Summary
The addition of IL-12p75 to naïve CD4+ T cells promotes their differentiation toward a TH1-type cytokine pattern. Dendritic cells stimulated by LPS generate IL-12p75 but only if the environment also contains IFN-γ. Thus, it appears that IFN-γ is needed to start the response that will result in further production of IFN-γ. We previously reported that paradoxically DCs produce IL-12p75 only after engaging primed, but not naïve T cells. This study examines the mechanism by which primed T cells trigger IL-12p75 secretion and asks whether this induction is also dependent on the presence of IFN-γ.
Here we show that, in contrast to LPS, primed T cells induce IL-12p75 in an IFN-γ-independent manner. Addition of rIFN-γ to cocultures of naïve T cells with DCs did not induce IL-12p75. Moreover, antigen-activated CD4+ T cells from wild type or IFN-γ-deficient mice both initiated IL-12p75 production from DCs. Surprisingly, we found that synergies between three T cell-derived factors –- CD40 Ligand, IL-4 and GM-CSF, was necessary and sufficient for IL-12p75 production.
These results suggest that there are at least two distinct pathways for IL-12p75 production in vivo. Furthermore, the T cell-dependent pathway of IL-12p75 production employs molecules that are not classically associated with a TH1-type response.
Keywords: TH0, TH1, CD40L, IFN-γ, IL-4, IL-12p70, IL-12p75, DCs
Introduction
A fundamental question in host responses to pathogens is how innate immune responses trigger and shape adaptive immunity. It is currently believed that a critical step in host defense against intracellular pathogens is the presence of IFN-γ. IL-12p75, which is secreted from DCs, is known to induce IFN-γ from NK or T cells [1]. IFN-γ alone does not induce IL-12p75 but can prime its production by antigen presenting cells in response to TLR agonists. Many studies have demonstrated that in vitro priming of DCs or monocytes/macrophages with recombinant IFN-γ (rIFN-γ) will dramatically enhance IL-12p75 production in the presence of bacteria/bacterial products. IFN-γ enhances transcription of both of the genes encoding the IL-12p75 heterodimer (p40 and p35), but it has a marked effect as a priming factor for the p35 subunit, which is a limiting factor for the amount of bioactive IL-12p75 production [2–4].
The prevailing view is that the initial host-pathogen interactions, which engages the Toll-like receptors (TLRs), will lead to the secretion of IL-12p75 from dendritic cells (DC), directing the naïve CD4+ T cells to differentiate into IFN-γ-producing TH1 cells [5]. Subsequently, positive feedback from the T cells (in the form of IFN-γ plus CD40L) will further enhance IL-12p75 production [6–8]. This has led to a model implicating IL-12p75 as an important initiation factor that bridge between innate and adaptive immunity [9]. However, we have previously reported that engagement of TLR alone, only led to the secretion of p40 and not the heterodimeric IL-12p75. We further demonstrated that in order to generate bioactive IL-12p75, DCs had to interact with antigen-activated, but not naïve T cells [10], suggesting that perhaps an ongoing TH1 responses will initiate IL-12p75 secretion from DCs (and not the TLR engagement). This was consistent with an alternative model of early host response to pathogens that we had previously proposed [11] and which has been extended by others [12] wherein IL-12p75 is not necessarily the primary inducer of the TH1 response, but rather an amplification loop that is generated after the first wave of IFN-γ production.
In support of this model, we have previously demonstrated that in vitro and in vivo stimulation of DCs with LPS alone was inadequate to induce IL-12p75 and the presence of IFN-γ was absolutely necessary for its induction [10]. Taken together, these data also suggest that perhaps the inability of naïve CD4+ T cells to induce IL-12p75 from DCs is related to their poor differentiation towards IFN-γ production as it has been reported previously [13]. The aim of the current study was therefore to elucidate mechanisms by which T cells (antigen-activated but not naïve) induce IL-12p75 production from DCs and gain insight into the relationship between endogenous IFN-γ secreted from antigen-activated T cells and the production of IL-12p75 by DCs.
We used a well-characterized T cell transgenic model, to show that the addition of IFN-γ to the coculture of naïve T cells with DCs did not reconstitute IL-12p75 production. We also found that antigen-activated CD4+ T cells from the spleen of wild type or IFN-γ deficient (IFN-γKO) 5C.C7 TCR transgenic mice were both capable of inducing IL-12p75 production from DCs. Furthermore, in contrast to mice that are challenged with LPS, where IFN-γ was essential for IL-12p75 production, high levels of IL-12p75 could be detected in the serum of IFN-γKO mice that were challenged with a superantigen (SAg). Superantigen-induced IL-12p75 production was T cell dependent, since RAG2KO mice did not produce measurable IL-12p75 when challenged with SAg.
Therefore, we demonstrate for the first time in an intact system (in vitro and in vivo) that there are two mechanistically distinct pathways, which lead to de novo secretion of IL-12p75. Innate stimuli induce IL-12p75 in an IFN-γ-dependent fashion. However, IL-12p75 production triggered by antigen-activated T cells requires a combination of CD40L, GM-CSF and IL-4 but not IFN-γ. The synergy between these molecules is necessary, since each individual molecule was unable to induce IL-12p75 on its own. Finally, although IFN-γ does not play a role in initiating IL-12p75 when antigen-activated T cells are involved, it plays a role in its subsequent amplifications.
Materials and Methods
Mice
Adult C57BL/6 mice (Jackson Laboratory, Bar Harbor), C57BL/6 IFN-γKO, B10.A RAG2KO and B10.A/SgSnAi TCR–Cyt 5C.C7-1 RAG2KO (5C.C7) mice (NIAID contract facility, Taconic Farms Germantown, NY), were kept in AAALAC accredited NIH, SPF barriers. The 5C.C7 CD40LKO and IFN-γKO mice were generated by crossing to C57BL/6 CD40LKO (Jackson Laboratory) and C57BL/6 IFN-γKO (gift of Dyana Dalton & Tim Stewart from Genentech) to a B10.A-RAG2KO 5C.C7 background.
Media and reagents
Bacterial LPS [Escherichia (E.) coli Serotype 0127:B8] (Sigma) was used at 100–200ng/ml, Staphylococcal enterotoxin B (SEB) (Sigma) was used at 50μg and Staphylococcal enterotoxin A (SEA) (Toxin Technology Sarasota, FL) was used at 10μg, Soluble Toxoplasma antigen (STAg), prepared from tachyzoites of the RH 88 strain of T. gondii, was a gift from Dr. Alan Sher, NIAID. Polyinosinic Acid-Polycytidylic Acid Poly (I:C) was from Calbiochem (La Jolla, CA). The immunostimulatory CpG-containing oligodeoxynucleotides (Hartmann G.), 1826, has the sequence 5'TCCATGACGTTCCGACGTT-3' (Invitrogen). Mycobacterium tuberculosis H37Rv whole cell lysates were from (MYCOS RESEARCH, LLC) were all used at 1μg/ml. Recombinant mouse GM-CSF, IL-4 and IFN-γ were from PreproTech Inc, and Goat anti-mouse IFN-γ or control IgG from R&D systems. Complete Iscove's Modified Dulbecco's Medium IMDM (GibcoBRL) was used with 10% heat-inactivated Fetal Bovine Serum (GibcoBRL), 2mM L-glutamine, 55μM 2ß-Mercaptoethanol, penicillin, streptomycin and gentamycin.
Generation of bone marrow-derived DCs (BM-DC)
As previously described [10] bone marrow cells from B10.A-RAG2KO or IFN-γKO-5C.C7 mice were erythrocyte depleted, washed and either cultured immediately or cryopreserved for later use. Cultures (1×106 cells/well in 24 well plate) in 2 ml of complete IMDM supplemented with GM-CSF (30U/ml) and IL-4 (3ng/ml) were maintained by regular medium changes. On the 7th day, the plate was chilled, and loosely adherent BM-DCs harvested. For pre-activated BM-DCs, LPS (100–200ng/ml) was added to the 24 well plate at day 6. Harvested cells were washed in cold medium before use. As previously described [10] splenic CD11c+ DCs were used for the IL-12p75 ELISPOT.
Generation of naïve T cells
Naïve 5C.C7 T cells were enriched by incubating splenocytes with a cocktail of monoclonal antibodies to B220 (RA3-6B2), CD11b (M1/70), and anti class-II (I-Ek) (14.4.4S) (BD Pharmingen), and binding to anti-Mouse/Rat Dynabeads (Dynal Biotech). In some experiments, T cells were further purified by FACS sorting to >99 % based on staining for CD4 and the absence of B220, pan-Class II, IAk, NK1.1, CD11c, CD11b, GR1 and CD21.
In vitro generation of antigen-activated/primed 5C.C7 CD4+ T cells
Splenocytes from 5C.C7 mice were depleted of erythrocytes by adding 5ml of ACK lysis buffer (10mM NH4Cl, 1M KHCO3 and 100mM Na2EDTA PH7.2) (Quality Biological Inc. Gaithersburg, MD) for 40 second and washed 3× in cold complete medium and then cultured in 1×106/ml of complete medium plus 1μM MCC 88–103. Fresh medium was added after 3 days. After 5 days, cells were harvested, washed 2× with medium and re-cultured with 10–20 U/ml of rmIL-2. These cultured T cells were maintained by adding fresh medium plus rmIL-2 every 4–7 days. The cells were used at day 10–90 without any antigen re-stimulation. They typically have cytokine phenotype of TH0 T cells (S5). For T-DC coculture experiments, such T cells were purified to >90% using a CD4+ T cell isolation kit (Miltenyi Biotech.) with the addition of anti-CD11c and anti-class-II to the manufacturer's cocktail and using 3–4 LS columns in tandem, instead of LD columns.
BM-DC/T cell co-culture
Purified naïve or in vitro cultured CD4+ T cells were incubated with 2×104 BM-DCs/well and ±0.1μM MCC (88–103) peptide in 200μl medium in 96 well U-bottom plates for 48 hrs. The supernatants were frozen at −30°C for subsequent cytokine assays. 5C.C7 T cell proliferation in such cultures was determined by adding 1μCi of [3H] thymidine and culturing for an additional 12–18 hrs followed by scintillation counting. Similarly, in some experiments, BM-DCs were cocultured in a 48 or 24 well plate with 5×105−1×106 mock transfected or CD40L-expressing NIH-3T3 cells for 48 hrs in the presence or absence of various cytokines.
Cytokine measurements
In most experiments, IL-12p75 was specifically measured using the ELISA kit from R&D system (Quantikine) with a sensitivity of 7.8 pg/ml. Cytokines in culture media or serum were also quantitated using SearchLight Multiplex cytokine array (Aushon Biosystem Billerica, MA) or Ray Biotech Quantibody mouse array (Ray Biotech Inc. Norcross, GA) was used in some experiments to quantitate various cytokines. Both platforms detect IL-12p75 specifically without cross reactivities with p40.
IL-12p75 ELISPOT
ELISPOT to specifically detect IL-12p75 was described previously [10]. Briefly, heterodimer specific mAbs (clone 9A5) were used to capture IL-12p75 and detection (clone C17.8-biotinylated). All were purchased from BD Pharmingen. Spots were developed using an ABC-alkaline phosphatase kit (Vector Laboratories). Multi Screen 96-well, PVDF-backed microtiter plates (MAIPS 4510) from Millipore (Billerica, MA). The spots were enumerated by an independent contractor (ZellNet Consulting Inc, Fort Lee NJ).
In vivo secretion of cytokines in response to injection of Superantigen
Mice were injected intravenously with 10μg of SEA dissolved in saline and injected in 100μl volume and control mice received saline alone. After 6 hours, blood was collected via intracardiac-puncture under anaesthesia. After coagulation for 1–2 hours at 4°C in Microtainer tubes (BD), serum was separated by centrifugation for 90 second at 13000 rpm, transferred to another tube and frozen at −80°C until subsequent ELISA
Results
Secretion of IL-12p75 from DC is dependent on the presence of antigen-activated T cells but independent of IFN-γ
To determine the role of IFN-γ from T cells on IL-12p75 secretion from DCs, sorted naïve or antigen-activated (cultured) CD4+ T cells from the PCC specific RAG.KO 5C.C7 TCR transgenic [Tg] mice were cocultured with bone marrow-derived dendritic cells (DCs) (obtained from RAG2KO mice, which are devoid of T or B cells). DCs were either in a “resting” state, LPS-preactivated, or received LPS at the time of coculture with T cells. Consistently, prior activation of DC with LPS and coculturing them with antigen-activated but not naïve T cells resulted in the highest levels of IL-12p75 production (Fig. 1 A). This is in agreement with a human study demonstrating that purified memory phenotype (CD45RO+) but not naïve (CD45RA+) human CD4+ T cells are capable of inducing IL-12p75 from human monocyte-derived DCs [14] and also is supported by a recent report showing that activated but not freshly isolated mouse splenic CD8+ T cells are capable of inducing IL-12p75 from DCs [15].
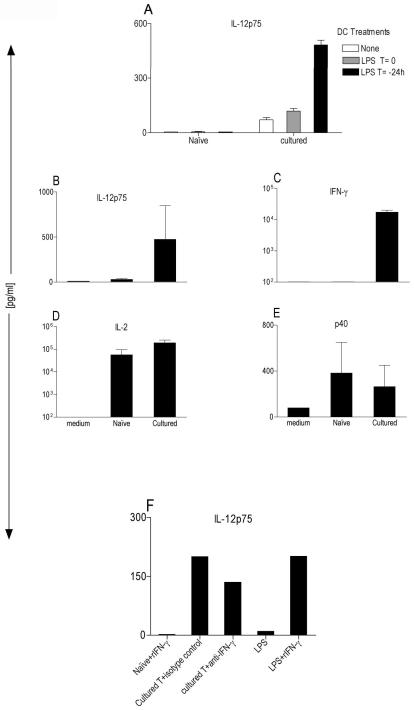
Figure 1. Antigen-activated but not naïve T cells induce IL-12p75 from DCs.
(A) BM-DCs at 2×104 cells/well from B10.A-RAG2KO mice were cocultured for 48 hr with 1×105 sorted naïve or antigen-activated 5C.C7 T cells +0.1μM MCC peptide in a 96-well U-bottom plate. LPS was added at 200ng/ml to DCs either at time zero (T=0) or 24h (T=−24h) and washed before the addition of T cells. ELISA (R&D system) specifically measured IL-12p75 in CSN after 48h. These data are expressed as the mean ± SD triplicates from a 96-well plate. (B–E) Multiplex cytokine array (SearchLight) measuring various cytokines in CSN obtained from LPS-activated (T=−24h) B10.A-RAG2KO. BMDCs at 2×104 cells/well cocultured for 48 hr with 1×105 naïve or antigen-activated 5C.C7 T cells +0.1μM MCC peptide in a 96 well U-bottom plate. These data are expressed as the mean ± SD composite of three independent experiments. (F) Same as (A) except two populations of DCs were used: 1-LPS-preactivated DCs (T=−24h) were either cocultured with naïve T cells plus 100ng/ml of rIFN-γ or with antigen-activated T cells plus 5μg/ml of neutralizing antibody for IFN-γ or 5μg/ml an isotype control Gt-anti-mouse. 2-“Resting” DC were stimulated with LPS or LPS plus IFN-γ stimulation.
We have previously observed a direct correlation between the presence of IFN-γ in the coculture of antigen-activated T cells and IL-12p75 production from DCs. Both of these cytokines are absent when naïve T cells were used [10]. Conceivably, the absence of IL-12p75 production from DCs cocultured with naïve T cells might be due to the absence of endogenous IFN-γ from these cells.
To further examine this possibility, we simultaneously measured IL-12p75, IFN-γ, IL-2 and p40 in the culture supernatant (CSN) obtained from the coculture of naïve or antigen-activated T cells with LPS-activated DCs by using a Multiplex Cytokine Array. The cytokine profile highlights the correlation between the presence of IFN-γ and IL-12p75 in antigen-activated but not naïve T cell cultures (Fig. 1 B & C). However, both naïve and antigen-activated T cells made high levels of IL-2 signifying that both are viable and functionally activated (Fig. 1 D). At the same time, naïve T cells also induced the p40 subunit of IL-12p75, demonstrating that the DCs are viable ruling out the possibility that the lack of IL-12p75 detection might be due to the death of the DCs (Fig. 1 E). This further strengthened the hypothesis that endogenous IFN-γ might be an important contributor in induction of IL-12p75 from DCs cocultured with antigen-activated T cells, since it is absent under similar conditions in naïve T cells (Fig. 1 C). To examine this question, we added recombinant IFN-γ (rIFN-γ) or a neutralizing antibody to IFN-γ to the coculture of DCs with either naïve or antigen-activated T cells respectively (Fig. 1 F). In contrast to a study that showed addition of rIFN-γ plus Superantigen to human CD4+CD45RA+ generated small amount (~100pg/ml) of IL-12p75 [13], in our system the addition of rIFN-γ was not able to reconstitute IL-12p75 in the coculture of sorted naïve T cells. In addition the neutralization of endogenous IFN-γ was inadequate to inhibit secretion of IL-12p75 in the presence of antigen-activated T cells (Fig. 1 F). Moreover, under the same conditions, “resting” DCs (we used resting DCs because LPS-preactivated DCs no longer secret IL-12p75 when re-stimulated with LPS plus rIFN-γ manuscript in preparation) induced similar levels of IL-12p75 secretion as did antigen-activated T cells, indicating that the rIFN-γ is active and that under these conditions the DCs can produce IL-12p75 (Fig. 1 F). Nevertheless, since antibodies might be ineffective at efficiently blocking the endogenous IFN-γ we decided to take a more definitive experimental approach to address the role of T cell derived IFN-γ.
Secretion of IL-12p75 from DC in the presence of antigen–activated T cells is IFN-γ-independent
We generated 5C.C7 TCR [Tg] mice, which lacked the IFN-γ gene. CD4+ T cells from these mice were sorted and used in the coculture assay. Consistent with the neutralizing antibody data, when various numbers of antigen-activated T cells from IFN-γ sufficient (WT) or IFN-γ deficient (IFN-γ KO) mice were cocultured with a fixed number of DCs that were either at “resting” state (no LPS), received LPS at the time of coculture (LPS t=0), or pre-activated with LPS for 24 hour prior to the coculture assay (LPS t=−24h), both WT and IFN-γKO T cells were able to induce IL-12p75 production from DCs (Fig. 2 A). As was shown earlier, an optimal DC treatment for IL-12p75 secretion was when they were pre-activated with LPS for 24 hour and washed before coculturing them with T cells (Fig. 2 A). These conditions had little effect on thymidine incorporation by T cells, confirming that the T cells are viable and functional (S 1).
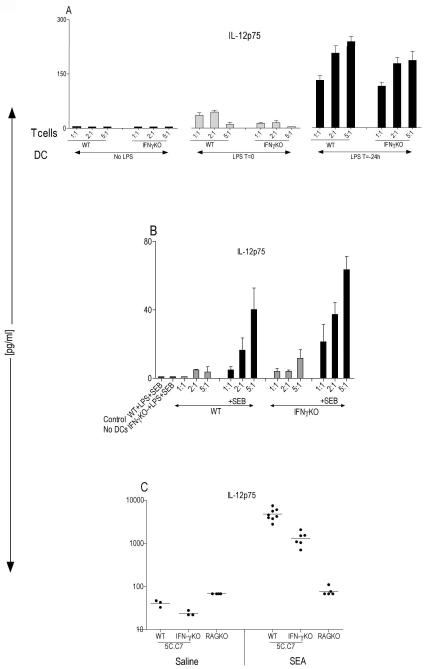
Figure 2. Secretion of IL-12p75 from antigen-activated T cells is IFN-γ independent.
(A) BM-DCs at 2×104 cells/well were obtained from 5C.C7 IFN-γKO, they were either untreated, received 200ng/ml of LPS at time zero (T=0) or they were preactivated with 200ng/m1 LPS for 24h (T=−24h) and washed before coculture with various numbers of sorted antigen-activated WT or IFN-γKO 5C.C7 splenic T cells plus 0.1μM MCC peptide. They were cocultured for 48 hours. IL-12p75 was specifically measured using ELISA (R&D System). (B) BM-DCs at 2×104 cells/well were obtained from 5C.C7 IFN-γKO mice, they were preactivated with 200ng/ml LPS for 24h (T=-24h) and washed before coculture with various numbers of sorted alloreactive WT or IFN-γKO splenic T cells (C57BL/6) in the presence or absence of 5μg/ml SEB. As a control, T cells were cultured with LPS plus SEB in the absence of DCs. These data are expressed as the mean ± SD triplicates from a 96 well U-bottom plate. (C) WT 5C.C7 TCR [Tg] (n=11) and IFN-γKO 5C.C7 TCR [Tg] (n=9) or B10.A-RAG2KO (n=9) mice were injected intravenously with either saline or 10μg of SEA and after 6 hrs serum was tested for the presence of IL-12p75 using a multiplex cytokine array (Ray Biotech).
To rule out the possibility that these results are unique to the 5C.C7 TCR transgenic with a monoclonal population of CD4+ T cells, various numbers of alloreactive B6 CD4+ T cells were cocultured with a fixed number of LPS-activated B10.A-RAGKO DCs. As is shown in Figure 2 B, consistent with data obtained from transgenic mice, both WT and IFN-γKO B6 splenic CD4+ T cells were able to induce secretion of IL-12p75 from DCs (Fig. 2 B). However, this induction was more pronounced in the presence of superantigen (SAg), a polyclonal stimulator of T cells (Fig. 2 B). Furthermore, the presence or absence of SAg had no effect on T cells proliferation, suggesting that the secretion of IL-12p75 from DCs does not correlate with their ability to activate T cell proliferation (S 2).
In vivo secretion of IL-12p75 in response to Superantigen is IFN-γ-independent
To examine the differences between WT or IFN-γKO mice and the role of endogenous IFN-γ in IL-12p75 production in vivo, a mouse model of sepsis was used. We injected WT or IFN-γKO 5C.C7 TCR [Tg] mice intravenously with SEA and after six-hour post injection, IL-12p75 was specifically measured in the serum obtained from these mice. Although the serum level of IL-12p75 is reduced (mean of 1324 pg/ml) in IFN-γKO mice relative to WT mice (mean of 4774 pg/ml), it is still produced in substantial quantities (Fig. 2 C). We also administered SEA to RAG2KO mice as a control to show that when these mice are challenged with SAg, the presence of T cells is absolutely necessary for IL-12p75 production (Fig. 2 C).
Collectively, these findings suggest that antigen-activated T cells can empower DCs for the secretion of IL-12p75 in the absence of IFN-γ pointing to two pathways of initiating IL-12p75 production, a TLR-dependent one that requires IFN-γ (IFN-γ-dependent pathway) and an IFN-γ–independent pathway that is T cell-dependent. Thus, we reasoned that perhaps there are other factor(s) (beside IFN-γ, which a naïve T cell must acquire through the process of activation and differentiation in order to become capacitated to induce IL-12p75 from DCs.
CD40L in combination with IL-4 and GM-CSF will induce IL-12p75 production
Since the addition of IFN-γ to the coculture of naïve T cells with DCs did not result in IL-12p75 production, we tested other products of activated/differentiated T cells. IL-4 and GM-CSF are growth factors for DCs and it has been shown that they enhance production of IL-12p75 from human and mouse DCs [16]. Furthermore, as shown in Figure 3 A & C, only antigen-activated but not naïve T cells made high levels of IL-4 and GM-CSF. Therefore, we added IL-4 and GM-CSF individually or as a mixture to naïve T cells cocultured with LPS-activated DCs and measured IL-12p75 produced. We detected only a small amount of IL-12p75 in the presence of IL-4, which was not comparable to the levels induced with antigen–activated T cells (Fig. 3 D). Furthermore, neither the presence of GM-CSF in combination with IL-4 nor the addition of IFN-γ to the mixture of IL-4 and GM-CSF reconstituted IL-12p75 to the levels detected when DCs were cocultured with antigen–activated T cells (Fig. 3 D).
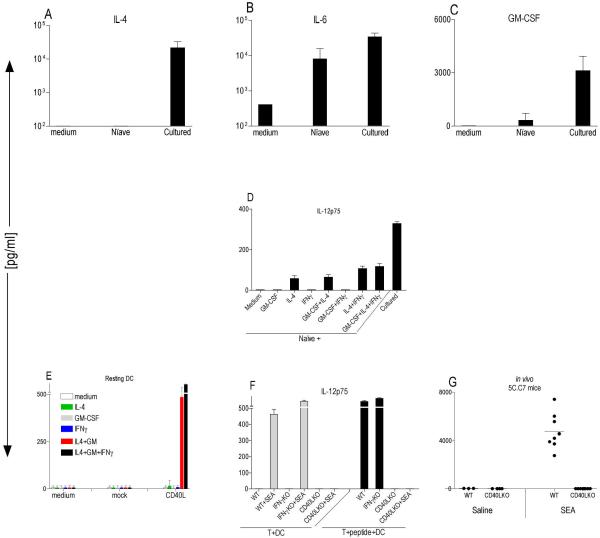
Figure 3. A combination of CD40L, IL-4 and GM-CSF will induce IL-12p75 production.
(A–C) B10.A-RAG2KO BM-DCs were preactivated with 200ng/ml LPS for 24h and washed before coculture at 2×104 cells/well with 1×105 naïve or antigen-activated 5C.C7 T cells +0.1μM MCC peptide in a 96 well U-bottom plate. After 48hrs, various cytokines were measured in the CSN using multiplex cytokine array (SearchLight). These data are expressed as the mean ± SD composite of three independent experiments. (D) BMDCs from B10.A-RAG2KO mice were preactivated with 200ng/ml LPS for 25hr then washed and cocultured at 2×104 cells/well with 2×105 sorted naïve or cultured 5C.C7 T [Tg] cells ±0.1μM MCC peptide in the presence or absence of 100ng/ml of various cytokines. CSN were tested for the presence of IL-12p75 with specific ELISA (R&D System) (E) Resting BM-DCs from B10.A-RAG2KO mice cocultured at 2×105 cells/well with 5×105 cell/well NIH-3T3 mock or NIH-3T3 CD40L expressing cells. Various cytokines were added to the coculture at final concentration of 100ng/ml in a 48-well plate. CSN weretested after 48hr coculture for the presence of IL-12p75 with specific ELISA (R&D System). These data are expressed as the mean ± SD composite of three independent experiments. (F) BM-DCs from B10.A-Rag2KO mice were preactivated with 100ng/ml LPS for 27hr then washed and cocultured at 2×104 cells/well with 2×105 WT, IFN-γKO and CD40L 5C.C7 T [Tg] cells ± 0.1μM MCC peptide and ±5μg/ml SEA in a 96 well U-bottom plate for 48 hr. CSN were tested for the presence of IL-12p75 with specific ELISA (R&D System). These data are expressed as the mean ± SD triplicates from a 96-well plate. (G) WT 5C.C7 TCR [Tg] (n=11), or CD40LKO 5C.C7 TCR [Tg] (n=12) mice were injected intravenously with either saline or 10μg of SEA and after 6 hrs serum was tested for the presence of IL-12p75 using a multiplex cytokine array (Ray Biotech).
Recently it was suggested that mouse CD4+ effector/memory but not naïve T cells express sufficient levels of CD40L to trigger phenotypic maturation of DCs [17]. Therefore, we asked if the presence of CD40L plus IL-4 and GM-CSF would be sufficient to induce IL-12p75 production from DCs. To test this, we used a fibroblast cell line that is transfected with CD40L. We found that, coculture of CD40L-expressing fibroblast with DCs in the presence of both IL-4 and GM-CSF but not each individual cytokine, led to substantial levels of IL-12p75 production, which was absent in the mock transfected control (Fig. 3 E). In contrast to previous studies that had demonstrated stimulation of unfractionated splenocytes with CHO cells transfected with CD40L alone and combination of CD40L plus IL-4 will induce IL-12p75 production [18] or stimulation of human monocytes-derived DCs with CD40L plus IL-4 leads to IL-12p75 production [19]; we found that the combination of IL-4, GM-CSF and CD40L was absolutely necessary, since under the same culture conditions, CD40L alone and mock-transfected control fibroblasts did not yield IL-12p75 (Fig. 3 E). Interestingly, a combination of CD40L plus IFN-γ was not able to initiate IL-12p75 production from DCs (Fig.3 E). However, addition of rIFN-γ to the combination of CD40L plus GM-CSF/IL-4 indeed increased IL-12p75 secretion (Fig.3 E).
Finally, we examined the contribution of the CD40-CD40L interaction and IL-12p75 production in an intact model by using a CD40LKO T cells. We generated 5C.C7 TCR [Tg] mice that are deficient in RAG2 and CD40L genes. Antigen-activated splenic T cells from WT or CD40LKO mice were cocultured with LPS-activated DCs in the presence or absence of cognate peptide and/or superantigen and the supernatant were tested for the presence of IL-12p75. We failed to detect measurable amounts of IL-12p75 in the coculture of CD40LKO T cells with DCs (Fig. 3 F). However, consistent with earlier results (Fig 2 A), we detected high levels of IL-12p75 in the presence of WT or IFN-γKO T cells (Fig. 3 F). Similarly, superantigen stimulated WT and IFN-γKO but not CD40LKO T cells also induced IL-12p75 in the DCs. (Fig. 3 F). The failure of antigen-activated CD40LKO T cells to induce IL-12p75 does not reflect a global impairment of T cell activation in these cells, since they proliferated as their WT counter parts (S 3) and were able to secrete key T cell cytokines (IL-2, IL-4, IFN-γ, IL-5 and IL-13) (S 5). Interestingly, coculture of WT and IFN-γKO T cells with DCs in the absence of cognate peptide did not result in IL-12p75 production indicating that its secretion is antigen-specific (Fig. 3 F). This finding is in agreement with a study showing that p35 mRNA accumulation in antigen presenting cells can be evoked by TCR/MHC interactions in a dose-dependent manner [20].
Finally, we could not detect IL-12p75 in the serum of CD40LKO mice challenged with SEA (Fig. 3 G). Thus, our in vitro and in vivo data suggests that when antigen-activated T cells are interacting with antigen-bearing DCs, a critical step for initiation of IL-12p75 production is a milieu that provides a combination of CD40L, GM-CSF and IL-4, independent of IFN-γ.
Discussion
It is widely believed that IL-12p75 is a key signal from dendritic cells that is secreted early during the innate host response to microbial pathogens, which instructs naïve T cells to differentiate towards a TH1 pathway initiating cell-meditated immunity through secretion of the cytokine IFN-γ [5]. Therefore, the induction of large amounts of IFN-γ at the site of infection is thought to depend on antigen-specific T cells, which rely on IL-12p75 for their differentiation into the IFN-γ-producing TH1 cells [21]. However, the regulation of IL-12p75 production in vivo is still poorly understood.
The current model emphasizes that the production of bioactive IL-12p75 by DCs can be initiated by innate signals through pattern recognition receptors (TLRs), while feedback from T cells are only instrumental in further amplifying these signals [7]. This view is based on a model proposed over a decade ago. The data supporting this model was obtained using reagents that did not discriminate between p40 subunit and the bioactive IL-12p75. We have recently shown that using appropriate reagents, only p40 (and not p75) is triggered by TLR stimulation alone. Therefore, our more up-to-date interpretation is that the initial engagement of DCs with pathogens (induction phase), which normally takes place in peripheral tissues, is insufficient to induce the production of IL-12p75.
In the earlier study, we also found that DCs stimulated with TLR agonists would make IL-12p75, when IFN-γ was available as a cofactor. This contrasted with studies arguing that the engagement of multiple TLR family members was sufficient to induce IL-12p75 [22, 23]. A potential explanation for these differences was that we used bone marrow from RAGKO mice to exclude any possible T cell involvement. In such a system we found that when cultured DCs are devoid of T cells, engagement of multiple TLRs are still insufficient to induce IL-12p75 from DCs. Furthermore, in our hands, stimulating CD11c+ splenic DCs with bacterial cell lysate or using multiple TLR agonists did not yield IL-12p75 (S 4). Interestingly, TLR activated DCs could make IL-12p75 in the absence of exogenous IFN-γ, if they engaged with antigen-activated T cells (but not naïve T cells) [10]. These findings raise an important conceptual question; how could IL-12p75 be the instigator of cell-mediated immunity to microbial pathogens if its induction is also dependent on the presence of IFN-γ and activated but not naïve T cells?
Our previous study did not elaborate the mechanism by which T cells induce IL-12p75 from DCs. Here we clearly demonstrate through in vitro and in vivo experiments (by using blocking antibodies, cytokine add back, as well as knockout mice) that IFN-γ does not play a role in induction of IL-12p75 from DCs when T cells are involved. Also, we discuss an unexpected T cell-dependent but IFN-γ-independent mechanism that requires a combination of CD40L, GM-CSF and IL-4 for IL-12p75 production. Thus, it seems that there are two distinct and independent pathways of IL-12p75 production: 1- A TLR-mediated pathway, which is IFN-γ-dependent and 2- A T cell-mediated pathway, which is IFN-γ-independent (see model, Figure 4). The requirement for IFN-γ to combine with TLR agonists to initiate IL-12p75 is unlikely to come from T cells; and it has been proposed that other innate cells (e.g. NK cells) can provide this signal [24].
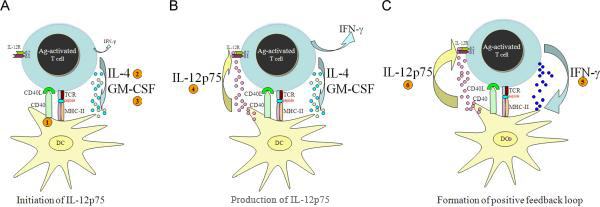
Figure 4.
(A)- To initiate IL-12p75 production during cognate interactions, DCs must first receive at least three signals: (1) CD40L, (2) IL-4 and (3) GM-CSF. Although, during the TDC interactions engagement of TCR/MHC will lead to IFN-γ production independent of IL-12p75, its presence is dispensable during the initiation of IL-12p75 production. (B) Once IL-12p75 is produced it will act on competent T cells that express functional IL-12 receptors (IL-12Rß1 & ß2), which will increase the level of IFN-γ that was already being produced as the result of TCR/MHC engagements. (C) The formation of a positive feedback loop created by IL-12→ IFN-γ will ensure their amplification, which leads to sustain IFN-γ–mediated immune responses. However, eventually, subsequent negative feedback (not shown) will inhibit the expansion of this loop.
The inability of naïve T cells to induce IL-12p75 from DCs was not a kinetic delay, since extended coculturing of naïve T cells and DCs together for up to six days did not result in the induction of IL-12p75 (data not shown). Our data with CD4+ T cells is also in agreement with a recent report showing that activated but not freshly isolated mouse splenic CD8+ T cells are capable of inducing IL-12p75 from DCs [15].
Although, the role of IFN-γ in IL-12p75 production from monocytes and macrophages has been extensively investigated in the context of various microbial stimuli [3, 4, 25–27], its role in the IL-12p75 induction by T cells has not been examined. Here by using antigen-activated T cells from WT or IFN-γKO mice stimulated by a cognate peptide, superantigen or allogeneic T cells, we demonstrate that both in vitro and in vivo, the absence of IFN-γ has no significant impact on the ability of antigen-activated T cells to induce IL-12p75 production.
These data suggest that the difference between the ability of naïve and antigen-activated T cells to induce IL-12p75 production is not simply related to their differential ability to secrete IFN-γ. Contrary to our findings, a study had suggested that naïve T cells were capable of inducing IL-12p75 when exogenous IFN-γ plus superantigen was added to cocultures [13]. This study used purified human naïve T cells enriched on surface markers (plate-bound anti-CD45RA). It is possible that their results stem from a minor contamination with recently activated T cells or does reflect a genuine mouse versus human difference. However, in our study, we can clearly distinguish naïve and antigen experienced T cells using TCR transgenic mice. Nevertheless, even in our experiments, the amount of IL-12p75 produced in the absence of IFN-γ is significantly reduced (~3 fold in the in vivo IFN-γKO model). This is consistent with the idea that IFN-γ provides positive feedback amplification for IL-12p75 production – but is not required for its de novo induction [11].
There are also recent reports using OVA-specific CD8+ T cells that also seem to be at odds with our findings. This study reports that the neutralization of endogenous IFN-γ in cocultures of PMA/ionomycin-activated CD8+ T cells with CD8α+ DCs dramatically reduced the level of IL-12p75 production, suggesting that IFN-γ was an important factor involved in CD8+ T cell-mediated IL-12p75 production from DCs [15]. Despite the differences in cell-types and activation stimuli used in that study, perhaps, these data suggest that in contrast to CD4+ T cells, CD8+ T cell-mediated effects may not trigger de novo IL-12p75 production, but only amplify it via IFN-γ, as we have discussed and has been reported [28, 29].
We also noticed that high levels of IL-4 and GM-CSF are present in cultures with activated but not naïve T cells (Fig. 3 A & C). Both IL-4 and GM-CSF are growth factors for in vitro generation of DCs and it has been shown that they enhance production of IL-12p75 from human and mouse DCs (when these cells are stimulated with TLR agonists; however, the role of intact T cells in IL-12p75 production has not been addressed) [16]. Therefore, it was reasonable to postulate that these cytokines may be critical for the ability of activated T cells to induce IL-12p75.
Addition of IL-4, GM-CSF or a combination of both plus IFN-γ to naïve T cells cocultured with DCs did not reconstitute IL-12p75 production from DCs at levels usually detected when antigen-activated T cells are used. Only a small amount of IL-12p75 could be detected when IL-4 was incorporated into the coculture assay (Fig. 3 D), suggesting that antigen-activated T cells are using other molecules, (which naïve T cells lack) to induce IL-12p75.
It has been recently reported that mouse CD4+ effector/memory but not naïve T cells express sufficient levels of CD40L to trigger phenotypic maturation of DCs [17]. Furthermore, several studies have reported a significant effect of CD40-CD40L interactions on IL-12p75 production from DCs in the presence of various stimuli (mostly TLR agonists) [7, 16, 30]. Therefore, it is conceivable that the inability of naïve T cells to induce IL-12p75 production from DCs might be due to the lack of sufficient levels of CD40L expression in addition to the absence of adequate amounts IL-4 and GM-CSF (Fig. 3 A & C).
To test this hypothesis, we took a reductionist approach and used a CD40L-transfected fibroblast cell line to simulate “resting” DCs (these cells are not truly “resting” as mechanical manipulation and in vitro culture conditions will cause them to upregulate low levels of costimulatory molecules, data not shown) in the presence or absence of IL-4 and GM-CSF and measured IL-12p75 secreted in the supernatant. Individually, neither IL-4 nor GM-CSF synergized with CD40L to induce IL-12p75. However, the full combination of IL-4, GM-CSF and CD40L was a potent inducer of IL-12p75 from DCs (Fig. 3 E).
The idea that CD40L, IL4 or GMCSF are relevant for DC activation and IL-12p75 production has been reported in the literature before. A major difference between our study and previous studies is the experimental approach. Previous studies have used CD40L transfected cells and anti-CD40 antibodies [7, 8, 16, 30] or soluble rCD40L trimer containing a modified leucine zipper sequence [6, 13, 31] to establish the current model of host response to pathogens and the role of IL-12p75 in this process. These studies did not specifically address the role of intact T cells in the induction of IL-12p75 from DCs, but rather they had emphasized the role of microbial stimuli in combination with a component of T cells (CD40L) involved in IL-12p75 production.
Our current in vitro and in vivo data suggests that T cell-mediate IL-12p75 production is more complex than it was previously thought. The T-cell-dependent but IFN-γ-independent pathway of IL-12p75 production requires a competent T cell that not only expresses CD40L but also must provide a combination of GM-CSF and IL-4 during cognate interaction in order to instruct a DC to secrete IL-12p75.
Collectively, in combination with our earlier report, we have now shown that both the microbial and T cell-mediated pathways for IL-12p75 induction, require “effector” molecules that are not usually present when a naïve T cell first interacts with an antigen-bearing DC. It is worth noting that the T cell-dependent pathway of IL-12p75 production also employs molecules that are not classically associated with a TH1 response but more with TH0 or even TH2 responses. Perhaps, this allows a T cell (TH0), early in the activation process to instruct DCs without fully committing and differentiating to a TH1-type cell. Nevertheless the activated T cells capable of making the combination of factors necessary to induce IL-12 p75 from DCs is unlikely to be available when the DCs first encounter the pathogen in peripheral tissues. Thus, perhaps, IL-12p75, which mostly serves to amplify IFN-γ, is only made when DCs migrate to the secondary lymphoid organs where adaptive immunity will be initiated. Therefore, IL-12p75 itself must play a role relatively late in the immune response – after a potent T cell response have been already initiated. In this case, IL-12p75 will function primarily to sustain the ongoing IFN-γ response (Figure 4 Model A, B and C).
These data further support our proposed model that innate signals are inadequate inducers of IL-12p75 production and signals that are generated by T cells are not simply amplifiers of IL-12p75 production. In fact, T cell signals initiate and instruct DCs to produce bioactive IL-12p75 to ensure continuos IFN-γ production. In contrast to a simple inductive pathway that requires only TLR ligand engagement, the necessity for multiple cell-cell interactions for the production of this heterodimeric protein, (encoded by two unrelated genes) is perhaps an evolutionary safeguard against sustaining a potentially undesirable TH1 immune response.
Supplementary Material
(S 1) same as (Fig. 2A) except 3H-thymidine was added to wells for the last 12 hours. These data are expressed as the mean ± SD triplicates from a 96-well plate (S 2) Same as (Fig. 2 B) except 3H-thymidine was added to wells for the last 12 hours. These data are expressed as the mean ± SD triplicates from a 96-well plate (S 3) same as (Fig. 3 F) CSN were tested for the presence of IL-12p75 with specific ELISA (R&D System). 3H-thymidine incorporation was measured during the last 12 hours. These data are expressed as the mean ± SD composite of four independent experiments. (S 4) ELISPOT splenic CD11c+ cells were stimulated with various bacterial products at 10μg/ml or cocultured with 1×105 cultured 5C.C7 +0.1μM MCC; IL-12p75 was captured by mAb recognizing only the IL-12p75 heterodimer (clone 9A5); resulting spots were counted (ZellNet Consulting). These data are expressed as the mean ± SD of triplicates wells.
(S 5) Cytokine Array (similar to Fig. 3 F) BM-DCs from B10.A-Rag2KO mice were preactivated with 200ng/ml LPS for 24hours then washed and cocultured at 2×104 cells/well with 1×105 WT, IFN-γKO and CD40L 5C.C7 T [Tg] cells±0.1μM MCC peptide in a 96 well U-bottom plate for 48 hours. CSN were tested for the presence of various cytokines using SearchLight cytokine array (Aushon Biosystems).
Acknowledgement
We would like to thank Dr. Ronald Schwartz for critical review of the manuscript. We would also like to thank Dr. Polly Matzinger for discussion and also her support, which made this work possible. This work was entirely supported by the intramural program of the NIAID, NIH.
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 2.Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–50. [PubMed] [Google Scholar]
- 3.Liu J, Cao S, Herman LM, Ma X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J Exp Med. 2003 Oct 20;198:1265–76. doi: 10.1084/jem.20030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snijders A, Hilkens CM, van der Pouw Kraan TC, Engel M, Aarden LA, Kapsenberg ML. Regulation of bioactive IL-12 production in lipopolysaccharide- stimulated human monocytes is determined by the expression of the p35 subunit. J Immunol. 1996;156:1207–12. [PubMed] [Google Scholar]
- 5.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007 Mar;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 6.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–8. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 7.Edwards AD, Manickasingham SP, Sporri R, et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J Immunol. 2002 Oct 1;169:3652–60. doi: 10.4049/jimmunol.169.7.3652. [DOI] [PubMed] [Google Scholar]
- 8.Schulz O, Edwards AD, Schito M, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000 Oct;13:453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003 Feb;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 10.Abdi K, Singh N, Matzinger P. T-cell control of IL-12p75 production. Scand J Immunol. 2006 Aug;64:83–92. doi: 10.1111/j.1365-3083.2006.01767.x. [DOI] [PubMed] [Google Scholar]
- 11.Abdi K. IL-12: the role of p40 versus p75. Scand J Immunol. 2002 Jul;56:1–11. doi: 10.1046/j.1365-3083.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- 12.Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007 Jan;28:33–8. doi: 10.1016/j.it.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997 Sep 1;90:1920–6. [PubMed] [Google Scholar]
- 14.Miro F, Nobile C, Blanchard N, et al. T cell-dependent activation of dendritic cells requires IL-12 and IFN-gamma signaling in T cells. J Immunol. 2006 Sep 15;177:3625–34. doi: 10.4049/jimmunol.177.6.3625. [DOI] [PubMed] [Google Scholar]
- 15.Wong KL, Lew FC, MacAry PA, Kemeny DM. CD40L-expressing CD8 T cells prime CD8alpha(+) DC for IL-12p70 production. Eur J Immunol. 2008 Aug;38:2251–62. doi: 10.1002/eji.200838199. [DOI] [PubMed] [Google Scholar]
- 16.Hochrein H, O'Keeffe M, Luft T, et al. Interleukin (IL)-4 is a major regulatory cytokine governing bioactive IL-12 production by mouse and human dendritic cells. J Exp Med. 2000;192:823–33. doi: 10.1084/jem.192.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin-Fontecha A, Baumjohann D, Guarda G, et al. CD40L+ CD4+ memory T cells migrate in a CD62P-dependent fashion into reactive lymph nodes and license dendritic cells for T cell priming. J Exp Med. 2008 Oct 27;205:2561–74. doi: 10.1084/jem.20081212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takenaka H, Maruo S, Yamamoto N, et al. Regulation of T cell-dependent and -independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J Leukoc Biol. 1997 Jan;61:80–7. doi: 10.1002/jlb.61.1.80. [DOI] [PubMed] [Google Scholar]
- 19.Ebner S, Ratzinger G, Krosbacher B, et al. Production of IL-12 by human monocyte-derived dendritic cells is optimal when the stimulus is given at the onset of maturation, and is further enhanced by IL-4. J Immunol. 2001 Jan 1;166:633–41. doi: 10.4049/jimmunol.166.1.633. [DOI] [PubMed] [Google Scholar]
- 20.Yamane H, Kato T, Nariuchi H. Effective stimulation for IL-12 p35 mRNA accumulation and bioactive IL- 12 production of antigen-presenting cells interacted with Th cells. J Immunol. 1999;162:6433–41. [PubMed] [Google Scholar]
- 21.Murphy EE, Terres G, Macatonia SE, et al. B7 and interleukin 12 cooperate for proliferation and interferon gamma production by mouse T helper clones that are unresponsive to B7 costimulation. J Exp Med. 1994 Jul 1;180:223–31. doi: 10.1084/jem.180.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005 Aug;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gautier G, Humbert M, Deauvieau F, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005 May 2;201:1435–46. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corthay A. A three-cell model for activation of naive T helper cells. Scand J Immunol. 2006 Aug;64:93–6. doi: 10.1111/j.1365-3083.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 25.Flesch IE, Hess JH, Huang S, et al. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. J Exp Med. 1995;181:1615–21. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayes MP, Murphy FJ, Burd PR. Interferon-gamma-dependent inducible expression of the human interleukin-12 p35 gene in monocytes initiates from a TATA-containing promoter distinct from the CpG-rich promoter active in Epstein-Barr virus-transformed lymphoblastoid cells. Blood. 1998 Jun 15;91:4645–51. [PubMed] [Google Scholar]
- 27.Bost KL, Clements JD. Intracellular Salmonella dublin induces substantial secretion of the 40- kilodalton subunit of interleukin-12 (IL-12) but minimal secretion of IL-12 as a 70-kilodalton protein in murine macrophages. Infect Immun. 1997;65:3186–92. doi: 10.1128/iai.65.8.3186-3192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park AY, Hondowicz BD, Scott P. IL-12 is required to maintain a Th1 response during Leishmania major infection. J Immunol. 2000 Jul 15;165:896–902. doi: 10.4049/jimmunol.165.2.896. [DOI] [PubMed] [Google Scholar]
- 29.Feng CG, Jankovic D, Kullberg M, et al. Maintenance of pulmonary Th1 effector function in chronic tuberculosis requires persistent IL-12 production. J Immunol. 2005 Apr 1;174:4185–92. doi: 10.4049/jimmunol.174.7.4185. [DOI] [PubMed] [Google Scholar]
- 30.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–52. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosca PJ, Hobeika AC, Clay TM, et al. A subset of human monocyte-derived dendritic cells expresses high levels of interleukin-12 in response to combined CD40 ligand and interferon-gamma treatment. Blood. 2000 Nov 15;96:3499–504. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(S 1) same as (Fig. 2A) except 3H-thymidine was added to wells for the last 12 hours. These data are expressed as the mean ± SD triplicates from a 96-well plate (S 2) Same as (Fig. 2 B) except 3H-thymidine was added to wells for the last 12 hours. These data are expressed as the mean ± SD triplicates from a 96-well plate (S 3) same as (Fig. 3 F) CSN were tested for the presence of IL-12p75 with specific ELISA (R&D System). 3H-thymidine incorporation was measured during the last 12 hours. These data are expressed as the mean ± SD composite of four independent experiments. (S 4) ELISPOT splenic CD11c+ cells were stimulated with various bacterial products at 10μg/ml or cocultured with 1×105 cultured 5C.C7 +0.1μM MCC; IL-12p75 was captured by mAb recognizing only the IL-12p75 heterodimer (clone 9A5); resulting spots were counted (ZellNet Consulting). These data are expressed as the mean ± SD of triplicates wells.
(S 5) Cytokine Array (similar to Fig. 3 F) BM-DCs from B10.A-Rag2KO mice were preactivated with 200ng/ml LPS for 24hours then washed and cocultured at 2×104 cells/well with 1×105 WT, IFN-γKO and CD40L 5C.C7 T [Tg] cells±0.1μM MCC peptide in a 96 well U-bottom plate for 48 hours. CSN were tested for the presence of various cytokines using SearchLight cytokine array (Aushon Biosystems).