Abstract
The Bdellovibrio are miniature “living antibiotic” predatory bacteria which invade, reseal, and digest other larger Gram-negative bacteria, including pathogens. Nutrients for the replication of Bdellovibrio bacteria come entirely from the digestion of the single invaded bacterium, now called a bdelloplast, which is bound by the original prey outer membrane. Bdellovibrio bacteria are efficient digesters of prey cells, yielding on average 4 to 6 progeny from digestion of a single prey cell of a genome size similar to that of the Bdellovibrio cell itself. The developmental intrabacterial cycle of Bdellovibrio is largely unknown and has never been visualized “live.” Using the latest motorized xy stage with a very defined z-axis control and engineered periplasmically fluorescent prey allows, for the first time, accurate return and visualization without prey bleaching of developing Bdellovibrio cells using solely the inner resources of a prey cell over several hours. We show that Bdellovibrio bacteria do not follow the familiar pattern of bacterial cell division by binary fission. Instead, they septate synchronously to produce both odd and even numbers of progeny, even when two separate Bdellovibrio cells have invaded and develop within a single prey bacterium, producing two different amounts of progeny. Evolution of this novel septation pattern, allowing odd progeny yields, allows optimal use of the finite prey cell resources to produce maximal replicated, predatory bacteria. When replication is complete, Bdellovibrio cells exit the exhausted prey and are seen leaving via discrete pores rather than by breakdown of the entire outer membrane of the prey.
The predatory bacterium Bdellovibrio bacteriovorus invades and grows within the periplasmic space of another prey bacterium, hydrolyzing the interior of that prey bacterium to provide a quantized meal, growing into long elongated cells, and using those resources and not external nutrients (18). Although Bdellovibrio bacteria were discovered in 1962, their small size (0.25 by 1 μm, compared to the more usual 1- by 3-μm dimensions of a typical Escherichia coli cell) and the very nature of their growth within the periplasm of another bacterium has made their growth and development recalcitrant to live microscopic studies. (25) Thus, we have not been able to observe how exactly a single predatory Bdellovibrio cell makes use of the finite resources of a single prey cell (called a bdelloplast, once invaded) to grow and then manages to coordinate the departure of its progeny from that bdelloplast once prey resources are exhausted. The conundrum of predatory, intrabacterial growth by Bdellovibrio bacteria, which seems at odds with the conventions of typical binary fission of simple, nonpredatory bacteria in limitless culture media, has interested microbiologists since the 1960s (12, 18, 21).
Early electron microscopic (EM) studies at time points throughout a predatory infection showed “attack-phase” Bdellovibrio cells entering prey by squeezing through a pore made in the outer membrane, with the prey cell wall then being partially digested, forming a rounded bdelloplast structure, followed by initiation of growth in a filamentous manner, forming an elongated “growth-phase” cell many times the length of a single Bdellovibrio cell (12). Studying the growth and sepatation of these filaments inside their prey was challenging, and early EM observations were limited by staining and fixing procedures that killed the growing Bdellovibrio cells. Thus, researchers arrived at a range of conclusions about predatory growth and septation, including both synchronous and sequential mechanisms (2, 4, 12, 20). Early researchers also studied the growth of HI (host- or prey-independent Bdellovibrio) on complex media; these strains grow erratically, probably due to diverse point mutational backgrounds (3, 6). These studies reported either sequential or synchronous filamentation of these HI-grown Bdellovibrio, outside of prey, without being able to directly conclude the mechanisms of predatory growth and septation inside bdelloplasts (5, 7, 11). This led to some reviewers avoiding the issue of septation by general statements about “multiple fission” of predatory Bdellovibrio cells inside prey (24) or by inferring synchronous septation without actually visualizing it (26, as cited in reference 19).
The most recent published experimental investigation of septation in predatorily growing Bdellovibrio cells was in a paper from 1989 by Gray and Ruby (10), which reported “apparent multiple elongation sites along the Bdellovibrio filament wall,” stated that the exact “kinetic mechanism” of Bdellovibrio growth and development within bdelloplasts remained unclear, and raised questions of previously proposed mechanisms of growth and division described for an HI strain by Eksztejn and Varon (7). Thus, in recent publications, predatory Bdellovibrio bacteria have been variously presumed to divide sequentially into multiple progeny once host resources were depleted (5, 8, 9, 17).
To address the issue of live predatory bdellovibrios developing within bdelloplasts, we have used recent advances in microscope technology coupled with a fluorescent prey backdrop. Our time-lapse microvideos, in combination with a detailed EM study, reveal many previously unknown events in Bdellovibrio predatory growth. Our main conclusions are firstly that septation events, along the elongated filamentous Bdellovibrio cell, occur synchronously, the first time such a growth pattern has been observed in vegetative bacteria. Secondly, this synchronicity of septation is maintained between two separate Bdellovibrio filaments, developing in doubly infected prey so that completion of division and bdelloplast lysis allows the progeny of both to simultaneously escape and seek further prey to invade. Thirdly, we definitively show that odd and even numbers of Bdellovibrio progeny are produced by predatory growth, something that is present in the data of previous investigators but which has not been commented upon (5, 12). Furthermore, we observe by using electron microscopy that the size of the progeny cell elongates upon exit from the bdelloplast. Lastly, we have been able to visualize that exit from the bdelloplast is by localized formation of a discrete pore or pores through which the Bdellovibrio progeny swim, rather than by degradation of the whole bdelloplast membrane. This latter observation helps inform the search for the enzymes responsible for predator exit from exhausted prey. Taken together with the attached video evidence, we have been able to shadow and reveal the developmental behavior of this bacterial predator as it goes about its cell cycle.
MATERIALS AND METHODS
Strains and growth conditions.
The wild-type genome-sequenced Bdellovibrio bacteriovorus strain HD100 (17, 25) was used throughout this study and was grown by predation on Escherichia coli S17-1 (23) in Ca-HEPES buffer (25 mM HEPES, 2 mM calcium chloride, pH 7.6) using standard culturing methods previously described in reference 14. E. coli cells were grown in YT medium (0.8% Bacto tryptone, 0.5% yeast extract, 0.5% NaCl, pH 7.5). A fluorescent E. coli S17-1::pMAL_p2-mCherry prey strain was used for green fluorescent protein (GFP) time-lapse work, and the flagellum-minus E. coli DFB225 strain was used as prey for all electron microscopic observations (15).
Construction of the fluorescently labeled E. coli strain.
The fluorescent E. coli S17-1::pMAL-p2_mCherry strain expresses the mCherry fluorescent protein exported to the periplasmic space using a malE signal sequence (22). This allows the prey to “backlight” the nonfluorescent Bdellovibrio cell, allowing it to be visualized while undergoing septation. The mCherry open reading frame (ORF) was amplified using the primers mCRYpMAL_F-ACTTGGATCCATGGTGAGCAAGGGCGAGGAGG and mCRYpMAL_R-AATCAAGCTTCGAATTCTTACTTGTACAGCTCGTCCATGC, which introduced the BamHI and HindIII sites used to ligate the product in frame with the malE signal sequence in the pMAL-p2 vector (New England Biolabs). The resulting plasmid was transformed into E. coli S17-1 by heat shock. Induction of the lacZ promoter upstream of the malE-mcherry ORF using isopropyl-β-d-1-thiogalactopyranoside (IPTG) resulted in periplasmic mCherry fluorescence; 200 μg/ml final concentrations of IPTG were used in all fluorescence imaging experiments.
Using time-lapse fluorescence microscopy to observe development of B. bacteriovorus growth-phase cells within bdelloplasts.
For live microscopy, synchronous predatory infections were set up using methods scaled down from those previously described in full in reference 9. Briefly, 50 ml of fresh attack-phase B. bacteriovorus cells subcultured at 24-h intervals for 3 days were filtered through two 0.45-μm-pore-size filters (to remove residual prey and produce pure Bdellovibrio cells, typically at 2 × 107 cells/ml), centrifuged at 5,525 × g for 20 min in a Sigma 4K15 centrifuge, resuspended in 10 ml Ca-HEPES, and allowed to recover for 30 min. Then 500 μl of that resulting Bdellovibrio suspension was added to 500 μl of the fluorescently tagged E. coli S17-1::pMAL-p2_mCherry prey and 500 μl Ca-HEPES buffer containing IPTG, resulting in a final concentration of 200 μg/ml. Subsequently, infections were incubated in a shaking 29°C incubator for 2 h. Typically this method led to a predator/prey ratio in excess of 3:1, shown previously to be sufficient for synchronous prey infection (1, 9); synchronicity was monitored by light microscopy after a 30-min incubation ensuring that >95% of prey cells had been infected and converted into bdelloplasts.
Infections were immobilized on a glass microscope slide coated with a 1% agarose-Ca-HEPES pad into which two reservoirs had been cut and filled with distilled water to replace moisture lost from the pad through dehydration. Immobilized bdelloplasts were visualized using a Nikon Eclipse E600 epifluorescence microscope using a 100× objective lens (numerical aperture [NA], 1.25), an hcRED filter block (excitation, 550 to 600 nm; emission, 610 to 665 nm), and an exposure time of 0.1 s. Images were acquired using a Hamamatsu Orca ER camera and the Simple PCI software (version 5.3.1.081004 from Digital Pixel). An H101A xy motorized stage (Prior Scientific) allowed precise revisiting of different locations on the slide (minimum step size, 0.01 μm), and a frictional z-axis controller (minimum step size, 2 nm) in conjunction with the Simple PCI software allowed fine autofocusing on immobilized developing bdelloplasts. Typically 10 fields of view were imaged sequentially every 2.5 min per experiment, as this provided a good tradeoff between temporal resolution and mCherry photobleaching. Time stamps embedded in image files were used for all measurements of time. Fluorescence mCherry activity in time-lapse movies was false-colored green and enhanced using either (or both) the “sharpen” and “smooth” tools in the Simple PCI software to provide additional clarity.
Electron microscopic (EM) observations of the B. bacteriovorus life cycle.
To start EM experiments, 50 μl of B. bacteriovorus predatory lysates and 150 μl of E. coli DFB225 culture were mixed in 2.5 ml Ca-HEPES buffer, and this mixture was incubated at 29°C with shaking at 200 rpm. Samples of the resulting predatory lysate were taken hourly; bdelloplasts were washed briefly with distilled water by centrifugation at low speeds (to prevent bursting) at 2,000 to 3,000 rpm and rapidly applied to grids. Samples were stained with 2% phosphotungstic acid (PTA) (pH 7.0) and observed with a JEOL 1200Ex electron microscope at 80 kV. The JW_cad version 5.02a (http://www.jwcad.net/) software was used to measure the cell lengths of Bdellovibrio cells as accurately as possible.
RESULTS
In this study we used a periplasmically, fluorescently labeled prey strain (E. coli S17-1::pMAL-p2_mCherry) that had been immobilized on Ca-HEPES 1% agarose pads and infected with Bdellovibrio cells. This let us study Bdellovibrio predatory development within bdelloplasts, as the prey fluorescence acted as a backlight to help visualize the Bdellovibrio cells. Using the Prior H101A motorized xy stage with an ultrafine z-axis control autofocusing apparatus in conjunction with our fluorescent microscope allowed us to accurately revisit and refocus on Bdellovibrio cells within bdelloplasts over several hours to generate the time-lapse movies. In fluorescent studies we used the E. coli B strain S17-1, which divides to produce cells with diverse cellular volumes, generating different sizes of metabolic resource pools for the invading Bdellovibrio cells to use for growth. E. coli prey were invaded by predatory cells of the nonfluorescent genome-sequenced Bdellovibrio bacteriovorus HD100 strain, which appear as dark-negative against the prey fluorescence background (Fig. 1) (17). Due to the protease-resistant nature of beta-barreled GFP structures, the fluorescent mCherry activity is not reduced by Bdellovibrio proteases within bdelloplasts, is not available for predatory growth, and apparently backlights the whole of the prey cell due to its high intensity (13). Fluorescence activity is rapidly lost when the bdelloplast lyses, allowing the precise time point of prey lysis to be recorded (Fig. 1B). We also carried out EM studies of the number of progeny in a different E. coli K12 strain, DFB225, which again has diverse cell volumes (see legends to Fig. 1 and 2) but for which the mean volume is smaller than that of E. coli S17-1; the use of two different strains precludes any prey-specific effects.
FIG. 1.
Bdellovibrio filamentous growth-phase cells septate synchronously within the bdelloplast, forming both odd and even numbers of progeny. (Aa) Frequency measurement of the number of mature Bdellovibrio cells within 146 E. coli S17-1::pMAL-p2_mCherry fluorescent bdelloplasts. Average prey cell length was 4.67 μm ± 1.37 and width was 0.97 μm ± 0.09 (n = 167). (Ab) Examples of septated and mature growth-phase Bdellovibrio cells within fluorescently labeled bdelloplasts, with images used to illustrate frequency plot shown in panel Aa and described above. (Ba) Sketch showing key points in Bdellovibrio cell development within a bdelloplast. (Bb and c) Selected frames from time-lapse movies showing synchronously dividing growth-phase cells in fluorescent bdelloplasts. (Bd) Selected frames from time-lapse movies showing two synchronously dividing growth-phase cells forming different amounts of progeny (3 and 4) within a fluorescent bdelloplast. Fluorescence mCherry activity is false-colored green for clarity. Bars = 1 μm. Selected time-lapse movies are provided in Movies S1 and S2 in the supplemental material.
FIG. 2.
Bdellovibrio cells produce both odd and even numbers of progeny within the bdelloplast, as visualized by electron microscopy. (a) Distribution of number of progeny within E. coli DFB225 bdelloplasts at 3 h postinfection (n = 77). (b) Examples of septated Bdellovibrio progeny within bdelloplasts at 3 h postinfection, showing (from left to right) 3, 4, and 5 progeny cells. Cells were stained with 2% PTA (pH 7.0). Bars = 500 nm. The average E. coli DFB225 prey cell length in this experiment was 2 μm.
Bdellovibrio cells enter prey cells using a type IV pilus system located at the nonflagellate pole of the cell (2, 8, 15a). In our experiments we allowed prey entry to occur and then studied the developmental fate of the Bdellovibrio organism within the infected fluorescent bdelloplasts. Analysis of 146 late-stage fluorescent bdelloplasts containing septated progeny revealed that Bdellovibrio growth-phase cells divide into both odd and even numbers of progeny (for examples, see Fig. 1Aa and b). EM studies also confirmed this observation for 77 bdelloplasts which had been formed by nonfluorescent prey (infected in liquid predatory cultures by Bdellovibrio cells) (Fig. 2). A graph showing the frequency of number of progeny within bdelloplasts showed no disinclination toward an odd number of progeny, as these data were normally distributed (Fig. 1Aa and 2a). The reduced maximal progeny yield in Fig. 2 versus Fig. 1 correlates with the smaller volume of the prey E. coli used, but it must be remembered that in fluorescent larger prey cells in Fig. 1 an unknown, but possibly significant, amount of cellular protein was being diverted into mCherry protein synthesis and so was unavailable for Bdellovibrio growth. Thus, had the Fig. 1 prey cells been nonfluorescent, the yield of Bdellovibrio cells from them might have been even higher.
An unexpected finding of this study (shown in Fig. 1A) was that a few Bdellovibrio cells entered prey and formed bdelloplasts, yet did not elongate or divide (see Movie S5 in the supplemental material). The small sizes of these prey bdelloplasts suggest that they may have contained insufficient resources to produce any Bdellovibrio progeny, yet these bdelloplasts lysed at time points of 4 to 5 h, releasing the single original Bdellovibrio cell. This time point was similar to the lysis time point for bdelloplasts yielding many progeny, and it suggests that those single Bdellovibrio cells may have gone through the hydrolytic host digestion program but simply had received insufficient nutrition to allow production of a whole Bdellovibrio genome and thus did not initiate elongation (see Movie S5). An alternative explanation is that the physically small size of the prey cell somehow constrained Bdellovibrio growth within it.
EM studies had suggested that the pili used for entry were still present inside prey bdelloplasts; it was proposed that Bdellovibrio cells continued to cling onto the prey cytoplasm (at the site of entry) and thus were predicted to grow unilaterally from the free pole (7, 20). Our new data show no evidence of this, and indeed both poles of the elongating Bdellovibrio organisms move freely within bdelloplasts from the outset of growth (see Movies S1 to S3 in the supplemental material) (Fig. 1Bb to d). Temporary obstructions of growing poles by prey cell contents may cause the elongation process to “stutter” slightly in a few frames of the videos (see Movie S3), but growth from both poles is clearly occurring.
In contrast to previous reports based on electron microscopy, and contrary to “normal” bacterial cell division dogma, Bdellovibrio growth-phase cells initiate and complete septation synchronously within prey, even when as many as 8 or 9 progeny are produced (see Movies S1 to S4 in the supplemental material) (Fig. 1B) (5, 20). Septation occurs only when the Bdellovibrio cell has reached its maximal length and initiates synchronously even when high numbers of progeny are formed (see Movie S3). In the time-lapse movies Bdellovibrio cells appear to be braced against prey internal structures, and dramatic shifts in progeny cell orientations are seen at septation, possibly caused when stored tension within the braced growth-phase cell is released during synchronous filament division (see subsequent positions in Fig. 1B and see Movie S4 in the supplemental material). These shifts allowed us to accurately measure the average septation time (from initiation to division) as 41 min (±4 min; 95% confidence interval [CI]; n = 47). This was independent of the number of progeny produced (Fig. 3), suggesting that diffusion of a septation signal, even in long filaments making many progeny, was not a limiting factor in producing the septation event.
FIG. 3.
Rate of septation of a growth-phase Bdellovibrio cell is independent of the number of progeny (n = 47) (A), whereas Bdellovibrio maturation and bdelloplast lysis time decrease as the number of progeny increases (n = 79) (B). Time points were calculated from time-lapse movies using image time stamp data from SimplePCI software. Error bars indicate standard deviations.
High numbers of Bdellovibrio cells in predatory cultures can sometimes lead to multiple infections of prey; in these cases invasion of the Bdellovibrio cell has had to be near simultaneous as the forming of the prey bdelloplast precludes other Bdellovibrio cells from entry (27). In double-infected bdelloplasts the two growing Bdellovibrio cells compete for resources, and as we chose to use an E. coli prey with diverse cell volumes (see legend to Fig. 1), double infections sometimes resulted in differing amounts of progeny, derived from two coinfecting Bdellovibrio cells in a single prey bdelloplast; for example Fig. 1Bd shows two coinfecting Bdellovibrio cells forming three and four progeny in a single bdelloplast. Conventional logic would suggest that the growth-phase cell forming three progeny should finish cell division first and lyse the bdelloplast; however, synchronicity of septation is maintained between the two different growing Bdellovibrio cells (see Movie S2 in the supplemental material) (Fig. 1Bd). This suggests that there is either a diffusible signal between the two Bdellovibrio cells, or that they are both reacting simultaneously to the final depletion of a key bdelloplast resource(s) beyond the critical level at which another whole progeny Bdellovibrio cell could have been produced.
Immature divided Bdellovibrio progeny must develop polar structures, such as flagella, as Bdellovibrio cells are seen to be motile as they lyse the bdelloplast (see Movie S7). Because prey lysis results in a loss of prey-derived fluorescence activity (Fig. 1B), we were able to determine that the time taken for Bdellovibrio cells to mature and lyse bdelloplasts after septation was 26 min (±3 min; 95% CI; n = 81). In contrast to the similarity in septation times for Bdellovibrio cells which were independent of filament length and thus progeny number, the time to lysis was inversely proportional to the number of progeny, indicating that the lysing power of multiple progeny in larger bdelloplasts was higher (Fig. 3B). This also showed that progeny departed bdelloplasts through one (88%) or occasionally two (12%) small discrete holes, rather than by catastrophic bdelloplast breakdown (percentages from 67 bdelloplasts) (see Movie S6).
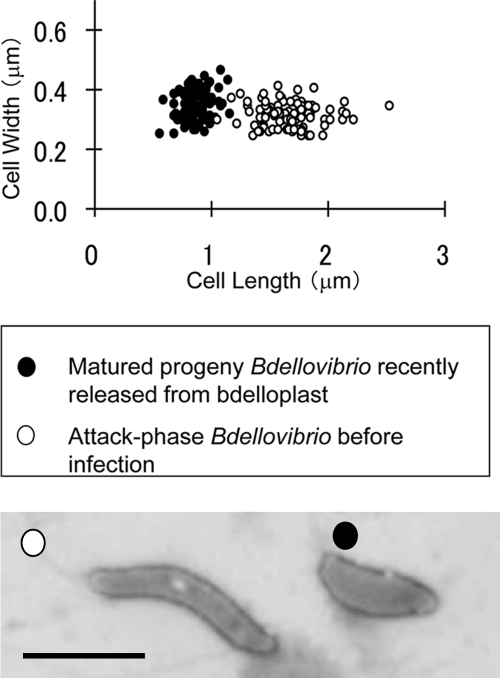
Newly formed Bdellovibrio progeny, having escaped the confines of the bdelloplast, go through a further phase where the cell length increases yet the cell width remains similar. This is observed as a near doubling in average progeny cell length, from 0.87 μm ± 0.12 μm (n = 89) within the bdelloplast to 1.66 μm ± 0.24 μm (n = 100) after bdelloplast escape, measured by electron microscopy (Fig. 4).
FIG. 4.
Cell length and width measurements of mature attack-phase cells and immature Bdellovibrio progeny from bdelloplasts. The average length of the cells before infection (white) was 1.66 ± 0.24 μm (n = 100), while that of progeny cells in bdelloplasts at 3 h after infection (black) was 0.87 ± 0.12 μm (n = 89). The average cell width is similar between recently divided progeny cells (0.350 ± 0.05 μm) and mature attack-phase cells (0.316 ± 0.04 μm). We also show two representative cells of the two types, side by side, under the same negative staining conditions and using a just-released cell as the immature progeny example to avoid bdelloplast debris. Bar = 1 μm.
DISCUSSION
This work describes in previously unseen detail how a predatory bacterium grows and divides atypically within another bacterium (the process is summarized in Fig. 1Ba). We have shown in live action that Bdellovibrio growth-phase cells within bdelloplasts grow bilaterally into elongated cells, and only after reaching the maximum length and receiving an unknown signal do they divide synchronously into both odd and even numbers of progeny (see Movies S1 to S5 in the supplemental material) (Fig. 1A and 2). This definitively shows what earlier researchers hypothesized for predatory Bdellovibrio cells from studies of HI strains and Bdellovibrio bacteria prematurely liberated from bdelloplasts by artificial lysis (7, 10). Also, our results quantify the yield of Bdellovibrio progeny possible from a single bdelloplast and provide temporal details of developmental events within predatory growth which greatly expand upon the previous observations made by Kessel and Shilo (12). Bdellovibrio septation, once initiated, takes 41 min independent of the number of progeny, and maturation of progeny and final bdelloplast lysis takes on average 26 min (Fig. 3A), depending on progeny number. Synchronicity of septation is maintained in long growth-phase cells and between two Bdellovibrio cells competing for resources in a single bdelloplast (see Movies S2 and S3). Progeny Bdellovibrio cells continue to elongate outside of the bdelloplast for a short period of time as they become nonreplicating, attack-phase cells (Fig. 4). Whether this is true differentiation is difficult to determine unless any key marker proteins associated with such changes can be discovered.
We speculate that filamentous growth has advantages in both coordinating prey digestion and maximizing division of prey resources among progeny that lead to net increases in Bdellovibrio fitness. Bdellovibrio cells must have unprecedented control over DNA replication to replicate an odd number of chromosomes from a single template and to maximize use of the quantized material available inside a dead prey bacterium to make the maximum number of progeny, something that Thomashow and Rittenberg, two fathers of Bdellovibrio research, were concerned with (26).
Bioinformatic studies of the Bdellovibrio sp. HD100 genome reveal that this bacterium encodes all of the components of regular bacterial septation machinery but no full complement of recognizable control systems, i.e., the MinCDE system (17). For an odd number of progeny to be formed from a filamentous cell, originally containing a single chromosome, one chromosomal DNA template must have replication repressed while another still replicates. Like other bacteria (such as E. coli), Bdellovibrio species are predicted to initiate DNA replication using the DnaA protein. The concentration of this protein in E. coli is finely controlled to initiate DNA replication only once from the origin of replication, and then its action is inhibited by a variety of mechanisms; for example, sequestering of oriC by the SeqA protein, making it unavailable for DnaA binding. Bdellovibrio DnaA must initiate replication multiple times in one cell, and therefore, additional and as-yet-unknown control systems must exist to control this process. IciA is a transcriptional regulator of the dnaA gene in other bacteria where transcription is known to be activated in vitro and in vivo by the binding of the IciA protein to two sites in the dnaA promoter region, and there is a homologue of the iciA gene, Bd3712, in Bdellovibrio species (16). Whether either of these control mechanisms acts to repress replication of one chromosomal origin when Bdellovibrio produces an odd progeny number has yet to be determined. As growth is bidirectional from both poles, and septation synchronous, it will be interesting to see if a regulatory factor controlling chromosome replication is sequestered at one of the poles, such as the pole where the flagellum was formerly located. Identifying the mechanisms that govern chromosome segregation and septation in the growing Bdellovibrio filament remains an exciting challenge being actively investigated.
Supplementary Material
Acknowledgments
This study was funded by a BBSRC Ph.D. Studentship for A.K.F. to R.E.S., an HFSP RGP57/2005 grant to R.E.S. and S.-I.A. for M.K., and a Daiwa Adrian Award to R.E.S. and S.-I.A. for R.D.W.
We acknowledge the Tsien laboratory as the source of the mCherry construct. We thank Marilyn Whitworth for technical assistance.
A.K.F. carried out all time-lapse fluorescence microscopic experiments and measurements, designed parts of the experimental program, and coauthored the manuscript. M.K. carried out all electron microscopic experiments and measurements and designed parts of the experimental program under the supervision of S.-I.A. R.D.W. constructed the fluorescent E. coli S17-1::pMAL-p2_mCherry strain. R.E.S. supervised the research, designed parts of the experimental program, and coauthored the manuscript. M.K., S.-I.A., and R.D.W. commented upon the manuscript.
Footnotes
Published ahead of print on 8 October 2010.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Abram, D., J. Castro e Melo, and D. Chou. 1974. Penetration of Bdellovibrio bacteriovorus into host cells. J. Bacteriol. 118:663-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abram, D., and B. K. Davis. 1970. Structural properties and features of parasitic Bdellovibrio bacteriovorus. J. Bacteriol. 104:948-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barel, G., and E. Jurkevitch. 2001. Analysis of phenotypic diversity among host-independent mutants of Bdellovibrio bacteriovorus 109J. Arch. Microbiol. 176:211-216. [DOI] [PubMed] [Google Scholar]
- 4.Burnham, J. C., T. Hashimoto, and S. F. Conti. 1968. Electron microscopic observations on the penetration of Bdellovibrio bacteriovorus into gram-negative bacterial hosts. J. Bacteriol. 96:1366-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnham, J. C., T. Hashimoto, and S. F. Conti. 1970. Ultrastructure and cell division of a facultatively parasitic strain of Bdellovibrio bacteriovorus. J. Bacteriol. 101:997-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotter, T. W., and M. F. Thomashow. 1992. Identification of a Bdellovibrio bacteriovorus genetic locus, hit, associated with the host-independent phenotype. J. Bacteriol. 174:6018-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eksztejn, M., and M. Varon. 1977. Elongation and cell division in Bdellovibrio bacteriovorus. Arch. Microbiol. 114:175-181. [DOI] [PubMed] [Google Scholar]
- 8.Evans, K. J., C. Lambert, and R. E. Sockett. 2007. Predation by Bdellovibrio bacteriovorus HD100 requires type IV pili. J. Bacteriol. 189:4850-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenton, A. K., C. Lambert, P. C. Wagstaff, and R. E. Sockett. 2010. Manipulating each MreB of Bdellovibrio bacteriovorus gives diverse morphological and predatory phenotypes. J. Bacteriol. 192:1299-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray, K. M., and E. G. Ruby. 1989. Unbalanced growth as a normal feature of development of Bdellovibrio bacteriovorus. Arch. Microbiol. 152:420-424. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz, A. T., M. Kessel, and M. Shilo. 1974. Growth cycle of predacious Bdellovibrios in a host-free extract system and some properties of the host extract. J. Bacteriol. 117:270-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessel, M., and M. Shilo. 1976. Relationship of Bdellovibrio elongation and fission to host cell size. J. Bacteriol. 128:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert, C., K. J. Evans, R. Till, L. Hobley, M. Capeness, S. Rendulic, S. C. Schuster, S. Aizawa, and R. E. Sockett. 2006. Characterizing the flagellar filament and the role of motility in bacterial prey-penetration by Bdellovibrio bacteriovorus. Mol. Microbiol. 60:274-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lambert, C., M. C. Smith, and R. E. Sockett. 2003. A novel assay to monitor predator-prey interactions for Bdellovibrio bacteriovorus 109 J. reveals a role for methyl-accepting chemotaxis proteins in predation. Environ. Microbiol. 5:127-132. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd, S. A., H. Tang, X. Wang, S. Billings, and D. F. Blair. 1996. Torque generation in the flagellar motor of Escherichia coli: evidence of a direct role for FliG but not for FliM or FliN. J. Bacteriol. 178:223-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Mahmoud, K. K., and S. F. Koval. 2010. Characterisation of type IV pili in the life cycle of the predator bacterium Bdellovibrio. Microbiology 156:1040-1051. [DOI] [PubMed] [Google Scholar]
- 16.Messer, W. 2002. The bacterial replication initiator DnaA. DnaA and oriC, the bacterial mode to initiate DNA replication. FEMS Microbiol. Rev. 26:355-374. [DOI] [PubMed] [Google Scholar]
- 17.Rendulic, S., P. Jagtap, A. Rosinus, M. Eppinger, C. Baar, C. Lanz, H. Keller, C. Lambert, K. J. Evans, A. Goesmann, F. Meyer, R. E. Sockett, and S. C. Schuster. 2004. A predator unmasked: life cycle of Bdellovibrio bacteriovorus from a genomic perspective. Science 303:689-692. [DOI] [PubMed] [Google Scholar]
- 18.Rittenberg, S. C., and R. B. Hespell. 1975. Energy efficiency of intraperiplasmic growth of Bdellovibrio bacteriovorus. J. Bacteriol. 121:1158-1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saier, M. H. J. 1994. Protein uptake into E. coli during Bdellovibrio infection: a process of reverse secretion? FEBS Lett. 337:14-17. [DOI] [PubMed] [Google Scholar]
- 20.Scherff, R. H., J. E. DeVay, and T. W. Carroll. 1966. Ultrastructure of host-parasite relationships involving reproduction of Bdellovibrio bacteriovorus in host bacteria. Phytopathology 56:627-632. [Google Scholar]
- 21.Seidler, R. J., and M. P. Starr. 1969. Factors affecting the growth of Bdellovibrio bacteriovorus developing within Escherichia coli. J. Bacteriol. 97:912-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaner, N. C., R. E. Campbell, P. A. Steinbach, B. N. Giepmans, A. E. Palmer, and R. Y. Tsien. 2004. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22:1567-1572. [DOI] [PubMed] [Google Scholar]
- 23.Simon, R., U. Preifer, and A. Puhler. 1983. A broad host range mobilisation system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. BioTechnology 1:784-791. [Google Scholar]
- 24.Starr, M. P. 1975. Bdellovibrio as a symbiont: the associations of bdellovibrios with other bacteria interpreted in terms of a generalised scheme for classifying organismic associations. Symp. Soc. Exp. Biol. 29:93-104. [PubMed] [Google Scholar]
- 25.Stolp, H., and M. P. Starr. 1963. Bdellovibrio Bacteriovorus gen. et sp. n., a predatory, ectoparasitic, and bacteriolytic microorganism. Antonie Van Leeuwenhoek 29:217-248. [DOI] [PubMed] [Google Scholar]
- 26.Thomashow, M. F., and S. C. Rittenberg. 1979. Descriptive biology of the bdellovibrios. In J. H. Parish (ed.), Developmental biology of prokaryotes, 9th ed. University of California Press, Berkeley, CA.
- 27.Thomashow, M. F., and S. C. Rittenberg. 1978. Intraperiplasmic growth of Bdellovibrio bacteriovorus 109J: attachment of long-chain fatty acids to escherichia coli peptidoglycan. J. Bacteriol. 135:1015-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.