Abstract
GnRH neurons follow a carefully orchestrated journey from their birth in the olfactory placode area. Initially, they migrate along with the vomeronasal nerve into the brain at the cribriform plate, then progress caudally to sites within the hypothalamus where they halt and send projections to the median eminence to activate pituitary gonadotropes. Many factors controlling this precise journey have been elucidated by the silencing or over expression of candidate genes in mouse models. Importantly, a number of these factors may not only play a role in normal physiology of the hypothalamic-pituitary-gonadal axis but also be mis-expressed to cause human disorders of GnRH deficiency, presenting as a failure to undergo normal pubertal development. This review outlines the current cadre of candidates thought to modulate GnRH neuronal migration. The further elucidation and characterization of these factors that impact GnRH neuron development may shed new light on human reproductive disorders and provide potential targets to develop new pro-fertility or contraceptive agents.
1.0 Introduction
Gonadotropin-releasing hormone (GnRH) is a hypothalamic releasing hormone that is synthesized in a small heterogenous neuronal population and secreted in an episodic fashion to control pituitary gonadotropin production and normal reproductive function(1-5). The GnRH neurons are unique among hypothalamic releasing factor neurons in that they originate in the olfactory placode/vomeronasal organ and migrate along vomeronasal nerves to the cribriform plate, the boundary between the peripheral olfactory system and the forebrain(1-5). Migrating GnRH neurons follow a branch of the vomeronasal nerve caudally into the hypothalamus(4). They then extend processes to the median eminence to release GnRH into the capillaries of the median eminence to modulate pituitary gonadotropin (luteinizing hormone, LH and follicle stimulating hormone, FSH) production and secretion. Loss of these neurons or misdirecting along the route results in failure of sexual maturation in mice and man. The underlying mechanisms that regulate the migration of GnRH neurons are incompletely understood. Recent studies have identified multiple factors extrinsic and intrinsic to GnRH neurons that control specific steps along the migratory route (discussed in (6-9)). This review updates the list of candidate proteins and pathways involved in GnRH neuronal migration and highlights those shown to be involved in human disorders of GnRH deficiency.
2.0 Stages of GnRH neuron migration
The process of GnRH neuronal migration can be divided into four specific stages which is useful to define candidate proteins involved in the movement and appropriate targeting of the neuronal population(6, 10). Analysis of this specific neuronal migratory process is limited by the fact that we have no marker of all GnRH neurons other than GnRH itself. No transcription factor or gene product has been demonstrated to mark all GnRH neurons early in development. The GnRH-GFP mice have green fluorescent protein expression driven by the GnRH promoter, but it is relatively weakly expressed early in development and while tracking GFP signal is useful, it does not reflect the total immunoreactive GnRH population(11). Since many external inputs inhibit GnRH gene and protein expression, the exact number of neurons and their location during development is not absolute but reflective of detected GnRH mRNA or protein. Future research is needed to identify markers of GnRH neurons. With this caveat, four steps of neuronal migration can be distinguished: 1)After their birth in the area of the olfactory placode in the mouse at approximately E10.5, GnRH neurons migrate together with vomeronasal axons across the nasal mesenchyme into the forebrain(1, 5). This initial step requires both the movement of GnRH neurons and the specific factors that promote the adherence of the neurons to axons of the vomeronasal nerve. 2) At the level of the cribriform plate, specific cues are needed as the vomeronasal nerve (VNN) divides with a branch that guides GnRH neurons turning caudally into the forebrain. 3) After crossing the cribriform plate and movement towards the hypothalamus, specific factors promote the extension of long processes through the basal forebrain toward the median eminence. 4). Lastly, the neurons detach from their axonal guides and disperse further in the hypothalamus and stop migrating. The exact steps may differ slightly across species, but most agree that a similar set of mechanistic steps are critical to target GnRH neurons to their appropriate destination in the hypothalamus so that the connection to the pituitary and ultimately reproductive competence can be achieved.
3.0 Factors involved in the initiation of GnRH Neuron Migration
Investigators have examined potential factors that may modulate GnRH neuron migration (reviewed in (6-10). Many studies have focused on factors that modify the cell migration along the vomeronasal nerve (see Table 1).
Table 1.
Factors implicated in GnRH neuron migration. List of matrix proteins, secreted proteins, transcription factors, membrane receptors and ligands and transcription factors implicated in GnRH neuron development.
| Cell Matrix/Adhesion | Neuro-transmitters | Growth Factors | G-Protein Coupled Receptors | Transcription Factors | Other |
|---|---|---|---|---|---|
| Neural cell adhesion molecule (NCAM) | GABA/GABA-A and GABA-B | FGF8/FGFR1 | PROK2/PROKR2 | Ebf2 | NELF |
| Cell surface proteoglycans (eg. B3GNT1) | Cholecystokinin-8 (CCK8)/ CCK1R | Gas6/ Axl and Tyro3 | SDF1/CXCR4 | Nhlh2 | |
| Anosmin (KAL1) | HGF/Met | Kiss/KissR | |||
| Heparan sulfate Proteoglycans | |||||
| Ephrins/Ephrin receptors | |||||
| Semaphorin 4D/PlexinB1 | |||||
| Semaphorin3a/Neuropilin2/PlexinA1 | |||||
| Netrin/DCC | |||||
| Reelin/ApoER2/Lrp8 |
B3GNT1- BetaGal beta-1,3-N-acetylglucosaminyltransferase 1; KAL 1 – Kallmann 1; DCC-Deleted in colorectal cancer gene; ApoER2- Apolipoprotein E receptor 2; Lrp8- Low density lipoprotein receptor-related protein 8; GABA- Gamma-Aminobutyric acid; CCK1R- Cholecystokinin-1 receptor; FGF8- Fibroblast growth factor 8; FGFR1- Fibroblast growth factor receptor 1; Gas6- Growth arrest specific gene 6; HGF- Hepatocyte growth factor; PROK2-Prokineticin 2; PROKR2-Prokineticin 2 receptor; SDF1 - Stromal cell derived factor 1; CXCR4- chemokine (C-X-C motif) receptor 4; Kiss- Kisspeptin; KissR- Kisspeptin receptor; Ebf2- Early B-cell factor 2; Nhlh2- Nescient helix loop helix 2; NELF- Nasal embryonic LHRH factor
3.1. Adhesion molecules tie the GnRH neurons to olfactory fibers
Molecules that are unique to the axon guides or to GnRH neurons would hypothetically restrict the neurons to follow these specific sets of axons into the forebrain. Identification of such factors has been has been complicated by the fact that GnRH neurons are phenotypically heterogeneous (12). The reason for this heterogeneity is not known, but it may be important for the regulation of the rate of migration, the ability to modulate migration at different times and locations along the route, and possibly to ensure that no single genetic mutation would prevent all or even most of these neurons from migrating to their intended destinations. The specific adhesion molecules elucidated to date include:
3.1.1. PSA-NCAM
GnRH neurons prefer to migrate along axons expressing a polysialic acid form of neural cell adhesion molecule (PSA-NCAM)(13, 14). Removal of the PSA by enzymatic digestion blocked GnRH neuron migration. Evaluation of NCAM and NCAM-180 null mice, however, revealed no significant disruption of GnRH neuron migration. These discrepant results may have been due to the redundancy of the NCAM subtype system, with GnRH neurons able to migrate instead with NCAM-140 positive axons (13, 14). No human NCAM mutations have been reported to date in patients with hypogonadotropic hypogonadism.
3.1.2. Glycoconjugates including cell surface glycoproteins
Bless and coworkers(15) showed that a cell surface glycoconjugate, carrying a lactosamine moiety, influences GnRH neuron migration. A glycosyltransferase ß1,3-N-acetylglucosaminyltransferase-1 (ß3GnT1) helps synthesize lactosamine addition on a protein for a subset of GnRH neurons. Its expression peaks at E13 then decreases by E18.5 consistent with a potential role in GnRH neuron migration. Mice null for ß3GnT1 show GnRH neurons retained in the nasal compartment at E15 with fewer neurons detected in the forebrain and a tendency for those neurons to be displaced in the dorsal rather than the ventral forebrain(15), suggesting misdirection. No human mutations have been reported to date.
3.1.3. Anosmin
The product of the Kallmann-1 (KAL1) gene, anosmin, was the first protein shown to be involved in normal GnRH neuronal migration and as a cause of X-linked form of Kallmann syndrome in humans, a combination of hypogonadotropic hypogonadism and deficient smell(16-18). The human fetus evaluated with Kallmann syndrome was noted to have olfactory, vomeronasal and terminalis nerves tangled in a web near the cribriform plate with absent olfactory tracts and bulbs (1, 19).
Anosmin is an extracellular matrix glycoprotein thought to be important in adhesion of the olfactory nerves along the initial migratory steps exiting the olfactory placode. It is a 680 amino acid secreted protein with motifs similar to those detected in axon guidance factors (20, 21). Putative roles for anosmin include as a cell matrix guide for axons, a chemoattractant that is secreted important in olfactory axon pathfinding, or a protein secreted to digest matrix to allow the olfactory axons to crosstalk with the olfactory bulb (reviewed in (22-24). Therefore, the effects of KAL1 mutations on GnRH neuron migration are indirect. Recent studies suggest that anosmin may also be involved in fibroblast growth factor receptor signaling, a growth factor pathway important in GnRH neuron development discussed below (25).
3.2. Guidance cues
3.2.1. EphA5
The ephrins are a group of cell surface molecules that signal through membrane tyrosine kinase receptors (EphA and EphB) and play a major role in axon guidance in many areas of brain development (26). The potential importance of this signaling system in GnRH neuron development was implicated by the analysis of the GN23 mutant mouse in which a 67 kb deletion downstream of the subtype 5 EphA receptor (EphA5) gene resulted in over-expression in GnRH neurons. These mice displayed a failure of normal neuronal migration with disordered clumps of GnRH neurons along the olfactory neurons. It is hypothesized that over-expression of EphA5 triggered abnormal adhesion of the GnRH neurons along the initial migratory route. In the adult, only 12% of the GnRH neurons reached a normal destination in the hypothalamus, yet surprisingly, the onset of sexual maturation occurred normally in these mice, suggesting a few appropriately targeted GnRH neurons are sufficient to promote the onset of reproductive function. However, more careful analysis showed that females were infertile or subfertile due to an abnormal LH surge mechanism (27). These mice over-expressing EphA5 receptor support the importance of the ephrin system in the early migration of GnRH neurons and the critical timing of turning on and off specific signals to ensure proper targeting of the population to ultimately allow normal reproductive function. In addition, these data suggest that because of the redundancy in the system, candidates which alter the number or location of GnRH neurons across development may result in subtle rather than absolute alterations in reproductive function. No human mutations in this system have been reported to date.
3.2.2. Nelf
Nasal embryonic LHRH factor (NELF) was isolated from expression profiling of migrating and non-migrating primary rodent GnRH neurons (28). Using an antiserum developed by the Wray group, studies suggested NELF was expressed at the membrane of GnRH and olfactory neurons during migration in the nasal region but then lost when they transitioned into the forebrain(28). Silencing of NELF transcripts by antisense oligomers in nasal explants resulted in a decrease in the number of GnRH neurons by 2/3 as well as the length and complexity of olfactory nerve fibers. Others have suggested NELF is a nuclear protein(29) whose role is implicated in neuronal migration. No knockout mouse model has been analyzed to date and the exact physiologic role of this protein awaits further studies. Recently human mutations in NELF have been identified in patients with normosmic idiopathic hypogonadotropic hypogonadism (nIHH) and KS(29-31).
3.3. Neurotransmitters that modulate GnRH neuronal migration
Slice cultures from transgenic mice expressing green fluorescent protein in GnRH neurons (GnRH-GFP mice)(11) showed that GnRH neurons move with greater frequency and with more changes in direction after they enter the brain. Perturbations of guiding fibers distal to moving GnRH neurons in the nasal compartment influenced movement without inducing detectable changes to the structure of the fibers in the immediate vicinity of moving GnRH neurons. These data suggest that the use of fibers by GnRH neurons for guidance may involve both specific signaling in addition to just mechanical guidance and supported the search for neurotransmitters involved in GnRH migration.
3.3.1. Gamma-aminobutyric acid (GABA
GABA may be prototypical for small secreted molecules that modulate GnRH neuronal migration (11, 32, 33). Consistent with the heterogeneity of the GnRH neuron population, only a subset of GnRH neurons (~30%) contain GABA during development (34). At the same time, almost all GnRH neurons contain GABAA receptors, but with heterogeneous complements of subunit composition (32, 33). One of the ways GABA is produced is via the 67 kDa form of glutamic acid decarboxylase (GAD67) (34). Transgenic mice with the GABA synthetic enzyme glutamic acid decarboxylase-67 (GAD-67) selectively over-expressed in GnRH neurons increased the number inhibited by GABA, but still only influenced a percentage of the total population(35). Over-expression of GAD67 in GnRH neurons did not disrupt the onset of sexual maturation, but the female mice displayed altered estrus cyclicity and rates of pregnancy (35). In contrast, GAD67 null mice displayed increased numbers of GnRH neurons out of the nasal placode at E14.5 and E17.5, although the changes were not balanced along the migratory route making an assessment of cell migration difficult to evaluate(36). GABA may also play a role in normal reproductive function in the adult through other receptors since examination of GABABR1 subunit null mice demonstrated again a normal onset of sexual maturation, but abnormal estrus cyclicity and impaired fertility (37, 38). No human mutations in the GABA system have been reported to date.
3.3.2. Cholecystokinin
Cholecystokinin (CCK)-8 is a widely distributed neuropeptide(39) that has been implicated in female reproductive behavior and modulation by sex steroids (40, 41). CCK acts via a G –protein coupled receptor, CCK-1R, expressed in GnRH neurons(42) and modulates GnRH neuronal migration. Mice null for CCK-1R at E14.5 displayed an increased number of GnRH neurons in the brain, suggesting it serves as an inhibitory modulator of GnRH neuron movement. However, there was compensation for the loss because in the adult the number and placement of GnRH neurons was normal and reproductive function was not impaired. Recent studies suggest that in the adult, CCK may also directly inhibit GnRH neuron firing (43). No human mutations have been detected.
3.4 Growth factors
3.4.1. FGF8/ FGFR1
Growth factors such as fibroblast growth factor 8 (FGF8) acting through fibroblast growth factor receptor 1 (FGFR1) play a role in GnRH neuron development and function (44). FGFs induce proliferation, differentiation and survival of many cell types. Mice null for FGFR1 are embryonic lethal at E9.5-12 related to altered early cell migration (45, 46) Over-expression of a dominant negative FGFR1 targeted to GnRH neurons resulted in decreased total number of GnRH neurons and abnormal projections to the median eminence suggesting that FGFs impact on multiple stages of GnRH neuron migration and development (47, 48). These mice had not only delayed puberty but with aging had premature ovarian failure (48, 49).
Multiple putative FGF ligands are expressed in forebrain development, but FGF8 was a potential candidate ligand because of its role in olfactory system development (50). Mice homozygous for a hypomorphic Fgf8 allele (51) had absent GnRH neurons in the hypothalamus with heterozygote mice showing 40% of the normal complement (52). Heterozygote mice were fertile. Consistent with these mouse models, humans with both anosmic and normosmic hypogonadotropic hypogonadism have been shown to have mutations in FGFR1 and/or FGF8 (52). Using the human data concerning the location of mutations in FGF8 found to date, the cognate ligands for FGFR1 in GnRH neurons are most likely the FGF8e and FGF8f splice variants(52). Therefore, careful phenotyping and genotyping of human patients with failure of pubertal development has given new insights into the basic studies of FGF/FGFR signaling pathway in forebrain development. Downstream components of the FGFR signaling pathway are additional potential candidates for hypogonadotropic hypogonadism that remain to be explored.
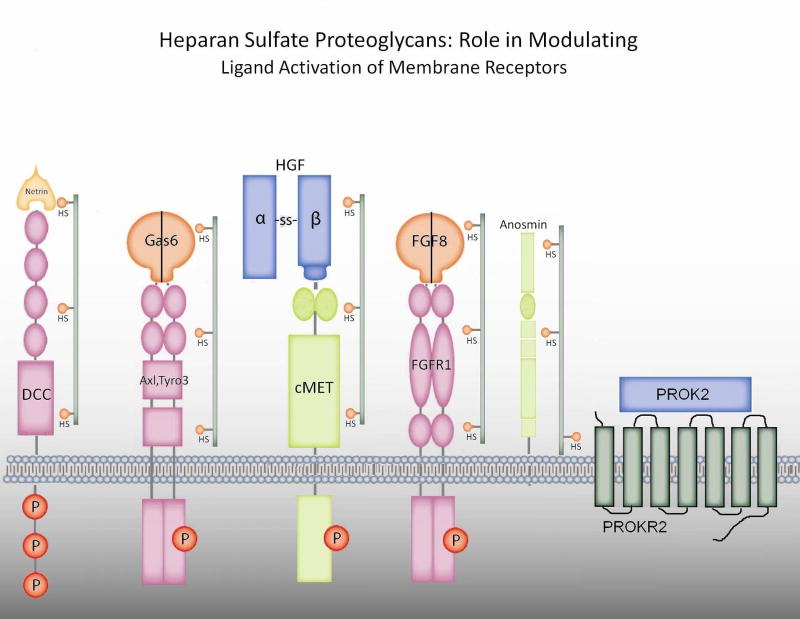
Recently investigators have suggested a functional tie between anosmin and the FGF signaling pathway. Heparan sulfate proteoglycans (HSPGs) are cell membrane and matrix-associated proteoglycans required for both anosmin and FGF action(44) (see Figure 1). In FNCB4 human fetal olfactory neuroepithlial cells, anosmin-1 triggered cytoskeletal rearrangement and neurite outgrowth via crosstalk with FGF/FGFR1/HSPG complex (53). In other studies in C. elegans, however, Hudson and coworkers suggest that the anosmin-1 ortholog, KAL-1, does not modulate FGF signaling(54). They identified that the heparan sulfate cores that modulate GnRH neuron migration include both syndecan(SDN-1) and glypican (GPN-1) and suggest that these many HSPG complexes modulate multiple factors involved in GnRH neuron migration such as anosmin-1/KAL-1 and FGFs/FGFR1 (see Fig 1). Review of the literature suggests that PROK2, HGF, netrin and perhaps Axl and Tyro3 may be modulated by HSPGs (see below). This raises the intriguing possibility of specificity in the type of HSPG to restrict or amplify crosstalk between cell surface or extracellular matrix factors to modulate GnRH neuron migration. To date, no human mutations in HSPGs have been reported.
Figure 1.
Heparan sulfate proteoglycans: role in modulating ligand activation of membrane receptors. Expanding data suggests that certain ligands or their receptors with fibonectin domains or similar regions are modulated by classes of heparan sulfate proteoglycans (HSPGs) (eg. in c. elegans, syndecan-1 or glipican-1) with specific HS to control ligand activation. These include potential interactions with Gas6/Axl or Tyro3 and documented interactions with HGF/cMET, netrin/DCC, FGF8/FGFR1, anosmin and PROK2/PROKR2.
3.5. G-protein coupled receptors
3.5.1 PROK2/PROKR2
Prokineticin 2(PROK2) and its receptor (PROKR2) were recently shown to modulate GnRH neuron migration and reproductive function with the analysis of mice mutant for the ligand and/or receptor (55, 56) and humans with absent or abnormal pubertal development (57, 58). PROK2 is an 81 amino acid ligand for PROKR2, a 384 amino acid G protein-coupled receptor (56, 59). Initial work focused on the role of this system in smooth muscle contraction of the gastrointestinal system, hematopoeisis, angiogenesis and circadian rhythms (59-61). However, mice null for PROKR2 were shown to have a loss of most of the GnRH neurons in the forebrain in adults and at E13.5, had formed a tangled web of olfactory vomeronasal axons that would be expected to alter GnRH neuron movement(55). Mice deficient in PROK2 also likely have abnormal GnRH neuron development indirectly because of altered olfactory bulb neurogenesis. Few GnRH neurons were detected in adult forebrain of PROK2 null mice and at E13.5 GnRH neurons were trapped in the tangle of olfactory axons after crossing the cribriform plate (56). PROK2 and PROKR2 are not expressed in GnRH neurons(58). These data are consistent with the idea that factors that impact olfactory bulb development are likely to impact GnRH neuron development at least through indirect influences on the fibers that guide GnRH neuron migration into the forebrain. Recent studies suggest that PROK2 also has heparan sulfate binding characteristics (62), raising the possibility that this system may also crosstalk with anosmin and FGF signaling pathways (see Fig 1).
Humans with mutations in this ligand and/or receptor have a variable clinical phenotype presenting with Kallmann syndrome, normosmic hypogonadotropic hypogonadism and some family members detected as asymptomatic carriers (57, 63, 64). This lack of correlation of genotype to phenotype has supported the hypothesis that hypogonadotropic hypogonadism may often reflect digenicity with heterozygous mutations in more than one candidate gene involved in GnRH neuron development causing the ultimate phenotypic presentation (57, 64).
3.6 Transcription factors: Ebf2
Ebf2 is a member of a helix loop helix transcription factor family implicated in neural development (65) and expressed in migrating GnRH neurons at E11 (66). Mice null for Ebf2 retained GnRH neurons clustered in the nasal mesenchyme(66). The effects appeared to be directly linked to GnRH neurons as the olfactory system development was not altered. The authors suggested a secondary loss of neurons although no studies of rates of apoptosis were shown. Since the only marker for GnRH neurons is either GnRH peptide or mRNA, failure to synthesize GnRH is difficult to differentiate from cell death and therefore many claims of cell loss remain ambiguous. At P0, the majority of detectable neurons were in the nasal mesenchyme or dorsal to the cribriform plate, but a small number of neurons was detected in the forebrain at the caudal edge of the olfactory bulb. The downstream effectors of the Ebf2 transcription factor in GnRH neurons are unknown. No mutations in Ebf2 were detected in a small number of KS and nIHH subjects analyzed (67).
4.0 Factors that guide the VNN and GnRH neurons toward the forebrain
4.1 Netrin 1/Deleted in colon cancer (DCC)
The turning of the caudal branch of the vomeronasal nerve toward the basal forebrain is regulated by netrin-1 chemoattraction (68). The caudal branch of the VNN expresses an Ig-superfamily protein, deleted in colon cancer (DCC). In other systems, DCC mediates netrin 1-dependent axon guidance. Analysis of the axon trajectories and position of GnRH neurons in DCC mutant mice showed that DCC negative c-VNN axons failed to turn ventrally in the forebrain, and instead were detoured into the cerebral cortex (68). The alterations in axon trajectories and cell migration seen in DCC and netrin-1 mutant mice are similar, suggesting that this receptor/ligand pairing is necessary and sufficient for regulating the guidance of the c-VNN towards the ventral forebrain (69). The loss of Unc5h3, the alternative netrin 1 receptor, did not affect the trajectory of DCC axons or GnRH neurons, showing the specificity of the ligand/receptor interaction. Netrin/DCC signaling is modulated by HSPG (70, 71) (see Fig 1). No human mutations in this pathway have been reported.
4.2. Semaphorins and Plexins
Semaphorin 4D (Sema4D) is a membrane-bound semaphorin that is proteolytically cleaved to bind Plexin B1 in the olfactory placode and along the GnRH neuron migratory route (72). Plexin B1 and NCAM expression colocalized in the olfactory system at E12.5 but investigators were unable to detect specific immunoreactivity for Plexin B1 along the VNN or in GnRH neurons at 14.5 or 17.5. Consistent with a localized effect early in GnRH neuron migration, in primary nasal explants cultures, PlexinB1 was detected in early cell divisions, but not later(72). Plexin B1 null mice showed a defect in GnRH neuron migration with less GnRH neurons at E14.5 and 20% less at P3 with accumulation in the olfactory bulb region. In adults, decreased GnRH fibers in the median eminence were observed (72). Studies by Prevot and coworkers have also implicated multiple semaphorins in GnRH secretion by the remodeling of GnRH fibers in the median eminence across the estrus cycle in response to sex hormones (73, 74). Together, these data suggest that the semaphorins may play specific roles in many aspects of GnRH neuron development and function.
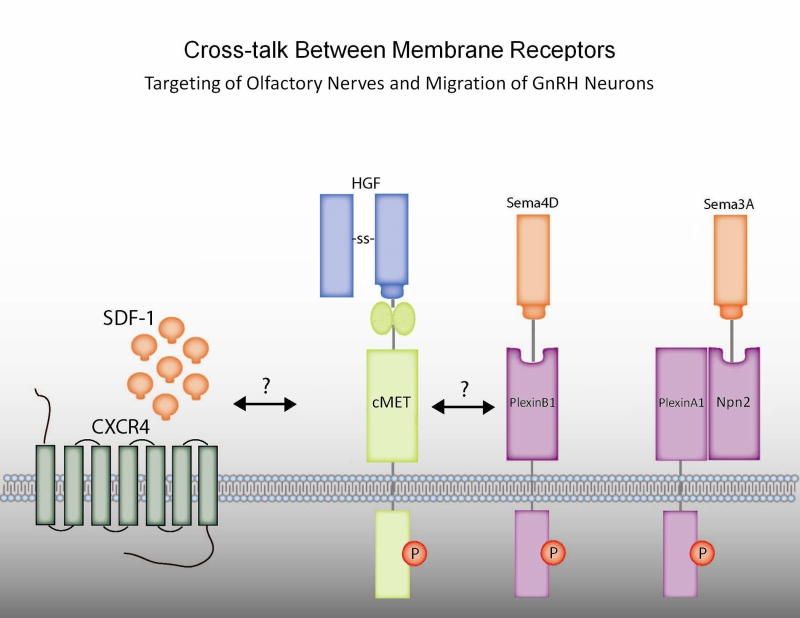
To understand potential mechanisms of semphorin/plexin signaling in neuronal migration, investigators performed experiments in Gn11 GnRH neuronal cells. The GnRH neuronal cells migrated either towards hepatocyte growth factor (HGF) or Sema4D in a Boyden chamber model. These effects could be blocked with either an HGF inhibitor or siRNA silencing of the HGF receptor, Met, suggesting that these ligands crosstalk via a plexin/Met interaction to modulate GnRH neuron migration (see Fig 2). Since Sema4D null mice do not have a reproductive phenotype, however, there may be compensation by different semaphorins in the knockout mice or that semaphorins other than 4D may be more physiologically relevant. No human mutations in this pathway have been documented to date.
Figure 2.
Crosstalk between membrane receptors targeting of olfactory nerves and migration of GnRH neurons. HGF/Met, chemokine and Semaphorin pathways have been implicated in both olfactory nerve targeting and GnRH neuron migration. SDF-1 (CXCL12) chemokine and its receptor CXCR4 may interact with HGF (hepatocyte growth factor)/cMET which in turn may interact with semaphorins and their plexin receptors in the absence or presence of docking intermediates NP2 (neuropilin 2).
4.3. Semaphorins/Neuropilin2
In addition to semaphorin 4D/plexin/HGF/Met story, the Class 3 semaphorins may impact on GnRH neuron development (see Fig 2). The Class 3 semaphorins are a group of inhibitory secreted and membrane bound proteins that bind the receptor, neuropilin-2 (NPN-2) and were initially shown to modulate axonal growth cone guidance (75). Examination of the Npn2 null mice showed a 25% loss of GnRH neurons in the adult, with increased neurons detected along the nasal septum (76). The authors suggest the defect in GnRH neuron migration is due to the defasciculation of the vomeronasal axons in these mice which disrupts the normal path and trajectory of the GnRH neurons. The location of the defect suggests that this system acts at a similar time to that of netrin/DCC signaling in GnRH neuron development. In addition to this indirect effect, a direct effect of semaphorins via Npn2 on GnRH neurons could contribute since endogenous GnRH neurons might express both the Npn2 receptor and the ligand, semaphorin3a (76). No human mutations have been reported in this ligand/receptor pair.
4.4. Reelin
Reelin is an extracellular glycoprotein shown to be involved in neuronal migration in several brain regions (77). Cariboni et al (78) showed that despite only 5% of GnRH neurons expressing one of the reelin receptors (ApoER2/Lrp8), that Reeler mice that lack Reelin had a decrease in forebrain GnRH neurons associated with their previously known decreased fertility (77). Reelin is expressed by the vomeronasal neurons but not GnRH neurons, suggesting the defects in GnRH neuronal migration are indirect(78). No human mutations in this pathway have been reported.
5.0 Growth factor pathways that move GnRH neurons through the cribriform plate and to the hypothalamus
5.1 Hepatocyte growth factor (HGF)/cMET
Met is a membrane tyrosine kinase receptor activated by the cytokine, HGF, shown to induce mitogenic, migratory and chemoattractant activities in multiple neuronal populations (79-81). HGF is a member of the plasminogen regulated growth factor family where proHGF is cleaved by uroplasminogen (uPA), tissue plaminogen (tPA) or coagulation factors to activate cMet. Giacobini and colleagues (82, 83) showed initially in GnRH neuronal cells, then in vivo, the potential importance of HGF/Met signaling to normal GnRH neuron development. In GnRH neuronal cells, HGF promoted cell migration and motility(82). HGF administration to embryonic nasal explants increased the distance that the cells migrated, whereas inhibition of HGF reduced both GnRH and olfactory axon outgrowth suggesting direct and indirect effects of this pathway. Since mice null for cMET are embryologically lethal, the investigators examined mice null for tPA/uPA (ie deficient in active HGF) and documented a 35% decrease in the number of GnRH neurons at 60-90d postnatally, associated with subfertility and decreased gonadotropin-induced frequency of ovulation(83). The exact timing of HGF/Met impact on GnRH neuron development awaits further study. The ability of Met to cross talk with other tyrosine kinases, G-protein coupled receptors and various docking proteins, however, suggests the potential interaction of this pathway with multiple other candidates discussed within this review (see Fig 1 and 2).
5.2. Axl and Tyro3
Axl, Tyro3 and Mer comprise the TAM family of receptor tyrosine kinases that play diverse roles in immune modulation, sexual function and tumorigenesis (84-86). We showed that Axl and Tyro3 were expressed in NLT cells as models for early migrating GnRH neurons while Tyro3 and Mer were expressed in GT1-7 cells as models for postmigratory GnRH neurons (87). In NLT GnRH neuronal cells, Growth arrest specific gene 6 (Gas6), the ligand for the TAM family, induced GnRH neuronal migration via a novel p38MAP kinase pathway(88) and protection from programmed cell death via ERK MAP kinase and PI3 kinase to Akt signaling(89), suggesting a potential role for Axl and Tyro3 in GnRH neuron development. Analysis of adult Axl and Tyro3 null mice showed a 25% decrease in the number of GnRH neurons overall, with a specific 34% loss in the preoptic area surrounding the OVLT coupled with a small increase in neuron numbers in rostral regions. Analysis of GnRH neuronal development in E15 embryos showed a 36% reduction in GnRH neurons reaching the ventral forebrain, whereas the population in the nose and dorsal forebrain were not altered. These data suggested a potential role for Axl and Tyro3 in GnRH neuron cell survival during the window of development where the neurons cross the cribriform plate region. Further studies showed increased incidence of apoptosis as indicated by activated caspase 3 amongst early migrating GnRH neurons (90). These migratory and survival defects are consistent with the mechanistic properties of this receptor kinase family in mediating movement and protection from programmed cell death(88, 89). The functional importance of this selective loss of GnRH neurons in Axl/Tyro3 null mice was suggested by analysis of the reproductive function of mice null for Axl and Tyro3 showing delayed first estrus and persistent abnormal estrus cyclicity (90) and abnormal LH surge mechanism (91). Examination of mice null for the ligand, Gas6 is underway to dissect if ligand is needed for Axl and Tyro3 effects on GnRH neuronal migration and survival in vivo. Initial analysis of 96 KS and nIHH patients demonstrated several heterozygous Axl mutations, suggesting the potential importance of this pathway in human reproduction (92). Gas6 is also a heparan sulfate proteoglycans activated ligand similar to FGFs and HGF (see Fig 1). Whether TAM family members crosstalk with other growth factor receptor pathways or other steps in GnRH neuronal migration is under active investigation (Fig 1 and Fig2).
5.3. Chemokine attractants: SDF1/CXCR4
Based on the hypothesis that chemoattraction would be an important mechanism for guiding GnRH neurons during their migration into the forebrain(6), stromal cell derived factor 1 (SDF-1 renamed as CXCL12) was identified as another potential candidate. It had been previously shown to impact migration of sensory cell (93), cerebral (94, 95) and cerebellar (96) precursors. CXCR4 is the G-protein coupled receptor for SDF-1(97). In the central nervous system, SDF-1 acts as a chemoattractant for granule cell precursors, and mice lacking either SDF-1 or CXCR4 show aberrant cerebellar development and neuronal proliferation(98-100). CXCR4 is also expressed by neurons in the embryonic olfactory system(101). SDF-1 is expressed in the nasal mesenchyme beginning at E10 in mice in a gradient spread across the caudal half of the nose(102), with highest levels in mesenchymal cells directly adjacent to the cribriform plate and forebrain where GnRH neurons migrate. CXCR4 is broadly expressed, and in many, but not all GnRH neurons by double-label in situ hybridization(102).
The migration of GnRH neurons was severely impaired in CXCR4 null mice (102). At E12, when 40% of GnRH neurons have migrated out of the VNO in control mice, almost all GnRH neurons in CXCR4 null mice still reside in the VNO. At E13, when about 50% of GnRH neurons have migrated into the forebrain in wild-type littermates, less than 3% of GnRH neurons migrated across the cribriform plate and none migrated caudally into the developing hypothalamus. There was also a significant increase in the number of TUNEL positive apoptotic cells in the VNO at E13, particularly along the rostral and ventral surface of the VNO. In CXCR4 null mice GnRH-1 neurons accumulated in the most rostral-ventral quadrant of the VNO. Likewise, TUNEL+ cells were found in the same location. These data suggested the importance of SDF-1/ CXCR4 signaling to mediate GnRH-1 neuron migration from their birthplace in the VNO to their ultimate destination in the forebrain. The recent suggestion of HGF signaling to Met receptors as synergistic with SDF-1 signaling to CXCR4 receptors (83), (see Fig 2) provides another example of the complex interactions among signaling systems during GnRH-1 neuron development. No human mutations in this system have been reported.
5.4. Transcription factor: Nhlh2
Nhlh2 is a helix-loop-helix protein expressed in the arcuate and anteroventral periventricular regions of the hypothalamus as well as the pituitary during development and adulthood(103). It is a member of a family of proteins expressed in postmitotic neurons acting as differentiating effectors late in development. During embryonic development Nhlh2 is expressed in a subset of GnRH neurons but then is absent later during development. Later in embryogenesis it was detected in Kisspeptin containing neurons in the hypothalamus and in the pituitary (104). Mice null for Nhlh2 had a loss of GnRH neurons in adulthood that occurred sometime between birth and adulthood (loss of 60% in females and 30% in males). At P0 there was a mild potential migratory defect with 16% more neurons in the olfactory/preoptic area and loss of neurons in the OVLT/caudal hypothalamic areas. Although these mice had delayed first estrus and estrus abnormalities, exposure of the females to males induced ovulation. LH expression was also decreased in the pituitaries of Nhlh2 null mice. These data suggested a complex role of Nhlh2 in the central reproductive axis, perhaps via Kisspeptin signaling as well as direct effects on later steps in GnRH neuron function postnatally and in addition pituitary defects which require further analysis.
Intriguingly, one of the many actions of Nhlh2 may be through necdin, a downstream target of Nhlh2 that was shown to augment GnRH gene transcription by interrupting Msx repression(105). Analysis of mice null for necdin demonstrated decreased hypothalamic neurons including GnRH neurons (by 30%) in the adult (106), with a selective loss in crossing the cribriform plate into the forebrain during embryogenesis (by 50% at E13.5 and by 30% at E 17.5), as well as a decrease in the extension of axons to the median eminence(105). No human mutations in Nhlh2 have been reported, but necdin has been implicated in Prader Willi, a syndrome of obesity and hypogonadotropic hypogonadism(107, 108).
6.0. Factors that halt GnRH neuronal migration
6.1. Kisspeptin/KissR
While GABA is thought to slow GnRH-1 neuron migration in the nasal compartment (32, 33) and it may function to alter GnRH-1 neuron associations with guiding fibers in the brain (11, 33), little is known about the factors that may contribute to the cessation of GnRH neuron migration once they have achieved their proper location within the hypothalamus. Kisspeptin is a 154 amino acid peptide with many shorter proteolytic cleavage products that is the product of the KiSS-1 gene, first shown to suppress metastasis of tumor cell lines (109). Kisspeptin binds to and activates GPR54(now called the Kiss receptor (KiSSR), a seven transmembrane, G-protein coupled receptor to mediate decreased cell motility(110-112). Although the majority of work in the field has focused on the role of kisspeptin/KiSSR signaling to promote GnRH secretion at the time of puberty (109, 113-115), its embryonic expression and potential function(s) has only recently been investigated. Preliminary studies suggest that Kisspeptin is detectable by in situ hybridization in a small number of neurons in the fetal arcuate nucleus of mice (116). Thus, Kisspeptin/KiSSR signaling may also play a role in the embryonic development of GnRH neurons in addition to its role in the onset of puberty.
7.0 Candidate genes that are mutated in humans to alter pubertal development
Nine genes have been reported in association with human hypogonadotrophic hypogonadism do date(30, 31, 92, 117-122). Table 2 illustrates the pattern of inheritance, the putative frequency of these genes found in patients with KS or nIHH and whether each has been noted to exhibit digenicity, ie., more than one mutation in an individual proband. The effects of KAL-1, FGF8, FGFR1, PROK2 and PROKR2 would be expected to alter the number and successful targeting of GnRH neurons, and the role of CHD7 in GnRH neuron development is not clear. Alterations in KiSSR would be expected to alter GnRH secretion at puberty but may have effects on early neuron migration or maturation since it is expressed in GnRH neurons across development, NELF and AXL have been recently described in a small number of patients. Phenotype, inheritance patterns and frequency of these genes are yet to be defined.
Table 2.
Candidates involved in GnRH neuron development mutated in human subjects. Names of genes shown to be mutated in probands with KS or nIHH with mode of inheritance, penetration, frequency of occurrence, phenotype and documentation of digenicity.
| Candidate | Pattern of inheritance | Phenotype | Frequency in IHH patients | Digenicity |
|---|---|---|---|---|
| KAL-1 (Anosmin) | X-linked | Anosmic, complete penetrance | 5-10% | Yes |
| FGF8 (KAL-6) | AD | Anosmic, normosmic NRP, FPP | 2% | Yes |
| FGFR1 (KAL-2) | AD | Variable expressivity AP, DP, PP | 10% | Yes |
| PROK2 (KAL-4) | AD, AR | Anosmic, normosmic Incomplete penetrance | 2% | Yes |
| PROKR2 (KAL-3) | AD, AR | Anosmic, normosmic Incomplete penetrance | 4% | Yes |
| CHD7 (KAL-5) | AD | Anosmic, normosmic CHARGE syndrome | 5% | Not reported |
| NELF | AR | Hyposmic | <1% | Yes |
| KISSR (GPR54) | AR | Normosmic | 2% | No |
| Axl | sporadic | Anosmic,normosmic | ? | Yes |
AD-autosomal dominant; AR-autosomal recessive; AP-absent puberty, DP-delayed puberty, DP-delayed pubery, NRP-normal reproductive phenotype, FPP-fully penetrant phenotype
CHARGE syndrome: coloboma, heart abnormalities, choanal atresia, retardation of growth and development, genital hypoplasia and ear abnormalities
KAL 1 – Kallmann 1; FGF8- Fibroblast growth factor 8; FGFR1- Fibroblast growth factor receptor 1; PROK2-Prokineticin 2; PROKR2-Prokineticin 2 receptor; CHD7- Chromodomain helicase DNA binding protein 7; NELF- Nasal embryonic LHRH factor; KISSR- Kisspeptin receptor
8.0 Summary
The number of factors involved in GnRH neuron migration and development continues to expand. Candidates range from transcription factors to multiple transmembrane tyrosine kinases or G-protein coupled receptors and their ligands to extracellular matrix proteins. Many of the factors identified to date influence GnRH neuron movement indirectly by altering the pace or targeting of the olfactory system. Candidates intrinsic to the GnRH neuron form a smaller subset of important guides. The inability to track endogenous GnRH neuron number independent of GnRH mRNA or protein remains a limitation to studies as to the effects of silencing of potential candidates in that one cannot distinguish between suppression of GnRH expression compared to loss of a subset of the neuronal population. Few of the candidates identified to date important in GnRH neuron development have been shown to be altered in patients with KS or nIHH. The lack of a definable molecular cause for the majority of subjects with delayed or absent puberty suggests the field will expand further in its attempts to define the constellation of mechanisms that drive GnRH neuron development and migration to ensure normal reproductive development.
Acknowledgments
We acknowledge the efforts of Kasey Maley-Combs for initial drafts of the figures and Mei Xu and Aaron Knox for review of the manuscript. Supported by NIH HD31191-12 and VA Merit Review (MEW) and NIH DC009034 (ST).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwanzel-Fukuda M, Pfaff DW. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989;338:161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- 2.Seeburg PH, Mason AJ, Stewart TA, Nikolics K. The mammalian GnRH gene and its pivotal role in reproduction. Recent Prog Horm Res. 1987;43:69–98. doi: 10.1016/b978-0-12-571143-2.50008-3. [DOI] [PubMed] [Google Scholar]
- 3.Wray S, Hoffman G. Postnatal morphological changes in rat LHRH neurons correlated with sexual maturation. Neuroendocrinology. 1986;43:93–97. doi: 10.1159/000124516. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K, Tobet SA, Crandall JE, Jimenez TP, Schwarting GA. The migration of luteinizing hormone-releasing hormone neurons in the developing rat is associated with a transient, caudal projection of the vomeronasal nerve. J Neurosci. 1995;15:7769–7777. doi: 10.1523/JNEUROSCI.15-12-07769.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wray S, Grant P, Gainer H. Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci U S A. 1989;86:8132–8136. doi: 10.1073/pnas.86.20.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobet SA, Schwarting GA. Minireview: recent progress in gonadotropin-releasing hormone neuronal migration. Endocrinology. 2006;147:1159–1165. doi: 10.1210/en.2005-1275. [DOI] [PubMed] [Google Scholar]
- 7.Wierman ME, Pawlowski JE, Allen MP, Xu M, Linseman DA, Nielsen-Preiss S. Molecular mechanisms of gonadotropin-releasing hormone neuronal migration. Trends Endocrinol Metab. 2004;15:96–102. doi: 10.1016/j.tem.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Cariboni A, Maggi R, Parnavelas JG. From nose to fertility: the long migratory journey of gonadotropin-releasing hormone neurons. Trends Neurosci. 2007;30:638–644. doi: 10.1016/j.tins.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Silveira LF, Trarbach EB, Latronico AC. Genetics basis for GnRH-dependent pubertal disorders in humans. Mol Cell Endocrinol. doi: 10.1016/j.mce.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Tobet SA, Bless EP, Schwarting GA. Developmental aspect of the gonadotropin-releasing hormone system. Mol Cell Endocrinol. 2001;185:173–184. doi: 10.1016/s0303-7207(01)00616-5. [DOI] [PubMed] [Google Scholar]
- 11.Bless EP, Walker HJ, Yu KW, Knoll JG, Moenter SM, Schwarting GA, Tobet SA. Live view of gonadotropin-releasing hormone containing neuron migration. Endocrinology. 2005;146:463–468. doi: 10.1210/en.2004-0838. [DOI] [PubMed] [Google Scholar]
- 12.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K, Rutishauser U, Crandall JE, Schwarting GA. Polysialic acid facilitates migration of luteinizing hormone-releasing hormone neurons on vomeronasal axons. J Neurosci. 1999;19:794–801. doi: 10.1523/JNEUROSCI.19-02-00794.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami S, Arai Y. Migration of LHRH neurons into the spinal cord: evidence for axon-dependent migration from the transplanted chick olfactory placode. Eur J Neurosci. 2002;16:684–692. doi: 10.1046/j.1460-9568.2002.02116.x. [DOI] [PubMed] [Google Scholar]
- 15.Bless E, Raitcheva D, Henion TR, Tobet S, Schwarting GA. Lactosamine modulates the rate of migration of GnRH neurons during mouse development. Eur J Neurosci. 2006;24:654–660. doi: 10.1111/j.1460-9568.2006.04955.x. [DOI] [PubMed] [Google Scholar]
- 16.Legouis R, Hardelin JP, Levilliers J, Claverie JM, Compain S, Wunderle V, Millasseau P, Le Paslier D, Cohen D, Caterina D, et al. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell. 1991;67:423–435. doi: 10.1016/0092-8674(91)90193-3. [DOI] [PubMed] [Google Scholar]
- 17.Ballabio A, Camerino G. The gene for X-linked Kallmann syndrome: a human neuronal migration defect. Curr Opin Genet Dev. 1992;2:417–421. doi: 10.1016/s0959-437x(05)80152-2. [DOI] [PubMed] [Google Scholar]
- 18.Franco B, Guioli S, Pragliola A, Incerti B, Bardoni B, Tonlorenzi R, Carrozzo R, Maestrini E, Pieretti M, Taillon-Miller P, et al. A gene deleted in Kallmann’s syndrome shares homology with neural cell adhesion and axonal path-finding molecules. Nature. 1991;353:529–536. doi: 10.1038/353529a0. [DOI] [PubMed] [Google Scholar]
- 19.Schwanzel-Fukuda M, Bick D, Pfaff DW. Luteinizing hormone-releasing hormone (LHRH)-expressing cells do not migrate normally in an inherited hypogonadal (Kallmann) syndrome. Brain Res Mol Brain Res. 1989;6:311–326. doi: 10.1016/0169-328x(89)90076-4. [DOI] [PubMed] [Google Scholar]
- 20.Albuisson J, Pecheux C, Carel JC, Lacombe D, Leheup B, Lapuzina P, Bouchard P, Legius E, Matthijs G, Wasniewska M, Delpech M, Young J, Hardelin JP, Dode C. Kallmann syndrome: 14 novel mutations in KAL1 and FGFR1 (KAL2) Hum Mutat. 2005;25:98–99. doi: 10.1002/humu.9298. [DOI] [PubMed] [Google Scholar]
- 21.Rugarli EI, Di Schiavi E, Hilliard MA, Arbucci S, Ghezzi C, Facciolli A, Coppola G, Ballabio A, Bazzicalupo P. The Kallmann syndrome gene homolog in C. elegans is involved in epidermal morphogenesis and neurite branching. Development. 2002;129:1283–1294. doi: 10.1242/dev.129.5.1283. [DOI] [PubMed] [Google Scholar]
- 22.Lutz B, Kuratani S, Rugarli EI, Wawersik S, Wong C, Bieber FR, Ballabio A, Eichele G. Expression of the Kallmann syndrome gene in human fetal brain and in the manipulated chick embryo. Hum Mol Genet. 1994;3:1717–1723. doi: 10.1093/hmg/3.10.1717. [DOI] [PubMed] [Google Scholar]
- 23.Rugarli EI. Kallmann syndrome and the link between olfactory and reproductive development. Am J Hum Genet. 1999;65:943–948. doi: 10.1086/302600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cariboni A, Pimpinelli F, Colamarino S, Zaninetti R, Piccolella M, Rumio C, Piva F, Rugarli EI, Maggi R. The product of X-linked Kallmann’s syndrome gene (KAL1) affects the migratory activity of gonadotropin-releasing hormone (GnRH)-producing neurons. Hum Mol Genet. 2004;13:2781–2791. doi: 10.1093/hmg/ddh309. [DOI] [PubMed] [Google Scholar]
- 25.Dode C, Hardelin JP. Kallmann syndrome: fibroblast growth factor signaling insufficiency? J Mol Med. 2004;82:725–734. doi: 10.1007/s00109-004-0571-y. [DOI] [PubMed] [Google Scholar]
- 26.Gamble JA, Karunadasa DK, Pape JR, Skynner MJ, Todman MG, Bicknell RJ, Allen JP, Herbison AE. Disruption of ephrin signaling associates with disordered axophilic migration of the gonadotropin-releasing hormone neurons. J Neurosci. 2005;25:3142–3150. doi: 10.1523/JNEUROSCI.4759-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149:597–604. doi: 10.1210/en.2007-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer PR, Wray S. Novel gene expressed in nasal region influences outgrowth of olfactory axons and migration of luteinizing hormone-releasing hormone (LHRH) neurons. Genes Dev. 2000;14:1824–1834. [PMC free article] [PubMed] [Google Scholar]
- 29.Xu N, Bhagavath B, Kim HG, Halvorson L, Podolsky RS, Chorich LP, Prasad P, Xiong WC, Cameron RS, Layman LC. NELF is a nuclear protein involved in hypothalamic GnRH neuronal migration. Mol Cell Endocrinol. 2009 doi: 10.1016/j.mce.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest. 2007;117:457–463. doi: 10.1172/JCI29884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura K, Acierno JS, Jr, Seminara SB. Characterization of the human nasal embryonic LHRH factor gene, NELF, and a mutation screening among 65 patients with idiopathic hypogonadotropic hypogonadism (IHH) J Hum Genet. 2004;49:265–268. doi: 10.1007/s10038-004-0137-4. [DOI] [PubMed] [Google Scholar]
- 32.Fueshko SM, Key S, Wray S. GABA inhibits migration of luteinizing hormone-releasing hormone neurons in embryonic olfactory explants. J Neurosci. 1998;18:2560–2569. doi: 10.1523/JNEUROSCI.18-07-02560.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bless EP, Westaway WA, Schwarting GA, Tobet SA. Effects of gamma-aminobutyric acid(A) receptor manipulation on migrating gonadotropin-releasing hormone neurons through the entire migratory route in vivo and in vitro. Endocrinology. 2000;141:1254–1262. doi: 10.1210/endo.141.3.7348. [DOI] [PubMed] [Google Scholar]
- 34.Tobet SA, Chickering TW, King JC, Stopa EG, Kim K, Kuo-Leblank V, Schwarting GA. Expression of gamma-aminobutyric acid and gonadotropin-releasing hormone during neuronal migration through the olfactory system. Endocrinology. 1996;137:5415–5420. doi: 10.1210/endo.137.12.8940365. [DOI] [PubMed] [Google Scholar]
- 35.Heger S, Seney M, Bless E, Schwarting GA, Bilger M, Mungenast A, Ojeda SR, Tobet SA. Overexpression of glutamic acid decarboxylase-67 (GAD-67) in gonadotropin-releasing hormone neurons disrupts migratory fate and female reproductive function in mice. Endocrinology. 2003;144:2566–2579. doi: 10.1210/en.2002-221107. [DOI] [PubMed] [Google Scholar]
- 36.Lee JM, Tiong J, Maddox DM, Condie BG, Wray S. Temporal migration of gonadotrophin-releasing hormone-1 neurones is modified in GAD67 knockout mice. J Neuroendocrinol. 2008;20:93–103. doi: 10.1111/j.1365-2826.2007.01623.x. [DOI] [PubMed] [Google Scholar]
- 37.Catalano PN, Di Giorgio N, Bonaventura MM, Bettler B, Libertun C, Lux-Lantos VA. Lack of functional GABA(B) receptors alters GnRH physiology and sexual dimorphic expression of GnRH and GAD-67 in the brain. Am J Physiol Endocrinol Metab. 298:E683–696. doi: 10.1152/ajpendo.00532.2009. [DOI] [PubMed] [Google Scholar]
- 38.Catalano PN, Bonaventura MM, Silveyra P, Bettler B, Libertun C, Lux-Lantos VA. GABA(B1) knockout mice reveal alterations in prolactin levels, gonadotropic axis, and reproductive function. Neuroendocrinology. 2005;82:294–305. doi: 10.1159/000093128. [DOI] [PubMed] [Google Scholar]
- 39.Vanderhaeghen JJ, Signeau JC, Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975;257:604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- 40.Vijayan E, Samson WK, McCann SM. In vivo and in vitro effects of cholecystokinin on gonadotropin, prolactin, growth hormone and thyrotropin release in the rat. Brain Res. 1979;172:295–302. doi: 10.1016/0006-8993(79)90539-0. [DOI] [PubMed] [Google Scholar]
- 41.Oro AE, Simerly RB, Swanson LW. Estrous cycle variations in levels of cholecystokinin immunoreactivity within cells of three interconnected sexually dimorphic forebrain nuclei. Evidence for a regulatory role for estrogen. Neuroendocrinology. 1988;47:225–235. doi: 10.1159/000124916. [DOI] [PubMed] [Google Scholar]
- 42.Giacobini P, Kopin AS, Beart PM, Mercer LD, Fasolo A, Wray S. Cholecystokinin modulates migration of gonadotropin-releasing hormone-1 neurons. J Neurosci. 2004;24:4737–4748. doi: 10.1523/JNEUROSCI.0649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giacobini P, Wray S. Cholecystokinin directly inhibits neuronal activity of primary gonadotropin-releasing hormone cells through cholecystokinin-1 receptor. Endocrinology. 2007;148:63–71. doi: 10.1210/en.2006-0758. [DOI] [PubMed] [Google Scholar]
- 44.Kim SH, Hu Y, Cadman S, Bouloux P. Diversity in fibroblast growth factor receptor 1 regulation: learning from the investigation of Kallmann syndrome. J Neuroendocrinol. 2008;20:141–163. doi: 10.1111/j.1365-2826.2007.01627.x. [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- 46.Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 47.Tsai PS, Moenter SM, Postigo HR, El Majdoubi M, Pak TR, Gill JC, Paruthiyil S, Werner S, Weiner RI. Targeted expression of a dominant-negative fibroblast growth factor (FGF) receptor in gonadotropin-releasing hormone (GnRH) neurons reduces FGF responsiveness and the size of GnRH neuronal population. Mol Endocrinol. 2005;19:225–236. doi: 10.1210/me.2004-0330. [DOI] [PubMed] [Google Scholar]
- 48.Gill JC, Tsai PS. Expression of a dominant negative FGF receptor in developing GNRH1 neurons disrupts axon outgrowth and targeting to the median eminence. Biol Reprod. 2006;74:463–472. doi: 10.1095/biolreprod.105.046904. [DOI] [PubMed] [Google Scholar]
- 49.Tsai PS, Gill JC. Mechanisms of disease: Insights into X-linked and autosomal-dominant Kallmann syndrome. Nat Clin Pract Endocrinol Metab. 2006;2:160–171. doi: 10.1038/ncpendmet0119. [DOI] [PubMed] [Google Scholar]
- 50.Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, Calof AL. Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development. 2005;132:5211–5223. doi: 10.1242/dev.02143. [DOI] [PubMed] [Google Scholar]
- 51.Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 52.Falardeau J, Chung WC, Beenken A, Raivio T, Plummer L, Sidis Y, Jacobson-Dickman EE, Eliseenkova AV, Ma J, Dwyer A, Quinton R, Na S, Hall JE, Huot C, Alois N, Pearce SH, Cole LW, Hughes V, Mohammadi M, Tsai P, Pitteloud N. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–2831. doi: 10.1172/JCI34538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gonzalez-Martinez D, Kim SH, Hu Y, Guimond S, Schofield J, Winyard P, Vannelli GB, Turnbull J, Bouloux PM. Anosmin-1 modulates fibroblast growth factor receptor 1 signaling in human gonadotropin-releasing hormone olfactory neuroblasts through a heparan sulfate-dependent mechanism. J Neurosci. 2004;24:10384–10392. doi: 10.1523/JNEUROSCI.3400-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hudson ML, Kinnunen T, Cinar HN, Chisholm AD. C. elegans Kallmann syndrome protein KAL-1 interacts with syndecan and glypican to regulate neuronal cell migrations. Dev Biol. 2006;294:352–365. doi: 10.1016/j.ydbio.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 55.Matsumoto S, Yamazaki C, Masumoto KH, Nagano M, Naito M, Soga T, Hiyama H, Matsumoto M, Takasaki J, Kamohara M, Matsuo A, Ishii H, Kobori M, Katoh M, Matsushime H, Furuichi K, Shigeyoshi Y. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci U S A. 2006;103:4140–4145. doi: 10.1073/pnas.0508881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ng KL, Li JD, Cheng MY, Leslie FM, Lee AG, Zhou QY. Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science. 2005;308:1923–1927. doi: 10.1126/science.1112103. [DOI] [PubMed] [Google Scholar]
- 57.Dode C, Teixeira L, Levilliers J, Fouveaut C, Bouchard P, Kottler ML, Lespinasse J, Lienhardt-Roussie A, Mathieu M, Moerman A, Morgan G, Murat A, Toublanc JE, Wolczynski S, Delpech M, Petit C, Young J, Hardelin JP. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006;2:e175. doi: 10.1371/journal.pgen.0020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pitteloud N, Zhang C, Pignatelli D, Li JD, Raivio T, Cole LW, Plummer L, Jacobson-Dickman EE, Mellon PL, Zhou QY, Crowley WF., Jr Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci U S A. 2007;104:17447–17452. doi: 10.1073/pnas.0707173104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheng MY, Bullock CM, Li C, Lee AG, Bermak JC, Belluzzi J, Weaver DR, Leslie FM, Zhou QY. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410. doi: 10.1038/417405a. [DOI] [PubMed] [Google Scholar]
- 60.Li M, Bullock CM, Knauer DJ, Ehlert FJ, Zhou QY. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 61.Ferrara N, LeCouter J, Lin R, Peale F. EG-VEGF and Bv8: a novel family of tissue-restricted angiogenic factors. Biochim Biophys Acta. 2004;1654:69–78. doi: 10.1016/j.bbcan.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 62.LeCouter J, Lin R, Tejada M, Frantz G, Peale F, Hillan KJ, Ferrara N. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc Natl Acad Sci U S A. 2003;100:2685–2690. doi: 10.1073/pnas.0337667100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monnier C, Dode C, Fabre L, Teixeira L, Labesse G, Pin JP, Hardelin JP, Rondard P. PROKR2 missense mutations associated with Kallmann syndrome impair receptor signalling activity. Hum Mol Genet. 2009;18:75–81. doi: 10.1093/hmg/ddn318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cole LW, Sidis Y, Zhang C, Quinton R, Plummer L, Pignatelli D, Hughes VA, Dwyer AA, Raivio T, Hayes FJ, Seminara SB, Huot C, Alos N, Speiser P, Takeshita A, Van Vliet G, Pearce S, Crowley WF, Jr, Zhou QY, Pitteloud N. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab. 2008;93:3551–3559. doi: 10.1210/jc.2007-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dubois L, Vincent A. The COE--Collier/Olf1/EBF--transcription factors: structural conservation and diversity of developmental functions. Mech Dev. 2001;108:3–12. doi: 10.1016/s0925-4773(01)00486-5. [DOI] [PubMed] [Google Scholar]
- 66.Corradi A, Croci L, Broccoli V, Zecchini S, Previtali S, Wurst W, Amadio S, Maggi R, Quattrini A, Consalez GG. Hypogonadotropic hypogonadism and peripheral neuropathy in Ebf2-null mice. Development. 2003;130:401–410. doi: 10.1242/dev.00215. [DOI] [PubMed] [Google Scholar]
- 67.Trarbach EB, Baptista MT, Garmes HM, Hackel C. Molecular analysis of KAL-1, GnRH-R, NELF and EBF2 genes in a series of Kallmann syndrome and normosmic hypogonadotropic hypogonadism patients. J Endocrinol. 2005;187:361–368. doi: 10.1677/joe.1.06103. [DOI] [PubMed] [Google Scholar]
- 68.Schwarting GA, Kostek C, Bless EP, Ahmad N, Tobet SA. Deleted in colorectal cancer (DCC) regulates the migration of luteinizing hormone-releasing hormone neurons to the basal forebrain. J Neurosci. 2001;21:911–919. doi: 10.1523/JNEUROSCI.21-03-00911.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwarting GA, Raitcheva D, Bless EP, Ackerman SL, Tobet S. Netrin 1-mediated chemoattraction regulates the migratory pathway of LHRH neurons. Eur J Neurosci. 2004;19:11–20. doi: 10.1111/j.1460-9568.2004.03094.x. [DOI] [PubMed] [Google Scholar]
- 70.Kastenhuber E, Kern U, Bonkowsky JL, Chien CB, Driever W, Schweitzer J. Netrin-DCC, Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. J Neurosci. 2009;29:8914–8926. doi: 10.1523/JNEUROSCI.0568-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bennett KL, Bradshaw J, Youngman T, Rodgers J, Greenfield B, Aruffo A, Linsley PS. Deleted in colorectal carcinoma (DCC) binds heparin via its fifth fibronectin type III domain. J Biol Chem. 1997;272:26940–26946. doi: 10.1074/jbc.272.43.26940. [DOI] [PubMed] [Google Scholar]
- 72.Giacobini P, Messina A, Morello F, Ferraris N, Corso S, Penachioni J, Giordano S, Tamagnone L, Fasolo A. Semaphorin 4D regulates gonadotropin hormone-releasing hormone-1 neuronal migration through PlexinB1-Met complex. J Cell Biol. 2008;183:555–566. doi: 10.1083/jcb.200806160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Seranno S, d’Anglemont de Tassigny X, Estrella C, Loyens A, Kasparov S, Leroy D, Ojeda SR, Beauvillain JC, Prevot V. Role of Estradiol in the Dynamic Control of Tanycyte Plasticity Mediated by Vascular Endothelial Cells in the Median Eminence. Endocrinology. doi: 10.1210/en.2009-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prevot V, Levine JE. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology. 2009;150:5509–5519. doi: 10.1210/en.2009-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Giger RJ, Cloutier JF, Sahay A, Prinjha RK, Levengood DV, Moore SE, Pickering S, Simmons D, Rastan S, Walsh FS, Kolodkin AL, Ginty DD, Geppert M. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. [DOI] [PubMed] [Google Scholar]
- 76.Cariboni A, Hickok J, Rakic S, Andrews W, Maggi R, Tischkau S, Parnavelas JG. Neuropilins and their ligands are important in the migration of gonadotropin-releasing hormone neurons. J Neurosci. 2007;27:2387–2395. doi: 10.1523/JNEUROSCI.5075-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caviness VS, Jr, So DK, Sidman RL. The hybrid reeler mouse. J Hered. 1972;63:241–246. doi: 10.1093/oxfordjournals.jhered.a108286. [DOI] [PubMed] [Google Scholar]
- 78.Cariboni A, Rakic S, Liapi A, Maggi R, Goffinet A, Parnavelas JG. Reelin provides an inhibitory signal in the migration of gonadotropin-releasing hormone neurons. Development. 2005;132:4709–4718. doi: 10.1242/dev.02033. [DOI] [PubMed] [Google Scholar]
- 79.Ebens A, Brose K, Leonardo ED, Hanson MG, Jr, Bladt F, Birchmeier C, Barres BA, Tessier-Lavigne M. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- 80.Maina F, Hilton MC, Ponzetto C, Davies AM, Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 1997;11:3341–3350. doi: 10.1101/gad.11.24.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ieraci A, Forni PE, Ponzetto C. Viable hypomorphic signaling mutant of the Met receptor reveals a role for hepatocyte growth factor in postnatal cerebellar development. Proc Natl Acad Sci U S A. 2002;99:15200–15205. doi: 10.1073/pnas.222362099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giacobini P, Giampietro C, Fioretto M, Maggi R, Cariboni A, Perroteau I, Fasolo A. Hepatocyte growth factor/scatter factor facilitates migration of GN-11 immortalized LHRH neurons. Endocrinology. 2002;143:3306–3315. doi: 10.1210/en.2002-220146. [DOI] [PubMed] [Google Scholar]
- 83.Giacobini P, Messina A, Wray S, Giampietro C, Crepaldi T, Carmeliet P, Fasolo A. Hepatocyte growth factor acts as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration. J Neurosci. 2007;27:431–445. doi: 10.1523/JNEUROSCI.4979-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Linger RMA, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2007 doi: 10.1016/S0065-230X(08)00002-X. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- 87.Fang Z, Xiong X, James A, Gordon DF, Wierman ME. Identification of novel factors that regulate GnRH gene expression and neuronal migration. Endocrinology. 1998;139:3654–3657. doi: 10.1210/endo.139.8.6221. [DOI] [PubMed] [Google Scholar]
- 88.Allen MP, Linseman DA, Udo H, Xu M, Schaack JB, Varnum B, Kandel ER, Heidenreich KA, Wierman ME. Novel mechanism for gonadotropin-releasing hormone neuronal migration involving Gas6/Ark signaling to p38 mitogen-activated protein kinase. Mol Cell Biol. 2002;22:599–613. doi: 10.1128/MCB.22.2.599-613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allen MP, Zeng C, Schneider K, Xiong X, Meintzer MK, Bellosta P, Basilico C, Varnum B, Heidenreich KA, Wierman ME. Growth arrest-specific gene 6 (Gas6)/adhesion related kinase (Ark) signaling promotes gonadotropin-releasing hormone neuronal survival via extracellular signal-regulated kinase (ERK) and Akt. Mol Endocrinol. 1999;13:191–201. doi: 10.1210/mend.13.2.0230. [DOI] [PubMed] [Google Scholar]
- 90.Pierce A, Bliesner B, Xu M, Nielsen-Preiss S, Lemke G, Tobet S, Wierman ME. Axl and Tyro3 modulate female reproduction by influencing gonadotropin-releasing hormone neuron survival and migration. Mol Endocrinol. 2008;22:2481–2495. doi: 10.1210/me.2008-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pierce ABB, Xu M, Tobet S, Lemke G, Wierman ME. Axl and Tyro3 regulate GnRH neuron migration and modulate female reproductive function. The Endocrine Society Meeting; San Francisco California. 2008. [Google Scholar]
- 92.Knox AJ, X M, Plummer L, Slavov D, Taylor D, Pitteloud N, Crowley WF, Wierman ME. Analysis of mutations in Axl and Tyro3 in probands with Kallman Syndrome and idiopathic hypogonadotropic hypogonadism. The Endocrine Society Meeting; Washington DC. 2009. [Google Scholar]
- 93.Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1-mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- 95.Daniel D, Rossel M, Seki T, Konig N. Stromal cell-derived factor-1 (SDF-1) expression in embryonic mouse cerebral cortex starts in the intermediate zone close to the pallial-subpallial boundary and extends progressively towards the cortical hem. Gene Expr Patterns. 2005;5:317–322. doi: 10.1016/j.modgep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 96.Zhu Y, Yu T, Zhang XC, Nagasawa T, Wu JY, Rao Y. Role of the chemokine SDF-1 as the meningeal attractant for embryonic cerebellar neurons. Nat Neurosci. 2002;5:719–720. doi: 10.1038/nn881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Toba Y, Tiong JD, Ma Q, Wray S. CXCR4/SDF-1 system modulates development of GnRH-1 neurons and the olfactory system. Dev Neurobiol. 2008;68:487–503. doi: 10.1002/dneu.20594. [DOI] [PubMed] [Google Scholar]
- 98.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 100.Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, Luster AD. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- 101.Tissir F, Wang CE, Goffinet AM. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res. 2004;149:63–71. doi: 10.1016/j.devbrainres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 102.Schwarting GA, Henion TR, Nugent JD, Caplan B, Tobet S. Stromal cell-derived factor-1 (chemokine C-X-C motif ligand 12) and chemokine C-X-C motif receptor 4 are required for migration of gonadotropin-releasing hormone neurons to the forebrain. J Neurosci. 2006;26:6834–6840. doi: 10.1523/JNEUROSCI.1728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Good DJ, Porter FD, Mahon KA, Parlow AF, Westphal H, Kirsch IR. Hypogonadism and obesity in mice with a targeted deletion of the Nhlh2 gene. Nat Genet. 1997;15:397–401. doi: 10.1038/ng0497-397. [DOI] [PubMed] [Google Scholar]
- 104.Cogliati T, Delgado-Romero P, Norwitz ER, Guduric-Fuchs J, Kaiser UB, Wray S, Kirsch IR. Pubertal impairment in Nhlh2 null mice is associated with hypothalamic and pituitary deficiencies. Mol Endocrinol. 2007;21:3013–3027. doi: 10.1210/me.2005-0337. [DOI] [PubMed] [Google Scholar]
- 105.Miller NL, Wevrick R, Mellon PL. Necdin, a Prader-Willi syndrome candidate gene, regulates gonadotropin-releasing hormone neurons during development. Hum Mol Genet. 2009;18:248–260. doi: 10.1093/hmg/ddn344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muscatelli F, Abrous DN, Massacrier A, Boccaccio I, Le Moal M, Cau P, Cremer H. Disruption of the mouse Necdin gene results in hypothalamic and behavioral alterations reminiscent of the human Prader-Willi syndrome. Hum Mol Genet. 2000;9:3101–3110. doi: 10.1093/hmg/9.20.3101. [DOI] [PubMed] [Google Scholar]
- 107.MacDonald HR, Wevrick R. The necdin gene is deleted in Prader-Willi syndrome and is imprinted in human and mouse. Hum Mol Genet. 1997;6:1873–1878. doi: 10.1093/hmg/6.11.1873. [DOI] [PubMed] [Google Scholar]
- 108.Jay P, Rougeulle C, Massacrier A, Moncla A, Mattei MG, Malzac P, Roeckel N, Taviaux S, Lefranc JL, Cau P, Berta P, Lalande M, Muscatelli F. The human necdin gene, NDN, is maternally imprinted and located in the Prader-Willi syndrome chromosomal region. Nat Genet. 1997;17:357–361. doi: 10.1038/ng1197-357. [DOI] [PubMed] [Google Scholar]
- 109.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 110.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 111.Hori A, Honda S, Asada M, Ohtaki T, Oda K, Watanabe T, Shintani Y, Yamada T, Suenaga M, Kitada C, Onda H, Kurokawa T, Nishimura O, Fujino M. Metastin suppresses the motility and growth of CHO cells transfected with its receptor. Biochem Biophys Res Commun. 2001;286:958–963. doi: 10.1006/bbrc.2001.5470. [DOI] [PubMed] [Google Scholar]
- 112.Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, Malli R, Sharabi A, Hiden U, Graier W, Knofler M, Andreae F, Wagner O, Quaranta V, Desoye G. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- 113.Tena-Sempere M. GPR54 and kisspeptin in reproduction. Hum Reprod Update. 2006;12:631–639. doi: 10.1093/humupd/dml023. [DOI] [PubMed] [Google Scholar]
- 114.Seminara SB. Converging at puberty’s hub. Endocrinology. 2007;148:5145–5146. doi: 10.1210/en.2007-0953. [DOI] [PubMed] [Google Scholar]
- 115.Plant TM. The role of KiSS-1 in the regulation of puberty in higher primates. Eur J Endocrinol. 2006;155(Suppl 1):S11–16. doi: 10.1530/eje.1.02232. [DOI] [PubMed] [Google Scholar]
- 116.Knoll JG, C C, Henion T, Schwarting GA, Tobet SA. Sex differences in kisspeptin mRNA expression during murine development; Endocrine Society; San Francisco, CA. 2008. pp. P1–680. [Google Scholar]
- 117.Oliveira LM, Seminara SB, Beranova M, Hayes FJ, Valkenburgh SB, Schipani E, Costa EM, Latronico AC, Crowley WF, Jr, Vallejo M. The importance of autosomal genes in Kallmann syndrome: genotype-phenotype correlations and neuroendocrine characteristics. J Clin Endocrinol Metab. 2001;86:1532–1538. doi: 10.1210/jcem.86.4.7420. [DOI] [PubMed] [Google Scholar]
- 118.Pitteloud N, Meysing A, Quinton R, Acierno JS, Jr, Dwyer AA, Plummer L, Fliers E, Boepple P, Hayes F, Seminara S, Hughes VA, Ma J, Bouloux P, Mohammadi M, Crowley WF., Jr Mutations in fibroblast growth factor receptor 1 cause Kallmann syndrome with a wide spectrum of reproductive phenotypes. Mol Cell Endocrinol. 2006:254–255. 60–69. doi: 10.1016/j.mce.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 119.Teles MG, Trarbach EB, Noel SD, Guerra-Junior G, Jorge A, Beneduzzi D, Bianco SD, Mukherjee A, Baptista MT, Costa EM, De Castro M, Mendonca BB, Kaiser UB, Latronico AC. A novel homozygous splice acceptor site mutation of KISS1R in two siblings with normosmic isolated hypogonadotropic hypogonadism. Eur J Endocrinol. 163:29–34. doi: 10.1530/EJE-10-0012. [DOI] [PubMed] [Google Scholar]
- 120.Pallais JC, Bo-Abbas Y, Pitteloud N, Crowley WF, Jr, Seminara SB. Neuroendocrine, gonadal, placental, and obstetric phenotypes in patients with IHH and mutations in the G-protein coupled receptor, GPR54. Mol Cell Endocrinol. 2006:254–255. 70–77. doi: 10.1016/j.mce.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 121.Kauffman A. Coming of age in theh Kisspeptin Era: Sex differences, development and puberty. Molecular and Cellularr Endocrinolology. 2010 doi: 10.1016/j.mce.2010.01.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pallais JC, A M, Pitteloud N, Seminara S, Crowley WF., Jr . Kallman Syndrome. Seattle Washington: University of Washington; 2010. Seattle. [Google Scholar]