1 Introduction
Anthrax is a severe infectious disease caused by Bacillus anthracis. Although primarily a disease of animals, it also infects humans and sometimes with fatal consequences [1]. Until recently the availability of effective animal vaccines coupled with the scarcity of human disease resulted in the organism receiving little attention outside of the military. The ability to form heat-resistant spores, which infect via the lungs, together with its presence in nature and the simplicity of the production technology makes the organism an attractive biological weapon for extremist groups [2]. The recent spread of anthrax spores through the U.S. postal system demonstrates the disruption and dramatic consequences that such an attack can inflict [2].
The capacity of B. anthracis to cause disease is dependent on the production of a polyglutamic acid capsule, which confers resistance to phagocytosis, and the expression of a tripartite toxin comprising protective antigen (PA, responsible for cell binding), edema factor (EF, a toxin acting via cAMP modulation) and lethal factor (LF, a metalloprotease which modulates MAP-kinase mediated signal transduction) [3]. The toxin follows the AB model: the A moiety contains the catalytic subunits LF and EF, while the B moiety, PA, serves to translocate either EF or LF into the cytosol of the cell [1].
LF is a 90,000 MW protein containing 776 residues which encompass four distinct domains [4]. The N terminal domain [domain 1 (LFD1)] facilitates binding of the protein to PA prior to membrane translocation. This region shares sequence homology with the N terminal domain of EF, which is not surprising given the fact that EF also binds to the same region of PA. The remaining regions of EF and LF mediate the catalytic activity of these enzymes. In the case of LF, domains 2 (LFD2), domain 3 (LFD3) and domain 4 (LFD4) form a long deep grove that holds the 16-residue N-terminal tail of MAPKK-2 prior to cleavage by the zinc metalloprotease catalytic centre located within domain IV [5].
PA is a 83,000 MW protein which also comprises four distinct regions [6]. The N terminal [domain 1 (PAD1)] region contains two calcium ions and a recognition site for protease activation. Cleavage of PA results in the release of a 20 K amino-terminal (PA20) and the subsequent assembly by PA63 of a heptamer, a ring-shaped structure with a negatively charged lumen, leading to the exposure of a large hydrophobic surface to which LF and EF binds. Currently, the contribution of the released PA20 to pathogenicity is unclear. Gene expression studies have shown that this fragment is able to induce apoptosis in human peripheral blood leukocytes [7] and recent studies by Reason and colleagues suggests that PA20 may have a role as an immune system decoy [8–9].
The cell surface bound PA63 fragment consists of a heptamerization domain [domain 2 (PAD2)] which contains a large flexible loop implicated in membrane insertion, a small domain of unknown function [domain 3 (PAD3)] and finally a 139 amino acid carboxy-terminal host cell receptor-binding domain [domain 4 (PAD4)] essential for host cell intoxication which is thought to contain dominant protective epitopes [10].
Numerous animal studies have confirmed the role of PA as the principal protective immunogen in the licensed US and UK human vaccines and have demonstrated its ability to elicit protective immunity against aerosol spore challenge [1]. While effective, these vaccines suffer from the requirement for a multiple dose priming series followed by yearly booster shots. In addition, adverse local reactions such as soreness, redness, itching and swelling at the site of injection have been observed, which have been attributed to trace amounts of LF and other bacterially derived, immunogenic antigens [11–14]. For this reason considerable effort is being directed towards developing a replacement, single protein vaccine comprising non-toxic recombinant PA.
Protective immunity against anthrax is thought to be primarily antibody mediated [15–16]; and strong correlation has been shown between PA-specific antibodies with toxin neutralizing activity (TNA) and protection in several animal models [17]. A similar association has also been found between PA-specific IgG and toxin neutralizing activity in serum from infected and vaccinated humans [18–19]. TNA antibodies are in fact considered to be a correlate of immunity for protection of vaccinated individuals.
Given the tripartite nature of the anthrax toxin one would also expect other components of the toxin, LF and EF, to stimulate the production of toxin neutralizing antibodies. Indeed, LF alone expressed from a DNA vaccine protected mice against a lethal toxin challenge and when given as a truncate protein, it provided some protection to rabbits against aerosol challenge with spores of the highly lethal Ames strain [20–21]. In addition to conferring protection, LF appears to be a more potent human immunogen than PA. Our group has shown that individuals with cutaneous anthrax had a much faster and robust antibody response to LF than to PA [22]. The UK human anthrax vaccine (AVP) also stimulates LF-specific antibodies albeit at a much lower level than that seen for PA, probably reflecting the relatively smaller amount of LF in the vaccine, i.e., the average concentration of PA and LF in AVP is 7.5 mg/ml and 2.5 mg/ml respectively [23, B. Hallis, HPA, UK, pers. comm.].
It has been suggested that vaccines such as AVP which contain both PA and LF would be able to confer protection against strains of B. anthracis in which PA has been genetically modified, either by nature or as a consequence of genetic engineering [24–26]. Further support for the inclusion of biologically inactive LF in a future anthrax vaccine is provided by the observation that when co-administered with PA, LF enhances the magnitude of the PA specific antibody response in mice [27–28]. The adjuvant effect of LF resides in the non-toxic N terminal domain (LFD1, amino acids 1–254). This region has been exploited by researchers as a means of delivering foreign antigens, leading to the stimulation of CD8+ and CD4+ T cells responses [28–30]. Surprisingly, however, little is known about the human T cell response to both PA and LF, following both infection and vaccination, other than that it is relatively long lived [31].
To determine the contribution of LF to the protective immune response stimulated by the current UK licensed human anthrax vaccine, we characterised the antibody and T cell responses of immunized volunteers. Animal studies were undertaken to identify the immunodominant and protective regions of the protein, and the effect of co-administering individual LF domains with PA. Finally a fusion protein comprising the N terminal domain of LF (LFD1) and the C terminal, immunodominant domain of PA (PAD4) was constructed and shown to confer complete protection against lethal challenge with anthrax.
2 Materials and methods
2.1 Construction of recombinant protein expression systems
Recombinant proteins including PA, the C terminal domain of PA (PAD4, amino acids 552–735), biologically inactive LF (LF7, a mutant in which glutamic acid replaces cysteine at position 687), the individual domains of LF (LFD1, LFD2, LFD3, LFD4 and LFD2–4 as described by Pannifer and colleagues [4] on the basis of the crystal structure), and a fusion protein (LFD1-PAD4) comprising the N terminal domain of LF (LFD1, amino acids 1–254) and the C terminal domain of PA (PAD4), were cloned and expressed from Escherichia coli as recombinant N terminal histidine tagged proteins using a commercially available expression system (pQE30 or pQE80L - QIAgen Inc). Due to the high AT nucleotide content of both PA and LF the corresponding gene sequences were codon optimised for expression in E. coli (GenScript Corp). Once constructed all expression vectors were stored at −70°C until required.
2.2 Expression of recombinant proteins
The individual protein expressions systems were optimised to obtain maximum protein yield. Briefly, cultures derived from a single colony were grown overnight at 37°C in LB broth supplemented with either ampicillin (100 µg/ml) or ampicillin plus kanamycin (100 µg/ml and 50 µg/ml, respectively), as appropriate. Overnight cultures were then diluted in fresh LB broth (containing appropriate antibiotics) and incubated at 37°C (250 rpm) until they reached an OD600 of 0.550–0.600. To induce protein expression, isopropyl β-D-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM. Cultures were then incubated at the optimum temperature and time period for maximum protein yield; LF7, LFD2, LFD4, LFD2–4 and LFD1-PAD4 were incubated overnight at 25°C, LFD3 was incubated overnight at 30°C, PA and LFD4 were incubated for 4 hours at 37°C. Cells were harvested by centrifugation at 10,000 g at 4°C for 20 minutes.
Bacterial pellets containing the recombinant protein were disrupted using a French Press (Thermo Scientific, Fisher) and centrifuged at 45,000 g at 4°C for 20 minutes. The resulting supernatant was then incubated with Talon® metal affinity resin (Clontech Laboratories) to bind the N terminal histidine tag. Following washing, the protein was recovered at 4°C using an elution buffer containing 150 mM imidazole, 50 mM sodium phosphate and 300 mM NaCl, (pH 7), and dialyzed against HEPES buffer (10 mM HEPES, 50 mM NaCl, pH 7.5) using a 10,000 MW cut off dialysis cassette (Pierce, Thermo Scientific).
The identity of the proteins was confirmed by SDS-PAGE (PhastGel; Amersham-Pharmacia) and Western Blot analysis. Protein bands were detected either by staining with PhastGel Blue R or, after electrophoretic transfer onto polyvinylidene difluoride membranes (Millipore) by using rabbit anti-LF or anti-PA sera. All exhibited the expected sizes and were recognized by specific antibodies. The endotoxin content of the different protein preparations was determined by the Limulus amoebocyte lysate linetic-QCL assay according to the manufacturer’s instructions (Lonza). Protein concentrations were determined using the BCA protocol (Pierce, Thermo Scientific) [32].
2.3 Human immune studies
2.3.1 Immune donors
Blood samples were taken with full informed consent, from four volunteers routinely vaccinated every 12 months for a minimum of 6 years with the UK Anthrax Vaccine Precipitated (AVP) vaccine (UK Department of Health) under approval by the CBD Independent Ethics Committee for the UK Ministry of Defence.
2.3.2 Antibody responses
Western blots were performed to confirm that subjects responded to both PA and LF. Recombinant PA and LF were produced as previously described [13, 33]. Endotoxin levels for the PA and LF proteins were <1 EU/mg and <4 EU/mg, respectively. Each protein (5 µg) and a 1 Kb size marker (Bio-Rad, California, USA) were loaded onto a 4–20% Tris-HCl polyacrylamide gel (Bio-Rad, California, USA) which was blotted onto nylon membranes. Membranes were washed with PBS (pH 7.2) (1% Tween-20), blocked overnight at 4°C with 1% PBS-Tween-20 (PBST) containing 5% non-fat dry milk (PBSTM) and incubated with sera (1:2,500) for 1h at 4°C. A secondary HRP-labeled goat anti-human antibody (diluted 1:10,000 in PBSTM) (Roche Diagnostics Corporation, Indianapolis, IN) was added for 30 min at 4°C. The membranes were developed with 3, 3', 5, 5' - tetramethylbenzidine (TMB) membrane peroxidase substrate (KPL, Maryland, USA) and the reaction was quenched with dH2O.
2.3.3 T cell analysis
Peripheral blood mononuclear cells (PBMC) were prepared from whole blood by Ficoll density-gradient centrifugation (Histopaque; Sigma, UK). The cells were washed and suspended in AIM-V serum-free medium (Invitrogen, UK). Proliferation in response to PA and LF was determined by 3H-thymidine incorporation. Cells, added in triplicate to 96-well flat-bottom plates at 3.5 × 105/well, were incubated with media only, 25 µg/ml of recombinant anthrax protein or 25 ng/ml of staphylococcal enterotoxin B (SEB) as positive control, for 5 days. 3H-thymidine (1 mCi/ well) was added 8 hours prior to cell harvest and incorporation was measured in a scintillation counter. Results are expressed in stimulation indices (SI) calculated as mean cpm measured in wells containing cells and antigens/ cpm in control wells containing cells and media alone.
2.4 Murine immune studies
2.4.1 Immunization
Immunogenicity studies were performed in female BALB/c mice (8–10 wks-old from Charles-River Laboratories) that were randomly allocated to different groups (10 mice per group) and immunized intramuscularly (i.m.) on days 0 and 28, with 10 µg of each of the following proteins: LFD1, LFD2, LFD3, LFD4, LFD2–4 and LF(LF7) alone and in combination with 10 µg of PA, in the presence of 0.5% alum (a 100 µl dose was given 50 µl in each hind leg). Serum samples were collected on days 0, 28, 56, 71 and 109 after vaccination. Additional groups were immunized with PAD4 and LFD1-PAD4 in a similar manner and sera were collected on days 0, 13, 27, 42 and 56. Protein-Alhydrogel adsorption was performed the day prior to vaccination; the protein was mixed with alhydrogel (Brenntag Stinnes Logistics, Frederikssund, Denmark) and incubated for 20 minutes at room temperature and then overnight at 4°C. For challenge experiments female A/J mice (8–12 wks-old obtained from Harlan UK, Ltd.) were immunized i.m. on days 0, 14 and 28 with 10 µg of either LF7 or LFD1 (first experiment) or PA, PAD4 LFD1, LFD1-PAD4 (second experiment) adsorbed to alum (alhydrogel), as described above. Serum samples were collected at different time points for antibody measurements. These experiments were approved by the University of Maryland or DSTL, Porton Down animal care and use committees.
2.4.2 Antibody responses
Serum IgG antibodies specific for B. anthracis LF and PA were measured by enzyme-linked immunosorbent assay (ELISA) [13]. Briefly, plates were coated with LF or PA (List Biological Laboratories) at 1 or 2 µg/ml in PBS, respectively, for 3 h at 37°C. Plates were washed with PBST and blocked overnight with PBS containing 10% dry milk (Nestle USA Inc., Glendale, Calif.). Serially diluted serum samples were incubated for 1 h at 37°C. PA and LF specific antibodies were revealed with HRP-labeled goat anti-mouse IgG (Roche Diagnostics Corporation, Indianapolis, IN) followed by TMB Microwell Peroxidase Substrate (KPL). The reaction was stopped by adding 100 µl of 1 M H3PO4 after 15 min incubation. Titers were reported as dilutional end-points calculated through linear regression equations as the inverse of the serum dilution that produced an A450 value of 0.2 above the blank (ELISA Units per ml), as previously described [34]. All samples were assayed in duplicate and a positive calibrated control included in each assay. For measurement of serum IgG specific for LF and PA in the protection experiments, ELISAs were performed as previously described [34], with titres calculated as the inverse of the serum dilution that produced an A414 value of 0.1 above the blank.
Anthrax toxin neutralizing activity (TNA) antibodies were measured as described previously [19]. Serially diluted serum samples were incubated with anthrax lethal toxin [PA + LF (List Biological Labs)] in 96-well plates for 1 h at 37°C and the mixture was transferred to a monolayer of J774A.1 cells. Viability was assessed by addition of tetrazolium salt MTT at 5 mg/ml. Titers were calculated as the reciprocal of a serum-sample dilution that resulted in 50% neutralization of toxin-mediated cytotoxicity (ED50), corresponding to the inflection point of a 4-parameter logistic-log fit curve. ED50 data were obtained using an end-point algorithm (Taylor method) developed by the Centers for Disease Control and Prevention (CDC) [35–36].
2.5 B. anthracis spore challenge
The challenge experiments were carried out blinded and adhered strictly to the UK Animals (Scientific Procedures) Act of 1986. B. anthracis STI (Tox+ Cap−) spores were prepared as described previously [34]. Female A/J mice were immunized i.m. on days 0, 14 and 28 with 10 µg of PA, LF, LFD1, PAD4 or LFD1-PAD4 adsorbed to alum, as described previously. For the LF protection experiment, serum samples were collected on day 73 and mice were inoculated intraperitoneally (i.p.) with 100 lethal doses (MLD) of B. anthracis STI spores (~105 CFU) on day 81. For the fusion protein experiment, serum samples were collected on day 62 and mice were inoculated i.p. with ~2×105 CFU STI spores on day 70. Following challenge, mice were closely observed for 14 days to determine their protective status. Humane endpoints were strictly observed; any animal that displayed a collection of clinical signs that indicated a lethal infection (e.g., piloerection, posture and mobility problems) was promptly euthanized.
2.6 Adsorption assay
ELISA plates were coated with 1 or 2 µg/ml of either LF or PA, respectively, or PBS alone (negative control) for 3 h at 37°C and washed with PBST. Serial dilutions of mouse sera were added and incubated overnight at 4°C. After incubation, 100 µl of adsorbed sera were transferred to a new antigen coated plate and a regular ELISA was run as described above. Positive anti-PA and anti-LF and negative controls were used in each assay.
2.7 Statistical analysis
Antibody titers were log transformed for calculation of geometric mean titer (GMT) and confidence intervals. Differences in titers among groups were assessed by Student’s t test and Mann Whitney Rank Sum Test when normality failed and by ANOVA with Bonferroni adjustment. For proliferation assays, mean cpm in experimental groups were compared to unstimulated controls using the Friedman test with Dunn's Multiple Comparison post-hoc testing. A P value of < 0.05 was considered statistically significant. Statistical analysis was performed using SigmaStat 3.0 (SPPS Inc. Chicago) and GraphPad® software (Prism, California, USA).
3 Results
3.1 Human immune response to B. anthracis PA and LF
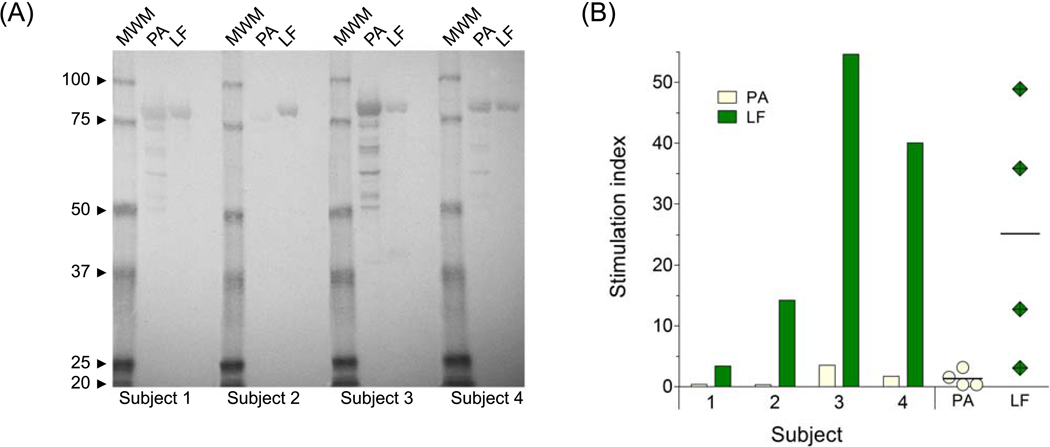
We examined the antibody and T cell responses to PA and LF of four volunteers immunized with the UK licensed human anthrax vaccine (Fig. 1). All four vaccinated subjects developed antibodies in response to LF and PA, with subjects 3 and 4 showing the highest responses against both proteins (Fig. 1A). These same individuals also exhibited robust proliferative T cell responses against both PA and LF, whereas the other two responded only to LF, and to a lower degree (Fig. 1B). Despite the variability, the magnitude of the serological responses to PA and LF, represented by the band intensity in the western blot analysis, followed the trend of the T cell proliferation. In all vaccinees, the cellular responses to LF were of a significantly greater magnitude than those to PA (P = 0.0046), with the mean stimulation index (SI) to PA being 1.49 compared to 28.08 for LF.
Figure 1.
Human immune responses to recombinant B. anthracis PA and LF. (A) Western blot analysis of PA and LF serum IgG in four AVP-vaccinated subjects. No responses were detected in an unvaccinated subject (negative control, not shown); (B) T cell proliferative responses in AVP-immunized individuals. Results are expressed in stimulation index (SI) comparing the responses to PA ( ) and LF (
) and LF ( ) for each vaccinated subject and displaying responses to PA (
) for each vaccinated subject and displaying responses to PA ( ) and LF (
) and LF ( ) as mean SI with range (individual points).
) as mean SI with range (individual points).
3.2 Cloning and expression of individual LF domains
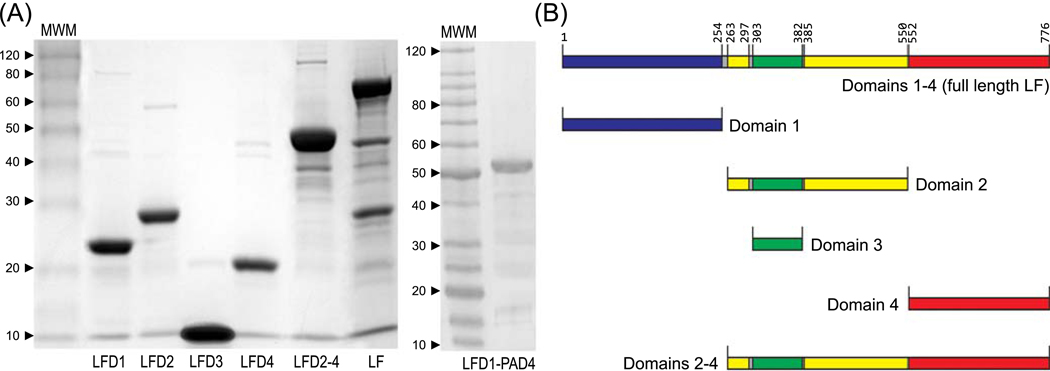
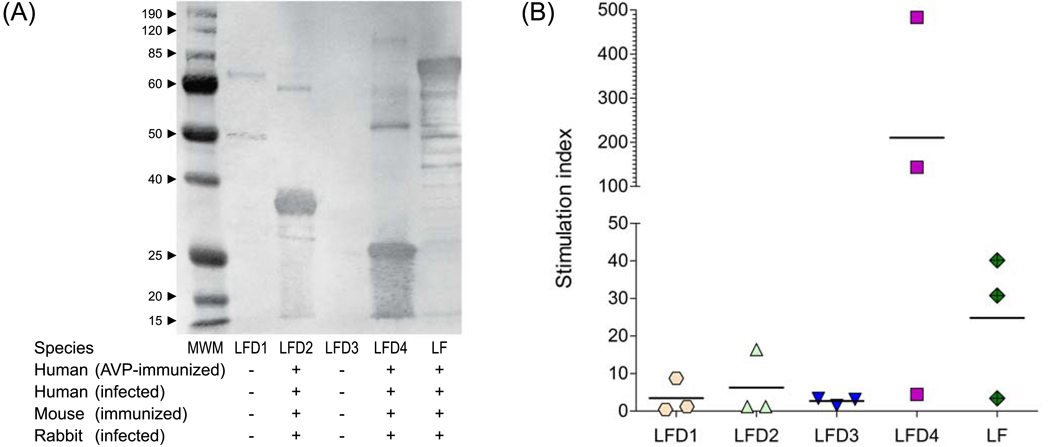
The analysis of the crystal structure of lethal factor revealed the presence of four distinct domains comprising a protein of 776 amino acids [4]. Domain 1 located at the N terminus (aa 1–254) mediates binding to PA, domain 2 (aa 263–297 and 385–550) shares structural homology with the catalytic domain of B. cereus toxin VIP2. Domain 3 (aa 303–382) is located within domain 2 and is involved in substrate recognition, while domain 4 contains a zinc binding metalloprotease catalytic motif. To determine the contribution of each of these regions to the immunogenicity and protective efficacy of the entire protein, the following recombination proteins were expressed from E. coli as histidine tagged proteins; LFD1 (aa 1–254), LFD2 (aa 263–550), LFD3 (aa 303–382), LFD4 (aa 552–776), LFD2–4 (aa 263–776) and finally full length biologically inactive LF [LF7 (aa 1–776)]. The size of each fragment was confirmed by SDS-PAGE gel analysis (Fig. 2); all proteins were recognized by LF-specific rabbit polyclonal antisera in a western blot analysis (data not shown). These recombinant domains represent a tool for characterisation of immune responses stimulated by the entire protein. Probing with polyclonal sera obtained from AVP-immunized and anthrax infected animals and humans revealed that domains 2 and 4 stimulated by far the strongest IgG antibody responses (Fig. 3A) and T cell proliferation in human AVP vaccines (Fig. 3B).
Figure 2.
SDS-PAGE analysis of purified E. coli expressed recombinant proteins. (A) LF, LF fragments and LFD1-PAD4 fusion protein; (B) Diagrammatic representation of the location of the individual LF domains within the full length protein.
Figure 3.
Antibody and T cell recognition of LF domains. (A) Recognition of individual LF domains by IgG antibodies produced in AVP-vaccinated humans. The same recognition pattern was observed in sera from humans or from different animal species following infection or vaccination; (B) Proliferative T cell responses to LF and LF domains in 3 humans volunteers immunized with the AVP vaccine; results are expressed as stimulation index (SI) as described above.
3.3 Serum IgG responses to individual LF domains
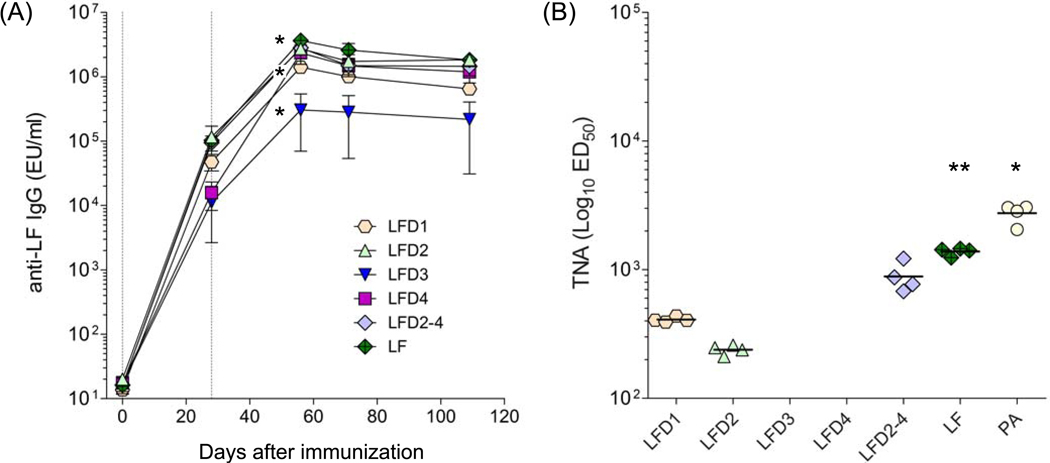
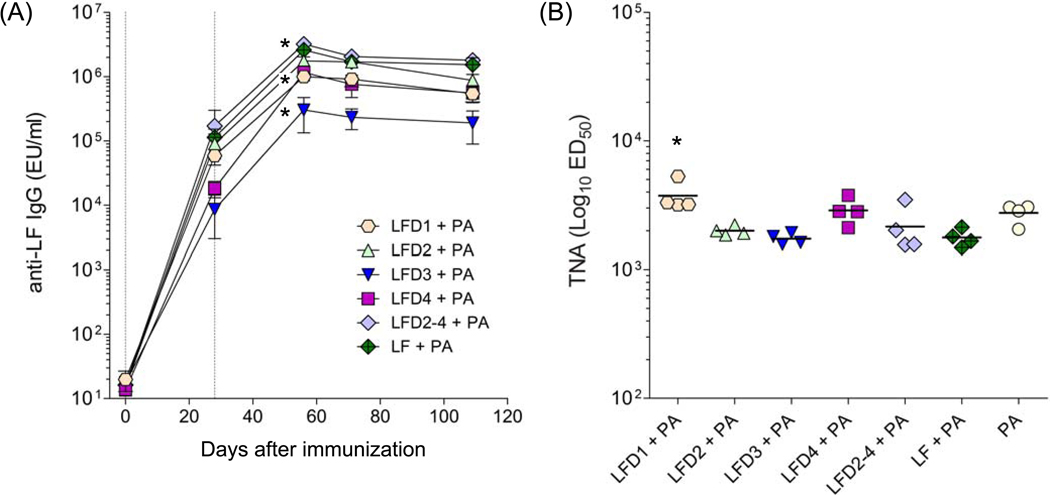
We next examined the immunogenicity of each recombinant LF domain when administered parenterally to mice, as subunit vaccines adsorbed to alum. The kinetics of the serum IgG responses to each domain are shown in Fig. 4A. All of the domains were found to be highly immunogenic. LFD3 elicited a robust IgG response but it was significantly lower compared with the other groups.
Figure 4.
Antibody responses in mice immunized with LF domains. (A) LF-specific IgG antibody and (B) toxin neutralizing activity (TNA) titers in mice immunized with LF domains. BALB/c mice (10 per group) received 10 µg of each domain protein adsorbed to alum via i.m. on days 0 and 28. Results are expressed as LF geometric mean IgG titers with 95% confidence intervals. TNA titers are shown as mean with range; ED50 was <100 for LFD3 and LFD4 groups. Vertical lines indicate immunization days. LF IgG titers among the different groups were compared at day 56 after immunization (peak); * denotes higher responses for LF vs. all other groups, higher responses for LFD1 vs. LFD3 but lower compared with all other groups, and lowest responses for LFD3 vs. all other groups (p<0.05). For TNA titers, * denotes higher responses to PA vs. LF (p<0.001) and ** higher responses to LF vs. all other domains (p<0.05)
3.4 Toxin neutralizing antibodies
It has been shown across different animal species, including mice, that the level of toxin neutralizing antibodies, a sub-component of the overall toxin specific response, correlates with protection. Hence, we measured the TNA responses in mice immunized with the individual LF domains and compared them with those induced by PA, approximately 3 months after the last immunization (Fig. 4B). LF domains 1 and 2 were able to stimulate TNA antibodies at levels which were previously shown (with serum from PA-immunized mice) to confer protection against a lethal spore challenge (GMT > 100) [13].
However, no such activity was seen for either domain 3 or domain 4. The failure of domain 3, which is located within domain 2 and is known to contain a linear TNA binding site, and domain 4 to stimulate TNA is not as easy to explain [37–39]. Indeed the ability of the fusion protein comprising domains 2, 3 and 4 to stimulate a much greater TNA response suggests that whole protein contains additional toxin neutralizing epitopes that are lost when the domains are expressed as individual proteins. Despite eliciting robust IgG responses, neither the LF fragments, nor the entire LF (LF7) protein, were as effective as PA at raising TNA antibodies.
3.5 Co-delivery of LF domains with PA
The ability of PA to enhance the host’s immune response to biologically inactive LF and fusion proteins has been demonstrated in animal studies and is a consequence of the ability of PA to transport LF into the cytosol of antigen presenting cells [27–28]. This system has been exploited to deliver payloads such as T cell epitopes to the cytosol of immune cells [29–30, 40] through fusion proteins which carry the desired antigen linked to the non-toxic, PA binding N terminal domain of LF (LFD1). To determine whether the magnitude of the domain specific antibody response could be enhanced in the presence of PA, mice were immunized as described above, with either 10 µg of the LF domain alone or in combination with 10 µg PA in the presence of alum. The kinetics of the specific IgG antibody responses determined by ELISA are shown in Fig. 5A. There was no obvious increase in LF-specific IgG antibody response when an LF domain was co-administered with PA (Fig 4A and 5A). There were no differences in the PA-specific IgG responses either (data not shown). It should be noted that the specific antibody responses to the individual domain stimulated during this study were extremely high and as a consequence any adjuvant effects may have been masked. The TNA antibody titers for the animals immunized with PA and LF domains are shown in Fig. 5B. Only LFD1, when administered in combination with PA, had enhanced levels of TNA. In fact, the presence of some of the LF domains appeared to reduce the toxin neutralizing response although the differences with the PA group were not statistically significant.
Figure 5.
Antibody responses in mice immunized with LF domains in combination with PA. (A) LF-specific IgG antibody and (B) toxin neutralizing activity (TNA) titers in mice immunized with LF domains alone or in combination with PA. BALB/c mice (10 per group) received 10 µg of domain protein or 10 µg of domain protein + 10 µg of PA together with alum on days 0 and 28. Results are expressed as LF geometric mean IgG titer with 95% confidence intervals. TNA titers represent mean with range. Vertical lines indicate immunization days. LF IgG titers were compared at day 56 (peak); * denotes higher responses to LF+PA and LFD2–4+PA vs. all other groups and lowest responses to LFD3+ PA vs. all other groups (p<0.05). For TNA titers, * denotes higher titers to LFD1+PA vs. all other domains (p<0.05).
3.6 LF-specific protection data
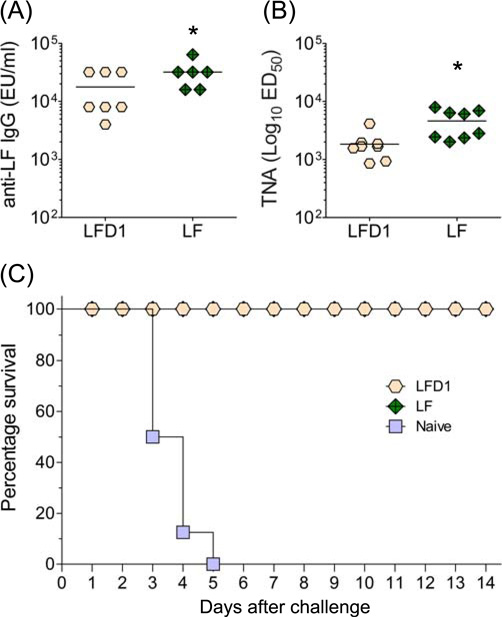
Because of the robust TNA responses elicited by LFD1, particularly when co-administered with PA, this domain became a lead component for a combined multi-subunit vaccine. Thus, we next examined the capacity of LFD1 and the biologically inactive LF (LF7) to protect against a lethal anthrax spore challenge. A/J mice were immunized i.m. on days 0, 14 and 28 with 10 µg of protein adsorbed to alum. Remarkable LF-specific serum IgG titers against both proteins (mean levels >10,000 µg/ml) were observed prior to challenge (Fig. 6A). The TNA responses (Fig. 6B) also exceeded levels previously shown to be protective [34]. All of the animals immunized with either full length LF or LFD1 survived a lethal i.p. challenge with ~1×105 CFU (100 MLDs) of B. anthracis STI spores, while mice in the naïve control group all died by day 5 (Fig. 6C).
Figure 6.
Protection mediated for antibodies against LFD1 or the full length protein (LF). A/J mice (8 per group) were immunized i.m. with 10 µg of protein adsorbed to alum on days 0, 14 and 28. Animals were subjected to lethal i.p challenge (~105 CFU) with B. anthracis STI spores on day 81. (A) Serum LF-specific IgG responses measured on day 73, (B) TNA titers elicited by LFD1 and LF, and (C) Survival curves after challenge. * denotes higher responses to LF compared with LFD1 (p<0.005).
3.7 LFD1-PAD4 fusion protein
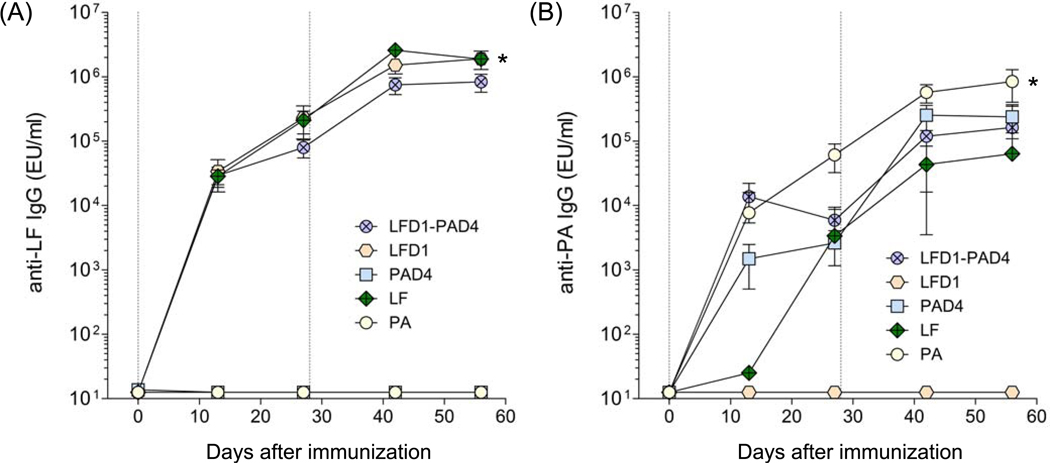
The results presented above support the supposition that the addition of biologically inactive LF or indeed domain 1 of LF to a vaccine comprising PA would enhance both the level and spectrum of protection. While it is technically feasible to express and individually purify each protein, a more elegant and cost effective approach would be to combine the essential regions from PA and LF into a single fusion protein. In a previous study we demonstrated that domain 4 of PA, the region of the protein which binds to a receptor on the surface of the target cell, is able to confirm protection when given as a recombinant protein [34]. Given that domain 1 of LF is also protective and represents the region through which LF binds to PA we reasoned that a fusion protein comprising domain 1 of LF and domain 4 of PA would confer a level of protection comparable to that achieved by PA. Therefore, a fusion protein comprising both domains was constructed and its immunogenicity in mice determined as previously described. High levels of LF and PA-specific IgG antibodies were produced after immunization (Fig. 7). The results suggest that the orientation of the LFD1-PAD4 fusion had no deleterious effect on its ability to stimulate antigen specific immune responses. Somewhat surprising was the finding that animals immunized with recombinant LF (LF7) mounted a weak cross reacting antibody response to PA, which was subsequently boosted by repeat immunization (Fig. 7B). Extensive characterisation of the LF used in these studies confirmed that the response was not due to contamination with PA (data not shown). We confirmed this observation by absorbing mouse sera with PA or LF, revealing the remaining antibodies by ELISA. Table 1 shows that mice immunized with LF mounted a PA specific response, the magnitude of which was substantially reduced following adsorption with PA. A similar response was not observed with LFD1 suggesting that the cross reaction is likely due to cryptic epitopes located within the remaining domains of LF.
Figure 7.
LF and PA-specific antibody responses in mice immunized with the fusion protein LFD1-PAD4. (A) LF-specific IgG and (B) PA-specific IgG. Mice were immunized i.m. with 10 µg of the fusion protein LFD1-PAD4 in the presence of alum. Data represents geometric mean titers and ±95% confidence intervals. Vertical lines indicate days of immunization. LF and PA titers were compared at day 56; * denotes higher LF IgG responses in mice immunized with LF and LFD1 vs. LFD1-PAD4 and all other groups; ** denotes higher PA responses in mice immunized with PA vs. all other groups (p<0,001).
Table 1.
Cross adsorption of LF and PA-specific antibodies using an ELISA based assay.
| Mouse | Immunogen | Anti-LF IgG (EU/ml) | Cross adsorption reduction (%) | Anti-PA IgG (EU/ml) | Cross adsorption reduction (%) | ||
|---|---|---|---|---|---|---|---|
| PA | LF | PA | LF | ||||
| 1045 | LF7 | 1,444,667 | 0 | 57 | 188,240 | 78 | 8 |
| 1046 | LF7 | 2,216,710 | 0 | 67 | 443,318 | 79 | 18 |
| 1056 | LFD1 | 211,557 | 0 | 58 | 0 | N.D. | N.D. |
3.8 Protection against injected B. anthracis STI challenge following immunization with recombinant fusion proteins
Finally, we determined the ability of the fusion protein (LFD1-PAD4), individual domains (LFD1 and PAD4), and PA to protect groups of A/J mice against a lethal anthrax spore challenge. Mice were immunized with the proteins adsorbed to alum, as described above. Two months after immunization, they were challenged i.p. with ~2×105 CFU (200 MLDs) of B. anthracis STI spores and monitored for 14 days for signs of morbidity. The unvaccinated (PBS) control group died by day 5 after challenge. In contrast, for all the protein tested, a high degree of protection (87.5 to 100%) against anthrax infection was seen in the immunized groups (Table 2). PA and LF-specific antibodies were measured prior to the challenge (Table 2). The antibody response stimulated by the LFD1-PAD4 fusion protein was lower compared to that induced by the individual domains, which probably reflects a difference in antigen content, 10 µg of LFD1-PAD4 would contain roughly half the amount of LFD1 or PAD4 present in 10 µg of each protein alone. In spite of the slightly lower antibody responses, the ability of the fusion protein to confer solid protection indicates its potential as a future vaccine candidate.
Table 2.
Antibody responses and protective efficacy of PA and LF domains. Groups of A/J mice were immunized with 10µg the different proteins on days 0, 14 and 28, as described in Section 2. Serum samples removed prior to challenge were pooled and assayed by ELISA for IgG to PA and LF. Mice were subjected to lethal i.p. challenge (~2×105 CFU) with B. anthracis STI spores on day 70.
| Antigen | Antibody Titer (EU/ml)a | Vaccine efficacy (%) survivors/total |
||
|---|---|---|---|---|
| PA | LF | |||
| PA | 1,280,000 | N.D. | 100% | (8/8) |
| PAD4 | 1,280,000 | N.D. | 87.5% | (7/8) |
| LFD1 | N.D. | 144,000 | 100% | (8/8) |
| LFD1-PAD4 | 320,000 | 52,000 | 100% | (8/8) |
| PBS | N.D. | N.D. | 0% | (0/8) |
Serum antibodies were measured on day 62 post immunization.
4 Discussion
The AVP vaccine has been in use for nearly 40 years [1] and while animal studies have identified the production of toxin neutralizing antibodies as the key protective component, surprisingly little attention has been paid to the contribution of the vaccine specific T cell response. Indeed memory CD4+ T cells are likely to be essential for the generation of neutralizing antibody, class switching, and affinity maturation [41]. A recent paper from Allen and colleagues reported that the memory T cell response to the whole vaccine of AVP immunized humans was long-lived and involved a high frequency of Th1 and Th2 cells [31]. To better understand the responses generated by the individual antigens which comprise AVP we examined the antibody and T cell responses to PA and LF in four volunteers immunized with AVP. Somewhat surprisingly, given the fact that AVP contains lower levels of LF than PA (2.5 mg/ml vs. 7.5 mg/ml, respectively), the LF specific T cell responses (mean SI = 28.1) were found to be significantly higher (P = 0.0046) than those seen for PA (SI = 1.49), suggesting that LF is able to stimulate robust memory T cell responses. As a matter of fact, 2 of the 4 vaccinated subjects failed to mount a detectable PA-specific T cell recall response, indicating that there may be some value in modifying PA to enhance its CD4 T cell composition, particularly if the protein is to be employed as the sole immunogen in a next generation human vaccine.
To further investigate the T cell and antibody responses invoked by LF we determined the ability of AVP immunized volunteers to respond to individual LF domains. While these vaccinees mounted strong antibody and T cell recall responses to domains 2 and 4, they showed relatively poor responses to domain 1 and 3. The lack of T cell response to domain 1 was unexpected given that this region contains at least one human toxin neutralizing B cell epitope and has recently been shown to contain a further five strong murine B cell epitopes [38–39]. Thus the context in which individual domains of LF, rather than the whole protein, are presented to the immune system may affect the quality of the resulting protective antibody response.
Numerous animal studies have confirmed the ability of PA, the principal immunogen of the US (AVA) and UK (AVP) human vaccines to stimulate protective TNA antibodies. While PA also invokes the production of antibodies in humans, the quality of this response has yet to be fully characterised. Reason and colleagues recently found that the PA specific antibody response of AVA immunized humans is biased towards the N terminus of the immunogen, specifically PA20, a region which is cleaved following cell surface binding and is thought to play no further role in intoxication [8–9]. It is yet to be determined if a similar bias is observed with AVP. Interestingly, only 18% of the antibodies generated by AVA which recognised PA20 possessed toxin neutralizing activity, while 31% of the antibodies directed against PA63, the region of the molecule that includes the cell surface binding region domain 4, were toxin neutralizing [8]. The role PA20, if any, in protection is presently unclear. While it is not thought to play a direct role in toxin uptake, it may act as an antibody decoy, allowing subsequently expressed PA free access to cell surface receptors. Thus the protective efficacy of PA could be further enhanced by altering the structure of PA to shift the epitope bias towards more functionally relevant regions of the molecule, such as domain 4, which as a recombinant protein can protect mice against a lethal spore challenge [8, 10].
In addition to PA the AVP vaccine also contains trace amounts of LF capable of stimulating T cell and antibody specific responses [23, 42, B. Hallis, HPA, UK, pers. comm.]. The ability of biologically inactive LF and individual domains of the protein alone to invoke protection against anthrax has been demonstrated in this and other studies [20, 22]. While all of the LF domains with the exception of domain 3 stimulated robust antibody responses, only domains 1 and 2 generated TNA titers comparable in magnitude to the protective levels achieved following immunization with PA [12]. While a direct correlation between LF-induced TNA and protection has yet to be reported, a number of studies, including our own, have demonstrated the ability of toxin neutralizing LF specific monoclonal antibodies to protect naive animals against lethal anthrax spore challenge, supporting the assumption that immunization with LF is able to stimulate a protective TNA response [37–39, 43]. Indeed the presence of linear B cell epitopes recognized by TNA antibodies within domains 1 and 2 may in part explain their neutralizing activity [37–39].
The failure of domain 3, which is located within domain 2 and is known to contain a linear TNA binding site, and of domain 4 to stimulate detectable levels of TNA was surprising [37, 39]. This lack of response could reflect a failure of the immune system to process the isolated recombinant domains in the same manner as it would if they were presented in the context of the complete protein [39]. Alternatively, whole protein contains additional linear and conformational toxin neutralizing epitopes which are lost when domains are expressed as individual recombinant proteins. The ability of a fusion protein comprising domains 2, 3 and 4 of LF to stimulate much greater TNA titers than seen with domain 2 alone lends support to this supposition.
The ability of PA to enhance the antibody response to biologically inactive LF and fusion proteins has been reported and thus we sought to determine if co-delivery of PA with individual LF domains would enhance the protective efficacy of the resulting antibody immune response, specifically the toxin neutralizing titer. Only domain 1, the region of LF which mediates binding to PA, was found to enhance the TNA response. Surprisingly, some combinations of PA and LF regions, such as domain 3, appeared to suppress the TNA titer. Indeed mice immunized with full length recombinant LF (LF7) but not LFD1 mounted a cross reacting antibody response to PA. It is possible that the process by which recombinant LF and its domains are expressed from E. coli resulted in recombinant proteins whose physical properties are sufficiently altered from those of native LF to result in the presentation of previously unseen B cells epitopes to the immune system [44]. Thus while these epitopes fail to stimulate an antibody response on primary immunization with PA they are recognised in animals given rLF. Conceivably cross reacting antibodies could hinder the binding of toxin neutralizing antibodies and thus account for the reduction in titer.
The results from this study suggest that a vaccine formulation comprising domain 1 of LF and a protective region of PA such as domain 4 would enhanced both the level and spectrum of protection compared to PA alone. While it is technically feasible to express and purify individual immunogens, a more elegant and cost effective approach would be to combine the protective regions into a single fusion protein. To this end we constructed a fusion construct comprising LFD1 and PAD4 which when administered to mice stimulated solid protection. Indeed it should be possible to further refine the composition of this vaccine by including only those B and T cell epitopes which are essential to protection [45–47]. Such an approach would enable researchers to maximize the epitope copy number (optimize the magnitude of the immune response) and edit out conflicting epitopes thereby preventing deleterious antigen competition.
In conclusion LF is immunogenic in humans and is likely to contribute to the protection stimulated by AVP. A single vaccine comprising protective regions from LF and PA would simplify production and confer a broader spectrum of protection then that seen with PA alone.
Acknowledgements
The authors would like to thank Tony Stagg and Joanne Thwaite from Dstl and Kitty Davis, Mardi Reymann and personnel from the CVD Applied Immunology Section for their technical expertise. This work was supported by NIH grants U19-AI-56578 to J. P. Nataro; AI-56578 to L.W. Baillie and AI-56578 and R01-AI065760 to M.F. Pasetti.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baillie LW. Is new always better than old?: The development of human vaccines for anthrax. Hum Vaccin. 2009;5(12):806–816. doi: 10.4161/hv.9777. [DOI] [PubMed] [Google Scholar]
- 2.Knight J. Bioweapons: Delivering death in the mail. Nature. 2001;414:837–838. doi: 10.1038/414837a. [DOI] [PubMed] [Google Scholar]
- 3.Baillie L, Read TD. Bacillus anthracis, a bug with attitude! Curr Opin Microbiol. 2001;4(1):78–81. doi: 10.1016/s1369-5274(00)00168-5. [DOI] [PubMed] [Google Scholar]
- 4.Pannifer AD, Wong TY, Schwarzenbacher R, Renatus M, Petosa C, Bienkowska J, et al. Crystal structure of the anthrax lethal factor. Nature. 2001;414(6860):229–233. doi: 10.1038/n35101998. [DOI] [PubMed] [Google Scholar]
- 5.Mock M, Fouet A. Anthrax. Annu Rev Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 6.Petosa C, Collier RJ, Klimpel KR, Leppla SH, Liddington RC. Crystal structure of the anthrax toxin protective antigen. Nature. 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 7.Hammamieh R, Ribot WJ, Abshire TG, Jett M, Ezzell J. Activity of the Bacillus anthracis 20 kDa protective antigen component. BMC Infect Dis. 2008;8:124. doi: 10.1186/1471-2334-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reason D, Liberato J, Sun J, Keitel W, Zhou J. Frequency and domain specificity of toxin-neutralizing paratopes in the human antibody response to anthrax vaccine adsorbed. Infect Immun. 2009;77(5):2030–2035. doi: 10.1128/IAI.01254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reason DC, Ullal A, Liberato J, Sun J, Keitel W, Zhou J. Domain specificity of the human antibody response to Bacillus anthracis protective antigen. Vaccine. 2008;26(32):4041–4047. doi: 10.1016/j.vaccine.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flick-Smith HC, Walker NJ, Gibson P, Bullifent H, Hayward S, Miller J, et al. A recombinant carboxy-terminal domain of the protective antigen of Bacillus anthracis protects mice against anthrax infection. Infect Immun. 2002;70(3):1653–1656. doi: 10.1128/IAI.70.3.1653-1656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baillie L, Hebdon R, Flick-Smith H, Williamson D. Characterisation of the immune response to the UK human anthrax vaccine. FEMS Immunol Med Microbiol. 2003;36(1–2):83–86. doi: 10.1016/S0928-8244(03)00085-3. [DOI] [PubMed] [Google Scholar]
- 12.Baillie L, Townend T, Walker N, Eriksson U, Williamson D. Characterization of the human immune response to the UK anthrax vaccine. FEMS Immunol Med Microbiol. 2004;42(2):267–270. doi: 10.1016/j.femsim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Baillie LWJ, Rodriguez AL, Moore S, Atkins HS, Feng C, Nataro JP, et al. Towards a human oral vaccine for anthrax: The utility of a Salmonella typhi Ty21a-based prime-boost immunization strategy. Vaccine. 2008;26(48):6083–6091. doi: 10.1016/j.vaccine.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasserman GM, Grabenstein JD, Pittman PR, Rubertone MV, Gibbs PP, Wang LZ, et al. Analysis of adverse events after anthrax immunization in US army medical personnel. J Occup Environ Med. 2003;45(3):222–233. doi: 10.1097/01.jom.0000058345.05741.6b. [DOI] [PubMed] [Google Scholar]
- 15.Beedham RJ, Turnbull PC, Williamson ED. Passive transfer of protection against Bacillus anthracis infection in a murine model. Vaccine. 2001;19(31):4409–4416. doi: 10.1016/s0264-410x(01)00197-9. [DOI] [PubMed] [Google Scholar]
- 16.Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J Hyg (Lond) 1956;54(1):28–36. doi: 10.1017/s0022172400044272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little SF, Ivins BE, Fellows PF, Pitt MLM, Norris SLW, Andrews GP. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. Vaccine. 2004;22(3–4):422–430. doi: 10.1016/j.vaccine.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Pittman PR, Leitman SF, Oro JGB, Norris SL, Marano NM, Ranadive MV, et al. Protective antigen and toxin neutralization antibody patterns in anthrax vaccinees undergoing serial plasmapheresis. Clin Diagn Lab Immunol. 2005;12(6):713–721. doi: 10.1128/CDLI.12.6.713-721.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quinn CP, Dull PM, Semenova V, Li H, Crotty S, Taylor TH, et al. Immune responses to Bacillus anthracis protective antigen in patients with bioterrorism-related cutaneous or inhalation anthrax. J Infect Dis. 2004;190(7):1228–1236. doi: 10.1086/423937. [DOI] [PubMed] [Google Scholar]
- 20.Galloway D, Liner A, Legutki J, Mateczun A, Barnewall R, Estep J. Genetic immunization against anthrax. Vaccine. 2004;22(13–14):1604–1608. doi: 10.1016/j.vaccine.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Hermanson G, Whitlow V, Parker S, Tonsky K, Rusalov D, Ferrari M, et al. A cationic lipid-formulated plasmid DNA vaccine confers sustained antibody-mediated protection against aerosolized anthrax spores. PNAS. 2004;101(37):13601–13606. doi: 10.1073/pnas.0405557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenneman K, Dognanay M, Akmal A, Goldman S, Galloway D, Mateczun A. The early humoral immune response to Bacillus anthracis. doi: 10.1111/j.1574-695X.2011.00800.x. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hepburn MJ, Hugh Dyson E, Simpson AJ, Brenneman KE, Bailey N, Wilkinson L, et al. Immune response to two different dosing schedules of the anthrax vaccine precipitated (AVP) vaccine. Vaccine. 2007;25(32):6089–6097. doi: 10.1016/j.vaccine.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 24.Pomerantsev AP, Staritsin NA, Mockov YV, Marinin LI. Expression of cereolysine AB genes in Bacillus anthracis vaccine strain ensures protection against experimental hemolytic anthrax infection. Vaccine. 1997;15(17–18):1846–1850. doi: 10.1016/s0264-410x(97)00132-1. [DOI] [PubMed] [Google Scholar]
- 25.Rosovitz MJ, Schuck P, Varughese M, Chopra AP, Mehra V, Singh Y. Alanine-scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J Biol Chem. 2003;278(33):30936–30944. doi: 10.1074/jbc.M301154200. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmaster AR, Ravel J, Rasko DA, Chapman GD, Chute MD, Marston CK, et al. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. PNAS. 2004;101(22):8449–8454. doi: 10.1073/pnas.0402414101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pezard C, Weber M, Sirard JC, Berche P, Mock M. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect Immun. 1995;63(4):1369–1372. doi: 10.1128/iai.63.4.1369-1372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Price BM, Liner AL, Park S, Leppla SH, Mateczun A, Galloway DR. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect Immun. 2001;69(7):4509–4515. doi: 10.1128/IAI.69.7.4509-4515.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ballard JD, Doling AM, Beauregard K, Collier RJ, Starnbach MN. Anthrax toxin-mediated delivery in vivo and in vitro of a cytotoxic T-lymphocyte epitope from ovalbumin. Infect Immun. 1998;66(2):615–619. doi: 10.1128/iai.66.2.615-619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Friedman R, Kushner N, Doling A, Thomas L, Touzjian N, et al. Genetically modified anthrax lethal toxin safely delivers whole HIV protein antigens into the cytosol to induce T cell immunity. PNAS. 2000;97(14):8027–8032. doi: 10.1073/pnas.97.14.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen JS, Skowera A, Rubin GJ, Wessely S, Peakman M. Long-lasting T cell responses to biological warfare vaccines in human vaccinees. Clin Infect Dis. 2006;43(1):1–7. doi: 10.1086/504806. [DOI] [PubMed] [Google Scholar]
- 32.Laird MW, Zukauskas D, Johnson K, Sampey GC, Olsen H, Garcia A, et al. Production and purification of Bacillus anthracis protective antigen from Escherichia coli. Protein Expr Purif. 2004;38(1):145–152. doi: 10.1016/j.pep.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Williamson ED, Flick-Smith HC, LeButt C, Rowland CA, Jones SM, Waters EL, et al. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect Immun. 2005;73(6):3598–3608. doi: 10.1128/IAI.73.6.3598-3608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stokes MG, Titball RW, Neeson BN, Galen JE, Walker NJ, Stagg AJ, et al. Oral administration of a Salmonella enterica-based vaccine expressing Bacillus anthracis protective antigen confers protection against aerosolized B. anthracis. Infect Immun. 2007;75(4):1827–1834. doi: 10.1128/IAI.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Soroka SD, Taylor TH, Jr, Stamey KL, Stinson KW, Freeman AE, et al. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J Immunol Methods. 2008;333(1–2):89–106. doi: 10.1016/j.jim.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 36.Taylor T, Quinn C, Schmidt D, Freeman A, Li H, Semenova V, et al. Novel mathematical approach to TNA endpoints. 5th International Conference on Anthrax; Nice, France. 2003. [Google Scholar]
- 37.Lim N-K, Kim J-H, Oh MS, Lee S, Kim S-Y, Kim K-S, et al. An anthrax lethal factor-neutralizing monoclonal antibody protects rats before and after challenge with anthrax toxin. Infect Immun. 2005;73(10):6547–6551. doi: 10.1128/IAI.73.10.6547-6551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albrecht MT, Li H, Williamson ED, LeButt CS, Flick-Smith HC, Quinn CP, et al. Human monoclonal antibodies against anthrax lethal factor and protective antigen act independently to protect against Bacillus anthracis infection and enhance endogenous immunity to anthrax. Infect Immun. 2007;75(11):5425–5433. doi: 10.1128/IAI.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen ML, Crowe SR, Kurella S, Teryzan S, Cao B, Ballard JD, et al. Sequential B-cell epitopes of Bacillus anthracis lethal factor bind lethal toxin-neutralizing antibodies. Infect Immun. 2009;77(1):162–169. doi: 10.1128/IAI.00788-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballard JD, Collier RJ, Starnbach MN. Anthrax toxin-mediated delivery of a cytotoxic T-cell epitope in vivo. PNAS. 1996;93(22):12531–12534. doi: 10.1073/pnas.93.22.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McHeyzer-Williams LJ, Pelletier N, Mark L, Fazilleau N, McHeyzer-Williams MG. Follicular helper T cells as cognate regulators of B cell immunity. Curr Opin Immunol. 2009;21(3):266–273. doi: 10.1016/j.coi.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baillie L, Townend T, Walker N, Eriksson U, Williamson D. Characterization of the human immune response to the uk anthrax vaccine. FEMS Immunol Med Microbiol. 2004;42(2):267–270. doi: 10.1016/j.femsim.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 43.Zhao P, Liang X, Kalbfleisch J, Koo HM, Cao B. Neutralizing monoclonal antibody against anthrax lethal factor inhibits intoxication in a mouse model. Hum Antibodies. 2003;12(4):129–135. [PubMed] [Google Scholar]
- 44.Gentile F, Conte M, Formisano S. Thyroglobulin as an autoantigen: What can we learn about immunopathogenicity from the correlation of antigenic properties with protein structure? Immunology. 2004;112(1):13–25. doi: 10.1111/j.1365-2567.2004.01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabhnani L, Manocha M, Sridevi K, Shashikiran D, Rayanade R, Rao DN. Developing subunit immunogens using B and T cell epitopes and their constructs derived from the F1 antigen of Yersinia pestis using novel delivery vehicles. FEMS Immunol Med Microbiol. 2003;38(3):215–229. doi: 10.1016/S0928-8244(03)00170-6. [DOI] [PubMed] [Google Scholar]
- 46.Sette A, Fleri W, Peters B, Sathiamurthy M, Bui H, Wilson S. A roadmap for the immunomics of category A–C pathogens. Immunity. 2005;22:155–161. doi: 10.1016/j.immuni.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Ingram RJ, Metan G, Maillere B, Doganay M, Ozkul Y, Kim LU, et al. Natural exposure to cutaneous anthrax gives long-lasting T cell immunity encompassing infection-specific epitopes. J Immunol. 2010;184(7):3814–3821. doi: 10.4049/jimmunol.0901581. [DOI] [PubMed] [Google Scholar]