured pulmonary vascular SMCs was enhanced by hypoxia (6). Other studies found that the mitogenic action of 5-HT was dependent upon 5-HTT activity in pulmonary artery SMCs from several species but not in systemic vessel SMCs (5). Hypoxia induces 5-HTT expression in cultured SMCs through a transcriptional mechanism and simultaneously increases the mitogenic activity of 5-HT (6). Exposure to hypoxia also increases 5-HTT expression in the rat lung, notably in the media of remodeled pulmonary vessels. The presence of two hypoxia-sensitive elements in the promoter region of the 5-HTT gene strongly suggests that 5-HTT may be an effector molecule for pulmonary vascular remodeling in response to hypoxia (6, 18). However, 5-HTT activity also depends upon the levels of 5-HT. In previous studies, we found that continuous intravenous infusion of 5-HT worsened PH in rats exposed to chronic hypoxia (19). This effect was prevented by administration of a 5-HT transport inhibitor despite further increases in plasma 5-HT levels (20). In aggregate, these observations suggest that 5-HTT activity may be involved in the response to exogenous 5-HT. However, they do not provide information on the role of 5-HTT activity in hypoxia-induced PH in the absence of pharmacological modulation of 5-HT.
We used 5-HTT knockout mice, which have been well characterized in terms of locomotor activity and behavior (8). Because 5-HTT is encoded by a single gene expressed in several cell types such as neurons, platelets, and pulmonary vascular endothelial cells and SMCs, 5-HTT deficiency can be expected to produce marked alterations not only in central serotoninergic neurotransmission but also in the metabolism and actions of indoleamine at the periphery. Under normal conditions, 5-HT is produced mainly by enterochromaffin cells in the gut, where it plays a role in mechano- and chemotransduction. A large part of the 5-HT released from enterochromaffin cells reaches the portal circulation, where it is avidly taken up by platelets and partly metabolized by the liver. The remaining free 5-HT in blood is cleared by the lung, a process that involves indoleamine uptake by pulmonary vascular endothelial and SMCs. In 5-HTT–/– mice, high-affinity [3H]5-HT uptake was absent in platelets in our study and in the brain in an earlier study (8), confirming the complete absence of 5-HTT gene activity in these mutants. 5-HT blood concentrations were also dramatically reduced in the mutants as compared with wild-type mice, an expected finding since platelet 5-HT is known to contribute at least 90% of whole blood 5-HT. In contrast, blood 5-HIAA levels did not differ significantly between 5-HTT–/– mutants and wild-type mice, suggesting an absence of marked adaptive changes in 5-HT production and/or degradation in the knockout animals.
The main finding from our study is that mice deficient in 5-HTT developed less PH than their littermate controls when exposed to hypoxia of various durations. Not only was pulmonary artery pressure lower, but pulmonary vessel muscularization and thickening were also less marked in 5-HTT–/– mice than in wild-type controls, suggesting that impaired pulmonary vascular SMC proliferation owing directly to the deficiency in 5-HTT–mediated 5-HT mitogenic activity was responsible for the attenuation of PH. In theory, protection from pulmonary vascular remodeling and PH could have resulted from other mechanisms. One is decreased pulmonary vasoreactivity to hypoxia. However, we found that the pulmonary pressor response to acute hypoxia as evaluated based on the RVSP increase was enhanced rather than blunted in 5-HTT–/– mice. The explanation of this finding remains speculative. One likely hypothesis is that the platelet uptake deficiency left more indoleamine available for binding to 5-HT receptors on pulmonary SMCs in 5-HTT–/– mice. In an earlier study, we found that 5-HT infusion in rats potentiated the in vivo acute pulmonary pressure response to hypoxia (19). Moreover, treatment with dexfenfluramine, which inhibits platelet 5-HT uptake and, additionally promotes 5-HT release from platelets, also potentiates in vivo acute hypoxic pulmonary vasoconstriction (20). It is therefore reasonable to assume that the deficiency in platelet 5-HT uptake in the 5-HTT–/– mice increases hypoxic pulmonary vasoreactivity through the same mechanism. Another possibility is that impaired 5-HT release by platelets or secondary platelet dysfunction may account for the attenuated pulmonary vascular remodeling in 5-HTT–/– mice. In this regard, a comparison of our results in 5-HTT–/– mice with those previously reported in the fawn-hooded rat is of interest. Fawn-hooded rats have a genetic deficiency in 5-HT platelet storage and a bleeding tendency owing to a defect in platelet aggregation (21), but have a normal 5-HTT amino acid sequence (22). Although fawn-hooded rats share with 5-HTT–/– mice an increase in pulmonary vasoreactivity to acute hypoxia, responses to chronic hypoxia are diametrically opposed: hypoxia-induced PH is facilitated in fawn-hooded rats, whereas it is attenuated in 5-HTT–/– mice. This militates against the possibility that attenuated remodeling in 5-HTT–/– mice was related to platelet dysfunction or to impaired 5-HT release. The increased vascular remodeling in fawn-hooded rats is believed to involve increased lung vessel exposure to 5-HT as a result of the inability of platelets to store 5-HT. Together with the fact that 5-HT infusion worsens hypoxic PH in rats, these findings support a key role for 5-HT in hypoxic PH, provided that 5-HTT expression and/or activity is present in pulmonary vascular SMCs. The observation of attenuated remodeling in our 5-HTT–deficient mice emphasizes the importance of 5-HTT expression by pulmonary SMCs for the development of hypoxic PH.
At present, the mechanisms by which 5-HT may exert its mitogenic effect after being transported inside SMCs remain speculative. Lee et al. observed that 5-HT–induced DNA synthesis was associated with tyrosine phosphorylation of GTPase-activating proteins and that both events were blocked by inhibitors of 5-HT transport or tyrosine kinase (23). Therefore, although 5-HT–induced mitogenesis of SMCs requires cellular internalization through 5-HTT rather than binding to a membrane receptor, tyrosine phosphorylation of GTPase-activating proteins appears to be a downstream intermediate in the signaling pathway. Recently, involvement of superoxide anion formation in association with 5-HT transport has also been suggested as a possible contributor to the mitogenic effects of 5-HT (24). Since reactive oxygen species are also considered to be potential mediators of vascular remodeling, we cannot exclude that protection against PH in 5-HTT–deficient mice was indirectly related to decreased formation of superoxide anions.
Interestingly, heart weight was lower in 5-HTT–/– mutants than in wild-type normoxic mice, although systemic arterial pressure did not differ between the two groups. Clinical studies have established that 5-HT promotes cardiac disease by causing fibroplasia, particularly in the valvular endocardium in the right side of the heart (25). In addition, patients treated with the 5-HT releaser fenfluramine in combination with phentermine have been reported to develop right and left valvular heart disease (26). In line with these observations, cardiac alterations may develop in 5-HTT–/– mice as a consequence of either an excessive amount of 5-HT reaching the heart or a deficiency in 5-HTT. Further studies are needed to explore the nature and the mechanisms of cardiac alterations in 5-HTT–deficient mice.
The importance of 5-HT in pulmonary vascular remodeling is generating renewed interest, in particular because several appetite suppressants that act by inhibiting 5-HT transport have been reported to increase the risk of PH in humans (7). However, further investigations are needed to determine whether a link exists between the mechanism of appetite suppressant–induced human PH and the present results demonstrating the importance of 5-HTT expression by pulmonary SMCs for the development of hypoxic PH in rodents. Recently, we found marked increases in 5-HTT binding and 5-HTT activity in platelets from patients with primary and secondary forms of PH (27). Therefore, one reasonable hypothesis is that human PH may be associated with 5-HTT overexpression in platelets and/or pulmonary artery SMCs and that appetite suppressants may act on this process. Further studies exploring 5-HTT expression in the pulmonary circulation of patients with primary or secondary PH are needed to extend the present findings to nonhypoxic forms of human PH.
Acknowledgments
This research was supported by grants from the Institut National de la Santé et de la Recherche Médicale and Bristol-Myers Squibb Foundation (Unrestricted Biomedical Research Grant program).
References
- 1.Rabinovitch M, Gamble W, Nadas AS, Miettinen O, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am J Physiol. 1979;236:H818–H827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- 2.Yu AY, et al. Impaired physiological response to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SL, Dunn J, Yu FS, Fanburg BL. Serotonin uptake and configurational change of bovine pulmonary artery smooth muscle cells in culture. J Cell Physiol. 1989;138:145–153. doi: 10.1002/jcp.1041380120. [DOI] [PubMed] [Google Scholar]
- 4.Lee SL, Wang WW, Moore BJ, Fanburg BL. Dual effect of serotonin on growth of bovine pulmonary artery smooth muscle cells in culture. Circ Res. 1991;68:1362–1368. doi: 10.1161/01.res.68.5.1362. [DOI] [PubMed] [Google Scholar]
- 5.Fanburg B, Lee S-L. A new role for an old molecule: serotonin as a mitogen. Am J Physiol. 1997;272:L795–L806. doi: 10.1152/ajplung.1997.272.5.L795. [DOI] [PubMed] [Google Scholar]
- 6.Eddahibi S, et al. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells: relationship with the mitogenic action of serotonin. Circ Res. 1999;84:329–336. doi: 10.1161/01.res.84.3.329. [DOI] [PubMed] [Google Scholar]
- 7.Abenhaim L, et al. Appetite-suppressant drugs and the risk of pulmonary hypertension. N Engl J Med. 1996;335:609–616. doi: 10.1056/NEJM199608293350901. [DOI] [PubMed] [Google Scholar]
- 8.Bengel D, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- 9.Adnot S, Raffestin B, Eddahibi S, Braquet P, Chabrier PE. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J Clin Invest. 1991;87:155–162. doi: 10.1172/JCI114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prina R, Dolfini E, Mennini T, Palermo A, Libertti A. Reduced serotonin uptake by spontaneously hypertensive rat platelets. Life Sci. 1981;29:2375–2379. doi: 10.1016/0024-3205(81)90473-2. [DOI] [PubMed] [Google Scholar]
- 11.Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Hamon M, et al. Alterations of central serotonin and dopamine turnover in rats treated with ipsapirone and other 5-hydroxytryptamine1A agonists with potential anxiolytic properties. J Pharmacol Exp Ther. 1988;246:745–752. [PubMed] [Google Scholar]
- 13.Mellilo BG, et al. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995;182:1683–1693. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee PJ, et al. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272:5375–5381. [PubMed] [Google Scholar]
- 15.Liu YL, Cox SR, Morita T, Kourembanas S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res. 1995;77:638–643. doi: 10.1161/01.res.77.3.638. [DOI] [PubMed] [Google Scholar]
- 16.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is upregulated by hypoxia. J Biol Chem. 1997;272:23659–23667. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor 1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245:894–899. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- 18.Bengel D, et al. Gene structure and 5′-flanking regulatory region of the murine serotonin transporter. Brain Res Mol Brain Res. 1996;44:286–292. doi: 10.1016/s0169-328x(96)00234-3. [DOI] [PubMed] [Google Scholar]
- 19.Eddahibi S, et al. Treatment with 5-HT potentiates development of pulmonary hypertension in chronically hypoxic rats. Am J Physiol. 1997;272:H1173–H1181. doi: 10.1152/ajpheart.1997.272.3.H1173. [DOI] [PubMed] [Google Scholar]
- 20.Eddahibi S, Adnot S, Launay JM, Sitbon MV, Raffestin B. Effects of dexfenfluramine on pulmonary hypertension during acute and chronic exposure to hypoxia in rats. Am J Respir Crit Care Med. 1998;157:1111–1119. doi: 10.1164/ajrccm.157.4.9704095. [DOI] [PubMed] [Google Scholar]
- 21.Tschopp TB, Zucher MB. Hereditary defect in platelet function in rats. Blood. 1986;40:217–226. [PubMed] [Google Scholar]
- 22.Gonzalez A-M, Smith APL, Emery CJ, Higenbottam TW. The pulmonary hypertensive fawn-hooded rat has a normal serotonin transporter coding sequence. Am J Respir Cell Mol Biol. 1998;19:245–249. doi: 10.1165/ajrcmb.19.2.3073. [DOI] [PubMed] [Google Scholar]
- 23.Lee SL, Wang WW, Fanburg BL. Association of Tyr phosphorylation of GTPase-activating protein with mitogenic action of serotonin. Am J Physiol. 1997;272:C223–C230. doi: 10.1152/ajpcell.1997.272.1.C223. [DOI] [PubMed] [Google Scholar]
- 24.Lee SL, Wang W, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol. 1999;277:L282–L291. doi: 10.1152/ajplung.1999.277.2.L282. [DOI] [PubMed] [Google Scholar]
- 25.Robiolio P, et al. Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation. 1995;92:790–795. doi: 10.1161/01.cir.92.4.790. [DOI] [PubMed] [Google Scholar]
- 26.Connolly HM, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 27.Eddahibi S, et al. Serotonin uptake and citalopram binding in platelets from patients with chronic pulmonary hypertension. Am J Respir Crit Care Med. 1999;159:A165.. (Abstr.) [Google Scholar]