Abstract
Background
Heart failure is a major contributor to cardiovascular morbidity and mortality in rheumatoid arthritis. However, little is known about myocardial structure and function in this population.
Methods
Using cardiac magnetic resonance imaging, measures of myocardial structure and function were assessed in men and women with rheumatoid arthritis enrolled in ESCAPE RA, a cohort study of subclinical cardiovascular disease in rheumatoid arthritis, and compared with controls without rheumatoid arthritis enrolled in the Baltimore cohort of the Multi-Ethnic Study of Atherosclerosis.
Results
Myocardial measures were compared between 75 rheumatoid arthritis patients and 225 matched controls. After adjustment, mean left-ventricular mass was 26 grams lower for the RA group compared to controls (p<0.001), an 18% difference. After similar adjustment, mean left-ventricular ejection fraction, cardiac output, and stroke volume were modestly lower in the rheumatoid arthritis group vs. controls. Mean left-ventricular end-systolic and end-diastolic volumes did not differ by rheumatoid arthritis status. Within the rheumatoid arthritis group, higher levels of anti-CCP antibodies and current use of biologics, but not other disease activity or severity measures, were associated with significantly lower adjusted mean left-ventricular mass, end-diastolic volume, and stroke volume, but not ejection fraction. The combined associations of anti-CCP antibody level and biologic use on myocardial measures were additive, without evidence of interaction.
Conclusions
These findings suggest that the progression to heart failure in RA may occur through reduced myocardial mass rather than hypertrophy. Both modifiable and non-modifiable factors may contribute to lower levels of left-ventricular mass and volume.
Keywords: myocardial dysfunction, heart failure, inflammation, cardiac imaging
INTRODUCTION
Rheumatoid arthritis (RA) is an inflammatory autoimmune disorder affecting approximately 1% of adults, frequently resulting in significant joint deformity and disability. Premature mortality, primarily due to cardiovascular disease (CVD) (1, 2), is a prominent feature of the disease. Average lifespan is reduced by 8–15 years in those with RA compared to age-matched controls (1). Evidence to date suggests that RA is an independent risk factor for heart failure (HF) (3) and that HF is a major contributor to overall CVD mortality in RA patients (4). However, the natural history of myocardial dysfunction leading to HF in RA has received little investigation.
While some established risk factors for HF, such as hypertension and diabetes, do not appear to be increased in RA patients (5), others, such as ischemic heart disease and insulin resistance, are more prevalent (6). However, these traditional risk factors do not appear to account for all of the excess risk for HF in RA patients (3). In the general population, elevated levels of cytokines, such as TNF-α and IL-6, in both serum and myocardium are predictive of, and associated with, severity of HF (7). Furthermore, cardiac-restricted overexpression of TNF-α in mice is associated with the spontaneous development of inflammatory myocarditis, leading to HF and death (8). Although there are no reports of myocardial cytokine expression in humans with RA, autopsy studies confirm the presence of inflammatory myocarditis and arteritis in a substantial fraction of patients (9). Furthermore, serum levels of TNF-α and IL-6 in patients with RA are considerably higher than those in individuals with HF (10, 11). Taken together, these data suggest that chronically high levels of systemic and/or myocardial inflammation may contribute to the increased rate of HF in RA.
Echocardiography and cardiac magnetic resonance (cMR) imaging have been successfully applied in the general population to identify early structural changes in the left ventricle (LV) that predate clinically overt HF. These studies have demonstrated that an increase in LV mass in asymptomatic patients is a potent predictor of incident HF, both systolic and diastolic, even in participants who remain free of obstructive coronary artery disease at follow-up (12, 13). We hypothesized that the prevalence of increased LV mass and of enlarged LV chamber volumes - myocardial parameters typically associated with progression to clinical HF – would be higher in RA compared to controls. We designed a comparative cohort study of LV structure and function in RA using the Multi-Ethnic Study of Atherosclerosis (MESA) (14) cohort as a control group. We selected cardiac MR as the methodology for measuring LV structure and function due to its superiority to echocardiography in resolution, sensitivity, and reproducibility (15–17).
METHODS
Participants and Enrollment
RA Participants
ESCAPE RA (Evaluation of Subclinical Cardiovascular disease And Predictors of Events in Rheumatoid Arthritis) is an ongoing cohort study investigating the prevalence, progression, and risk factors for subclinical CVD in men and women with RA (18). It was designed with identical inclusion and exclusion criteria (except for RA diagnosis) to MESA, a population-based cohort study of subclinical CVD with similar objectives. Enrollment in ESCAPE required a diagnosis of RA ≥ 6 months (American College of Rheumatology criteria for the classification of RA (19)) and an age between 45–84 years. Individuals with prevalent CVD, defined as self-reported physician-diagnosed myocardial infarction (MI), heart failure, coronary artery revascularization, angioplasty, peripheral arterial disease or procedures, pacemaker or defibrillator devices, and current atrial fibrillation, were excluded. Additional exclusions were weight exceeding 300 pounds (due to imaging equipment restrictions) and a computed tomography (CT) scan of the chest within six months prior to enrollment (to limit radiation exposure). Participants were recruited from among patients followed at the Johns Hopkins Arthritis Center and by referral from local rheumatologists. The study was approved by the Johns Hopkins Hospital Institutional Review Board. All participants provided written consent prior to enrollment occurring from October 2004 through May 2006.
Control Participants
Non-RA controls were Baltimore MESA participants. A description of the MESA study design and methods has been published (14). In brief, MESA enrolled a multiethnic cohort of 6,814 participants from six U.S. communities between 2000 and 2002, among whom 1,086 were enrolled by the Johns Hopkins (Baltimore) Field Center. A total of 788 of these underwent cMR at baseline, with the remainder not scanned due to contraindications to cMR. Thirteen controls who reported use of disease-modifying anti-rheumatic drugs (DMARDs) typically prescribed for the treatment of RA were excluded. From these 775, three controls were frequency matched at random to each RA patient based on gender, age, and ethnicity (Caucasian vs. non-Caucasian).
Assessments
Cardiac MR
All consenting MESA participants without contraindications underwent cMR imaging at the Johns Hopkins Cardiac MR Research Suite. Contraindications to cMR included mobile implanted metallic devices, reported metallic particles in eye, exposure to metalworking or welding in past without available skull radiographs, and claustrophobia. A subset of ESCAPE RA participants (predefined as 40% of the RA patient sample) were also scanned using the same MESA equipment, protocols, and personnel. RA patients were selected at random for scanning, without consideration of clinical characteristics. Characteristics of the subset undergoing cMR scanning were not significantly different from those of the full ESCAPE RA cohort. Electrocardiography-triggered contiguous breath-hold gradient echo cine imaging was performed on 1.5 T magnet scanners (GE Healthcare or Siemens Medical Solutions) as previously described (20). Calibration studies revealed no significant differences in the measurement of LV mass or volumes between scanners. Cine imaging was used to determine volumes (end-systolic and end-diastolic) and LV mass, with imaging data analyzed using commercially available software (MASS, version 4.2, Medis, Leiden, the Netherlands) by MESA trained readers. Cardiac output was defined as (end-diastolic volume – end-systolic volume) × heart rate. Ejection fraction was defined as stroke volume/end-diastolic volume. MESA inter-reader intraclass coefficients for LV mass, end-diastolic volume, and end-systolic volume were 0.97, 0.98, and 0.95, respectively (21).
Covariate Assessment
ESCAPE utilized identical questionnaires, equipment, methods, and quality control procedures as MESA. Study coordinators were trained and certified by MESA trainers. Information on demographics, smoking, and family history was collected by questionnaire. Resting blood pressure (BP) was measured three times in the seated position, and the average of the last two measurements was used in the analysis. Hypertension was defined by systolic BP ≥ 140 mmHg, diastolic BP ≥ 90, or antihypertensive medication use. Diabetes was defined as a fasting serum glucose ≥ 126 mg/dL or use of anti-diabetic medications. Physical activity was assessed using a modified 7-Day Physical Activity Recall questionnaire (7-Day PAR) (22). Body mass index (BMI) was calculated as weight (kg) divided by height2 (meters2). Body surface area (BSA) was calculated using the formula:
Prescription and over-the-counter medications used in the preceding two weeks were documented from containers supplied by the participant.
All participants underwent cardiac multidetector CT scanning using methodology described previously (23). Coronary calcification was quantified using the Agatston method (24), with a phantom of known calcium density scanned along with the participant to ensure standardization across scans.
RA-Specific Covariates
In RA patients, 44 joints were examined by a single trained assessor for swelling, tenderness, deformity, and surgical replacement or fusion. RA disease duration was calculated based on self-report from time of physician diagnosis. RA activity was calculated using the Disease Activity Score for 28 joints (DAS28) with CRP (25). Functional limitation was assessed with the Stanford Health Assessment Questionnaire (HAQ) (26). Current and past use of glucocorticoids, and biologic and non-biologic DMARDs were ascertained by interviews. Single view, anterior-posterior radiographs of the hands and feet were obtained and scored for radiographic damage of RA by using the Sharp-van der Heijde method (27) by a single, trained radiologist blinded to patient characteristics. For five subjects with incomplete radiographic assessments, the missing score (hand or foot) was imputed from the available data based on a regression equation using data from the remaining subjects in the cohort.
Laboratory Covariates
Fasting sera and plasma were separated by centrifugation, and stored at −70°C. All assays (except RA autoantibodies) were performed at MESA designated laboratories using MESA quality control procedures. High sensitivity C-reactive protein (CRP) and IL-6 were measured as previously described (28). LDL-cholesterol was estimated in plasma specimens having a triglyceride value <400 mg/dL using the Friedewald equation. The RA autoantibodies, rheumatoid factor (RF) and anti-cyclic citrullinated peptide (anti-CCP) antibodies, were defined as positive at concentrations of ≥ 60 and ≥ 40 units, respectively.
Statistical Analysis
Summary statistics for outcome and predictor variables were calculated for the RA and control groups with means and standard deviations for normally distributed continuous variables, medians and interquartile ranges for non-normally distributed continuous variables, and counts and percentages for categorical variables. Differences in means between the RA and control groups were compared using t-tests. When departures from normality for continuous variables were detected, differences in distributions were compared with the Kruskal-Wallis test. Differences in proportions for categorical variables were compared using the chi-square goodness-of-fit test or Fisher's exact test.
Linear regression was used to explore the association of MR-derived cardiac measures with RA status. First, in unadjusted models, only RA status was included as a predictor variable. Next, in multivariate models, sociodemographic and CV risk factors associated with both the exposure and outcome (or with strong biologic plausibility in relation to the outcome) at the p < 0.15 level in univariate analyses were included as potential confounders, with adjusted means, 95% confidence intervals, and p-values calculated for the groups according to RA status. The Shapiro-Wilk test was used to check for normality of distribution for the MR outcomes vis-à-vis the predictor variables, and no departures from normality were found. Variance inflation factors were calculated to ensure that variables with excessive collinearity were not co-modeled. Differences in cMR measures by RA status were explored in subgroups defined by gender and age. Analysis of covariance (ANCOVA) was used to test interactions between gender and RA status with regard to cardiac MR outcome measures.
Within the RA group, the associations of RA characteristics with cMR outcomes were explored, first in simple linear regression models with the individual RA characteristic of interest as the only variable in the model. Next, multivariate linear regression was used to adjust for pertinent demographic and CV risk factors. Finally, a complex model including all the RA characteristics identified at the p < 0.15 level from the adjusted models was constructed for each cMR outcome. The potential for interaction of RA characteristics with significant associations with the cMR outcome from the complex model was explored in stratified analyses. ANCOVA was used to test statistical significance of potential interactions.
Throughout, statistical calculations were performed using Intercooled Stata 9 (StataCorp, College Station, TX). In all tests, a two-tailed α of 0.05 was defined as the level of statistical significance.
RESULTS
Seventy-five RA patients and 225 frequency matched non-RA controls were studied. Participant characteristics according to RA status are summarized in Table 1. Compared to controls, mean systolic and diastolic blood pressures and heart rate were higher in RA patients. Diabetes and current smoking did not significantly differ by RA status. Whereas median CRP did not differ between groups; median IL-6 levels were significantly higher in RA patients. Although the prevalence of any coronary calcium did not differ between groups, median Agatston calcium scores among persons with any coronary calcium were higher in RA patients than in controls (p=0.023), consistent with results previously reported for the complete ESCAPE cohort (18). Within the RA group, median disease duration was 7 years, with 25% of patients having disease of less than four years. Mean current RA disease activity, based on the DAS28, was in the moderate range. All but one RA patient had evidence of radiographic damage (i.e. a total modified Sharp score > 0). Among the 66 patients (88%) treated with non-biologic DMARDs, methotrexate was prescribed in 53 (80%). Among the 37 patients receiving current biologic treatment, 36 (97%) were prescribed TNF inhibitors; 19 (51%) etanercept, 11 (30%) adalimumab, and 6 (16%) infliximab. The remaining patient had received a single treatment course of rituximab, but had been treated with TNF inhibitors prior.
Table 1.
Participant Characteristics According to Rheumatoid Arthritis Status
| Characteristic | RA n = 75 | Control n = 225 | p-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 59.1 ± 9.1 | 59.3 ± 8.9 | 0.87 |
| Female gender; n (%) | 39 (52.0) | 117 (52.0) | 1.00 |
| Caucasian; n (%) | 66 (88.0) | 198 (88.0) | 1.00 |
| Body surface area, m2 | 1.88 ± 0.22 | 1.91 ± 0.22 | 0.37 |
| Cardiovascular Risk Factors | |||
| Diabetes; (%) | 3 (4.0) | 13 (5.8) | 0.77 |
| Hypertension; n (%) | 41 (54.7) | 94 (41.8) | 0.052 |
| Systolic blood pressure, mm | 129 ± 17 | 123 ± 18 | 0.012 |
| Diastolic blood pressure, mm | 77 ± 9 | 71 ± 10 | <0.001 |
| Use of anti-hypertensives; n (%) | 27 (36.0) | 72 (32.0) | 0.52 |
| Heart rate, beats per minute | 70 ± 11 | 65 ± 11 | 0.003 |
| Current smoking; n (%) | 12 (16.0) | 28 (12.4) | 0.43 |
| HDL-C, mg/dL | 55 ± 20 | 51 ± 14 | 0.12 |
| LDL-C, mg/dL | 116 ± 33 | 120 ± 28 | 0.33 |
| Triglycerides, mg/dL | 113 ± 63 | 127 ± 75 | 0.11 |
| Current statin use; n (%) | 10 (13.3) | 37 (16.4) | 0.52 |
| Intentional exercise, min/day; median (IQR) | 21 (0 – 77) | 32 (9 – 79) | 0.081 |
| C-reactive protein, mg/L; median (IQR) | 2.0 (1.0 – 4.6) | 1.7 (0.8 – 4.1) | 0.35 |
| Interleukin-6, pg/mL; median (IQR) | 2.4 (1.5 – 6.0) | 1.1 (0.7 – 1.8) | <0.001 |
| Coronary calcium score >0; n (%) | 42 (56.0) | 114 (50.7) | 0.42 |
| Coronary calcium score*; median (IQR) | 190 (104 – 540) | 93 (22 – 279) | 0.023 |
| Rheumatoid Arthritis Characteristics | |||
| RA duration, years; median (IQR) | 7 (4 – 17) | -- | -- |
| Rheumatoid factor seropositivity, n (%) | 50 (66.7) | -- | -- |
| anti-CCP seropositivity, n (%) | 53 (70.7) | -- | -- |
| Any HLA-DRB1 shared epitope alleles, n (%) | 58 (77.3) | -- | -- |
| Disease Activity Score (28 joints with CRP) | 3.51 ± 1.10 | -- | -- |
| HAQ score; median (IQR) | 0.63 (0 – 1.25) | -- | -- |
| Total modified Sharp score; median (IQR) | 49 (19 – 110) | -- | -- |
| Current rheumatoid nodules, n (%) | 25 (33.3) | -- | -- |
| Current prednisone use, n (%) | 29 (38.7) | -- | -- |
| Cumulative prednisone**, grams; median (IQR) | 5.0 (1.8 – 10.4) | -- | -- |
| Current NSAID use, n (%) | 49 (65.3) | -- | -- |
| Any current non-biologic DMARDs, n (%) | 66 (88.0) | -- | -- |
| Current methotrexate, n (%) | 53 (70.7) | -- | -- |
| Any current biologic DMARD use, n (%) | 37 (49.3) | -- | -- |
| TNF inhibitors, n (%) | 36 (48.0) | -- | -- |
| Rituximab, n (%) | 1 (1.3) | -- | -- |
values presented are mean ± standard deviation unless otherwise noted
For patients with a coronary calcium score>0 only
For current or past users only
RA=rheumatoid arthritis; HAQ=Health Assessment Questionnaire; NSAID=non-steroidal anti-inflammatory drug; TNF=tumor necrosis factor
Association of RA Status with Cardiac MR Measures of Structure and Function
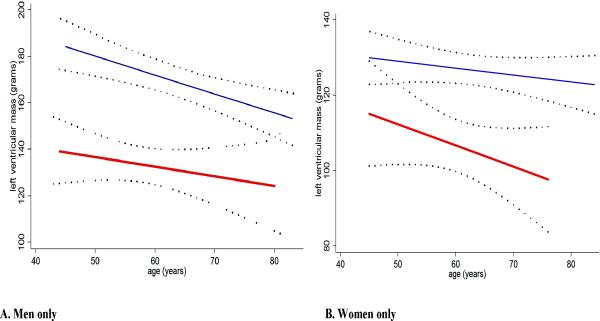
In univariate analyses of the combined RA and control groups, LV mass was positively and significantly associated with male gender, increasing BMI and body surface area, diabetes, hypertension, current smoking, increasing exercise, and prevalent coronary calcification, and inversely associated with increasing age, heart rate, and HDL cholesterol (data summarized in supplemental table). There was no statistical interaction of RA status on the association of any of these variables with LV mass (supplemental table). Further, in unadjusted analyses, mean LV mass was 26 grams lower in the RA compared to the control group (p<0.001), representing an 18% difference (Table 2). After adjusting for unbalanced clinical characteristics (systolic and diastolic blood pressures, heart rate, HDL, triglycerides, exercise, and coronary calcium) mean LV mass remained 26 grams lower for the RA group (p<0.001). Further adjustment for IL-6 or CRP did not change this association (data not shown). Similar differences were observed for LV mass indexed to body surface area and LV mass indexed to LV end-diastolic volume (Table 2). Furthermore, significantly lower mean LV mass was observed in both men and women with RA (Figure 1), although the relative reduction in mean LV mass was greater for men than women when compared to their respective controls (20% and 14% lower, respectively) (p for heterogeneity=0.028). For both genders, mean LV mass declined with advancing age regardless of RA status (Figure 2).
Table 2.
Crude and Adjusted Measures of Cardiac Structure and Function According to RA Status
| Unadjusted Mean (95% CI) |
Adjusted* Mean (95% CI) |
||||||
|---|---|---|---|---|---|---|---|
| Cardiac MR Measure | RA | Control | p-value | RA | Control | Difference** | p-value |
| LVM, grams | 120 (111, 129) | 146 (141, 150) | <0.001 | 119 (112, 127) | 145 (141, 149) | − 17.9% | <0.001 |
| LVMI, grams per m2 of BSA | 63 (60, 67) | 76 (74, 78) | <0.001 | 64 (61, 67) | 75 (74, 77) | − 14.7% | <0.001 |
| LVM/LVEDV, grams per mL | 0.99 (0.93, 1.06) | 1.16 (1.13, 1.19) | <0.001 | 0.98 (0.92, 1.04) | 1.16 (1.13, 1.19) | − 15.5% | <0.001 |
| LVEF, % | 66 (64, 67) | 68 (67, 69) | 0.006 | 65 (64, 67) | 68 (67, 69) | − 4.4% | 0.003 |
| Cardiac Output, L/min | 5.5 (5.2, 5.8) | 5.6 (5.4, 5.8) | 0.58 | 5.3 (5.0, 5.6) | 5.7 (5.5, 5.9) | − 7.7% | 0.034 |
| LVSV, mL | 80 (75, 84) | 87 (84, 89) | 0.012 | 80 (75, 85) | 86 (84, 89) | − 7.0% | 0.019 |
| LVSVI, mL per m2 of BSA | 42 (40, 44) | 46 (44, 47) | 0.012 | 43 (41, 45) | 45 (44, 47) | − 5.6% | 0.041 |
| LVEDV, mL | 122 (115, 130) | 128 (124, 132) | 0.18 | 123 (116,131) | 127 (124,131) | − 3.2% | 0.34 |
| LVEDVI, mL per m2 of BSA | 65 (62, 68) | 67 (65, 69) | 0.23 | 66 (63, 69) | 67 (65, 68) | − 1.2% | 0.67 |
| LVESV, mL | 40 (36, 44) | 42 (39, 44) | 0.66 | 41 (38, 45) | 41 (39, 43) | -- | 0.92 |
| LVESVI, mL per m2 of BSA | 21 (20, 23) | 22 (21, 23) | 0.92 | 22 (20, 24) | 22 (20, 22) | -- | 0.56 |
Adjusted for systolic and diastolic blood pressures, heart rate, HDL cholesterol, triglycerides, habitual exercise, and coronary calcium score
Percent difference in the adjusted mean values of the indicated variable for the RA group compared with the control group.
LVM=left ventricular mass; LVMI=left ventricular mass index; LVEF=left ventricular ejection fraction; LVSV=left ventricular stroke volume; LVSVI=left ventricular stroke volume index; LVEDV=left ventricular end diastolic volume; LVEDVI=left ventricular end diastolic volume index
Figure 1. Adjusted Mean Left Ventricular Mass by Cardiac Magnetic Resonance Imaging According to RA Status and Gender.
Square = adjusted mean for the RA group. Diamond = adjusted mean for the control group. Limits represent 95% confidence intervals for the calculated adjusted means. Adjusted for systolic and diastolic blood pressure, heart rate, HDL cholesterol, triglycerides, exercise, and coronary calcium score. RA=rheumatoid arthritis
Figure 2. Linear Association of Left-Ventricular Mass with Age According to RA Status, by Gender.
A=men only; B=women only. RA group = thick red line; non-RA control group = thin blue line; Dotted lines= 95% confidence intervals about the least squares estimates of the linear association of left ventricular mass with age RA=rheumatoid arthritis
After adjustment, mean LV ejection fraction, cardiac output, and LV stroke volume were modestly, but significantly, lower in the RA group than in the control group. In contrast, adjusted mean LV end-diastolic and systolic volumes (including these measures indexed to body surface area) did not significantly differ according to RA status (Table 2).
Association of RA Characteristics with Measures of Cardiac Structure and Function
In analyses adjusted only for body surface area, increasing anti-CCP antibody level and the use of biologic DMARDs were significantly inversely associated with measures of cardiac structure, in particular with lower mean LV stroke and end-diastolic volumes (Table 3, Model 1). The association with anti-CCP antibody remained significant after adjusting for the demographic and cardiovascular risk factors that had the strongest associations with the outcomes (age, gender, systolic blood pressure, use of anti-hypertensive medications, and current smoking) (Table 3, Models 2 and 3); however, the adjusted association of biologic DMARD use and lower mean LV mass was only of borderline significance. Importantly, neither RF seropositivity, increasing RF titer, nor for the presence of the HLA-DRB1 shared epitope were associated with decreases in measures of cardiac structure (data not shown). Likewise, other measures of RA disease activity (DAS28, physician global assessment), severity (e.g. total modified Sharp score, HAQ), systemic inflammation (log CRP and IL-6 levels), and other treatment (use of non-biologic DMARDs, current and cumulative use of glucocorticoids, and NSAIDs) were not significantly associated with mean measures of LV structure (data not shown). Despite associations with measures of cardiac structure, anti-CCP antibody level and biologic DMARD use were not significantly associated with cardiac function measures (i.e. LV ejection fraction or cardiac output), in either crude or adjusted analyses.
Table 3.
Crude and Adjusted Associations of Selected RA Characterisitics with Cardiac MR Measures of Left-Ventricular Structure and Function
| Adjusted Model 1† |
Adjusted Model 2‡ |
Adjusted Model 3§ |
|||||
|---|---|---|---|---|---|---|---|
| Outcome | Characteristic | β | (95% CI) | β | (95% CI) | β | (95% CI) |
| LVM | |||||||
| Anti-CCP, per 10 units | −0.35 | (−0.89, 0.20) | −0.52* | (−0.97, −0.06) | −0.46* | (−0.91, −0.01) | |
| Any biologic DMARD | −6.54 | (−14.69, 1.62) | −6.69** | (−13.48, 0.10) | −5.75** | (−12.44, 0.95) | |
| LVEF | |||||||
| Anti-CCP, per 10 units | −0.005 | (−0.02, 0.02) | −0.02 | (−0.22, 0.19) | −0.01 | (−0.33, 0.20) | |
| Any biologic DMARD | −0.06 | (−3.15, 3.04) | −0.58 | (−3.60, 2.44) | −0.23 | (−3.35, 2.90) | |
| LVSV | |||||||
| Anti-CCP, per 10 units | −0.54* | (−0.97, −0.10) | −0.55* | (−1.00, −0.10) | −0.48* | (−0.92, −0.04) | |
| Any biologic DMARD | −7.32* | (−13.98, −0.67) | −8.37* | (−15.07, −1.68) | −7.39* | (−13.97, −0.82) | |
| LVEDV | |||||||
| Anti-CCP, per 10 units | −0.87* | (−1.52, −0.21) | −0.84* | (−1.53, −0.16) | −0.75* | (−1.42, −0.07) | |
| Any biologic DMARD | −11.04* | (−21.05, −1.02) | −11.80* | (−22.02, −1.58) | −10.28* | (−20.31, −0.24) | |
RA characteristics tested without significant associations with cardiac MR outcomes included RA duration, rheumatoid factor seropositivity, presence of the HLA-DRB1 “shared epitope”, DAS28, HAQ, total modified Sharp score, physician global assessment of disease activity, presence of rheumatoid nodules, log CRP, log IL-6, current prednisone use, dose of current prednisone, cumulative prednisone dose, use of non-biologic DMARDs, and use of NS AIDs (non-selective and COX-2 selective)
p < 0.05
0.10 > p < 0.05
RA characterisitics were individually adjusted only for body surface area except for the outcome of LVEF, in which only crude associations are shown
RA characteristics listed were individually adjusted for age, gender, body surface area, systolic blood pressure, antihypertensive use, and current smoking
RA characteristics listed were modeled simultaneously and adjusted for Model 2 demographic and cardiovascular risk covariates
Because of the potential association of biologic DMARD use with anti-CCP antibody level, models were constructed that simultaneously included both these variables with adjustment for demographic and cardiovascular risk factors (Table 3, Model 3); no substantial differences were seen when compared to the model including these variables separately.(i.e., Table 3, model 2). Models considering the combined associations of these variables (Figure 3) in mutually exclusive groups defined by current biologic use and anti-CCP antibody levels (above or below the median of 128 units) demonstrated additive effects without evidence of significant interactions (i.e. p>0.05 for all tests of statistical interaction) between current biologic use and anti-CCP antibody levels. The number of RA patients with former, but not current, exposure to biologics (n=4) was not sufficient to analyze this group separately. However, excluding these four from the analyses did not significantly alter the associations of current biologic use with LV structure measures (data not shown).
Figure 3. Adjusted Individual and Combined Associations of anti-CCP Antibody Level and Biologic DMARD Use with Cardiac Magnetic Resonance Measures of Left Ventricular Structure and Function.
A. LV mass (grams); B. LV stroke volume (mL); C. LV end-diastolic volume (mL). Open square = anti-CCP < median (128 units), no current biologics; Solid square = anti-CCP < median, current biologics used; Open diamond = anti-CCP > median, no current biologics; Solid diamond = anti-CCP > median, current biologics used. Means and 95% confidence intervals are depicted. Red Solid horizontal line = Adjusted mean for the control group; Red horizontal dashed line = 95% confidence limit for the adjusted mean for the control group. Adjusted for body surface area, age, gender, systolic blood pressure, antihypertensive use, and current smoking. DMARD=disease modifying anti-rheumatic drug; LV=left-ventricular
DISCUSSION
To our knowledge, this study is the first to utilize cMR to investigate cardiac structure and function in RA. We found that mean LV mass was strikingly lower in RA vs. controls, even after accounting for demographic and cardiovascular risk factors, with differences more pronounced in men than women. Two RA characteristics, increasing titer of anti-CCP antibodies and use of biologic DMARDs, were significantly and independently associated with lower adjusted mean LV mass, stroke volume, and end-diastolic volume but not with ejection fraction. Other RA disease activity and severity measures, systemic inflammatory markers, and other RA therapies were not associated with cardiac measures.
Our finding of lower LV mass was unexpected and contrary to our original hypothesis that RA patients without clinical CV disease would exhibit myocardial features that antedate clinical HF in the general population, such as increased LV mass, LV chamber dilation, and decreased ejection fraction (29, 30). Although the etiology and implications of our finding of lower LV mass in RA patients are not readily apparent, it is tempting to speculate that the lower LV mass observed in RA patients could be related to chronic myocarditis and/or microvascular hypoperfusion resulting in myocyte loss and/or fibrosis. Supporting this, Raza et al (31) reported a case of microvascular dysfunction in an RA patient that was reversed with immunosuppression. Alternatively, lower LV mass could reflect reduced physical conditioning in RA patients; although this is less likely since adjustment for exercise did not alter the relationship between RA status and LV mass. While macrovascular ischemia might contribute to myocardial abnormalities (32), adjustment for coronary calcification did not alter the differences in myocardial measures between RA patients and controls. Our data raise the hypothesis that RA associated HF may progress along a different pre-clinical pathway than HF due to coronary artery disease or chronic hypertension. Longitudinal follow-up and histopathological studies are needed to evaluate these etiologic possibilities. Our observation that age-associated declines in LV mass were exaggerated in RA patients compared to controls lends credence to the concept of the RA disease state as one of “accelerated aging” with the myocardia of RA patients effectively older than those of their non-RA counterparts.
Two categories of HF are recognized clinically. Systolic HF is characterized by contractile dysfunction with eccentric LV remodeling, ventricular dilation, reduced ejection fraction, and decreased mass/volume ratio. In contrast, non-systolic (or diastolic) HF is characterized by concentric remodeling resulting in a stiff ventricle with relatively preserved ejection fraction and chamber volumes. It has been suggested that diastolic dysfunction is a precursor to both types of HF (33). A number of two dimensional echocardiographic studies have explored LV function in RA, with most reporting increased diastolic dysfunction with preserved systolic function, while a minority report systolic dysfunction (reviewed in (34) also (35)). While our cMR sequences did not allow for investigation of diastolic function, our results do suggest that systolic function may be very modestly reduced in asymptomatic RA patients.
A single study, recently reported by Rudominer et al (36), evaluated LV mass in RA patients vs. controls. In contrast to our finding of a lower mean LV mass index, Rudominer et al detected a higher mean LV mass index in their RA group compared to a control group matched on demographics and hypertension status. There are several potential explanations for this discrepancy. For one, the RA populations studied are quite dissimilar. The Rudominer cohort was considerably younger than ours (mean age of 47 years) and almost exclusively female (99%). Because differences in LV mass between RA patients and controls in our study were more pronounced in men, and increased with age in women, both the absence of men and older women in the Rudominer study could have resulted in missing these preferentially affected individuals. However, the more likely explanation relates to the different imaging techniques utilized. The formulas for calculating LV mass using M-mode and two-dimensional echocardiography utilized by Rudominer et al extrapolate measures of myocardial thickness and chamber size obtained in one or two planes, relying on assumptions of ventricular morphology and wall thickness. This method overestimates LV mass in the setting of eccentric LV hypertrophy (37, 38). Thus, bias in comparisons might be introduced if eccentric morphology is more prominent in the RA group. This was noted in the Rudominer study, in which 15 of 16 patients with LV hypertrophy were found to have eccentric remodeling. cMR avoids this potential error, as measurement of ventricular mass is three-dimensional and does not rely on assumptions of myocardial morphology. However, additional studies in a larger group of RA patients are required to reconcile the divergent findings between our cMR and the echocardiographic study of Rudominer et al.
Although our initial hypothesis was that chronic elevation of inflammatory cytokines would result in greater LV mass (hypertrophy) in the RA group, we found no significant relationship between current levels of inflammation and measures of LV structure or function. However, because of wide variations in CRP and IL-6 levels over time in RA patients, particularly with treatment, current levels may not be reflective of cumulative exposure. Longitudinal studies will be needed to evaluate the cumulative effect of cytokines the myocardium.
Interestingly, treatment with biologics (predominantly anti-TNF therapy) and titer of anti-CCP antibody were associated with lower LV mass and smaller LV volumes in the RA group. It is possible that these variables are a proxy for RA disease severity and that disease severity accounts for the cardiac abnormalities observed. However, we did not observe similar associations with other RA severity measures, such as RF, presence of the shared epitope, or degree of radiographic joint damage. There is biologic plausibility for both of these factors to mediate the observed outcomes. Augmenting myocardial TNF-α in experimental animals induces LV hypertrophy and dilation (39), and anti-TNF treatment limits these changes. Thus, anti-TNF therapy could be protective in RA by reducing the pathogenic effects of TNF-α. However, a minimum level of TNF-α may actually be required to maintain myocardial structural homeostasis, as suggested by studies in another mouse model in which myocardial TNF-α is rendered completely inactive (40). Such mice demonstrate restrictive cardiomyopathy and dramatically reduced ventricular volumes. Thus, anti-TNF therapy in RA patients may reduce TNF-α levels below those required to maintain normal myocardial structure. The cross-sectional nature of our study does not enable us to distinguish between these two possibilities. Incidentally, studies in humans receiving anti-TNF therapy have yielded conflicting results. In persons without RA with advanced HF, TNF inhibition was not effective in reducing morbidity or mortality (41, 42), with a suggestion of worsening in one trial (41). In RA patients, case reports and case series of new onset or worsening HF after anti-TNF therapy have been published (43). In contrast, population-based studies of RA patients have reported lower (44), similar (45), and higher (46) rates of incident HF in anti-TNF treated vs. non-anti-TNF treated RA patients. Thus, definitive conclusions on the effect of TNF inhibitors on the myocardium will require a prospective interventional study. Furthermore, whether a reduction in LV mass and ventricular volumes is ultimately protective or pathogenic in the RA myocardium will also require extended observation.
Anti-CCP antibodies were also a potent predictor of lower LV mass and ventricular chamber volumes in our RA patients, suggesting a potential pathophysiological link to HF. Anti-CCP antibodies are directed against citrullinated proteins such as vimentin and fibronectin. Interestingly, anti-vimentin and -fibronectin, antibodies have been identified and associated with induction of inflammatory myocarditis and cardiac transplant rejection in animals (47, 48), and were predictive of transplant rejection in humans (49). However, antibodies against the citrullinated homologs were not investigated. Citrullination of skeletal muscle has been demonstrated in patients with rheumatic disease (50), but we are not aware of any studies that have investigated citrullination in the rheumatoid myocardium. It is tempting to speculate that anti-CCP antibodies may be directed against myocardial proteins and that these immune processes are involved causally in the cardiac abnormalities observed in our study.
There are notable limitations to our study. Despite being the first study utilizing cMR in RA patients, the sample of RA patients was relatively small. However, as differences between RA patients and controls for the outcome measures were generally large with limited variability, even small differences were detectable within the bounds of statistical significance. We were, however, limited in our ability to perform many subgroup analyses. We plan to incorporate additional patients into future studies in order to validate these findings. In addition, our cross-sectional design does not permit conclusive inference regarding cause and effect. Longitudinal follow-up with repeat cMR on these patients is underway to identify predictors of change in myocardial measures.
In summary, RA patients demonstrated markedly lower LV mass compared with controls and modest decreases in ejection fraction, cardiac output, and stroke volume. Differences in LV mass between RA patients and controls were greater for men than women. Higher titer of anti-CCP antibodies and use of biologics were associated with lower LV mass, and LV stroke and end-diastolic volumes after accounting for pertinent confounders. Further investigation is underway to better understand the implications of these findings on the development of clinical HF in RA.
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to the ESCAPE RA Staff, Marilyn Towns, Michelle Jones, Patricia Jones, Marissa Hildebrandt, and Shawn Franckowiak, for their dedication and hard work and to the staffs of the field center of the Baltimore MESA cohort and the MESA Coordinating Center at the University of Washington, Seattle.
Drs. Uzma Haque, Clifton Bingham III, Carol Ziminski, Jill Ratain, Ira Fine, Joyce Kopicky-Burd, David McGinnis, Andrea Marx, Howard Hauptman, Achini Perera, Peter Holt, Alan Matsumoto, Megan Clowse, Gordon Lam and others generously recommended their patients for this study.
FUNDING SOURCES This work is supported by Grant Number AR 050026 from the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases and contracts N01-HC-95159 through N01-HC-95166 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. Additional funding was provided by grant support from the Arthritis National Research Foundation.
REFERENCES
- 1.Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol. 2003;30(1):36–40. [PubMed] [Google Scholar]
- 2.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52(3):722–32. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 3.Nicola PJ, Maradit-Kremers H, Roger VL, Jacobsen SJ, Crowson CS, Ballman KV, et al. The risk of congestive heart failure in rheumatoid arthritis - A population-based study over 46 years. Arthritis Rheum. 2005;52(2):412–20. doi: 10.1002/art.20855. [DOI] [PubMed] [Google Scholar]
- 4.Nicola PJ, Crowson CS, Maradit-Kremers H, Ballman KV, Roger VL, Jacobsen SJ, et al. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum. 2006;54(1):60–7. doi: 10.1002/art.21560. [DOI] [PubMed] [Google Scholar]
- 5.del Rincon ID, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44(12):2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 6.Dessein PH, Stanwix AE, Joffe BI. Cardiovascular risk in rheumatoid arthritis versus osteoarthritis: acute phase response related decreased insulin sensitivity and high-density lipoprotein cholesterol as well as clustering of metabolic syndrome features in rheumatoid arthritis. Arthritis Res. 2002;4(5):R5. doi: 10.1186/ar428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 8.Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, Demayo FJ, Spinale FG, et al. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104(7):826–31. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- 9.Lebowitz WB. Heart in Rheumatoid Arthritis (Rheumatoid Disease) - A Clinical and Pathological Study of 62 Cases. Ann Intern Med. 1963;58(1):102. doi: 10.7326/0003-4819-58-1-102. [DOI] [PubMed] [Google Scholar]
- 10.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated Circulating Levels of Tumor-Necrosis-Factor in Severe Chronic Heart-Failure. N Engl J Med. 1990;323(4):236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 11.Dibbs Z, Thornby J, White BG, Mann DL. Natural variability of circulating levels of cytokines and cytokine receptors in patients with heart failure: implications for clinical trials. Journal of the American College of Cardiology. 1999;33(7):1935–42. doi: 10.1016/s0735-1097(99)00130-8. [DOI] [PubMed] [Google Scholar]
- 12.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990 May 31;322(22):1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 13.Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87(9):1051–7. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Reichek N, Helak J, Plappert T, Sutton MS, Weber KT. Anatomic validation of left ventricular mass estimates from clinical two-dimensional echocardiography: initial results. Circulation. 1983;67:348–52. doi: 10.1161/01.cir.67.2.348. [DOI] [PubMed] [Google Scholar]
- 16.Katz J, Malloy CR, Filipchuk NG, Peshock RM. Left ventricular volumes measured by MR imaging. Radiology. 1985;156:717–9. doi: 10.1148/radiology.156.3.4023232. [DOI] [PubMed] [Google Scholar]
- 17.Katz J, Milliken MC, Peshock RM. Estimation of human myocardial mass with MR imaging. Radiology. 1988;169(2):495–8. doi: 10.1148/radiology.169.2.2971985. [DOI] [PubMed] [Google Scholar]
- 18.Giles JT, Szklo M, Post W, Petri M, Blumenthal RS, Lam G, et al. Coronary arterial calcification in rheumatoid arthritis: comparison to the multi-ethnic study of atherosclerosis. Arthritis Res Ther. 2009;11(2):R36. doi: 10.1186/ar2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 20.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, et al. Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006;186(6 Suppl 2):S357–65. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 21.Heckbert SR, Post W, Pearson GD, Arnett DK, Gomes AS, Jerosch-Herold M, et al. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48(11):2285–92. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair SN, Haskell WL, Ho P, Paffenbarger RS, Jr, Vranizan KM, Farquhar JW, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. doi: 10.1093/oxfordjournals.aje.a114163. [DOI] [PubMed] [Google Scholar]
- 23.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR, Jr, et al. Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology. 2005;234(1):35–43. doi: 10.1148/radiol.2341040439. [DOI] [PubMed] [Google Scholar]
- 24.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of Coronary-Artery Calcium Using Ultrafast Computed-Tomography. J Am Coll Cardiol. 1990;15(4):827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 25.Prevoo ML, van' t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe F, Kleinheksel SM, Cathey MA, Hawley DJ, Spitz PW, Fries JF. The clinical value of the Stanford Health Assessment Questionnaire Functional Disability Index in patients with rheumatoid arthritis. J Rheumatol. 1988;15(10):1480–8. [PubMed] [Google Scholar]
- 27.van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol. 2000;27(1):261–3. [PubMed] [Google Scholar]
- 28.Nettleton JA, Steffen LM, Mayer-Davis EJ, Jenny NS, Jiang R, Herrington DM, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2006;83(6):1369–79. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left Ventricular Dilatation and the Risk of Congestive Heart Failure in People without Myocardial Infarction. N Engl J Med. 1997;336(19):1350–5. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 30.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Reiss CK, Levy D. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction : Prevalence and mortality in a population-based cohort. J Am Coll Cardiol. 1999;33(7):1948–55. doi: 10.1016/s0735-1097(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 31.Raza K, Banks M, Kitas GD. Reversing myocardial microvascular disease in a patient with rheumatoid arthritis. J Rheumatol. 2005 Apr;32(4):754–6. [PubMed] [Google Scholar]
- 32.Elhendy A, Schinkel AFL, van Domburg RT, Bax JJ, Poldermans D. Incidence and predictors of heart failure during long-term follow-up after stress Tc-99m sestamibi tomography in patients with suspected coronary artery disease. J Nucl Cardiol. 2004;11(5):527–33. doi: 10.1016/j.nuclcard.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: The Cardiovascular Health Study. J Am Coll Cardiol. 2001;37(4):1042–8. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 34.Giles JT, Fernandes V, Lima JA, Bathon JM. Myocardial dysfunction in rheumatoid arthritis: epidemiology and pathogenesis. Arthritis Res Ther. 2005;7(5):195–207. doi: 10.1186/ar1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatia GS, Sosin MD, Patel JV, Grindulis KA, Khattak FH, Hughes EA, et al. Left ventricular systolic dysfunction in rheumatoid disease: an unrecognized burden? J Am Coll Cardiol. 2006;47(6):1169–74. doi: 10.1016/j.jacc.2005.10.059. [DOI] [PubMed] [Google Scholar]
- 36.Rudominer RL, Roman MJ, Devereux RB, Paget SA, Schwartz JE, Lockshin MD, et al. Independent association of rheumatoid arthritis with increased left ventricular mass but not with reduced ejection fraction. Arthritis Rheum. 2009;60(1):22–9. doi: 10.1002/art.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellenger NG, Marcus NJ, Davies C, Yacoub M, Banner NR, Pennell DJ. Left ventricular function and mass after orthotopic heart transplantation: a comparison of cardiovascular magnetic resonance with echocardiography. J Heart Lung Transplant. 2000;19(5):444–52. doi: 10.1016/s1053-2498(00)00079-6. [DOI] [PubMed] [Google Scholar]
- 38.Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19(7):1550–8. doi: 10.1016/0735-1097(92)90617-v. [DOI] [PubMed] [Google Scholar]
- 39.Bozkurt B, Kribbs SB, Clubb FJ, Michael LH, Didenko VV, Hornsby PJ, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97(14):1382–91. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 40.Diwan A, Dibbs Z, Nemoto S, DeFreitas G, Carabello BA, Sivasubramanian N, et al. Targeted Overexpression of Noncleavable and Secreted Forms of Tumor Necrosis Factor Provokes Disparate Cardiac Phenotypes. Circulation. 2004;109(2):262–8. doi: 10.1161/01.CIR.0000109642.27985.FA. [DOI] [PubMed] [Google Scholar]
- 41.Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a Chimeric Monoclonal Antibody Tumor Necrosis Factor - {alpha}, in Patients With Moderate-to-Severe Heart Failure: Results of the Anti TNF Therapy Against Congestive Heart Failure (ATTACH) Trial. Circulation. 2003;107(25):3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 42.Mann DL, McMurray JJV, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted Anticytokine Therapy in Patients With Chronic Heart Failure: Results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109(13):1594–602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 43.Kwon HJ, Cote TR, Cuffe MS, Kramer JM, Braun MM. Case reports of heart failure after therapy with a tumor necrosis factor antagonist. Ann Intern Med. 2003;138(10):807–11. doi: 10.7326/0003-4819-138-10-200305200-00008. [DOI] [PubMed] [Google Scholar]
- 44.Wolfe F, Michaud K. Congestive Heart Failure in Rheumatoid Arthritis: Rates, Predictors, and the Effect of Anti-TNF Therapy. Am J Med. 2004;116(5):305–11. doi: 10.1016/j.amjmed.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 45.Listing J, Strangfeld A, Kekow J, Schneider M, Kapelle A, Wassenberg S, et al. Does tumor necrosis factor alpha inhibition promote or prevent heart failure in patients with rheumatoid arthritis? Arthritis Rheum. 2008;58(3):667–77. doi: 10.1002/art.23281. [DOI] [PubMed] [Google Scholar]
- 46.Setoguchi S, Schneeweiss S, Avorn J, Katz JN, Weinblatt ME, Levin R, et al. Tumor necrosis factor-alpha antagonist use and heart failure in elderly patients with rheumatoid arthritis. Am Heart J. 2008;156(2):336–41. doi: 10.1016/j.ahj.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahesh B, Leong HS, McCormack A, Sarathchandra P, Holder A, Rose ML. Autoantibodies to vimentin cause accelerated rejection of cardiac allografts. Am J Pathol. 2007;170(4):1415–27. doi: 10.2353/ajpath.2007.060728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azimzadeh AM, Pfeiffer S, Wu GS, Schroder C, Zhou H, Zorn GL, 3rd, et al. Humoral immunity to vimentin is associated with cardiac allograft injury in nonhuman primates. Am J Transplant. 2005;5(10):2349–59. doi: 10.1111/j.1600-6143.2005.01022.x. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez-Marquez A, Aguilera I, Blanco RM, Pascual D, Encarnacion-Carrizosa M, Alvarez-Lopez MR, et al. Positive association of anticytoskeletal endothelial cell antibodies and cardiac allograft rejection. Hum Immunol. 2008;69(3):143–8. doi: 10.1016/j.humimm.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Makrygiannakis D, af Klint E, Lundberg IE, Lofberg R, Ulfgren AK, Klareskog L, et al. Citrullination is an inflammation-dependent process. Ann Rheum Dis. 2006;65(9):1219–22. doi: 10.1136/ard.2005.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.