Abstract
The NSABP B-32 trial is examining whether patients with initially negative sentinel lymph nodes (SLNs) who have occult metastases detected on deeper levels and cytokeratin immunohistochemistry (CK-IHC) stains are at risk for regional or distant metastases. The experimental B-32 protocol was designed to detect metastases larger than 1.0 mm by examining sections approximately 0.5 and 1.0 mm deeper into the paraffin blocks (2 levels; wide spacing). This pilot quality assurance study compares detection rates to a comprehensive protocol designed to detect metastases larger than 0.2 mm (multilevel; narrow spacing). All SLNs were sectioned grossly at close to 2.0 mm and all sections embedded in paraffin blocks. For clinical treatment, a single H&E section was examined from each block. For 54 cases with 1–5 SLNs and all SLNs negative, additional CK-IHC sections were evaluated every 0.18 mm through the block until no tissue remained. 20 of 176 (11.4%) blocks harbored occult metastases; the B-32 protocol detected metastases in 11 blocks (6.3%) and 9 additional blocks (5.1%) with metastases were detected on sections that would not have been evaluated (p=0.002; correlated proportions). Median number of levels examined per block on the comprehensive protocol was 11 (range 3–26); the B-32 protocol was fixed at 2 levels (median 2; range 1–2). Median thickness of node sections in the block was 2.1 mm (range 0.7–4.8 mm) and the modal thickness was 2.3 mm. Although more comprehensive sectioning of SLNs detects additional micrometastases, the data suggest diminishing returns and reduced cost effectiveness for the comprehensive strategy.
Keywords: Sentinel lymph nodes, breast cancer, micrometastases, occult metastases
Introduction
The primary aim of the National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol B-32 is to determine whether patients undergoing sentinel lymph node (SLN) biopsy alone have an increased risk for axillary recurrence and decreased overall survival compared to patients undergoing complete axillary dissection (12, 13). A secondary aim of this randomized prospective trial is to determine whether women with occult metastases detected on deeper levels into initially negative SLN paraffin blocks are a population at risk for axillary recurrence or decreased overall survival.
A critical component of protocol B-32 was standardizing the pathologic assessment of SLNs for clinical treatment decisions (10). Pathologists were instructed to thinly slice SLNs, embed all slices in paraffin tissue blocks, and evaluate a single section from the surface of each block with routine hematoxylin and eosin (H&E) stained sections. Routine cytokeratin immunohistochemistry (CK-IHC) stains were prohibited except to confirm or refute suspicious findings on the initial H&E stain. The B-32 protocol identified approximately 1600 women with positive nodes and 4000 women with negative lymph nodes: half with only SLNs removed and half with SLNs plus axillary nodes removed. The initial pathologic evaluation was designed to exclude all patients with macrometastases from the “node negative” group. The node positive cases are excluded from outcome analysis and represent a heterogeneous mixture of patients with macrometastases (larger than 2.0 mm), micrometastases (larger than 0.2 mm and no larger than 2.0 mm), and isolated tumor cell clusters (no larger than 0.2 mm). The 4000 “node negative” cases include women with occult micrometastases smaller than 2.0 mm; however, this group is relatively free of contaminating macrometastases that would bias the outcome studies. The use of quotes in referring to “node negative” is deliberate; every “negative” SLN has the potential to harbor occult metastases. A more accurate term would be “no metastases detected.”
Following pathologic examination by the treating institution, paraffin blocks of all SLN negative cases from B-32 were sent to a central laboratory at the University of Vermont for a clinically blinded search for occult metastases. Additional sections approximately 0.5 mm and 1.0 mm deeper into each paraffin block were evaluated with H&E and CK-IHC stains. Assuming each node slice is no thicker than 2.0 mm, this experimental protocol leaves no more than 1.0 mm of unexamined tissue in the block and thus would be expected to identify a very high proportion of metastases larger than 1.0 mm that remained undetected in the paraffin block after the clinical evaluation. Since a histologic section is two dimensional, the true maximum dimension of any identified metastasis is unknown, somewhat analogous to the tip of the iceberg principle. The additional levels evaluated on the experimental B-32 sectioning protocol further divide the initially “node negative” group. The “initially negative, occult metastasis positive” subset includes metastases ranging from single cells to as large as 2.0 mm while the “initially negative, occult metastasis negative” subset includes a mixture of true node negative cases and cases with undetected occult metastases theoretically ranging from single cells to as large as 1.0 mm. When the outcome analysis data for the B-32 trial is available, it will be important to understand how the various groups were generated – in other words, how the pathology sectioning protocol impacts what is detected and what is missed – and the degree to which the “node negative” subsets are contaminated with potential occult (undetected) metastases. It is critical for clinicians and pathologists to understand the inherent imprecision in determining the true micrometastatic tumor burden in lymph nodes.
The current quality assurance pilot study investigates two major issues. The first is a comparison of the occult metastases detected by the two-level widely spaced sectioning protocol used for the experimental component of the B-32 trial to those detected by a more comprehensive multilevel narrowly spaced sectioning protocol all the way through the paraffin block. The comprehensive sectioning protocol was designed to detect all micrometastases larger than 0.2 mm (3, 6). This aspect of the pilot study design provides an estimate of residual undetected micrometastases in the “occult metastasis negative” group for the B-32 trial. The second issue is to determine the thickness of the node sections submitted in the paraffin blocks. Excluding macrometastases from the “node negative” SLN cases is the single most important quality assurance component of the SLN pathologic examination. This aspect of the pilot study helps to define additional needed education regarding SLN gross evaluation. The data generated also allow a limited comparison of detection rates for other potential sectioning strategies.
Materials and Methods
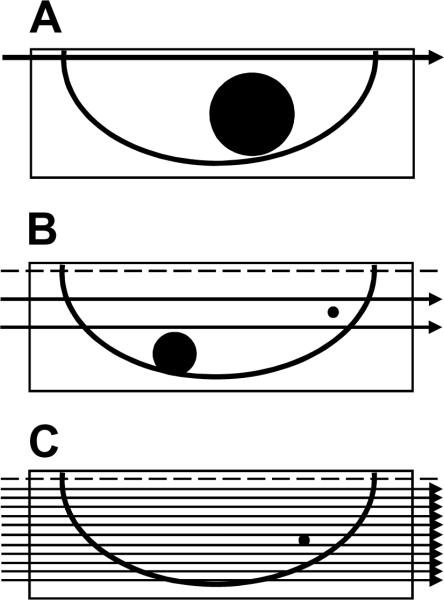
A series of 54 node negative patients sequentially enrolled at the University of Vermont on NSABP protocol B-32 was selected for more comprehensive pathologic evaluation of SLNs. Intraoperative frozen section analysis was prohibited so as to preserve all tissue for permanent section histopathologic examination. Intraoperative cytologic evaluation by touch or scrape and smear technique was performed on patients having SLN biopsy without axillary dissection. SLNs were bisected or sliced to produce gross sections approximately 2.0 mm in maximum thickness and each slice was embedded in paraffin for microscopic evaluation. A single section from the surface of each paraffin block was stained with hematoxylin and eosin (H&E) then evaluated for nodal metastases. These results were documented in the pathology report and used for clinical treatment decisions. This protocol is referred to as the B-32 clinical sectioning strategy and was designed to exclude macrometastases larger than 2.0 mm from the “node negative” group (Figure 1).
Figure 1. Paraffin block sectioning protocols.
All sentinel nodes were either bisected or sectioned to produce gross node sections approximately 2.0 mm thick. All gross sections were embedded in paraffin blocks. A. A single hematoxylin and eosin (H&E) stained section was evaluated from the surface of each paraffin block and the results documented in the pathology report for clinical treatment decisions. This protocol is referred to as the B-32 clinical sectioning strategy and was designed to exclude macrometastases larger than 2.0 mm from the “node negative” group. Note that metastases up to 2.0 mm may be missed. B. One H&E and one cytokeratin immunohistochemial (CK-IHC) stained section were evaluated at two levels - approximately 0.5 mm and 1.0 mm - deeper into the paraffin block (four sections total). This two-level, widely spaced protocol is referred to as the B-32 experimental strategy and was designed to exclude micrometastases larger than 1.0 mm from the “occult metastases negative” group. Note that isolated tumor cell clusters (ITC) and micrometastases up to 1.0 mm may be missed. C. One CK-IHC stained section was evaluated every 0.18 mm completely through the paraffin block. This multilevel, narrowly spaced protocol is referred to as the comprehensive strategy and was designed to exclude micrometastases larger than 0.2 mm from the “occult metastases negative” group. Note that ITCs up to 0.18 mm may be missed. Occult metastases identified on sections at approximately 0.5 mm and 1.0 mm into the paraffin block - the sections evaluated for the B-32 occult metastasis study - were compared to the occult metastases identified on the entire set of CK-IHC stained sections. The dotted line represents the initial section used for clinical treatment; all SLN blocks were required to be negative on this initial section to be included in the pilot study.
All cases included in this quality assurance study were required to have invasive carcinoma larger than 0.1 cm and smaller than 3.0 cm and to be node negative on initial pathology examination as documented in the pathology report. Cases with more than 5 SLNs were excluded to reduce histology expenses. Each paraffin embedded tissue block was sectioned entirely through the block at a microtome setting of 0.006 mm (6 μm; 6 microns). Sections every 0.18 mm (every 30th section) were mounted and stained with anti-cytokeratin immunohistochemistry (CK-IHC). This multilevel, narrowly spaced protocol is referred to as the comprehensive strategy (Figure 1) and was designed to exclude micrometastases larger than 0.2 mm from the “occult metastases negative” group (3, 6).
The AE1–AE3 anti-cytokeratin antibody (Chemicon International, Temecula, CA) was used followed by a polymer detection kit (Dako North America, Carpinteria, CA). Target antibody was visualized with diaminobenzidene (DAB) and slides were counterstained with hematoxylin. Two additional sections immediately preceding the CK-IHC slides corresponding to approximately 0.5 mm and 1.0 mm from the surface of the paraffin block were also stained with H&E. The two CK-IHC stained and two H&E stained slides at 0.5 and 1.0 mm comprised the two-level widely spaced experimental B-32 protocol sections. Results from these four slides were recorded on standard pathology forms developed for the B-32 trial and entered into the pathology database for the main B-32 pathology outcome analysis of occult metastases. This two-level, widely spaced protocol is referred to as the B-32 experimental strategy and was designed to exclude micrometastases larger than 1.0 mm from the “occult metastases negative” group (Figure 1).
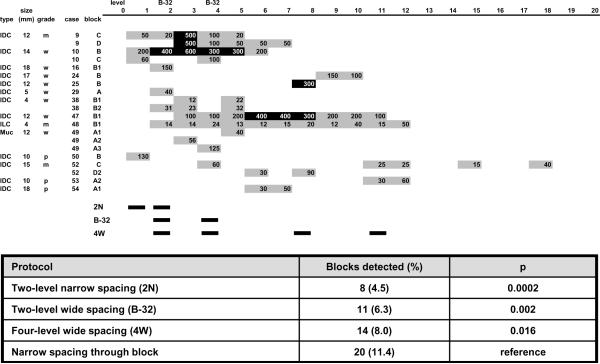
Two additional strategies were statistically evaluated. A logical extension of the B-32 experimental strategy is to examine additional levels at 0.5 mm intervals. This approach decreases the likelihood of missing macrometastases in lymph node sections that are thicker than 2.0 mm. A popular strategy used by laboratories in the United States is to perform one initial level and two additional deeper levels without specifying deliberate wide spacing (personal communication). Detection rates for both of these potential sectioning strategies were evaluated and are depicted as “two level narrow spacing (2N)” and “four-level wide spacing (4W)” in Figure 2.
Figure 2. Location by level and size of occult metastases detected in sentinel lymph nodes.
A comprehensive sectioning strategy evaluating cytokeratin immunohistochemical stains every 0.18 mm entirely through the paraffin block was used. Occult metastasis detection rates for two-level narrow spacing (2N), two-level wide spacing (B-32), and four-level wide spacing (4W) protocols were compared to the reference comprehensive multilevel narrow spacing protocol. The comprehensive protocol is statistically significantly different from the remaining sampling protocols. Note that the smallest p-value and lowest rate of metastasis detection is associated with the 2N protocol sampling the most superficial levels of the paraffin block indicating the two-level narrow spacing protocol is the least effective at detecting occult micrometastases. Gray boxes indicate occult metastases no larger than 0.2 mm (isolated tumor cell clusters; ITCs) and black boxes indicate occult metastases larger than 0.2 mm (micrometastases). Each box contains the size in μm (microns) of the largest occult metastasis identified on the level. Blocks 10B and 47B1 contained contiguous micrometastases present on several levels with non-contiguous ITCs on sections superficial and deep to the micrometastasis.
Data from the more comprehensive pilot analysis were separately recorded and by design include the results of the B-32 experimental protocol sections for comparison. However, any metastases identified exclusively on slides not designated as B-32 sections are not linked to the main B-32 outcome analysis. For each case, the arbitrarily assigned case number, tumor type, tumor size, tumor grade (Nottingham system), block identifiers, number of SLNs, and number of SLN blocks was recorded. Data collected for each lymph node section included presence or absence of occult metastases and size of largest metastasis identified on each positive section. The total number of sections evaluated for each block was noted; any section containing exclusively adipose following the final section containing lymph node tissue was not included. For depth calculations, histology estimated that a maximum of 0.15 mm had been removed from the block for initial facing and again for subsequent facing for a total of 0.3 mm removed relative to the original lymph node cut surface prior to the first CK-IHC section examined.
The protocols that used entirely overlapping sectioning strategies of the same samples, including the multi-level narrowly spaced pilot protocol and the two-level widely spaced experimental B-32 protocol, result in paired or correlated data samples. These paired data were compared for marginal homogeneity using a test of correlated proportions. Comparison of other potential sectioning strategies that were not completely overlapping resulted in unpaired data that were compared using a binomial test. Data from similar sectioning strategies was tested with a kappa statistic (1).
Both the B-32 protocol and the pilot study were approved by the University of Vermont institutional review board for protection of human subjects in medical research.
Results
The 54 cases with initially negative SLNs on this pilot study included: 1 mucinous; 5 lobular; and 48 ductal NST (no special type) invasive breast carcinomas. There were 27 well, 18 moderately, and 9 poorly differentiated carcinomas. Tumor size ranged from 0.2 cm to 2.5 cm with an average and median size of 1.1 cm and 1.0 cm, respectively. A total of 138 sentinel lymph nodes were sectioned, embedded in 176 paraffin tissue blocks, and evaluated with a mean of 2.6 and a median of 2 SLNs per case. The mean number of sections (levels) examined per paraffin block was 10.8 and the median was 11 (range 3–26). A total of 1901 CK-IHC sections were evaluated. The total number of sections evaluated for this pilot does not include the 176 H&E sections prepared for initial pathology interpretation or the 352 H&E sections evaluated for the B-32 experimental protocol.
The comprehensive multi-level narrowly spaced protocol detected occult metastases in 20 (11.4%) of the 176 blocks, 17 (12.3%) of the 138 SLNs, and 14 (25.9%) of the 54 cases evaluated (Figure 2). The largest occult metastasis identified on any single section ranged from 0.012 mm to 0.6 mm. The two-level widely spaced B-32 protocol detected significantly fewer occult metastases in 11 (6.3%) of the blocks (p=.002), 11 (8.0%) of the nodes (p=.016), and 9 (16.7%) of the cases (p=.031); the largest occult metastasis on any single section ranged from 0.02 mm to 0.4 mm. A two-level narrowly spaced protocol – evaluating only the first two levels of the comprehensive protocol – detected occult metastases in 8 (4.5%) of the blocks (p=.0002) and the largest occult metastasis on any single section ranged from 0.02 mm to 0.4 mm. A four-level widely spaced protocol – 0.5 mm spacing – detected occult metastases in 14 (8.0%) of the blocks (p=.016) and the largest occult metastasis on any single section ranged from 0.02 mm to 0.4 mm. The size of the largest metastasis detected in each block by the B-32 experimental protocol and the comprehensive protocol is presented in Figure 3.
Figure 3. Comparison of maximum metastasis size detected by a two-level wide spaced and a multilevel narrow spaced sectioning protocol.
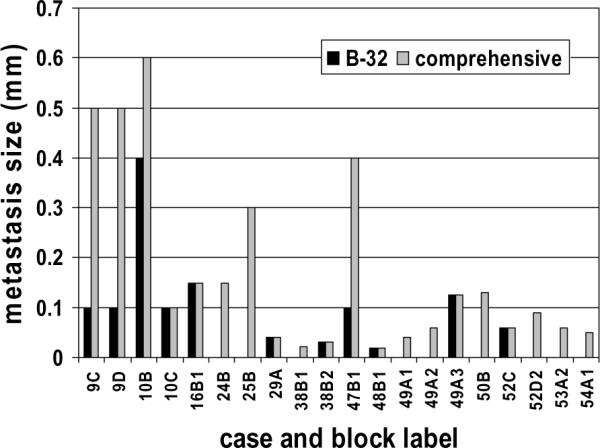
The wide spaced (B-32) protocol examines only two sections at approximately 0.5 mm and 1.0 mm deeper into the paraffin block. The narrow spaced (comprehensive) protocol examines sections every 0.18 mm through the block producing a median of 11 sections per block. Occult metastases were identified in nine additional blocks by the comprehensive protocol (one micrometastasis (25B); 8 isolated tumor cell clusters). Three micrometastases were misclassified as isolated tumor cell clusters by the B-32 protocol (9C, 9D, 47B1).
Compared to the comprehensive multilevel protocol, the B-32 protocol failed to detect occult metastases in 9 blocks and the missed occult metastases ranged from 0.04 mm to 0.3 mm. The comprehensive protocol identified micrometastases in 3 of 11 blocks (27.3%) and 2 of 9 cases (22.2%) that were misclassified as isolated tumor cell clusters (ITCs) on the B-32 protocol. The two-level narrowly spaced protocol failed to detect occult metastases in 12 blocks and the missed occult metastases ranged from 0.04 mm to 0.5 mm. The misclassification rates for micrometastases were the same as for the B-32 protocol. When independently comparing the two-level narrowly spaced protocol to the two-level widely spaced B-32 protocol, occult metastases were detected in 7 blocks by both protocols, in 1 block exclusively by the two-level narrowly spaced protocol, and 4 blocks exclusively by the B-32 protocol (p=.375). Agreement between the two-level narrow and wide spaced protocols was good (kappa=.722; p<.0001).
The median calculated thickness of the lymph node sections in the paraffin blocks was 2.1 mm (range 0.7 to 4.8 mm) and the modal thickness was 2.3 mm. A total of 54.5% of lymph node sections in paraffin blocks were thicker than 2.0 mm, 17.0% were thicker than 2.5 mm, and 8.5% were thicker than 3.0 mm (Figure 4).
Figure 4. Frequency distribution of maximum lymph node section thickness in 176 paraffin tissue blocks.
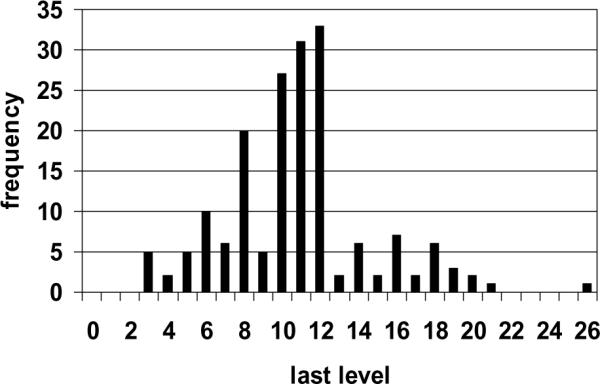
Lymph nodes were bisected or sectioned grossly at approximately 2.0 mm maximum thickness, embedded in paraffin blocks, and microscopic sections mounted on slides at 0.18 mm intervals through the block. Histology technicians were instructed to mount sections until no nodal tissue remained visible in the block. The last level containing any microscopic nodal tissue was recorded; any further sections containing only adipose were not included. In general and when possible, two levels were mounted on each slide for cytokeratin staining to reduce expense. Note the higher frequency of the last level occurring on an even numbered section. For thickness calculation, it was assumed that 0.3 mm of tissue had been removed for initial and subsequent block facing prior to the first experimental cytokeratin stain examined. Calculated median and modal thickness was 2.1 and 2.3 mm, respectively.
Discussion
National guidelines from the College of American Pathologists (CAP) and the American Society for Clinical Oncology (ASCO) recommend slicing sentinel nodes from breast cancer patients as close to 2.0 mm as possible and submitting all the slices for microscopic examination (8, 14). For clinical decision making, a single routinely stained section from the surface of each paraffin block is sufficient for pathologic evaluation of SLNs. However, the ASCO guideline recognizes that examining additional sections deeper into the paraffin block or using CK-IHC stains will detect additional metastases. Despite attempts toward standardization, the European Working Group for Breast Screening Pathology has documented existing heterogeneity in microscopic SLN evaluation protocols (4, 5). Heterogeneity also exists in the United States but has not been formally documented.
The value of identifying macrometastases larger than 2.0 mm is well established and integrated into the AJCC/UICC staging systems (7, 9, 11, 16). A more recent analysis of data from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) national cancer database has demonstrated that the presence of micrometastases no larger than 2.0 mm in lymph nodes is associated with an overall decrease in survival at 10 years of 1%, 6%, and 2% for T1 (no larger than 2.0 cm), T2 (larger than 2.0 cm but no larger than 5.0 cm), and T3 tumors (larger than 5.0 cm), respectively, compared to patients with no nodal metastases detected (2). This SEER analysis included years prior and subsequent to the advent of sentinel node biopsy. In contrast, a large retrospective analysis of data from California and Massachusetts demonstrated no impact on 15-year mortality estimates in any tumor size category when only a single lymph node contained any size metastasis (15). This study emphasizes the importance of primary tumor characteristics when minimal nodal tumor burden is present. There are currently no randomized clinical outcome data on the significance of micrometastases in sentinel lymph nodes, especially when a concerted effort has been made to prospectively exclude macrometastases from the study population. The NSABP B-32 trial was designed to address this question.
For pathology laboratories that have decided to evaluate SLNs with deeper levels and CK-IHC stains, despite guideline recommendations that do not endorse this practice, there is no established standard protocol in the United States for microscopic evaluation other than a single section from each paraffin block. At the extreme end of the spectrum, the most thorough evaluation would include CK-IHC stains at 0.006 mm intervals all the way through the block. This approach is labor intensive and prohibitively expensive requiring more than 330 sections to evaluate a 2 mm thick section of lymph node (17). A common protocol (personal communication) is to evaluate three superficial levels of the block with CK-IHC; however, this approach leaves a large volume of unexamined lymph node in the paraffin block while emphasizing detection of the smallest metastases secondary to the high sensitivity of CK-IHC. Most histology technicians will mount tissue at 0.02 to 0.06 mm intervals when levels are requested and this is very narrow spacing when the goal is statistical sampling of lymph node sections. A more logical approach involves evenly spaced sections through the paraffin block. The B-32 experimental protocol for detection of occult metastases used two widely spaced levels at approximately 0.5 mm and 1.0 mm deeper into the paraffin block. This NCI funded protocol had to be cost effective while maximizing the likelihood of detecting the largest and most significant metastases. The current pilot quality assurance study tests the protocol design hypothesis on a subset of patients enrolled on the B-32 trial. It also partly defines the degree to which the comparison “node negative” group is contaminated with undetected occult metastases since the current pilot protocol was designed to detect virtually all metastases larger than 0.2 mm: the lower limit of the micrometastasis range. The comprehensive pilot protocol is also similar in design to protocols used in Europe (5).
The first observation from this pilot study is that no occult metastases larger than 1.0 mm were identified by the comprehensive protocol and missed by the more economical two-level widely spaced B-32 protocol. This observation is encouraging but limited by the size of the pilot study; undoubtedly with a large sample it would have been demonstrated that a few 1.0 mm metastasases would be missed.
The second, and fully expected, observation is that the B-32 detection strategy missed occult metastases in 9 blocks from 5 cases (figure 2) and in one of these cases the metastasis was 0.3 mm. The B-32 strategy identified 9 cases with occult metastases and classified the remaining 45 cases as “node negative”. This suggests that in the larger B-32 trial, patients classified as “negative for occult metastases” have at least an 8.9% chance of having undetected ITCs and a 2.2% chance of having undetected micrometastases. In addition, when only ITCs are detected, there is a 22% chance the ITCs are misclassified micrometastases secondary to failure to detect the largest dimension of the metastasis. These are unavoidable consequences of applying statistical sampling strategies to detection of ITCs and micrometastases.
The third observation is that the two-level widely spaced B-32 strategy has a higher detection rate than the two-level narrowly spaced strategy. This empiric data supports the mathematical model predictions that superficial levels have poorer performance for detecting micrometastases than wide spaced levels (3, 6). The pilot study did not accumulate a large enough number of positive blocks to statistically demonstrate a difference between the two strategies but the 6.3% detection rate for the widely spaced strategy compared to the 4.5% detection rate for the narrowly spaced strategy supports the logical hypothesis that sampling more of the lymph node volume by widening the sectioning interval increases detection rates. Thus, it is not surprising that the four-level widely spaced sectioning strategy had an 8.0% detection rate. It must be emphasized that this pilot study only examines detection rates not clinical significance. Until the B-32 outcome analysis is available, we would only recommend the standard clinical B-32 protocol of thin sectioning of lymph nodes at 2.0 mm intervals, embedding all slices, and examining one section from each block.
The fourth observation is that many sentinel lymph node sections are thicker than 2.0 mm. This pilot study determined that, on average, gross node sections were 2.1 mm thick and that the most common thickness was 2.3 mm (figure 4). A sharp decrease in the observed frequency of sections thicker than 2.3 mm indicates that personnel are making a concerted effort to slice nodes at 2.0 mm intervals in the gross dissection room but that this is not an easy task in its practical application. In addition, many but not all of the thicker sections were composed predominantly of adipose tissue with only a small amount of microscopically recognized nodal tissue. It is probable that some of these tissue slices were considered lymph nodes surrounded by adipose on gross examination when in reality they were nodes largely replaced by adipose. It should be noted that occult metastases were detected as deep as 3.4 mm into one gross lymph node section and in one case the first occult metastasis was 2.1 mm from the surface of the block. Clearly, careful attention to gross node section thickness can enhance detection rates and this should be communicated to surgical pathology prosectors. Alternatively, widely spaced sections through the block may compensate for submitting node sections thicker than 2.0 mm.
Our most important objective in pathologic evaluation of sentinel nodes is to identify all macrometastases. The clinical B-32 sampling strategy (thin node sections, one section from the top of each block) was designed to exclude macrometastases larger than 2.0 mm from the “node negative” group. The B-32 experimental protocol (two additional levels at 0.5 mm and 1.0 mm deeper into the negative node blocks) was designed to exclude micrometastases larger than 1.0 mm from the “node negative” group. A defensible but slightly more expensive extension of the B-32 experimental protocol is to prepare sections at 0.5 mm intervals through the block. In the current pilot study no metastases larger than 0.6 mm were identified using a comprehensive strategy of sections every 0.18 mm through the block. This supports the theory behind the B-32 experimental design objective of excluding 1.0 mm metastases from the “node negative” group and verifies the practical, although imperfect, application of that design.
In summary, this pilot quality assurance companion study to the B-32 trial demonstrates once again that more metastases are detected when more levels are examined. More importantly, the pilot study demonstrates that logical statistically based sampling strategies are effective at excluding metastases of pre-defined sizes from “node negative” populations and that the size of undetected metastases are related to the thickness of unexamined nodal tissue. If outcome studies indicate the oncology and pathology community should ultimately adopt recommendations for additional levels on negative sentinel node blocks, any new protocol standard must consider a limited number of evenly and widely spaced sections deeper into the block rather than asymmetric sampling of the superficial portion of the block. This concept has both statistical and economic justification.
Acknowledgement
This work was supported by Public Health Service grants (UO1-CA65121; P30-CA22435; 10CA-12027; U10CA-69974; U10CA-37377; U10CA-69651) from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD. The views expressed in this article are solely those of the authors and do not necessarily represent the official views of the National Cancer Institute or the U.S. federal government. This work was presented in part at the 98th annual meeting of the United States and Canadian Academy of Pathology, March 10, 2009. Ms. Dupuis and Ms. Weaver began this project as part of an Advanced Biology Course at South Burlington High School (Curtis Belton, instructor) with presentation of preliminary data at the Vermont State Science Fair. Ms. Dupuis is currently employed by the Vermont State Office of the Chief Medical Examiner; Ms. Weaver is a student at the University of Vermont.
References
- 1.Agresti A. Analysis of Ordinal Categorical Data. John Wiley & Sons; New York: 1984. [Google Scholar]
- 2.Chen SL, Hoehne FM, Giuliano AE. The prognostic significance of micrometastases in breast cancer: A SEER population-based analysis. Ann Surg Oncol. 2007;14:3378–3384. doi: 10.1245/s10434-007-9513-6. [DOI] [PubMed] [Google Scholar]
- 3.Cserni G. A model for determining the optimum histology of sentinel lymph nodes in breast cancer. J Clin Pathol. 2004;57:467–471. doi: 10.1136/jcp.2003.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cserni G, Amendoeira I, Apostolikas N, et al. Discrepancies in current practice of pathological evaluation of sentinel lymph nodes in breast cancer. Results of a questionnaire-based survey by the European Working Group for Breast Screening Pathology. J Clin Pathol. 2004;57:695–701. doi: 10.1136/jcp.2003.013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cserni G, Bianchi S, Vezzosi V, et al. Variations in sentinel node isolated tumour cells/micrometastasis and non-sentinel node involvement rates according to different interpretations of the TNM definitions. Eur J Cancer. 2008;44:2185–2191. doi: 10.1016/j.ejca.2008.06.033. [DOI] [PubMed] [Google Scholar]
- 6.Farshid G, Pradhan M, Kollias J, Gill PG. Computer simulations of lymph node metastasis for optimizing the pathologic examination of sentinel lymph nodes in patients with breast carcinoma. Cancer. 2000;89:2527–2537. [PubMed] [Google Scholar]
- 7.Fisher E, Palekar A, Rockette H, et al. Pathologic Findings From the National Surgical Adjuvant Breast Project (Protocol No. 4): V. Significance of Axillary Nodal Micro- and Macrometastases. Cancer. 1978;42:2032–2038. doi: 10.1002/1097-0142(197810)42:4<2032::aid-cncr2820420453>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6th ed. Springer; New York: 2002. [Google Scholar]
- 10.Harlow SP, Krag DN, Julian TB, et al. Prerandomization surgical training for the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial: a randomized phase III clinical trial to compare sentinel node resection to conventional axillary dissection in clinically node-negative breast cancer. Ann Surg. 2005;241:48–54. doi: 10.1097/01.sla.0000149429.39656.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huvos AG, Hutter RVP, Berg JW. Significance of Axillary Macrometastases and Micrometastases in Mammary Cancer. Ann Surg. 1971;173:44–46. doi: 10.1097/00000658-197101000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krag DN, Anderson SJ, Julian TB, et al. National Surgical Adjuvant Breast and Bowel Project. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: Results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8(10):881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 13.Krag DN, Julian TB, Harlow SP, et al. NSABP-32: Phase III randomized trial comparing axillary resection with sentinel lymph node dissection: a description of the trial. Ann Surg Oncol. 2004;113(Suppl):208s–210s. doi: 10.1007/BF02523630. [DOI] [PubMed] [Google Scholar]
- 14.Lyman GH, Giuliano AE, Somerfield, et al. American Society of Clinical Oncology Guideline Recommendations for Sentinel Lymph Node Biopsy in Early-Stage Breast Cancer. J Clin Oncol. 2005;23(30):7703–7720. doi: 10.1200/JCO.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Michaelson JS, Silverstein M, Sgroi D, et al. The effect of tumor size and lymph node status on breast carcinoma lethality. Cancer. 2003;98:2133–2143. doi: 10.1002/cncr.11765. [DOI] [PubMed] [Google Scholar]
- 16.Sobin LH, Wittekind Ch, editors. UICC TNM classification of malignant tumours. 6th ed. John Wiley and Sons; New York: 2002. [Google Scholar]
- 17.Weaver DL. Sentinel Lymph Nodes and Breast Carcinoma: Which micrometastases are clinically significant? Am J Surg Pathol. 2003;27:842–845. doi: 10.1097/00000478-200306000-00018. [DOI] [PubMed] [Google Scholar]