SUMMARY
A large body of work spanning 25+ years provides compelling evidence for the involvement of the basal ganglia-superior colliculus pathway in the initiation of rapid, orienting movements of the eyes, called saccades. The role of this pathway in saccade control is similar to the role of the basal ganglia-thalamic pathway in the control of skeletal movement: a transient cessation in tonic inhibition supplied by the basal ganglia to motor structures releases movements via the direct pathway whereas a transient increase in inhibition by the basal ganglia to motor structures prevents movements via the indirect pathway. In parallel with recent advances in the study and treatment of patients with basal ganglia disease and in animal experiments in the skeletal motor system, the results of studies exploring the role of the basal ganglia-superior colliculus pathway in saccades highlight the need for a revisiting of our understanding of the role of this pathway in saccades. The discovery of many different response profiles of neurons in the substantia nigra pars reticulata of the basal ganglia and in the superior colliculus, coupled with advances in experimental and statistical techniques including sophisticated behavioral procedures and multiple neuron recording and analysis, point toward a role for the basal ganglia-superior colliculus pathway in cognitive events intervening between vision and action, such as memory, target selection and saccade choice and valuation.
Keywords: saccades, action selection, substantia nigra, choice, decision-making, population coding, electrophysiology, cognition, inhibition
INTRODUCTION
A number of lines of investigation have converged on the idea that areas of the brain controlling eye movements play an active role in cognitive events - such as selection for perception and action, rather than being passive recipients of processed information from sensory areas. For example, altering frontal eye field cortical neuronal activity with electrical stimulation enhances the sensory responses of V4 neurons [1]. Altering superior colliculus neuronal activity with electrical stimulation enhances motion detection and discrimination abilities [2,3]. The command to make a saccadic eye movement to a visual target likely determines which stimulus becomes the target for a smooth pursuit eye movement [4,5] and a cue to make an eye movement enhances contrast sensitivity of visual responses of saccade-related neurons [6]. Moreover, a recent re-assessment of anatomical evidence reveals that many primary afferents also have branching projections to motor areas [7]. Therefore, the long-held view of motor areas as passive recipients of information processed largely within sensory areas should be re-evaluated.
The basal ganglia are a set of forebrain nuclei associated with debilitating disorders of movement such as Parkinson’s disease, Huntington’s disease and dystonia. Consistent with a role for motor areas in cognition, the neurons in many basal ganglia nuclei show activity that is modulated by reward or reward expectancy, perhaps linking information about reward to motor systems for the guidance of saccades [8]. The original recordings of basal ganglia neurons in monkeys revealed a preferential modulation of neuronal activity for eye movements guided by memory [9,10]. In light of the increasing evidence that motor areas play a role in cognitive processes and that the basal ganglia are a set of structures receiving input from virtually all areas of cerebral cortex and projecting back to motor and association areas [11], we review recent work suggesting that the role of the basal ganglia and its inputs to the superior colliculus extend beyond saccade initiation. We briefly review the current understanding of the role of the basal ganglia - superior colliculus pathway in saccade initiation. Then we highlight recent work in the superior colliculus and the substantia nigra pars reticulata showing that neuronal activity in each of these areas is correlated with events that precede the onset of saccades. Such events include memory, selection, and action choice. These experimental results underscore the fact that not all substantia nigra neuronal activity is a mirror-image of superior colliculus neuronal activity. We then discuss anatomical and physiological evidence showing that the pathway from the basal ganglia to the superior colliculus extends further than just inputs to saccade-related burst neurons of the superior colliculus. In particular, new evidence in the rodent shows that the substantia nigra pars reticulata targets inhibitory interneurons in addition to targeting excitatory output neurons of the superior collliculus. Next, we highlight clinical evidence showing that patients with Parkinson’s disease have disordered cognitive processes in addition to movement disturbances and indeed, experimental work in monkeys reveals a preferential role for basal ganglia pathways in the cognitive control of movement. Taken together, these results suggest a refined hypothesis for the role of the basal ganglia in saccade control: the basal ganglia come on-line for processes leading to a saccade choice when sensory information is absent or provides no new information. Conversely, when salient sensory information is present, the basal ganglia are less critical.
OVERVIEW OF THE BASAL GANGLIA-SUPERIOR COLLICULUS PATHWAY IN SACCADES: THE SUBSTANTIA NIGRA
The substantia nigra is comprised of a compact and a diffuse clustering of neurons - the pars compacta and the pars reticulata respectively. The compact and diffuse clusters of neurons making up the substantia nigra lie dorsal to the cerebral peduncle in the ventral midbrain. The pars compacta contains large cells that provide the dopaminergic input to the striatum also referred to as the nigro-striatal dopamine system. A growing body of evidence points toward a role for this pathway in mechanisms of reward. For recent reviews, see [12,8]. In what follows we focus on the role of the GABAergic neurons of the pars reticulata (nigra) in visual and oculomotor behavior.
The nigra is one of two output nuclei of the basal ganglia (see Figure 1; pallidal output not shown) and consists largely of GABAergic projection neurons that inhibit target neuronal activity in the superior colliculus (colliculus) and thalamus [13–15]. Nigral neurons display short-duration action potentials and spontaneous firing in vivo and in vitro with mean rates of 12–100 sp/s [16]. The tonic discharge of nigral neurons is thought to provide a sustained hyperpolarization of target neurons.
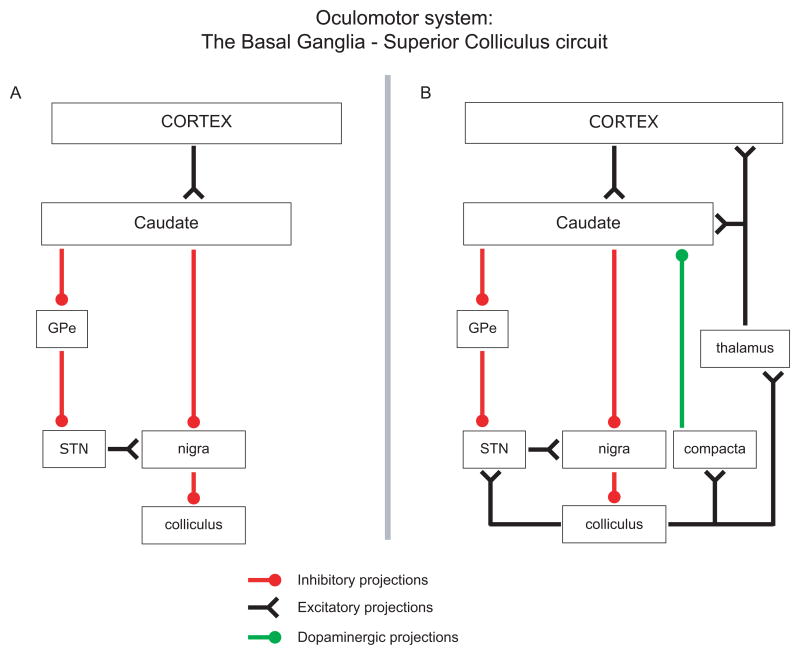
Figure 1.
Schematic diagrams of the basal ganglia-superior colliculus circuit. (A) Classical model of the basal ganglia - superior colliculus pathway. (B) Updated model in light of new evidence (not complete). As the only glutamatergic projection in the basal ganglia, the subthalamic nucleus can act to prevent a saccade by activating the nigra. This is the indirect pathway. By inhibiting the nigra, the striatum can cause disinhibition of the colliculus, thus activating a saccade. Newly revealed projections from the colliculus to the subthalamic nucleus and to the dopaminergic neuron containing pars compacta and ventral tegmental area, suggest a need to revise theories related to these areas. Red lines terminating in filled circles denote inhibitory projections. Black lines ending in inverted arrowheads denote excitatory projections. Green lines terminating in filled circles denote dopaminergic projections. Abbreviations: GPe = Globus Pallidus external; STN = subthalamic nucleus; nigra = subtantia nigra pars reticulata; compacta = subtsantia nigra pars compacta.
The relationship between saccadic eye movements and neuronal activity in the nigra was first identified from recordings performed in cat and monkey [9,17–20]. Studies of saccadic eye movements emphasize the nigra due to its direct projections to the colliculus [21]. The nigra is thought to act as a gate permitting collicular burst neurons (predorsal bundle neurons) to signal the brainstem thereby generating saccadic eye movements [22,23]. Support for this model is derived from several lines of evidence. First, electrical stimulation of the colliculus activates nigral neurons antidromically [20]. Second, nigral neurons have GABA-positive terminals surrounding the somas of predorsal bundle neurons within the colliculus [24,25]. Third, electrical stimulation of the nigra evokes IPSPs (inhibitory post synaptic potentials) in collicular predorsal bundle neurons [26]. These anatomical and physiological findings are consistent with the notion that the nigra provides strong synaptic inhibition of SC predorsal bundle neurons. Fourth, injection of the GABA antagonist, bicuculline, into the colliculus results in irrepressible saccadic eye movements in monkeys [27]. Likewise, injection of the GABA agonist, muscimol, into the nigra also produces irrepressible saccades in monkeys [28] and orienting head and body movements in cats [29]. Together these results are strong support for an inhibitory influence of the nigra on saccade-related burst neurons in the colliculus and therefore a role for this pathway in the initiation of saccadic eye movements.
Based on these findings and those in cerebral cortex [30], the current model of saccade generation is as follows: the drive to make a voluntary saccade presumably originates from neuronal activity within the eye fields of the cerebral cortex in the frontal and parietal lobes. Cortical neuronal discharge activates the medium spiny GABAergic neurons of the caudate nucleus, which in turn inhibit the tonic activity of GABAergic neurons in the nigra [31,32]. The pause in nigral activity results in a transient disinhibition of the colliculus driving the brainstem saccade generating machinery. This series of events results in a saccade [33].
DIVERSITY IN NEURONAL RESPONSE TYPES
Since the original work on the nigro-collicular pathway, a number of different neuronal response profiles in both the nigra (Figure 2A–F) and the colliculus (Figure 2G–N) have been described. In addition to the well-known decrease in neuronal discharge associated with saccade onset in the nigra of monkeys [19] or head movements in cats [17], some nigral neurons decrease their discharge rate either transiently or in a sustained manner with the appearance of visual stimuli, even if a saccade is not made [34,35]. Some neurons in the nigra increase their activity in association with visual and oculomotor events [34,36,37]. The increases, sustained pauses or transient visual pauses in nigral activity provide a wide dynamic range for signal transmission in the generation or control of saccades in this pathway. Although precisely how this dynamic range is used remains unexplored. One hypothesis is that nigral neurons showing pauses in discharge disinhibit collicular circuitry releasing a saccade whereas nigral neurons showing increases in discharge suppress SC circuitry preventing a saccade. Evidence in the cat showing opposite influences of these different nigral neurons on the ipsilateral and contralateral colliculus is consistent with this hypothesis [38]. Implicit in this hypothesis however, is that nigral neurons are segregated according to their inputs from the direct or indirect pathways. Evidence for this in monkey is unavailable currently. Nevertheless, the basal ganglia may suppress saccades through activation of nuclei in the indirect pathway such as the globus pallidus and the subthalamic nucleus (Figure 1). Recent recordings from the pallidum and the subthalamic nucleus in switching tasks and the anti-saccade task are consistent with this hypothesis [39,40].
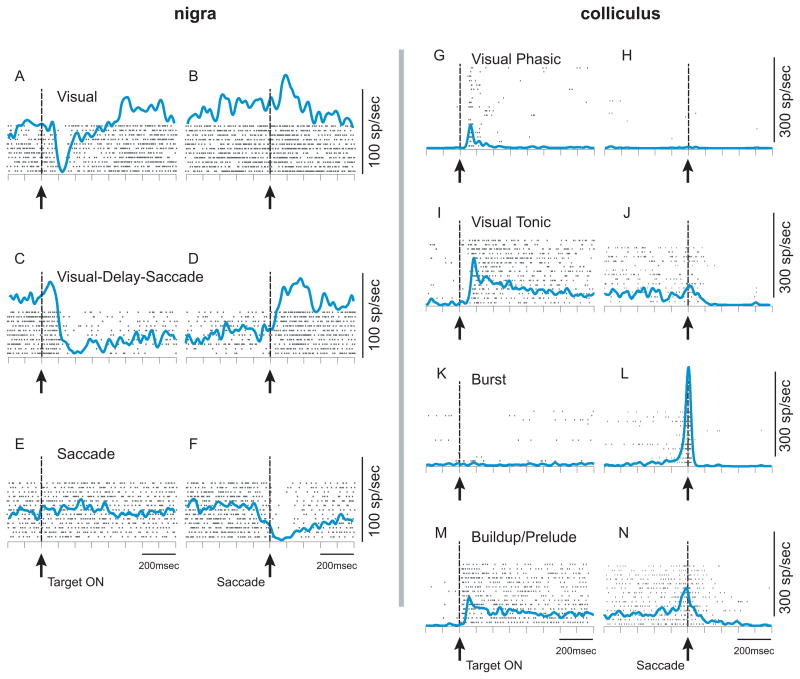
Figure 2.
Response profiles of nigral and collicular neurons are varied. Each tick represents the time of occurrence of an action potential. Each row of ticks indicates an individual trial. The blue lines show the spike density functions (σ = 12ms for the nigra and 10ms for the colliculus). The vertical dashed lines and upward arrows in each panel indicate the alignment times. Panels A, C, E, G, I, K, M are aligned on the onset of the visual stimulus labeled ‘Target on’. Panels B, D, F, H, J, L, N are aligned on saccade onset, labeled ‘Saccade’. Panels A–F are from nigral neurons. Panels G–N are collicular neurons. These data were collected while monkeys performed a visually-guided delayed saccade task. The neurons are not all from the same monkeys. Note that at the relevant phases of the task – visual target onset, delay-period and saccade, the nigral response is a reduction in firing rate, whereas that of the colliculus is an elevation in firing rate.
Our current understanding of the role of the nigro-collicular pathway in gating saccades relies exclusively on the connections between the nigral neurons that show a transient pause in activity around the time of saccade onset and the temporally correlated activity of the saccade-related burst neuron in the colliculus (cf., Figure 2E, F and K, L). Burst neuron activity is characterized by a high frequency, transient increase in discharge associated with the onset and duration of saccades and, as indicated above, is thought to be the command signal for saccades. [41–43]. However, like the nigra, recordings in the intermediate and deep layers of the monkey colliculus reveal a variety of neuronal response profiles in addition to the saccade-related burst. Some of the collicular response profiles can be seen in panels G–N of Figure 2. The response type heavily studied recently is the buildup neuron or prelude burst neuron [44,45]. This neuronal response profile is characterized by a discharge above baseline during the delay-period of a delayed-saccade task, while monkeys wait for a cue to make a saccade (Figure 2M,N). The delay-period activity is likely to be involved in processes that intervene between vision and action such as target selection for saccades [46,47] for smooth pursuit eye movements [5], shifts of attention [6,48–51], decision-making and saccade choice [52–56]. Even among neurons with delay-period activity in the colliculus, there may be functional differences that are only revealed when the appropriate task is used. For example, using a motion direction discrimination task, prelude bursters were further divided into two groups [52]. Among other differences, one group of neurons showed direction-selectivity and was rapidly predictive of the target choice whereas the second was not direction-selective and more slowly predictive of target choice. Recent advances in multi-neuron recording techniques allowing for simultaneously recording from multiple neuron types may help assay how each of these types contributes to cognitive events leading to oculomotor task performance [56,57].
TRANSFORMING SENSATION INTO ACTION: THE NIGRA
In much the same way that delay-period activity in the colliculus implicates this discharge in selection, nigral neuronal activity has also been implicated in selection based on the modulation of neuronal activity in advance of the onset of saccades [35]. In a simple task that manipulated the probability that any one of eight possible stimuli would be selected for a saccade, collicular neurons showed high levels of activity when only one target was available for selection. However, as the number of possible targets increased, this activity decreased, consistent with the reduced probability of any stimulus being the target. The sensory responses of neurons in the nigra exhibited a similar pattern, except these neurons showed pauses in discharge. For example, when only one target appeared, nigral neurons showed a maximal pause in activity. When multiple stimuli appeared, the pause in activity was reduced. Thus, nigral neurons, like neurons in the colliculus, reflect changes in the probability that a particular stimulus will be identified as a target for a saccadic eye movement. Therefore, these neurons signal events that precede saccadic eye movement generation, possibly saccade choice.
The larger and longer pause in nigral neuronal activity occurring with a single saccade target in the selection task would result in a larger disinhibition of colliculus when the probability of selecting a particular target is higher. Note that the latencies of visual responses in nigral neurons are slightly longer than those of collicular neurons [19,35] so the initial decrease in nigral activity could not contribute to the initial visual response changes in the colliculus (cf., Figure 2A,M). During the delay-period, before the saccade target was identified, collicular neurons showed a dramatic modulation of activity, whereas the nigral activity was modulated very little. This indicates that the suppressive effect of the multiple stimuli seen in the colliculus during the delay-period in this task does not result from an increased inhibition from the nigra. This is an example in which the nigral and the collicular neuronal activities are not mirror images of one another and it conflicts with proposed mechanisms of saccade initiation in which the nigra holds off the production of a saccade.
REFINING THE ROLE OF THE NIGRO-COLLICULAR PATHWAY: FROM CIRCUITS TO BEHAVIOR
The focus of the original nigro-collicular experiments in monkeys was on saccade-related burst neurons. In the cat, studies of the nigro-collicular projection emphasized tectoreticulospinal neurons [26] or neurons with visual responses [38]. It is likely that the tectoreticulospinal neurons in cat are homologous to the burst and buildup neurons in monkey [44,58]. Anatomical experiments in rat, cat and monkey suggest that the nigro-collicular pathway extends beyond just burst neurons. Indeed, anatomical results demonstrate that nigral neurons target multiple layers within the colliculus and that these inputs arise from different regions of the nigra [59–61]. Recent evidence in the monkey demonstrates that electrical activation of the nigra suppresses the activity of buildup neurons in both the ipsilateral and contralateral colliculus [62]. Taken together, these results demonstrate that the influence of the nigra on the colliculus extends beyond the saccade-related burst neuron.
Evidence that the nigra has a broad influence on collicular neuronal populations is revealed in recent work by the Isa group. They developed an in vitro preparation to study the nigro-collicular pathway in mice that were genetically-modified to express green fluorescent protein in GABAergic neurons of the colliculus and used it to test whether the nigra influences different neurons of the colliculus [63]. Stimulation of the dorso-lateral nigra produced monosynaptic IPSPs in both GABAergic and non-GABAergic neurons of the intermediate colliculus. Although GABAergic interneurons of the colliculus project within and between collicular layers [64,65], the projections of the nigro-recipient GABAergic neurons of the colliculus remain within the intermediate layer [63]. This elegant study reveals that the basal ganglia may also influence the spatiotemporal properties of neuronal activity within the colliculus by regulating inhibitory circuitry.
The role of the intrinsic inhibitory circuitry of the colliculus in behavior is poorly understood, particularly for monkeys [65,66]. However, recent experiments in monkeys are consistent with a role for the nigra in modulating inhibitory circuits within the colliculus leading to saccades [67]. Trained monkeys performed visually-guided and memory-guided saccades to locations throughout the visual field. On randomly interleaved trials, electrical stimulation of the nigra occurred. Some evidence suggests that stimulation reduces the activity of nigral cell bodies [68], whereas axonal processes are thought to increase activity with stimulation. [69]. A simple hypothesis therefore is that electrical stimulation of the nigra should increase efferent axonal discharge, further inhibiting the colliculus and suppressing saccades. At the very least, the latency of saccades should increase. In contrast to the prediction, nigral stimulation decreased the latency of visually-guided saccades and had varied effects on the latency of memory-guided saccades. Additionally, electrical stimulation of the nigra decreased the variability of visually-guided saccade latency. Since the activity of the colliculus buildup neurons consistently showed suppression with electrical stimulation of the nigra it is unlikely that the decrease in saccade latency resulted from antidromic activation of collicular neurons [62]. Rather, inhibition of local inhibitory interneurons analogous to those described by Isa and colleagues may underlie this result.
The observation that nigral stimulation decreases the variability of the latencies of saccades is hard to understand from a mechanism based simply on disinhibition. Work in the avian pallidal-thalamic pathway may shed light on alternative mechanisms [70]. Using whole-cell patch recordings of avian thalamic neurons, the investigators presented electrical stimulation to the avian pallidal nucleus mimicking realistic synaptic stimulation. These simulated spike trains resulted in precisely timed, single IPSPs and rebound action potentials in thalamic neurons. A similar mechanism might underlie synchronization of collicular activity leading to the reduction in variability of saccade onset times. Future work exploring the biophysics of collicular circuitry and the relationship of nigral inputs to the activity of collicular neuronal populations will provide a deeper insight into the mechanisms underlying how this pathway regulates behavior. In vitro studies in rodents and monkeys will be integral in elucidating this circuitry.
HINTS FROM THE CLINIC: MOVEMENT, COGNITION AND PARKINSON’S DISEASE
Akinesia or lack of movement, tremor and rigidity are hallmark symptoms seen in patients with Parkinson’s disease (PD). Another common characteristic is a shuffling pattern of gait. Patients often walk with very small and frequent steps. Interestingly, if these patients are given visual cues such as high contrast lines on the floor, the gait pattern can be normalized. This phenomenon is called paradoxical movement [71,72] Paradoxical movement exists in the saccadic system as well as the skeletal motor system. For example, it is generally understood that visually-guided saccades in patients with PD are difficult to distinguish from those of healthy controls. Memory-guided saccades in contrast, are often impaired compared to those of healthy controls [73]. Work in the monkey basal ganglia confirms these clinical observations. For example, electrical stimulation of the nigra preferentially alters memory-guided saccades but leaves visually-guided saccades relatively unaltered [67]. Recordings in the nigra reveal that neuronal activity is preferentially linked to memory-guided elbow movements [74]. In pallidal neurons, decreases in activity are more commonly seen during sensory-guided reaching whereas increases are more commonly seen during memory-guided reaching [75]. Thus, the basal ganglia of both patients and monkeys appear to play a preferential role in cognitive processes leading to action.
It is easy to imagine that alterations in the neuronal activity of basal ganglia neurons such as those described above might underlie some of the deficits seen in patients with basal ganglia disease, in particular the inability to produce movement guided by mechanisms associated with memory. What is less clear is through what circuitry visual information arises to provide support for movement in the diseased basal ganglia. One prominent hypothesis is that the cerebellum compensates for a damaged basal ganglia and assists in sensory-guided movement [71]. A second possibility that arises in light of recent anatomical studies is that visual information reaches the basal ganglia via the projection from the superior colliculus to the dopamine containing neurons of the pars compacta, the ventral tegmental area and the subthalamic nucleus [76–80; Figure 1B]. Visual information could reach the striatum via the nigrostriatal pathway, but also through thalamic inputs to the striatum [81]. Understanding the functional significance of these newly identified loops through the basal ganglia involving subcortical areas is an important area for future investigation.
In addition to basic science informing clinical medicine, the study of the basal ganglia is one area in which insights obtained from the clinic also directly impact basic science. For example, a number of lines of evidence support the hypothesis that oscillations in the β band among neurons within basal ganglia circuits underlie the symptoms of akinesia and rigidity in PD [82]. Using advances in multiple neuron recording techniques and statistical techniques, it was shown that neurons in the striatum as well as the external and internal divisions of the pallidum in monkeys display synchronous activity occurring within the β band frequency (12–30Hz). The synchronized activity of neurons is more likely to occur in the Parkinsonian state than in the healthy state [83,84]. However, work in the caudate of monkeys performing a saccade task shows that β band oscillations are present even in the healthy caudate [85]. Interestingly, the synchronous activity among neurons was decreased around the time of a saccade. These new approaches to understanding brain activity highlight the possible role of temporal information contained in the trains of action potentials rather than rate information. Although interesting, it remains to be explored what relationship these synchronous discharges have to oculomotor behavior and more importantly, how timing information is ultimately converted to the rate code required by motoneurons for all types of action.
CONCLUSIONS
Since the original work in the nigro-collicular pathway of monkeys, the results of basic science and clinical experiments reveal a much more nuanced picture. In addition to showing pauses in activity associated with saccade onset, nigral neurons decrease their activity well in advance of the onset of saccades. The modulation in activity of nigral neurons is predictive of an upcoming saccade choice implicating these neurons in cognitive processing leading to movement. Nigral neurons as well as neurons of other basal ganglia nuclei such as the pallidum and the subthalamic nucleus also likely contribute to the suppression of unwanted saccades. Whether these neurons are linked differently to the indirect and direct pathway of the basal ganglia is unknown. Moreover, it is still unclear whether the direct and indirect pathways operate in a competitive nature or cooperatively. The classical model suggests competition but an alternative is that selective populations of neurons are recruited from both pathways, or from one or the other pathway, but only in specific behavioral contexts. With the recent improvements in methods for simultaneous neuronal recordings, we are well-poised to begin addressing such questions.
The combination of state of the art molecular, anatomical and physiological techniques reveals that nigral inputs to the colliculus do more than simply gate saccade occurrence. This input likely plays a role in sculpting the shape of the population activity across the map of the colliculus. Bringing novel techniques in molecular biology together with those in electrophysiology and imaging to bear on circuit questions is bound to provide important new insights into this pathway and its role in cognition and movement control.
Finally, perhaps the best example of the confluence of clinical and basic science research is found in studies of basal ganglia disease processes. A continued emphasis on studying the basal ganglia and its outputs at all levels in multiple systems from in vitro and in vivo experiments in rodents and monkeys to investigations of patients with disease will no doubt continue to provide important insights into the role of the nigro-collicular pathway in health and disease.
Acknowledgments
The work in our laboratory is supported by NIH EY13692 (MAB), the Alice McPherson Eye Research Institute (MAB) and NCRR P51 RR000167 awarded to the Wisconsin National Primate Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
• of special interest
•• of outstanding interest
- 1.Armstrong KM, Fitzgerald JK, Moore T. Changes in visual receptive fields with microstimulation of frontal cortex. Neuron. 2006;50:791–798. doi: 10.1016/j.neuron.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller JR, Philiastides MG, Newsome WT. Inaugural Article: Microstimulation of the superior colliculus focuses attention without moving the eyes. PNAS. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner JL, Lisberger SG. Linked target selection for saccadic and smooth pursuit eye movements. J Neurosci. 2001;21:2075–2084. doi: 10.1523/JNEUROSCI.21-06-02075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krauzlis RJ, Dill N. Neural correlates of target choice for pursuit and saccades in the primate superior colliculus. Neuron. 2002;35:355–363. doi: 10.1016/s0896-6273(02)00756-0. [DOI] [PubMed] [Google Scholar]
- 6.Li X, Basso MA. Preparing to move increases the sensitivity of superior colliculus neurons. J Neurosci. 2008;28:4561–4577. doi: 10.1523/JNEUROSCI.5683-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guillery RW. Branching thalamic afferents link action and perception. J Neurophysiol. 2003;90:539–548. doi: 10.1152/jn.00337.2003. [DOI] [PubMed] [Google Scholar]
- 8•.Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. This review describes results of experiments from basal ganglia nuclei supporting the hypothesis that the neuronal activity in these basal ganglia structures related to eye movements can be best understood in the framework of reinforcement learning theory. [DOI] [PubMed] [Google Scholar]
- 9.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–1284. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- 10.Bayer HM, Handel A, Glimcher PW. Eye position and memory saccade related responses in substantia nigra pars reticulata. Exp Brain Res. 2004;154:428–441. doi: 10.1007/s00221-003-1735-7. [DOI] [PubMed] [Google Scholar]
- 11.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 12•.Schultz W. Behavioral theories and the neurophysiology of reward. Ann Rev Psych. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. This review describes some of the knowledge on brain mechanisms related to rewarding outcomes. It focuses on the activity of single neurons studied by neurophysiological techniques in behaving animals, in particular monkeys, and emphasizes the role of behavioral theories, such as animal learning theory and microeconomic utility theory, on the understanding of these brain mechanisms. [DOI] [PubMed] [Google Scholar]
- 13.DiChiara G, Porceddu ML, Morelli ML, Mulas ML, Gessa GL. Evidence for a GABAergic projection from the substantia nigra to the ventromedial thalamus and to the superior colliculus of the rat. Brain Res. 1979;176:273–284. doi: 10.1016/0006-8993(79)90983-1. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida M, Omata S. Blocking by picrotoxin of nigra-evoked inhibition of neurons of ventromedial nucleus of the thalamus. Experientia. 1979;35:794. doi: 10.1007/BF01968253. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier G, Thierry AM, Shibazaki T, Feger J. Evidence for a GABAergic inhibitory nigrotectal pathway in the rat. Neurosci Lett. 1981;21:67–70. doi: 10.1016/0304-3940(81)90059-8. [DOI] [PubMed] [Google Scholar]
- 16••.Deniau JM, Mailly P, Maurice N, Charpier S. The pars reticulata of the substantia nigra: a window to basal ganglia output. Prog Brain Res. 2007;160:151–172. doi: 10.1016/S0079-6123(06)60009-5. In this very nice review of the substantia nigra pars reticulata, the authors describe the data underlying contemporary hypotheses for the role of the nigra in behavior. The ideas are based on recent physiological and anatomical work primarily in the rodent. Work by this author shows that there is an elegant, onion-like topographical organization of the nigra linking the different cognitive and motor regions of the cerebral cortex with the rest of the basal ganglia. [DOI] [PubMed] [Google Scholar]
- 17.Joseph JP, Boussaoud D. Role of the cat substantia nigra pars reticulata in eye and head movements. I. Neural activity. Exp Brain Res. 1985;57:286–296. doi: 10.1007/BF00236534. [DOI] [PubMed] [Google Scholar]
- 18.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. II. Visual responses related to fixation of gaze. J Neurophysiol. 1983;49:1254–1267. doi: 10.1152/jn.1983.49.5.1254. [DOI] [PubMed] [Google Scholar]
- 19.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- 20.Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol. 1983;49:1285–1301. doi: 10.1152/jn.1983.49.5.1285. [DOI] [PubMed] [Google Scholar]
- 21.Jayaraman A, Batton RR, 3rd, Carpenter MB. Nigrotectal projections in the monkey: an autoradiographic study. Brain Res. 1977;135:147–152. doi: 10.1016/0006-8993(77)91058-7. [DOI] [PubMed] [Google Scholar]
- 22.Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- 23.Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. Rev Oculomot Res. 1989;3:213–255. [PubMed] [Google Scholar]
- 24.Behan M, Lin CS, Hall WC. The nigrotectal projection in the cat: an electron microscope autoradiographic study. J Neurosci. 1987;21:529–539. doi: 10.1016/0306-4522(87)90139-4. [DOI] [PubMed] [Google Scholar]
- 25.Bickford ME, Hall WC. The nigral projection to predorsal bundle cells in the superior colliculus of the rat. J Comp Neurol. 1992;319:11–33. doi: 10.1002/cne.903190105. [DOI] [PubMed] [Google Scholar]
- 26.Karabelas AB, Moschovakis AK. Nigral inhibitory termination on efferent neurons of the superior colliculus: an intracellular horseradish peroxidase study in the cat. J Comp Neurol. 1985;239:309–329. doi: 10.1002/cne.902390305. [DOI] [PubMed] [Google Scholar]
- 27.Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J Neurophysiol. 1985;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- 28.Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. II. Effects of muscimol in monkey substantia nigra pars reticulata. J Neurophysiol. 1985;53:292–308. doi: 10.1152/jn.1985.53.1.292. [DOI] [PubMed] [Google Scholar]
- 29.Boussaoud D, Joseph JP. Role of the cat substantia nigra pars reticulata in eye and head movements. II. Effects of local pharmacological injections. Exp Brain Res. 1985;57:297–304. doi: 10.1007/BF00236535. [DOI] [PubMed] [Google Scholar]
- 30.Schall JD, Thompson KG. Neural selection and control of visually guided eye movements. Ann Rev Neurosci. 1999;22:241–259. doi: 10.1146/annurev.neuro.22.1.241. [DOI] [PubMed] [Google Scholar]
- 31.Hikosaka O, Sakamoto M, Miyashita N. Effects of caudate nucleus stimulation on substantia nigra cell activity in monkey. Exp Brain Res. 1993;95:457–472. doi: 10.1007/BF00227139. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida M, Precht W. Monosynaptic inhibition of neurons of the substantia nigra by caudato-nigral fibers. Brain Res. 1971;32:225–228. doi: 10.1016/0006-8993(71)90170-3. [DOI] [PubMed] [Google Scholar]
- 33.Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- 34.Handel A, Glimcher PW. Quantitative analysis of substantia nigra pars reticulata activity during a visually guided saccade task. J Neurophysiol. 1999;82:3458–3475. doi: 10.1152/jn.1999.82.6.3458. [DOI] [PubMed] [Google Scholar]
- 35.Basso MA, Wurtz RH. Neuronal activity in substantia nigra pars reticulata during target selection. J Neurosci. 2002;22:1883–1894. doi: 10.1523/JNEUROSCI.22-05-01883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin S, Sommer MA. Activity of neurons in monkey globus pallidus during oculomotor behavior compared with that in substantia nigra pars reticulata. J Neurophysiol. 2010;103:1874–1887. doi: 10.1152/jn.00101.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basso MA, Pokorny JJ, Liu P. Activity of substantia nigra pars reticulata neurons during smooth pursuit eye movements in monkeys. Eur J Neurosci. 2005;22:448–464. doi: 10.1111/j.1460-9568.2005.04215.x. [DOI] [PubMed] [Google Scholar]
- 38•.Jiang H, Stein BE, McHaffie JG. Opposing basal ganglia processes shape midbrain visuomotor activity bilaterally. Nature. 2003;423:982. doi: 10.1038/nature01698. This very nice paper exploits electrophysiological and anatomical techniques to demonstrate that different classes of nigral neurons have different projection patterns to the colliculus in the cat. Whether the nigral neurons identified here in the anesthetized cat have homologies to those in the nigra of behaving monkeys remained to be determined. [DOI] [PubMed] [Google Scholar]
- 39.Isoda M, Hikosaka O. Role for subthalamic nucleus neurons in switching from automatic to controlled eye movement. J Neurosci. 2008;28:7209–7218. doi: 10.1523/JNEUROSCI.0487-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshida A, Tanaka M. Enhanced modulation of neuronal activity during antisaccades in the primate globus pallidus. Cereb Cortex. 2009;19:206–217. doi: 10.1093/cercor/bhn069. [DOI] [PubMed] [Google Scholar]
- 41.Wurtz RH, Goldberg ME. Activity of superior colliculus neurons in behaving monkeys. 3. Cells discharging before eye movemets. J Neurophysiol. 1972;35:575–686. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]
- 42.Sparks DL. Response properties of eye movement-related neurons in the monkey superior colliculus. Brain Res. 1975;90:147–152. doi: 10.1016/0006-8993(75)90690-3. [DOI] [PubMed] [Google Scholar]
- 43.Schiller PH, Stryker M. Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol. 1972;35:915–924. doi: 10.1152/jn.1972.35.6.915. [DOI] [PubMed] [Google Scholar]
- 44.Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J Neurophysiol. 1995;73:2313–2333. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- 45.Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature. 1992;355:542–545. doi: 10.1038/355542a0. [DOI] [PubMed] [Google Scholar]
- 46.McPeek RM, Keller EL. Saccade target selection in the superior colliculus during a visual search task. J Neurophysiol. 2002;88:2019–2034. doi: 10.1152/jn.2002.88.4.2019. [DOI] [PubMed] [Google Scholar]
- 47.Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci. 1998;18:7519–7534. doi: 10.1523/JNEUROSCI.18-18-07519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7:56. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- 49.Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- 50.Lovejoy LP, Krauzlis RJ. Inactivation of primate superior colliculus impairs covert selection of signals for perceptual judgments. Nat Neurosci. 2010;13:261–266. doi: 10.1038/nn.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X, Basso MA. Competitive stimulus interactions within single response fields of superior colliculus neurons. J Neurosci. 2005;25:11357–11373. doi: 10.1523/JNEUROSCI.3825-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horwitz GD, Newsome WT. Target selection for saccadic eye movements: Prelude activity in the superior colliculus during a direction-discrimination task. J Neurophysiol. 2001;86:2543–2558. doi: 10.1152/jn.2001.86.5.2543. [DOI] [PubMed] [Google Scholar]
- 53.Ratcliff R, Cherian A, Segraves M. A comparison of macaque behavior and superior colliculus neuronal activity to predictions from models of two-choice decisions. J Neurophysiol. 2003;90:1392. doi: 10.1152/jn.01049.2002. [DOI] [PubMed] [Google Scholar]
- 54.Ratcliff R, Hasegawa YT, Hasegawa RP, Smith PL, Segraves MA. Dual diffusion model for single-cell recording data from the superior colliculus in a brightness-discrimination task. J Neurophysiol. 2007;97:1756–1774. doi: 10.1152/jn.00393.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim B, Basso MA. A probabilistic strategy for understanding action selection. J Neurosci. 2010;30:2340–2355. doi: 10.1523/JNEUROSCI.1730-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim B, Basso MA. Saccade target selection in the superior colliculus: A signal detection theory approach. J Neurosci. 2008;28:2991–3007. doi: 10.1523/JNEUROSCI.5424-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Port NL, Wurtz RH. Sequential activity of simultaneously recorded neurons in the superior colliculus duirng curved saccades. J Neurophysiol. 2003;90:1887–1903. doi: 10.1152/jn.01151.2002. [DOI] [PubMed] [Google Scholar]
- 58.Moschovakis AK, Scudder CA, Highstein SM. The microscopic anatomy and physiology of the mammalian saccadic system. Prog Neurobiol. 1996;50:133–254. doi: 10.1016/s0301-0082(96)00034-2. [DOI] [PubMed] [Google Scholar]
- 59.Beckstead RM, Edwards SB, Frankfurter A. A comparison of the intranigral distribution of nigrotectal neurons labeled with horseradish peroxidase in the monkey, cat, and rat. J Neurosci. 1981;1:121–125. doi: 10.1523/JNEUROSCI.01-02-00121.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harting JK, Huerta MF, Hashikawa T, Weber JT, Van Lieshout DP. Neuroanatomical studies of the nigrotectal projection in the cat. J Comp Neurol. 1988;278:615–631. doi: 10.1002/cne.902780412. [DOI] [PubMed] [Google Scholar]
- 61.May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- 62.Liu P, Basso MA. Substantia nigra stimulation influences monkey superior colliculus neuronal activity bilaterally. J Neurophysiol. 2008;100:1098–1112. doi: 10.1152/jn.01043.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci. 2008;28:11071–11078. doi: 10.1523/JNEUROSCI.3263-08.2008. In this elegant study the authors developed a novel in vitro slice preparation containing the nigro-collicular pathway from GAD-67-GFP knock-in mice. Electrophysiological recording of identified GABAergic neurons in the colliculus combined with electrical stimulation showed that nigral axons contact inhibitory interneurons local to the intermediate layers of the colliculus. This nigral input is in addition to the well-known input to the excitatory, output neurons of the intermediate layers of the colliculus. This novel result points toward a role for these inputs in modulating inhibitory circuits of the colliculus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee PH, Sooksawate T, Yanagawa Y, Isa K, Isa T, Hall WC. Identity of a pathway for saccadic suppression. PNAS. 2007;104:6824–6827. doi: 10.1073/pnas.0701934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Behan M, Steinhacker K, Jeffrey-Borger S, Meredith MA. Chemoarchitecture of GABAergic neurons in the ferret superior colliculus. J Comp Neurol. 2002;452:334–359. doi: 10.1002/cne.10378. [DOI] [PubMed] [Google Scholar]
- 66.Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res. 1992;90:219–248. doi: 10.1016/s0079-6123(08)63616-x. [DOI] [PubMed] [Google Scholar]
- 67•.Basso MA, Liu P. Context-dependent effects of substantia nigra stimulation on eye movements. J Neurophysiol. 2007;97:4129–4142. doi: 10.1152/jn.00094.2007. Using electrical stimulation of the nigra in behaving monkeys, the authors demonstrated that alterations in the activity of the nigra preferentially influence the performance of memory-guided compared to visually-guided saccades. [DOI] [PubMed] [Google Scholar]
- 68.Lafreniere-Roula M, Hutchison WD, Lozano AM, Hodaie M, Dostrovsky JO. Microstimulation-induced inhibition as a tool to aid targeting the ventral border of the subthalamic nucleus Clinical article. J Neurosurg. 2009;111:724–728. doi: 10.3171/2009.3.JNS09111. [DOI] [PubMed] [Google Scholar]
- 69.McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol. 2004;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- 70••.Person AL, Perkel DJ. Unitary IPSPs drive precise thalamic spiking in a circuit required for learning. Neuron. 2005;46:129–140. doi: 10.1016/j.neuron.2004.12.057. The Area X - DLM projection is the avian homologue of the mammalian pallido-thalamic pathway. Introduction of simulated spike trains to this pathway resulted in precisely timed activation of DLM neurons. This experiment demonstrated excitation resulting from activating an inhibitory (Calyceal) synapse in the absence of excitatory input. This result suggests that the basal ganglia may assist in the regulation of the timing of synchronous activity within target structures via post-inhibitory rebound mechanisms. [DOI] [PubMed] [Google Scholar]
- 71.Glickstein M, Stein J. Paradoxical movement in Parkinson’s disease. Trends in Neurosciences. 1991;14:480. doi: 10.1016/0166-2236(91)90055-y. [DOI] [PubMed] [Google Scholar]
- 72.Morris M, Iansek R, Matyas T, Summers J. Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain. 1996;119:551–568. doi: 10.1093/brain/119.2.551. [DOI] [PubMed] [Google Scholar]
- 73.Kennard C, Lueck CJ. Oculomotor abnormalities in diseases of the basal ganglia. Reviews of Neurology. 1989;145:587–595. [PubMed] [Google Scholar]
- 74.Wichmann T, Kliem MA. Neuronal activity in the primate substantia nigra pars reticulata during the performance of simple and memory-guided elbow movements. J Neurophysiol. 2004;91:815–827. doi: 10.1152/jn.01180.2002. [DOI] [PubMed] [Google Scholar]
- 75.Turner RS, Anderson ME. Context-dependent modulation of movement-related discharge in the primate globus pallidus. J Neurosci. 2005;25:2965–2976. doi: 10.1523/JNEUROSCI.4036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coizet V, Comoli E, Westby GW, Redgrave P. Phasic activation of substantia nigra and the ventral tegmental area by chemical stimulation of the superior colliculus: an electrophysiological investigation in the rat. Eur J Neurosci. 2003;17:28–40. doi: 10.1046/j.1460-9568.2003.02415.x. [DOI] [PubMed] [Google Scholar]
- 77•.Comoli E, Coizet V, Boyes J, Bolam JP, Canteras NS, Quirk RH, Overton PG, Redgrave P. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6:974–980. doi: 10.1038/nn1113. Using a combination of anatomical and physiological techniques, these investigators revealed a short latency pathway arising from the colliculus and targeting both dopaminergic and non-dopaminergic neurons of the substantia nigra pars compacta. This discovery provides a novel pathway through which visual information can influence neurons in the ventral midbrain and has implications for the role of dopamine neurons in models of reinforcement learning. [DOI] [PubMed] [Google Scholar]
- 78.Dommett E, Coizet V, Blaha CD, Martindale J, Lefebvre V, Walton N, Mayhew JEW, Overton PG, Redgrave P. How Visual Stimuli Activate Dopaminergic Neurons at Short Latency. Science. 2005;307:1476–1479. doi: 10.1126/science.1107026. [DOI] [PubMed] [Google Scholar]
- 79•.May PJ, McHaffie JG, Stanford TR, Jiang H, Costello MG, Coizet V, Hayes LM, Haber SN, Redgrave P. Tectonigral projections in the primate: a pathway for pre-attentive sensory input to midbrain dopaminergic neurons. Eur J Neurosci. 2009;29:575–587. doi: 10.1111/j.1460-9568.2008.06596.x. This study establishes the existence of the collicular-nigral pathway in primates and also has implications for the role of monkey dopamine neurons in reinforcement learning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McHaffie JG, Jiang H, May PJ, Coizet V, Overton PG, Stein BE, Redgrave P. A direct projection from superior colliculus to substantia nigra pars compacta in the cat. J Neurosci. 2006;138:221–234. doi: 10.1016/j.neuroscience.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 81.Schulz JM, Redgrave P, Mehring C, Aertsen A, Clements KM, Wickens JR, Reynolds JNJ. Short-latency activation of striatal spiny neurons via subcortical visual pathways. J Neurosci. 2009;29:6336–6347. doi: 10.1523/JNEUROSCI.4815-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kühn AA, Tsui A, Aziz T, Ray N, Brücke C, Kupsch A, Schneider G-H, Brown P. Pathological synchronisation in the subthalamic nucleus of patients with Parkinson’s disease relates to both bradykinesia and rigidity. Exp Neurol. 2009;215:380–387. doi: 10.1016/j.expneurol.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 83.Raz A, Frechter-Mazar V, Feingold A, Abeles M, Vaadia E, Bergman H. Activity of pallidal and striatal tonically active neurons is correlated in MPTP-treated monkeys but not in normal monkeys. J Neurosci. 2001;21:128RC. doi: 10.1523/JNEUROSCI.21-03-j0006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raz A, Vaadia E, Bergman H. Firing patterns and correlations of spontaneous discharge of pallidal neurons in the normal and the tremulous 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine vervet model of Parkinsonism. J Neurosci. 2000;20:8559–8571. doi: 10.1523/JNEUROSCI.20-22-08559.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Courtemanche R, Fujii N, Graybiel AM. Synchronous, focally modulated {beta}-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci. 2003;23:11741–11752. doi: 10.1523/JNEUROSCI.23-37-11741.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]